Abstract
Objective. To evaluate clustering patterns of prevalent infection with multiple human papillomavirus (HPV) types in 3677 men from the HPV in Men (HIM) study.
Methods. HPV testing was performed in samples combined from the glans penis/coronal sulcus, the shaft, and the scrotum by Linear Array methodology. Linear Array uses a mixed probe to assess HPV52 positivity, which limits the assay’s ability to determine HPV52 status in the presence of HPV33, 35, or 58. Logistic regression was used to model type-specific HPV positivity, adjusted for age, study area, lifetime number of sexual partners, and specific HPV type prevalence. Participant-level random effects were added to represent unobservable risk factors common to all HPV types.
Results. The observed-to-expected ratio for infections with ≥ 3 types was 1.09 (95% credible interval, 1.04–1.14). For the majority of 2-type combinations, no evidence was found of a significant departure of the observed from the expected number. An apparent clustering of HPV52 with HPV35 or 58 was observed, because of limitation in the ability of Linear Array to define HPV52 positivity.
Conclusions. Our study showed that, despite obvious anatomical differences, HPV coinfections do seem to occur at random in the male external genitalia as in the female cervix.
Human papillomavirus (HPV) is a necessary cause for cervical cancer [1] and is the most common sexually transmitted infection [2, 3]. In men, HPV infection contributes to the burden of such diseases as genital warts and several cancers, including cancer of the anus and of the penis [2]. Although multiple HPV types are often found in the external genitalia of men [4–6], to our knowledge, no information is available on whether certain combinations of HPV types might be found together in a coinfection more or less often than what would be expected by sexual transmission and common risk factors. This issue is particularly relevant to inform the monitoring of benefits of vaccination of women or men on the incidence of HPV infection in men. The removal of certain HPV types through vaccination could, in theory, indirectly increase or decrease the prevalence of other untargeted types.
Previous studies have attempted to assess clustering patterns of multiple HPV types in women [7–15]. Clustering of HPV types has been assessed using statistical methods based on mixed models or similar approaches [7, 14–18]. These methods allow the investigator to control for the apparent positive association between all HPV types induced by common acquisition modalities. Overall, studies in women suggested that HPV coinfections in the cervix occur at random. In the present analysis, we investigated the patterns of HPV coinfections among men from the HPV in Men (HIM) study, a population-based cohort study of the natural history of anogenital HPV infection.
MATERIALS AND METHODS
Study Population and Specimen Collection
Men (mean age, 32.3 years [range, 18–70 years]) were recruited in Brazil, Mexico, and the United States during the period 2005 through 2009, as described elsewhere [19–21]. Different prewetted Dacron applicators were used to sample 3 sites of the external genitalia, the glans penis/coronal sulcus, the shaft, and the scrotum [19]. Among uncircumcised men, the foreskin was sampled at the time of collection of the glans penis/coronal sulcus sample. Prior to DNA extraction, the 3 genital samples were combined to produce 1 DNA extract per participant at each clinic visit.
HPV DNA Detection Techniques
HPV testing of the combined DNA extract was conducted using an L1 gene consensus polymerase chain reaction (PCR) that amplifies a broad spectrum of HPV genotypes [22]. DNA extraction was performed using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. HPV genotyping was conducted by using the Linear Array methodology of Roche to detect the following 38 HPV types: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39, and CP6108 [23].
The Roche Linear Array test can only indirectly measure HPV52 by using a mixed probe [24]. HPV33, 35, and 58 are the types that, together with HPV52, are capable of cross-reacting with the mixed probe. Positivity for HPV52 was defined as positivity of the mixed probe when HPV33, 35, or 58 were not detected by their specific probes. If the mixed probe had positive results in a sample that was also positive for 33, 35, or 58, positivity for HPV52 could not be determined. To overcome this problem and to consider the widest possible range of prospects for HPV52 positivity, we envisaged 2 scenarios. In scenario A, all samples positive for the mixed probe and for either HPV33, 35, or 58 were considered HPV52 positive; in scenario B, all samples positive for the mixed probe and for either HPV33, 35, or 58 were considered HPV52 negative.
Statistical Analyses
The present analysis was restricted to HPV infections that were positive by means of genotyping by Linear Array only and with a prevalence in the whole population of at least 1%. Samples that were HPV positive by means of consensus PCR but negative by means of Linear Array genotyping (n = 569) were considered HPV negative. Similarly, participants infected only with HPV types with a prevalence <1.0% (ie, HPV26, 33, 44, 64, 67, 69, 82, or IS39) were also considered HPV negative (n = 104). Overall, the analysis of clustering HPV infections was restricted to the following 30 HPV types: 6, 11, 16, 18, 31, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 68, 70, 71, 72, 73, 81, 83, 84, and CP6108.
A logistic regression model was fitted with type-specific HPV positivity as an outcome, controlling for age, study area, lifetime number of sexual partners, and specific HPV type prevalence, as previously described [7]. Participant-level random effects were included, which represent the effect of unobserved risk factors (sexual habits of the men and of their partners, the ability to clear HPV infection, and so forth). A Bayesian approach with Markov Chain Monte Carlo (MCMC) simulation was used. Estimates are reported as posterior means and 95% credible intervals (CIs), based on 10 000 MCMC iterations following a burn-in period of 1000 iterations. Discrepancies between the data and the model were assessed by means of posterior predictive 2-sided P values and measured by observed-to-expected (O/E) ratios for the different combinations of HPV types [25]. To minimize errors due to multiple comparisons and for consistency with previous analyses on women [7], the threshold for significance of P values to test type-type associations was set to .01.
RESULTS
Only results from the baseline visit were considered in the present report. Of 4075 men recruited into the HIM study, 3899 had β-globin–positive genital specimens to include in this analysis. Overall positivity for any of the 30 HPV types considered was 51.3%, and HPV prevalence was 43.5% in the United States, 49.5% in Mexico, and 59.7% in Brazil.
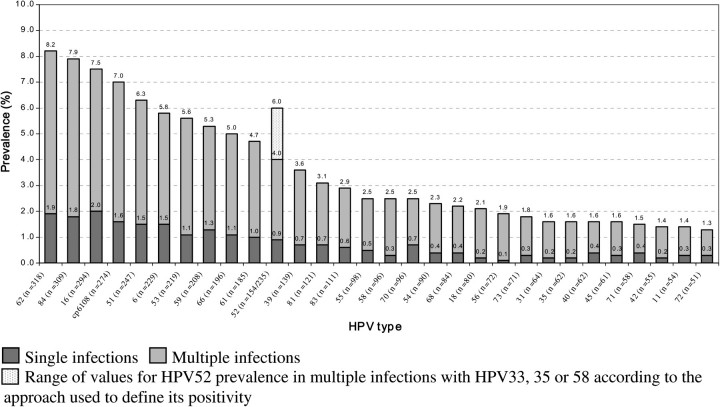
Figure 1 shows type-specific HPV prevalence. The most prevalent HPV types were HPV62 (8.2%, n = 318), HPV84 (7.9%, n = 309), HPV16 (7.5%, n = 294), CP6108 (7.0%, n = 274), and HPV51 (6.3%, n = 247). HPV52 prevalence ranged from 4.0% (n = 154) in scenario B to 6.0% (n = 235) in scenario A. Type-specific prevalences were comparable to those from a previous report in the same study populations [19]. HPV type distribution was similar in men from Brazil, Mexico, and the United States, with the possible exception of a lower prevalence of HPV16 in Mexico (5.3%) than elsewhere (9.2% in Brazil and 8.0% in the United States).
Figure 1.
Type-specific human papillomavirus (HPV) prevalence in 3899 men—the HPV in Men (HIM) study.
Table 1 displays the observed and expected numbers of men with single and multiple HPV infections (1 type, 2 types, and ≥ 3 types), when HPV52 was considered positive every time the mixed probe had a positive result (scenario A). The analysis on clustering patterns of HPV infections was restricted to 3677 men for whom the information on lifetime number of sexual partners was available. The O/E ratio for infections with ≥ 3 types was 1.54 (95% CI, 1.44–1.65) when the model was adjusted only for age and study area, 1.39 (95% CI, 1.30–1.48) when the model was further adjusted for lifetime number of sexual partners, and 1.09 (95% CI, 1.04–1.14) after the inclusion of participant-random effects. Although the discrepancy between observed and expected counts was substantially reduced by the introduction of participant-random effects, there was still a small excess of infections with ≥ 3 types. The results of Table 1 are substantially similar when HPV52 was considered positive only if the mixed probe was positive and none of HPV33, 35, or 58 was detected by their specific probe (scenario B, data not shown).
Table 1.
Observed-to-Expected Ratio (O/E) of Multiple Infections With the 30 Most Common Human Papillomavirus (HPV) Types, According to Various Models—the HPV in Men (HIM) study
| Basic modelb |
Adjusted modelc |
Full modeld |
|||||
| No. of HPV typesa | Observed, no. (%) | Expected | O/E (95% CI) | Expected | O/E (95%CI) | Expected | O/E (95% CI) |
| 0 | 1804 (49.1) | 1269.9 | 1.42 (1.37–1.47) | 1347.0 | 1.34 (1.30–1.38) | 1750.5 | 1.03 (1.01–1.06) |
| 1 | 866 (23.6) | 1334.1 | 0.65 (.64–.65) | 1262.9 | 0.69 (.68–.69) | 961.1 | 0.90 (.88–.92) |
| 2 | 467 (12.7) | 722.2 | 0.65 (.63–.67) | 678.4 | 0.69 (.67–.71) | 469.4 | 1.00 (.96–1.03) |
| 3+ | 540 (14.7) | 350.8 | 1.54 (1.44–1.65) | 388.7 | 1.39 (1.30–1.48) | 496.0 | 1.09 (1.04–1.14) |
Abbreviation: CI, credible interval.
Samples positive for the mixed probe and for either HPV33, 35, or 58 are considered HPV52 positive.
Controlling for age and study area.
As b plus lifetime number of female sexual partners.
As c plus individual random effects.
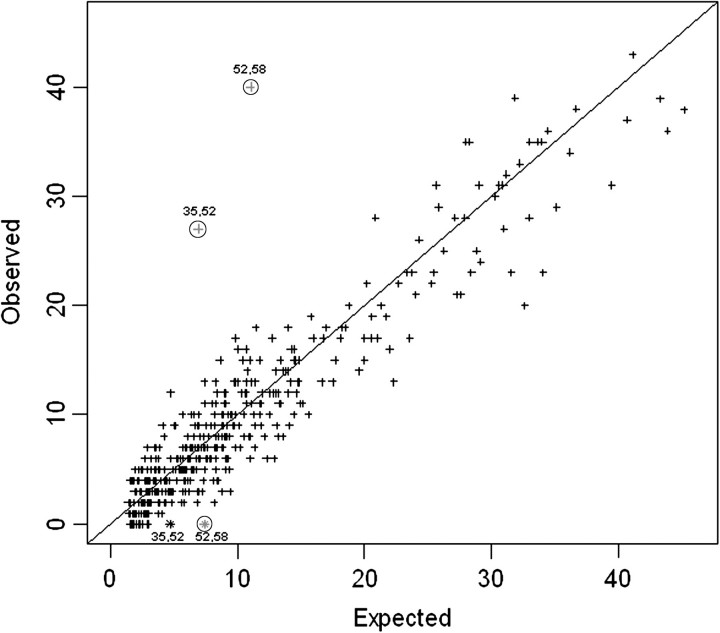
Figure 2 displays the observed versus expected occurrence for each 2-type combination of HPV types, under the full model and according to scenario A. For the majority of 2-type combinations, no evidence was found of a significant departure of the observed from the expected number. However, HPV pairs 35/52 and 52/58 were found in combination more often than expected. These associations showed a high level of statistical significance (HPV pair 35/52: P = 2.6 × 10−7; O/E ratio, 3.97 [99% CI, 2.13–7.25]; HPV pair 52/58: P = 2.3 × 10−9; O/E ratio, 3.68 [99% CI, 2.21–6.03]). Figure 2 also shows observed versus expected occurrence for HPV pairs 35/52 and 52/58 when scenario B was assumed. In this latter case, the number of joint infections with HPV pairs 35/52 and 52/58 was 0, and 2 HPV pairs showed an inverse association that was significant at the 0.01 level for HPV pair 52/58 (P = .002; O/E ratio, 0.00 [99% CI, .00–.75]) but not for 35/52 (P = .023; O/E ratio, 0.00 [99% CI, .00–1.19]). The results of the analyses were substantially similar when stratified according to circumcision status (data not shown).
Figure 2.
Observed versus expected occurrence for 2-way combinations of 30 human papillomavirus (HPV) types—the HPV in Men (HIM) study. Plus signs represent occurrences of HPV pairs under scenario A (all samples positive for the mixed probe and for either HPV33, 35, or 58 are considered HPV52 positive). HPV pairs located in the upper triangle indicate positive clustering, whereas those in the lower triangle represent negative clustering between the HPV types involved. Two asterisks represent occurrences of joint infections with HPV types 35/52 and 52/58 under scenario B (samples positive for the mixed probe and for either HPV33, 35, or 58 are considered HPV52 negative). Significance for independence of joint infections at 0.01 level is indicated by encircled symbols.
DISCUSSION
This analysis of HPV coinfections in men extends previous observations in women and provides a broader picture on the clustering patterns of HPV infections. Similarly to the findings in women, our results suggest that, despite a general tendency of all HPV types to cluster together because of the shared routes of transmission and common risk factors, HPV coinfections occur at random.
It has been previously shown, however, that the presence of certain HPV types in multiple infections could be considerably overestimated as a result of an artifact of the HPV genotyping method [7]. In this study, the apparent clustering of HPV52 with HPV35 or 58 is a direct consequence of the extreme assumptions used to define HPV52 positivity in multiple infections with the other types included in the mixed probe. The opposite direction of the associations in the 2 scenarios reflects the fact that, necessarily, scenario A leads to an overestimate and scenario B to an underestimate of HPV52 positivity in multiple infections with HPV35 or 58.
The PCR assay included in our study cannot provide a precise estimate of HPV52 prevalence but only a range of possible estimates. This range was not negligible, varying from 4.0% to 6.0% on the overall study population. Some authors have attempted to overcome the impossibility of directly measuring HPV52 when using Linear Array by using a separate PCR primer specific for this type [24]. It is, however, not clear how other studies on men or women that used Linear Array have dealt with this issue.
In the present study, participant-level random effects were used to account for sources of correlation between all HPV types due to unobservable risk factors shared by all HPV infections. Participant-level random effects do not distinguish between unobserved risk factors due to sexual behavior or partner’s behavior and other individual risk factors, such as immunological susceptibility. This statistical approach has been previously used in studies of women to diminish the spurious excess of multiple types that derives from the common modes of infection acquisition (Table 1). Although a small excess of HPV infections with 3 or more types was observed even after adjustment for age, study area, lifetime number of sexual partners, and inclusion of participant-random effects, no evidence of a significant departure of the observed from the expected counts was found for the majority of 2-type combinations. The significant positive and negative clusters involving HPV52 were due to the different ways of defining HPV52 positivity in this study, envisaging the most extreme possible values for HPV52 positivity in multiple infections. The contrast between these associations and the weak or null associations for all other HPV pairs provides reassurance on the appropriateness of the statistical approach used. Overall, these results provide additional evidence for the lack of biological interaction between type-type combinations of HPV types. A limitation of this study is that the findings were based on combined samples from different penile sites and that, therefore, the behavior of HPV types in each of those anatomical sites could not be evaluated.
In summary, the elucidation of whether HPV positivity for certain HPV types is not measured precisely and whether preferential combinations of HPV types exist has relevant implications for evaluations of HPV vaccines and for studies that require a precise measurement of HPV, including clinical studies of HPV persistence. In conclusion, our findings showed that, despite obvious anatomical differences, HPV coinfections do seem to occur at random in the male external genitalia as in the female cervix [7, 15–17].
Notes
Acknowledgments.
We thank Qiagen for kindly providing STM for the collection and storage of samples at no charge to the study.
Financial support.
The HPV in men (HIM) study is funded by the National Institutes of Health (R01 CA 098803).
Potential conflicts of interest.
L. V. is a consultant of MSD for the quadrivalent vaccine and has participated in expert meetings organized by Roche. A. R. G. is on the speakers Bureau of Merck and has received consulting fees and a medical grant from Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.IARC. Human Papillomaviruses. Lyon, France: International Agency for Research on Cancer; 2007. Monographs on the evaluation of carcinogenic risks to humans. Vol 90. [Google Scholar]
- 3.Vaccarella S, Franceschi S, Herrero R, et al. Sexual behavior, condom use and HPV: pooled analysis of the International Agency for Research on Cancer HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15:326–33. doi: 10.1158/1055-9965.EPI-05-0577. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–9. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- 5.Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–6. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 6.Nielson CM, Harris RB, Flores R, et al. Multiple-type human papillomavirus infection in male anogenital sites: prevalence and associated factors. Cancer Epidemiol Biomarkers Prev. 2009;18:1077–83. doi: 10.1158/1055-9965.EPI-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccarella S, Franceschi S, Snijders PJ, Herrero R, Meijer CJ, Plummer M. Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2010;19:503–10. doi: 10.1158/1055-9965.EPI-09-0983. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Myers L, Hammons AF, et al. Prevalence and clustering patterns of human papillomavirus genotypes in multiple infections. Cancer Epidemiol Biomarkers Prev. 2005;14:2439–45. doi: 10.1158/1055-9965.EPI-05-0465. [DOI] [PubMed] [Google Scholar]
- 9.Méndez F, Muñoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158–65. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 10.Thomas KK, Hughes JP, Kuypers JM, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182:1097–102. doi: 10.1086/315805. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 12.Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–23. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 13.Molano M, van den Brule AJ, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi A, Katki H, Hildesheim A, et al. Human papillomavirus infection with multiple types: pattern of co-infection and risk of cervical disease. J Infect Dis. 2011;203:910–20. doi: 10.1093/infdis/jiq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccarella S, Franceschi S, Herrero R, et al. Clustering of multiple human papillomavirus infections in women from a population-based study in Guanacaste, Costa Rica. J Infect Dis. 2011;204:385–90. doi: 10.1093/infdis/jir286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 17.Plummer M, Vaccarella S, Franceschi S. Multiple human papillomavirus infections: the exception or the rule? J Infect Dis. 2011;203:891–3. doi: 10.1093/infdis/jiq146. [DOI] [PubMed] [Google Scholar]
- 18.Xue X, Gange SJ, Zhong Y, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev. 2010;19:159–69. doi: 10.1158/1055-9965.EPI-09-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyitray AG, Smith D, Villa L, et al. Prevalence of and risk factors for anal human papillomavirus infection in men who have sex with women: a cross-national study. J Infect Dis. 2010;201:1498–508. doi: 10.1086/652187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HPV in Men Study Group. Human papillomavirus infection in men residing in Brazil, Mexico, and the USA. Salud Publica Mex. 2008;50:408–18. doi: 10.1590/s0036-36342008000500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks M, Gupta SB, Liaw KL, et al. Confirmation and quantitation of human papillomavirus type 52 by Roche Linear Array using HPV52-specific TaqMan E6/E7 quantitative real-time PCR. J Virol Methods. 2009;156:152–6. doi: 10.1016/j.jviromet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analyses. 2nd ed. New York: Chapman and Hall/CRC; 2004. [Google Scholar]