Abstract
Low-avidity serotype–cross-reactive antibodies are hypothesized to play a key role in triggering severe disease in patients with secondary dengue virus (DENV) infection. However, there is little systematic information about the frequency, avidity, and cross-reactivity of DENV-specific B cells in individuals experiencing primary instead of secondary infection. We compared DENV-specific B-cell responses in a cohort of Thai children with primary or secondary DENV infection. B cells specific for DENV precursor membrane protein, envelope (E) protein, and nonstructural protein 1 were detectable in immune peripheral blood mononuclear cells with the highest frequencies of DENV E-specific B cells detected in patients experiencing primary DENV-1 infections. DENV E-specific B cells were highly serotype-specific after primary DENV infections, whereas most E-specific B cells in patients with secondary infection were serotype–cross-reactive and secreted antibodies with higher avidity to heterologous DENV serotypes. Our data suggest that the minor populations of serotype–cross-reactive B cells generated by primary DENV infection are preferentially expanded during secondary DENV infection.
Dengue viruses (DENV) are responsible for millions of cases of infection each year, with 500000 hospitalizations mainly in the tropical and subtropical areas of the world [1]. Epidemiologic studies indicate that serious manifestations of dengue disease (dengue hemorrhagic fever [DHF]) are more likely to occur in children and adults experiencing secondary infections than in those with primary infections [2] and in cases of primary infection in infants born to immune mothers. Plasma leakage, the hallmark of DHF, occurs late during the acute infection at or near defervesence and is coincident with the clearance of virus [3, 4]. These findings suggest that plasma leakage is mediated by host responses rather than by direct virally mediated tissue damage. Antibody-dependent enhancement (ADE) of infection—whereby anti-DENV immunoglobulin G (IgG) acquired from a previous heterologous infection or passively acquired by an infant from the mother can enhance viral uptake into Fcγ receptor-positive cells—is thought to trigger the immunological cascade responsible for DHF [5, 6].
Dengue viruses belong to the family Flaviviridae, and the genome encodes 3 structural proteins (the capsid [C], precursor membrane [prM], and envelope [E]) and 7 nonstructural proteins (NS1–NS5). Robust antibody responses are generated to 3 proteins: E, which contains 3 distinct domains, I, II, and III (potent neutralizing activity); prM, which augments infectivity of poorly infectious immature virions; and NS1, which directs complement-mediated lysis of infected cells [7–11]. As determined by experiments using mouse monoclonal antibodies, antibodies to E domain III, which binds to the cellular receptor, have the most potent neutralizing activity. [12–16]. Serological studies in humans have suggested, however, that antibodies to domain III are a small portion of the overall antibody response to the E protein [17, 18].
Recently, several groups have generated monoclonal antibodies from human peripheral blood mononuclear cells (PBMCs) obtained from DENV-immune donors. Antibodies against the envelope protein with poor, moderate, and potent neutralizing activity and antibodies against prM that were poorly neutralizing but highly cross-reactive were isolated and characterized [19–21]. These studies did not compare antibody profiles in children with primary versus secondary infections or mild as opposed to severe disease. Furthermore, the studies included patients infected with only 1 or 2 of the DENV serotypes and used PBMCs collected at a single time point, usually several years after infection.
We sought to address these gaps by assessing B-cell responses in PBMCs obtained early and late during convalescence in 28 donors from a cohort of Thai children undergoing primary or secondary DENV infection, using enzyme-linked immunospot (ELISpot) and enzyme-linked immunosorbent assays (ELISAs) on culture supernatants. We found higher frequencies of DENV-specific B cells in early convalescent samples; responses in cases of primary infection were serotype-specific, whereas responses in cases of secondary infection were serotype–cross-reactive.
MATERIALS AND METHODS
Study Subjects and Blood Samples
The study design for patient recruitment and collection of blood samples has been reported in detail elsewhere [22]. All research involving human participants was approved by the institutional review boards of the Thai Ministry of Public Health, the Office of the US Army Surgeon General, and the University of Massachusetts Medical School (UMMS). Written informed consent was obtained from each subject and/or his or her parent or guardian. Briefly, Thai children 6 months to 14 years of age with acute febrile illnesses were enrolled. Serology and virus isolation were used to confirm acute DENV infections, and primary and secondary infections were distinguished on the basis of serologic responses (Table 1). Blood samples were obtained during acute illness, in early convalescence, and at intervals during late convalescence (6 months after study entry). Frozen PBMCs were shipped on dry ice to UMMS for analysis.
Table 1.
Summary of Donor Information and Clinical Diagnosis
| Subject | Serology | Serotype | Diagnosis | Days tested |
| CHD96068 | Primary | DENV-1 | DF | 9;189 |
| CHD95058 | Primary | DENV-1 | DF | 10;182 |
| CHD96057 | Primary | DENV-1 | DF | 9;184 |
| CHD95039 | Primary | DENV-1 | DF | 11;184 |
| CHD96041 | Primary | DENV-1 | DHF II | 9;190 |
| CHD96050 | Primary | DENV-3 | DF | 9;183 |
| CHD96092 | Primary | DENV-3 | DF | 9;184 |
| CHD96097 | Primary | DENV-3 | DF | 9;178 |
| CHD96114 | Primary | DENV-3 | DHF II | 10;210 |
| CHD95026 | Primary | DENV-3 | DF | 11;182 |
| CHD96120 | Primary | DENV-3 | DHF II | 10;182 |
| CHD95062 | Secondary | DENV-1 | DF | 9;183 |
| KPP95022 | Secondary | DENV-1 | DHF III | 9;183 |
| CHD94045 | Secondary | DENV-1 | DHF II | 9 |
| KPP96019 | Secondary | DENV-1 | DHF II | 9;176 |
| CHD96015 | Secondary | DENV-1 | DHF I | 9;329 |
| KPP95023 | Secondary | DENV-1 | DHF I | 9 |
| CHD96094 | Secondary | DENV-1 | DF | 9;178 |
| CHD96067 | Secondary | DENV-2 | DF | 9;180 |
| CHD96032 | Secondary | DENV-2 | DF | 11;193 |
| KPP96008 | Secondary | DENV-2 | DHF II | 9;199 |
| CHD96085 | Secondary | DENV-3 | DF | 9;210 |
| CHD96107 | Secondary | DENV-3 | DHF II | 9;205 |
| KPP96021 | Secondary | DENV-3 | DHFII | 9;206 |
| KPP95007 | Secondary | DENV-3 | DHF I | 9;206 |
| CHD96100 | Secondary | DENV-4 | DHF II | 9;185 |
| CHD96074 | Secondary | DENV-4 | DF | 9;181 |
| CHD94043 | Secondary | DENV-4 | DF | 9 |
Abbreviations: DENV-1, dengue virus type 1; DENV-2, dengue virus type 2; DENV-3, dengue virus type 3; DENV-4, dengue virus type 4; DF, dengue fever; DHF I, dengue hemorrhagic fever grade I; DHF II, dengue hemorrhagic fever grade II; DHF III, dengue hemorrhagic fever grade III.
B-Cell Bulk Culture
Frozen PBMCs were thawed and washed twice. Cells were counted and diluted to 2 × 106 cells/mL in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), Pen/Strep (100 U/mL; Gibco), and l-glutamine (200 mmol/L; Gibco). PBMCs were stimulated with 1000 U/mL rHuIL2 (Peprotech) and 2.5 μg/mL R848 (Invivogen). Cells were added to a 24-well plate and incubated for 7 days at 37°C in 5% CO2. Supernatants from stimulated PBMCs were used for avidity assays and ELISA assays with prM peptides.
ELISpot Assay
Millipore ELISpot plates (catalog no. MAIPSWU10) were prewetted with 70% ethanol and washed 5 times with distilled water. Wells were coated with 100 μL of DENV E or NS1 proteins (15 μg/mL; Hawaii Biotech). To detect total IgG, wells were coated with 100 μL of anti-human IgG (15 μg/mL; MT91/145; Mabtech). Plates were stored at 4°C overnight, washed with phosphate-buffered saline (PBS; Gibco), and blocked with 100 μL RPMI 1640 with 10% FBS for 30 minutes at room temperature. The cells were counted and 100 μL of cells were added to each well, in duplicate. Cell concentrations varied depending on the cell recovery. The plate was incubated at 37°C overnight. After washing, 100 μL of biotinylated monoclonal antibody directed against human IgG (1 μg/mL in PBS with 0.5 % FCS; MT78/145; Mabtech) was added. Plates were incubated for 2 hours at room temperature and washed, and streptavidin horseradish peroxidase (HRP; 100 μL of 1:1000 dilution in PBS with 0.5% FBS; BD Pharmingen) was added. Plates were incubated at room temperature for 1 hour, developed with 3,3',5,5'-tetramethylbenzidine (TMB) substrate (100 μL/well; Calbiochem), and analyzed using an ELISpot plate reader (CTL Immunospot). ELISpot responses were considered to be positive if the number of spots was >1000 spots per 1 × 106 cells for total IgG spots and >50 spots per 1 × 106 cells for DENV-specific responses.
Hemagglutination Inhibition and 50% Plaque Reduction Neutralization Titers
Plaque reduction neutralizing antibody titers against reference strains of DENV types 1–4 were performed on the plasma samples obtained, by use of standard methods. The 50% plaque reduction neutralization titer (PRNT50) was calculated by use of a log probit regression method (SPSS software; version 10.0; SPSS) and was reported as a reciprocal titer [23]. Hemagglutination inhibition antibody against DENV types 1–4 were measured in all specimens up to a reciprocal titer of 10240 [3].
Avidity Assays
Two sets of 96-well microplates were coated overnight with 20 ng/well of DENV E proteins. Plates were blocked with 1% bovine serum albumin for 90 minutes and incubated with 100 μL of serially diluted supernatants from stimulated PBMCs in duplicate for 1 hour at 37°C. Plates were washed with PBS with 0.1% Tween-20. One set of plates was incubated with 8 mol/L urea for 10 minutes at 37°C. Both sets of plates were washed and incubated with HRP-labeled goat anti-human IgG (catalog no. A80-100P; Bethyl Laboratories, Montgomery, TX) for 1 hour at 37°C. TMB solution was added and the enzyme reaction was stopped by addition of 1 mol/L HCl. Absorbance values >2-fold above the background level were considered to be positive. The avidity index was calculated as the ratio of the optical density with urea to the optical density without urea.
Statistical Analysis
Differences in frequencies of B cells were assessed by viral serotype and serologic response. Statistical differences were analyzed using linear regression models. We used the Pearsons correlation coefficient test to examine associations between hemagglutination inhibition (HAI) titers, PRNT50 titers, and antibody-secreting cells (ASCs) by ELIspot assay. The statistical significance level was at P < .05. Analysis was performed using Stata MP (version 11.0; Stata, College Station, TX) and Prism (GraphPad, La Jolla, CA).
RESULTS
Higher Frequencies of DENV-1 E-Specific B Cells in PBMCs From Donors With Primary DENV-1 Infection
Our study cohort included 28 children, 11 with primary infection (5 with DENV-1 and 6 with DENV-3) and 17 with secondary infections; there were no subjects with primary DENV-2 or DENV-4 infections (Table 1). The 17 children with secondary DENV infections included infections with all 4 DENV serotypes (7 with DENV-1, 3 with DENV-2, 4 with DENV-3, and 3 with DENV-4). We used a B-cell ELIspot assay to enumerate ASCs in PBMCs stimulated in vitro that were obtained approximately 9 days and 6 months after children were enrolled.
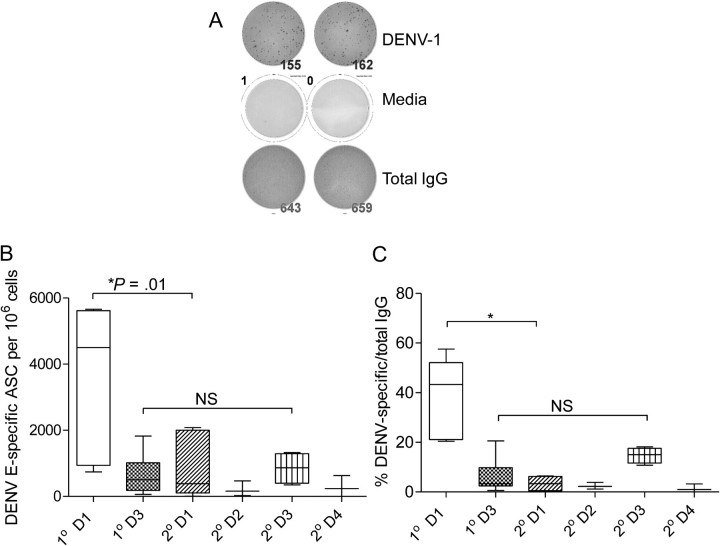
We detected DENV E-specific ASCs in 26 of 28 PBMC samples obtained 9–11 days after study entry (Figure 1). The 2 samples that did not have detectable DENV-specific ASCs also had low total IgG responses, suggesting less efficient activation of memory B cells (data not shown). Figure 1 shows a representative ELIspot response in PBMCs from a patient with DENV-1 infection. We first analyzed the frequency of B cells that secreted antibodies corresponding to the currently infecting DENV serotype. We compared the frequency of ASCs in primary DENV infections with that in secondary DENV infections in donors infected with the same serotype of DENV. We found significantly higher frequencies of DENV-specific ASCs in children with primary DENV-1 infection compared with children with secondary DENV-1 infection (mean, 3523 vs 790 ASCs per 106 cells, respectively; P = .019), whereas no differences were detected between children with primary and secondary DENV-3 infection (mean, 645 vs 852 ASCs per 106 cells, respectively; P = .592) (Figure 1). When comparisons were made regardless of serotype, frequencies of ASCs in primary infections were significantly higher than frequencies of ASCs in secondary infections (P = .02). Among children with secondary infections, no significant differences in B-cell frequencies were observed according to serotype of infection.
Figure 1.
High frequencies of E-specific B cells in patients with primary dengue virus type 1 (DENV-1) infection. Frequencies of antigen-specific B cells were measured by enzyme-linked immunospot (ELIspot) assay in the peripheral blood mononuclear cells (PBMCs) of patients with primary (1° D1, 1° D3) and secondary (2° D1, 2° D2, 2° D3, 2° D4) DENV infections after in vitro stimulation for 7 days with R848 and interleukin 2 as described in the Materials and Methods. Plates were coated with the indicated recombinant DENV E proteins or a capture antibody for human immunoglobulin G (IgG). A, Representative ELIspot responses in PBMCs of a patient with primary DENV-1 infection. The top row shows 2 wells with DENV-specific IgG spots, the middle row shows spots detected in 2 wells after medium stimulation, and the bottom row shows total IgG spots. B, C, Box and whisker (5th–95th percentile) plots showing the frequency of B cells that responded to the currently infecting serotype of DENV E protein per 106 cells and the frequency of DENV-specific B cells as a percentage of total IgG-secreting cells, respectively. Abbreviations: ASCs, antibody-secreting cells; D1, DENV-1; D2, DENV-2; D3, DENV-3; D4, DENV-4; NS, not significant.
Because the cell recovery and frequency of total ASCs differed among the subjects, we also assessed DENV-specific ASCs as a percentage of total ASCs in the same sample. DENV E-specific cells constituted a mean of 11.7% of total ASCs (range, 0.32%–57.5%) in samples obtained at the early convalescence time point. When compared in this fashion, B-cell responses in children with primary DENV-1 infection were still significantly higher than in children with secondary DENV-1 infection (Figure 1).
We compared correlations between frequencies of ASCs by ELIspot assay to PRNT50 and HAI titers (Figure S1; available online). The data show a significant association between HAI titers and ASC frequencies in patients with primary DENV-3 infection (P = .02) and between PRNT50 titers and ASC frequencies in patients with primary DENV-1 infection (P = .01). In patients with secondary infections, PRNT50 titers and HAI titers were high for multiple serotypes, as is well known. ASC frequencies in donors with secondary infection were lower (as shown in Figure 1).
These data indicate that B cells that secrete virus-specific antibodies are readily detectible in PBMCs from Thai children experiencing natural primary and secondary DENV infections, and that E-specific B-cell frequencies were highest in primary DENV-1 infections.
B-Cell Responses Are Serotype-Specific in Primary DENV Infections and Serotype–Cross-Reactive in Secondary Infections
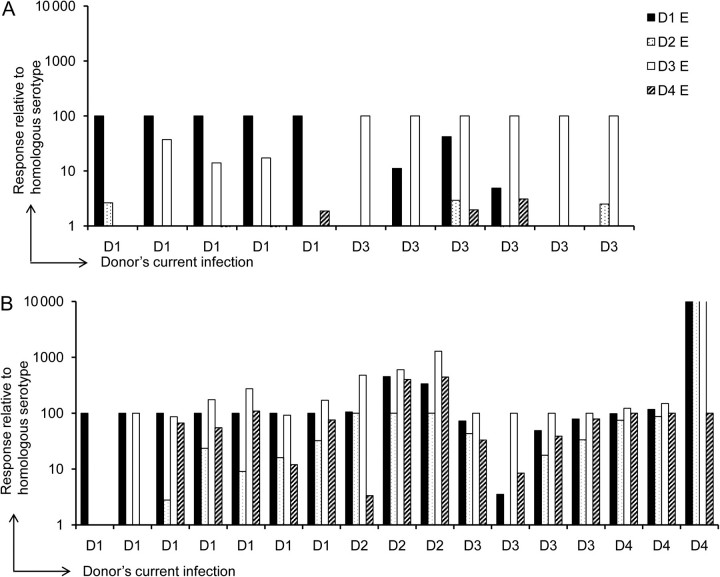
Cross-reactive antibodies have been proposed to play a major role in enhancing DENV infection through ADE. We therefore measured the serotype specificity of E-specific B-cell responses in children with primary or secondary infections. Responses to the currently infecting serotype were assigned a value of 100, and we expressed the frequency of ASCs to heterologous serotypes relative to that to the homologous serotype (Figure 2). In children with primary DENV-1 infections, modest responses were detected to DENV-3 in 3 of 5 samples (10%–30% of the response to DENV-1). Similarly, in children with primary DENV-3 infection, 3 of 6 samples had ASCs to DENV-1 (10%–40% of the response to DENV-3) as well as lower responses to DENV-2 and DENV-4 (<5% of the response to DENV-3) (Figure 2). In contrast, B-cell responses to heterologous serotypes were similar to or higher than responses to the homologous DENV serotype in 16 of the 17 samples obtained from children experiencing secondary infections (Figure 2). Furthermore, 14 of 17 children with secondary infections had ASCs that recognized 3 of the 4 serotypes of DENV E. These results indicate that B-cell responses detected early after primary infection were predominantly serotype-specific whereas responses detected early after secondary infection were predominantly serotype–cross-reactive.
Figure 2.
Serotype-specific B-cell responses during primary dengue virus (DENV) infection. The relative frequencies of B cells that responded to all 4 serotypes of DENV E protein were assessed in a B-cell enzyme-linked immunospot assay. Frequencies of B cells that responded to the homologous (currently infecting) serotype of DENV E protein were assigned a value of 100, and responses to the other 3 serotypes were calculated relative to the responses to the homologous serotype in donors with primary (A) or secondary (B) DENV infection. Abbreviations: D1, DENV-1; D2, DENV-2; D3, DENV-3; D4, DENV-4.
Higher Avidity of E-Specific Antibodies to Heterologous Serotypes After Secondary Infection
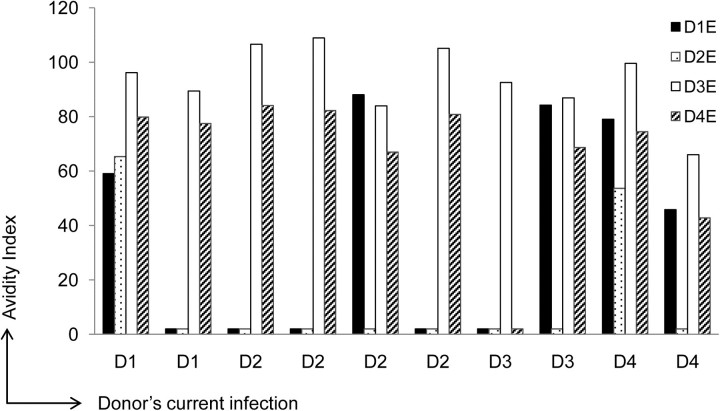
The ELISpot data demonstrated a higher frequency of serotype–cross-reactive B cells after secondary DENV infection. We speculated that these antibodies would have a higher avidity to heterologous serotypes of DENV E protein, reflecting both affinity maturation and original antigenic sin [24]. The IgG antibody avidity test has been used to differentiate primary from secondary DENV infections [25, 26]. Here we used avidity assays on supernatants generated from stimulated PBMCs of subjects with secondary DENV infections. We calculated the avidity index and present the values obtained at the highest sample dilution showing a positive routine ELISA result for each serotype (Figure 3). In 9 of 10 supernatants tested, the avidity index was higher to serotypes other than the currently infecting serotype. These data suggest that antibodies detected during secondary infection recognize multiple serotypes of DENV E protein and have higher avidity to heterologous epitopes.
Figure 3.
Antibody avidity to homologous and heterologous dengue virus (DENV) serotypes in secondary infection. Supernatants from stimulated peripheral blood mononuclear cells from donors with secondary DENV infections were analyzed by enzyme-linked immunosorbent assay with the 4 DENV E proteins with and without addition of urea. The avidity index was calculated as the ratio of the optical density with urea to the optical density without urea times 100. Abbreviations: D1, DENV-1; D2, DENV-2; D3, DENV-3; D4, DENV-4.
Distinct Specificities of DENV-Specific B-Cell Responses During Early and Late Convalescence
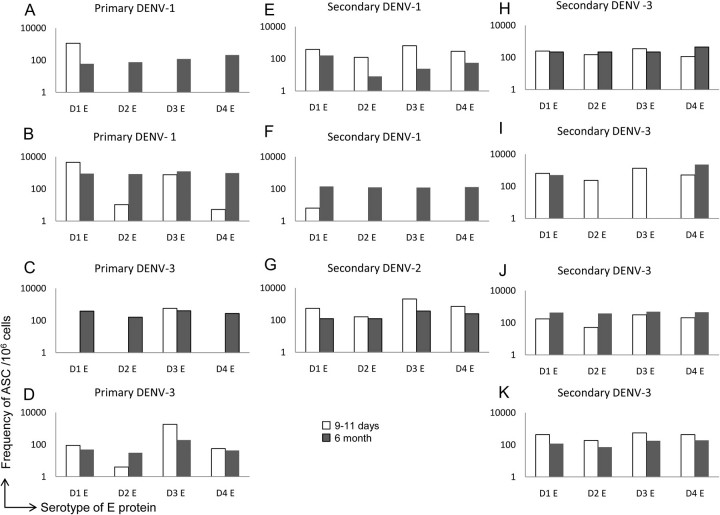
The data presented above were derived from PBMCs obtained 9–11 days after study entry. Previous studies have shown that the frequency of influenza-specific ASCs in blood peaks at 5–8 days after vaccination whereas the frequency of respiratory syncytial virus–specific ASCs following natural infection has a more prolonged window of up to 1 month following infection [27–29]. Individuals are protected from infection with other DENV serotypes for several months after primary infection [30], and the period of risk for symptomatic secondary infection is ≥6 months after infection. We therefore compared the memory B-cell responses in PBMCs obtained early during convalescence to samples obtained 6 months after infection. Frequencies of DENV E-specific ASCs declined to undetectable levels in 8 subjects. In the remaining 11 patients, we detected virus-specific ASCs in the 6-month sample. PBMCs from several subjects had lower or similar frequencies of DENV E-specific ASCs in the 6-month sample compared with the early time point (Figure 4), but the pattern of serotype reactivity was similar. In contrast, in 3 subjects with primary infection and 1 with secondary infection we detected B cells reactive to heterologous E proteins at the late time point that were not observed earlier in these subjects (Figure 4). In 1 donor with secondary DENV-3 infection, no responses were detected to DENV-3 at the 6-month time point (Figure 4) but responses to DENV-1 and DENV-4 were detected. The data indicate that ASCs detected by ELIspot assay at late time points did not always reflect responses detected in early convalescence.
Figure 4.
B-cell enzyme-linked immunospot responses in peripheral blood mononuclear cells (PBMCs) obtained early and late during convalescence. Shown are frequencies of B cells in PBMCs of patients at early (9–11 days) and late (6 months) time points after primary dengue virus type 1 (DENV-1) infection (A, B), primary DENV-3 infection (C, D), secondary DENV-1 infection (E, F), secondary DENV-2 infection (G), and secondary DENV-3 infection (H–K). Abbreviations: ASCs, antigen-secreting cells; D1, DENV-1; D2, DENV-2; D3, DENV-3; D4, DENV-4.
Serotype–Cross-Reactive Responses to NS1 and prM Proteins
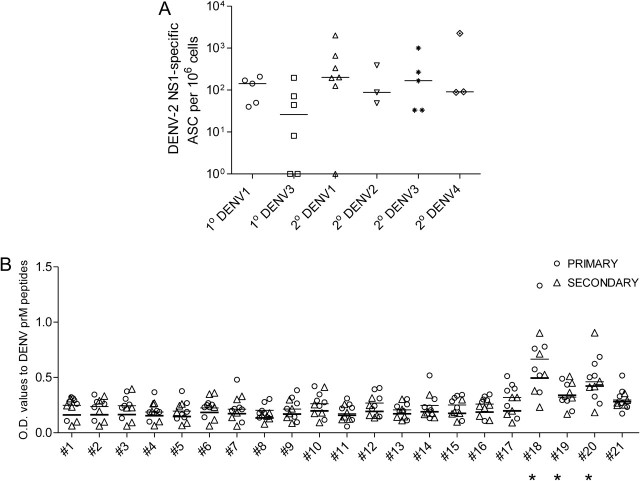
Recent data suggest that antibodies to proteins other than E are a major component of the response of humans to DENV. We therefore assessed responses to the NS1 and prM proteins. Responses to purified recombinant DENV-2 NS1 protein were measured by ELISpot assay. We detected frequencies of >50 ASCs per 106 cells to the DENV-2 NS1 protein in 20 of 28 donors (Figure 5). Because the vast majority of positive responses were obtained from donors that were not currently infected with DENV-2, these responses reflect cross-reactive antibody responses to NS1 (Figure 5).
Figure 5.
Serotype–cross-reactive responses to nonstructural protein 1 (NS1) and precursor membrane protein (prM) of dengue virus (DENV). Frequencies of B cells that responded to DENV-2 NS1 were assessed in a B-cell enzyme-linked immunospot assay (A). Enzyme-linked immunosorbent assay responses were assessed in supernatants from DENV-immune stimulated PBMCs to the indicated overlapping 17-mer peptides within the prM protein (B). Open circles and open triangles represent values obtained in supernatants from peripheral blood mononuclear cells of children with primary (1°) or secondary (2°) DENV infection. *P = .006, P = .002, and P = .02 for peptides 18, 19, and 20, respectively. Abbreviations: ASCs, antigen-secreting cells; OD, optical density.
Purified prM protein was unavailable; therefore, we measured responses to 21 linear DENV-2 17-mer overlapping peptides covering the entire prM protein by ELISA, using the culture supernatants of B cells stimulated in vitro. We found that responses to peptides 18 (WILRHPGFTIMAAILAY), 19 (FTIMAAILAYTIGTTHF), and 20 (LAYTIGTTHFQRALIFIL) were significantly higher than those to other peptides (Figure 5). No responses to these peptides were detected in B-cell supernatants from DENV-naive subjects (data not shown). Our data demonstrate antibody responses to both the prM and NS1 proteins in DENV-immune subjects.
DISCUSSION
We analyzed the frequency and serotype-specificity of DENV-specific B cells following acute DENV infection by use of ELIspot assays [31]. Our data show that B cells are readily activated in vitro to secrete antibodies at early and late time points in children with primary and secondary infection. Other recent studies have examined antibody responses to DENV infection [19–21]. However, our study is novel in several respects. First, we used ELIspot assays to assess the frequency of DENV-specific memory B cells; other studies examined polyclonal antibody responses in serum samples or studied monoclonal antibodies, which may be a less accurate representation of the B-cell repertoire available to respond to a subsequent infection. Second, we used samples from children with primary and secondary DENV infection. Third, we included infections with all 4 serotypes of DENV. Fourth, we tested PBMCs obtained at early and late convalescence, thus allowing us to examine the repertoire of B cells available to respond over time.
Our analysis revealed significantly higher frequencies of DENV-1–specific B cells in PBMCs of subjects with primary DENV-1 infection during early convalescence. Although the comparison of subjects infected with different serotypes was planned, no differences have previously been reported in antibody responses in primary cases according to serotype. We utilized purified recombinant proteins produced under the same conditions in the ELIspot assay, which measures only the frequency of antigen-specific ASCs; it is therefore unlikely that this finding is an artifact of the assay conditions. Our finding is consistent with the results of a recent study by Huynh et al [32], who reported that viral RNA levels in primary DENV-1 cases were significantly higher than those in primary DENV-2 or DENV-3 cases or in secondary DENV-1 cases. Another possible explanation for these results is a longer duration of viremia in primary infections compared with secondary infections, which is supported by our previous findings in this cohort [3]. The main objective in this study was to compare the frequency of antigen-specific ASCs in primary versus secondary DENV infections. We selected a representative subset of subjects from our clinical study to include all serotypes and both dengue fever (DF) and DHF. Mean frequencies of E-specific B cells were not significantly different in patients with mild disease (DF) compared with those with severe disease (DHF; mean, 1,330 vs 867 APCs per 106 cells, respectively). However, the small number of subjects with the same DENV serotype, serologic response (primary vs secondary), and clinical outcome (DF vs DHF) limits the statistical power for important subgroup analyses.
We found striking serotype–cross-reactivity to the E protein (with B cells responding to heterologous serotypes as well as or better than to homologous serotypes) in children experiencing secondary DENV infections, in contrast to serotype-specific responses in primary infections. When detectable, DENV-specific B cells in many subjects had a different profile of serotype–cross-reactivity at the 6-month time point compared with the same subjects early during convalescence. Despite the higher frequency of DENV-1–specific B cells in early convalescence in children with primary DENV-1 infection, this higher frequency was not maintained 6 months later. Memory B cells that were present earlier may have trafficked to tissues such as bone marrow during late convalescence [33]. Increased serotype–cross-reactivity at the later time point could indicate either preferential survival of the cross-reactive B cells or maturation of the B-cell response. Although we are unable to exclude the possibility that another exposure occurred between early and late convalescence, we believe that it is unlikely that some subjects may have been exposed to another DENV infection between the 2 time points. First, DENV infections tend to be seasonal in Thailand. Because we enrolled patients during the peak transmission season, the 6-month interval would tend to fall outside the peak season. In addition, prior studies have shown a period of resistance to re-infection lasting 3–6 months after an acute DENV infection. Our data suggest that DENV-specific memory B-cell responses in humans evolve over the first 6 months after infection.
Our finding that culture supernatants from PBMCs stimulated in vitro react with prM peptides indicates that some antibodies bind linear epitopes. Of particular interest were responses to the linear peptide prM 18 (WILRHPGFTIMAAILAY). A commonly used monoclonal antibody 2H2 recognized amino acids within the first membrane-spanning domain of the M protein (GFTVMAAILAYIIGTT) [9]. To our knowledge, this is the first report of human antibodies directed against a linear prM epitope. Most known neutralizing epitopes are conformational, but continuous amino acid sequences corresponding to antigenic determinants of the proteins that elicit antibodies in DENV-infected patients have only been mapped to the E protein [34]. Dejnirattisai et al [21] found a large number of monoclonal antibodies directed against prM, which together with our findings indicates that both conformational and linear epitopes need to be further defined on the prM protein.
We used a B-cell ELIspot assay in these studies to measure the frequency and specificity of DENV-specific B cells in the peripheral blood. The development of B-cell ELIspot-based assays has only recently facilitated the study of human B-cell immune responses in the peripheral blood [31]. The B-cell ELIspot assay detects B cells that secrete IgG capable of binding to DENV antigens, and it does not give further information about antibody function (eg, neutralization or enhancement). We found a trend toward significance in correlation between ASCs detected by ELIspot assay and HAI titers in patients with secondary DENV-1 infection and between ASCs detected by ELIspot assay and PRNT50 titers in patients with primary DENV-1 infection. Our data indicate that the relationship between DENV-specific antibody titers in the serum and the frequency of DENV-specific memory B cells in the blood is still not well defined and warrants further investigation. Halliley et al [27] found that peak frequencies of ASCs following influenza virus immunization correlated with the increase in serum HAI titers.
We chose an antigen-independent method used by several groups to stimulate B cells in the peripheral blood, because frequencies of ASC precursors in PBMCs are extremely low [27, 31, 35]. The predictive value of B-cell ELIspot assays for protection from infection remains to be established. The technical problems with neutralizing antibody assays for DENV, and the poor performance of these assays as a correlate of protective immunity, have been well described elsewhere [36]. With the advent of clinical vaccine trials, there is considerable interest in additional measures of humoral immunity to DENV, and the B-cell ELIspot assay warrants further study as a potential biomarker of protective immunity.
Notes
Acknowledgments.
We thank the donors who generously provided PBMCs for use in our studies. We thank Suchitra Nimmannitya and David Vaughn, as well as the staff of the Queen Sirikit National Institute for Child Health and the Department of Virology, Armed Forces Research Institute of Medical Sciences, for patient recruitment, blood collection, clinical data collection, virology, and HLA information. We thank Wenjun Li for performing statistical analysis for the data provided in this manuscript.
Financial support.
This work was supported by the National Institutes of Health (P01 AI34533); and the US Army Military Infectious Disease Research Program.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pinheiro FP, Corber SJ. Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q. 1997;50:161–9. [PubMed] [Google Scholar]
- 2.Thein S, Aung MM, Shwe TN, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 4.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–30. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11:S830–9. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 6.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–67. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 7.Beasley DW, Holbrook MR, Travassos Da Rosa AP, et al. Use of a recombinant envelope protein subunit antigen for specific serological diagnosis of West Nile virus infection. J Clin Microbiol. 2004;42:2759–65. doi: 10.1128/JCM.42.6.2759-2765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falconar AK. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesion proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol. 1997;142:897–916. doi: 10.1007/s007050050127. [DOI] [PubMed] [Google Scholar]
- 9.Falconar AK. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch Virol. 1999;144:2313–0. doi: 10.1007/s007050050646. [DOI] [PubMed] [Google Scholar]
- 10.Falconar AK. Monoclonal antibodies that bind to common epitopes on the dengue virus type 2 nonstructural-1 and envelope glycoproteins display weak neutralizing activity and differentiated responses to virulent strains: implications for pathogenesis and vaccines. Clin Vaccine Immunol. 2008;15:549–61. doi: 10.1128/CVI.00351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falconar AK, Young PR. Miles MA. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch Virol. 1994;137:315–26. doi: 10.1007/BF01309478. [DOI] [PubMed] [Google Scholar]
- 12.Ludolfs D, Reinholz M. Schmitz H. Highly specific detection of antibodies to tick-borne encephalitis (TBE) virus in humans using a domain III antigen and a sensitive immune complex (IC) ELISA. J Clin Virol. 2009;45:125–8. doi: 10.1016/j.jcv.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Oliphant T, Nybakken GE, Engle M, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–59. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukupolvi-Petty S, Austin SK, Purtha WE, et al. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol. 2007;81:12816–26. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol. 2001;75:7769–73. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roehrig JT. Antigenic structure of flavivirus proteins. Adv Virus Res. 2003;59:141–75. doi: 10.1016/s0065-3527(03)59005-4. [DOI] [PubMed] [Google Scholar]
- 17.Crill WD, Hughes HR, Delorey MJ. Chang GJ. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One. 2009;4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA. de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–13. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltramello M, Williams KL, Simmons CP, et al. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–83. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schieffelin JS, Costin JM, Nicholson CO, et al. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejnirattisai W, Jumnainsong A, Onsirisakul N, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalayanarooj S, Vaughn DW, Nimmannitya S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–21. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 23.Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of pre-existing dengue virus (DV) neutralizing antibody levels to viremia and disease severity in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 24.Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2010;85:410–21. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Souza VA, Fernandes S, Araujo ES, et al. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J Clin Microbiol. 2004;42:1782–4. doi: 10.1128/JCM.42.4.1782-1784.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Souza VA, Tateno AF, Oliveira RR, et al. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J Clin Virol. 2007;39:230–3. doi: 10.1016/j.jcv.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Halliley JL, Kyu S, Kobie JJ, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–7. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee FE, Falsey AR, Halliley JL, Sanz I, Walsh EE. Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults. J Infect Dis. 2010;202:1659–66. doi: 10.1086/657158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suryani S, Tangye SG. Therapeutic implications of advances in our understanding of transitional B-cell development in humans. Expert Rev Clin Immunol. 2010;6:765–75. doi: 10.1586/eci.10.55. [DOI] [PubMed] [Google Scholar]
- 30.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 31.Crotty S, Aubert RD, Glidewell J. Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Duyen HT, Ngoc TV, Ha DT, et al. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis. 2011;203:1292–300. doi: 10.1093/infdis/jir014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, Mei H, Dorner T, et al. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–39. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 34.da Silva AN, Nascimento EJ, Cordeiro MT, et al. Identification of continuous human B-cell epitopes in the envelope glycoprotein of dengue virus type 3 (DENV-3) PLoS One. 2009;4:e7425. doi: 10.1371/journal.pone.0007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baer J, Santiago F, Yang H, et al. B cell responses to H5 influenza HA in human subjects vaccinated with a drifted variant. Vaccine. 2010;28:907–15. doi: 10.1016/j.vaccine.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roehrig JT, Hombach J. Barrett AD. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008;21:123–32. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]