Translocation, the directional movement of transfer RNA (tRNA) and messenger RNA (mRNA) substrates on the ribosome during protein synthesis, is regulated by dynamic processes intrinsic to the translating particle. Using single-molecule fluorescence resonance energy transfer (smFRET) imaging, in combination with site-directed mutagenesis of the ribosome and tRNA substrates, it is shown that peptidyl-tRNA within the aminoacyl site of the bacterial pretranslocation complex can adopt distinct hybrid tRNA configurations resulting from uncoupled motions of the 3′-CCA terminus and the tRNA body. The hybrid configuration where both the 3′-CCA end and body of peptidyl-tRNA have moved in the direction of translocation exhibits dramatically enhanced puromycin reactivity, an increase in the rate at which EF-G engages the ribosome, and accelerated rates of translocation. These findings provide compelling evidence that the substrate for EF-G catalyzed translocation is an intermediate wherein the bodies of both tRNA substrates adopt hybrid positions within the translating ribosome.
Keywords: translocation, peptidyl-tRNA, EF-G, ribosome, A-site finger, hybrid states, single-molecule FRET
Abstract
Translocation, the directional movement of transfer RNA (tRNA) and messenger RNA (mRNA) substrates on the ribosome during protein synthesis, is regulated by dynamic processes intrinsic to the translating particle. Using single-molecule fluorescence resonance energy transfer (smFRET) imaging, in combination with site-directed mutagenesis of the ribosome and tRNA substrates, we show that peptidyl-tRNA within the aminoacyl site of the bacterial pretranslocation complex can adopt distinct hybrid tRNA configurations resulting from uncoupled motions of the 3′-CCA terminus and the tRNA body. As expected for an on-path translocation intermediate, the hybrid configuration where both the 3′-CCA end and body of peptidyl-tRNA have moved in the direction of translocation exhibits dramatically enhanced puromycin reactivity, an increase in the rate at which EF-G engages the ribosome, and accelerated rates of translocation. These findings provide compelling evidence that the substrate for EF-G catalyzed translocation is an intermediate wherein the bodies of both tRNA substrates adopt hybrid positions within the translating ribosome.
INTRODUCTION
Protein synthesis is carried out by the ribosome, a universally conserved, 2.4 MDa RNA-protein assembly comprised of two distinct subunits (30S and 50S in prokaryotes). Elongation, the synthesis phase of translation, requires repetitive cycles of aminoacylated tRNA selection, peptide bond formation, and the directional translocation of tRNA and mRNA substrates with respect to the ribosome. These processes are catalyzed by elongation factor Tu (EF-Tu), the peptidyltransferase center (PTC) of the ribosome, and elongation factor G (EF-G), respectively. However, despite extensive investigations, (Rodnina and Wintermeyer 2001; Ogle and Ramakrishnan 2005; Zaher and Green 2009), the molecular mechanism of these translational events remains unclear.
Atomic resolution structures of the ribosome have revealed the molecular basis of “classical” tRNA binding sites—the aminoacyl (A), peptidyl (P), and exit (E) sites (Korostelev et al. 2008). In their “classical” positions, the L-shaped A- and P-site tRNA molecules orient perpendicular to the subunit interface maintained by the pairing of the tRNA anticodons with mRNA codons on the small subunit and Watson-Crick base-pairing interactions between the universally conserved 3′-CCA ends of tRNA and ribosomal RNA (rRNA) within the large subunit PTC (Fig. 1; Samaha et al. 1995; Kim and Green 1999). While the specific interactions of tRNAs in classical positions are critical for both peptide bond formation (Lieberman and Dahlberg 1994; Weinger et al. 2004; Brunelle et al. 2006; Dorner et al. 2006) as well as peptide release (Feinberg and Joseph 2006), such contacts must be extensively remodeled for tRNA substrates to move directionally through the ribosome during active translation (Agirrezabala and Frank 2009; Munro et al. 2009).
FIGURE 1.
Structural model of the bacterial ribosome with three classically bound tRNAs. Crystal structure (left) of the Thermus thermophilus ribosome (Protein Data Bank accession codes: 2J00, 2J001) showing tRNAs (red) bound in the aminoacyl (A), peptidyl (P), and exit (E) sites, as well as ribosomal RNA (gray) and ribosomal proteins (blue and tan) for the large and small subunit, respectively. The peptidyl transferase center is shown enlarged with the P and A loops (cyan) interacting with the CCA termini of the P- and A-site tRNAs, respectively.
EF-G (GTP) operates on the pretranslocation (PRE) ribosome complex containing deacylated tRNA in the P site and peptidyl-tRNA in the A site in a manner that depends strongly on GTP hydrolysis (Rodnina et al. 1997; Pan et al. 2007; Munro et al. 2010c). In so doing, EF-G mediates the translocation of mRNA and tRNA substrates with respect to the ribosome to generate the post-translocation (POST) complex in which deacylated tRNA occupies the E site and peptidyl-tRNA occupies the P site. The A site, vacated by EF-G (GDP) release, is then competent for downstream reactions. Precision in these movements is critical to synthesis of the mRNA-encoded polypeptide product. A deeper understanding of tRNA-ribosome remodeling events and their relationship to EF-G catalyzed translocation is, therefore, critical to understanding both the fidelity and regulation of translation.
It has long been hypothesized that large-scale conformational rearrangements in both the ribosome and aa-tRNA substrates underpin the translocation mechanism (Bretscher 1968; Spirin 1968). Consistent with this notion, early biochemical studies demonstrated that A- and P-site tRNAs adopt “hybrid” configurations (A/P, P/E) within the PRE complex (Moazed and Noller 1989). Such configurations arise from the spontaneous movement of A- and P-site tRNA 3′-CCA termini with respect to the large subunit, movements that are independent of anticodon translocation with respect to the small subunit. Hybrid tRNA configurations are speculated to lower the activation barrier for substrate movement by serving as intermediates in the translocation process (Semenkov et al. 2000). Elegant mutagenesis studies, where tRNA hybrid states were stabilized through specific manipulations of tRNA-ribosome interactions at the PTC, suggest that a PRE complex in which the 3′-CCA end of peptidyl-tRNA occupies the large subunit P site, (the A/P hybrid state) is an authentic translocation intermediate (Dorner et al. 2006).
Further studies of the translocation mechanism through single-molecule FRET (smFRET) imaging revealed that the PRE complex is intrinsically dynamic in nature, exhibiting a range of spontaneous, large-scale conformational changes critical to the translocation reaction coordinate (Munro et al. 2007; Cornish et al. 2008; Fei et al. 2008, 2009; Munro et al. 2010a,c; Dunkle et al. 2011). Of most direct relevance to the present study, A- and P-site tRNAs within the PRE complex, labeled at their elbow domains via naturally occurring modified nucleotides (Blanchard et al. 2004), were shown to dynamically exchange between classical (A/A; P/P) and two kinetically and structurally distinct hybrid states (Munro et al. 2007). Based on supporting structural and mutagenesis data, these distinct configurations were posited to arise from coupled and uncoupled movements of A- and P-site tRNAs with respect to each other and the ribosome. As earlier biochemical data (Moazed and Noller 1989) had anticipated that coupled tRNA motions arise from both A- and P-site tRNAs simultaneously adopting hybrid positions (A/P; P/E), the configuration characterized as arising from the coupled movement of both tRNAs to their hybrid positions was termed the H1 hybrid state. Correspondingly, the second hybrid state, arising from uncoupled motion of deacylated P-site tRNA into its hybrid position while peptidyl-tRNA remained classically configured (P/E; A/A) within the A site was termed the H2 state.
The spontaneous exchange of A- and P-site tRNA between classical and hybrid states within the PRE complex has since been confirmed through two independent cryo-electron microscopy (EM) investigations (Agirrezabala et al. 2008; Julian et al. 2008). Such studies provided the first three-dimensional structural information reporting on the nature of the hybrid PRE complex configuration. In line with the earlier smFRET studies (Munro et al. 2007), a comparison of classical and hybrid PRE complex configurations revealed that the formation of tRNA hybrid states is accompanied by large scale rearrangements in the ribosome. Such changes included an 8° rotation of the small subunit with respect to the large, a 4° rotation of the small subunit head in the direction of translocation, and an ∼20 Å closure of the flexible L1 stalk domain within the large subunit E site, where it comes into contact with the P/E hybrid tRNA elbow domain. Notably, however, only a single hybrid state of tRNA binding was observed in which both A- and P-site substrates occupied hybrid configurations, defined by the 3′-CCA ends of both A- and P-site tRNA being present in the P and E sites of the large subunit, respectively. Here, the P/E hybrid configuration was achieved by an ∼15–20 Å motion of the deacylated P-site tRNA body toward the E site. By contrast, the A/P hybrid state was achieved by motions of the single-stranded 3′-CCA end of peptidyl-tRNA within the A site, while the tRNA acceptor stem, body, and anticodon domains remained classically configured. In such a configuration, the spacing between A- and P-site tRNAs was found to be consistent with the H2 hybrid state (Munro et al. 2009). Support for this assignment was later provided by cryo-EM studies of a PRE complex stabilized in the H2 state by a single point mutation in the P loop of the large subunit (Fu et al. 2011). Collectively, such findings question the nature of the H1 hybrid state and its role, if any, in the mechanism of translocation.
To further examine the relationship and influence of peptidyl-tRNA position on the mechanism of translocation, we have undertaken a mutagenesis approach to probe structural features of the PRE complex and their relationship to translocation using single-molecule FRET techniques. Following methods analogous to those previously described (Dorner et al. 2006), specific configurations of the PRE complex were enforced and their impact on the rates of translocation examined. Through such efforts, we show that mutations in the inter-subunit bridge B1a element, known as the A-site finger (ASF), specifically enhance H1 state occupancy. Complexes that occupy this hybrid tRNA configuration exhibit an increase in the rate at which EF-G engages the ribosome and faster rates of translocation. These findings suggest that a PRE complex configuration in which both tRNA bodies occupy hybrid configurations is preferentially engaged by EF-G and, correspondingly, that movement of the peptidyl-tRNA body into the P site is a critical determinant of the mechanism of translocation.
RESULTS
As previously described (Blanchard et al. 2004; Munro et al. 2007), the motions of tRNAs between classical and hybrid states within the bacterial PRE complex were quantified through prism-based smFRET imaging of surface-immobilized ribosome complexes bearing deacylated (Cy3-s4U8) tRNAfMet in the P site and (Cy5-acp3U47) fMet-Phe-tRNAPhe in the A site. Imaging experiments were performed at a frame rate of 25 sec−1 in Tris-Polymix buffer containing 15 mM [Mg2+] (Materials and Methods). The single-molecule data obtained were analyzed using hidden Markov modeling (HMM) procedures (Munro et al. 2007), from which the rates and amplitudes of tRNA motions could be assessed. Site-specific mutations were introduced into the ribosome at the tRNA binding sites utilizing procedures that enable mutant ribosomes expressed and assembled in vivo to be purified to homogeneity (Youngman and Green 2005). Mutation of the ASF was performed using standard site-directed mutagenesis procedures.
Compensatory mutational analysis confirms the assignment of hybrid tRNA configurations in the PRE complex
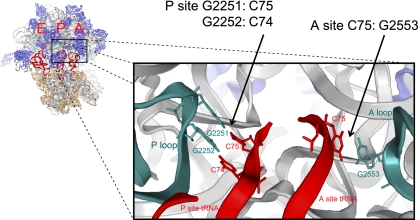
As anticipated from previous studies, wild-type (WT) PRE complexes were observed to predominantly occupy (∼65%) the classical (C) configuration, with transient excursions to two distinct hybrid tRNA configurations (H1 and H2) identified as having low- (H2) and intermediate- (H1) FRET values (∼0.23 and ∼0.39, respectively) (Supplemental Fig. S1). Site-directed mutagenesis of single residues within the A and P loops (G2553C and G2252C, respectively) suggest that these states correspond to PRE complex configurations in which only the P-site tRNA body adopts a hybrid configuration (H2: P/E; A/A) and where both tRNA bodies adopt hybrid configurations (H1: P/E; A/P) (Munro et al. 2007).
The dynamic behaviors of individual PRE complexes were recapitulated at the ensemble level, where population FRET histograms showed a dominant peak at high-FRET (mean FRET value ∼0.55) and a broadened lower-FRET distribution representing both hybrid tRNA configurations (H1+H2) (Supplemental Fig. S2A,B, left panels). As previously reported (Munro et al. 2007), dramatically reduced classical state occupancy and significantly increased H1 and H2 state occupancies were observed when analogous experiments were performed on PRE complexes bearing a G2553C mutation in the A loop of 23S rRNA (Supplemental Fig. S2A,B, middle panels) or a G2252C mutation in the P loop of 23S rRNA (Supplemental Fig. S2A,B, right panels).
In order to specifically examine whether such FRET perturbations manifest solely from the disruption of interactions between A- and P-site tRNAs and the ribosome (G2553-C75 and G2252-C74 base pairs, respectively), a compensatory mutagenesis strategy was employed in which mutations were introduced into A- and P-site tRNAs to restore Watson-Crick pairing. Although previous studies employed analogous methods to investigate how tRNA-ribosome base-pairing interactions affect the translocation mechanism (Dorner et al. 2006), smFRET has yet to be used to directly probe how such mutations affect dynamic processes within the PRE complex.
To do so, native tRNA molecules containing specific mutations in the 3′-CCA end were generated, which could then be fluorescently labeled at naturally occurring modified nucleotides as previously described (Blanchard et al. 2004; Munro et al. 2007). Native Escherichia coli tRNAs were cleaved with snake venom phosphodiesterase to liberate specific RNA fragments from the tRNA 3′ end, followed by T4 RNA ligase-mediated ligation of “WT” or “mutant” synthetic oligonucleotides to restore the molecule to its full length (Supplemental Fig. S3). For A-site tRNA, this procedure resulted in the exchange of the native acceptor stem of tRNAPhe with either a WT or C75G mutant fragment. For P-site tRNAfMet, the acceptor stem was substituted with either a WT or C74G mutant fragment. Subsequent to reconstitution, tRNAfMet and tRNAPhe molecules were labeled with Cy3 and Cy5 fluorophores, respectively. Following aminoacylation and formylation, Cy3-labeled fMet-tRNAfMet was enzymatically delivered to the P site of mRNA-programmed, tight-coupled 70S ribosome particles. As summarized in Fig. 2, “cut and ligated” WT tRNAs bound within either the A or P site of wild-type (left panels) and mutant (either G2553C or G2252C) PRE complexes (center panels) exhibited behaviors that were comparable to those of native tRNA (cf. Supplemental Fig. S2).
FIGURE 2.
Compensatory mutations in P- and A-site tRNA CCA ends restore tRNA equilibrium on the pretranslocation ribosome. Cartoons (far left panels) illustrate tRNA dynamics (red stick) on the ribosome, with the 50S (purple) and 30S (orange) subunit. The green and red stars represent the approximate positions of Cy3 and Cy5 fluorophores on tRNA, respectively. The Exit (E), Peptidyl (P), and Aminoacyl (A) sites are labeled below the 30S subunit. (A) Histograms of single-molecule FRET trajectories reveal the tRNA equilibrium at the population level. Left: wild-type (WT) ribosomes with native WT tRNAfMet in the P site and “cut and ligated” WT tRNAPhe in the A site. Center: G2553C ribosomes with native WT tRNAfMet in the P site and “cut and ligated” WT tRNAPhe in the A site. Right: G2553C ribosomes with WT tRNAfMet in the P site and C75G tRNAPhe in the A site. (B) Histograms— Left: WT ribosomes with “cut and ligated” WT tRNAfMet in the P site and native WT tRNAPhe in the A site. Center: G2252C ribosomes with “cut and ligated” WT tRNAfMet in the P site and native WT tRNAPhe in the A site. Right: G2252C ribosomes with C74G tRNAfMet in the P site and native WT tRNAPhe in the A site. The color scale at the bottom of the panel represents the relative populations.
To assess whether the hybrid tRNA configurations (lower FRET states) observed in the mutant ribosome PRE complexes were due solely to the disruption of individual base pairs between tRNA and the ribosome, experiments were repeated using tRNAs bearing compensatory mutations in the 3′ ends of A- and P-site tRNA. Indeed, delivery of C75G mutated Phe-tRNAPhe to the A site of G2553C PRE complexes returned the system to a wild-type distribution of FRET configurations (Fig. 2A, right panel). HMM analysis revealed that five of the six rate constants defining A- and P-site tRNA motions were restored to within 12% of those observed for the WT complex (Supplemental Table S1). Notably, the classical to H2 transition (kC→H2) was found to be ∼50% below that of the wild-type system. Reductions in the rate of this specific transition are consistent with the classical state being modestly stabilized by the C75G tRNA mutation in the context of the G2553C A loop mutation and/or the H2 state being modestly destabilized due to disruption of peptidyl-tRNA's native interactions with the P loop. These data demonstrated that the G2553C mutation specifically altered tRNA configurations within the PRE complex as a direct result of the C75-G2553 base pair disruption.
Analogous experiments performed on PRE complexes bearing a G2252C mutation in the P loop of 23S rRNA and the compensatory C74G mutation in P-site tRNAfMet also exhibited a wild type-like distribution of tRNA configurations (Supplemental Fig. S2B, right panel). Here, the classical state showed somewhat higher occupancy than observed for the WT system (59% vs. 42%). This distinction could be attributed to a ∼35% decrease in the rate at which P-site tRNA exits the classical state (kC→H2 = 0.82 sec−1 vs. 1.25 sec−1) and somewhat accelerated rates exiting the H1 hybrid state (kH1→C = 10.8 sec−1 vs. 7.7 sec−1; kH1→H2 = 5.0 sec−1 vs. 3.3 sec−1) (Supplemental Table S1). Such deviations from WT behavior are likely explained by the destabilization of A-site peptidyl-tRNA interactions with the mutated P loop in the hybrid (A/P) state.
Mutations designed to favor hybrid tRNA configurations stabilize the H2 hybrid state
Having validated the present mutagenesis approach, we next examined the model that the intermediate for translocation specifically corresponds to a PRE complex configuration in which both tRNAs occupy hybrid configurations (A/P; P/E) (Dorner et al. 2006). According to the established classical-hybrid equilibrium model (Munro et al. 2007), this configuration should correspond to a PRE complex in the H1 hybrid state. Following logic analogous to that reported through bulk translation studies (Dorner et al. 2006), we set out to promote H1 state occupancy by forming PRE complexes with ribosomes bearing two P loop mutations (G2251C, G2252C), WT P-site tRNAfMet, and A-site dipeptidyl tRNAPhe bearing compensatory C75G and C74G mutations.
As expected, the classical state was observed to be highly destabilized (8 ± 1% vs. 42 ± 2%) (Supplemental Table S1). However, contrary to expectation, the predominant FRET state observed was the H2 hybrid state, a configuration where the established model predicts only P-site tRNA occupies a hybrid configuration (A/A, P/E) (Fig. 3, right panel). Kinetic analysis revealed that classical state destabilization stemmed from a two- to threefold increase in the rate exiting the classical state (kC→H2 and kC→H1) and a more than eightfold decrease in the rates exiting hybrid states (kH2→C and kH1→C) (Supplemental Table S1). The observed preference for H2 occupancy (59 ± 5%) was the consequence of an apparent stabilization of the H2 hybrid state, stemming principally from a reduction in the rate at which the system returned to the classical state from H2 (kH2→C). H2 stabilization also manifested from a ∼30% increase and 40% decrease in the rates of transition between H1 and H2 hybrid states (kH1→H2 and kH2→H1), respectively. Such observations argue for complexities in the nature of tRNA motions within the PRE complex exceeding those previously anticipated (Munro et al. 2007). In line with the conclusions of recent cryo-EM investigations, such data suggest that the transition of peptidyl-tRNA from its classical to hybrid (A/P) configuration only requires motions of the 3′-CCA end of peptidyl-tRNA into the P site of the large subunit, while the A-site tRNA body remains classically configured (Agirrezabala et al. 2008; Julian et al. 2008). Thus, the present observations suggest that the H2 FRET state, originally defined as representing an A/A; P/E hybrid PRE complex configuration (Munro et al. 2007), may actually represent an ensemble of configurations that also includes the A/P; P/E hybrid configuration in which only the 3′-CCA end of peptidyl-tRNA has moved into the P site.
FIGURE 3.
Enforcing the A/P hybrid state through compensatory mutations in the P loop (G2251C G2252C) and peptidyl-tRNA (C75G C74G) stabilizes the H2 hybrid state. Histograms are displayed as in Figure 2. Left: wild-type (WT) ribosomes with native WT tRNAfMet in the P site and “cut and ligated” WT tRNAPhe in the A site. Right: G2251C G2252C ribosomes with native WT tRNAfMet in the P site and “cut and ligated” C75G C74G tRNAPhe in the A site.
Such findings suggest that the prevailing model of tRNA dynamics within the PRE complex must be extended to include the notion that the single-stranded 3′-CCA ends of tRNA may move independently of the tRNA bodies within the PRE complex. These motions are unlikely to be observed with the present FRET labeling strategy, as the donor and acceptor fluorophores are positioned distally to these sites of movement. Thus, the changes in FRET presently observed likely report on relatively slow, global conformational remodeling events in the PRE complex that result in a substantial repositioning of the tRNA body. In this model, the H1 FRET state must, therefore, reflect a PRE complex configuration in which the A-site tRNA body has moved toward the P site.
Truncation of the A-site finger promotes the H1 hybrid state
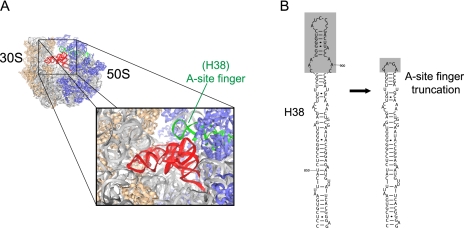
On the basis of kinetic and structural arguments, it had been previously speculated that transitions from the H2 to H1 hybrid states result from remodeling events at the inter-subunit bridge, B1a (Munro et al. 2007). Bridge B1a, which includes the ASF [Helix 38 (H38) of 23S rRNA; nucleotides 827–942], extends from a position proximal to the large subunit central protuberance between the A- and P-site tRNA to contact the small subunit head domain (Fig. 4A). In the PRE complex, the ASF comes into direct contact with A-site tRNA where the D- and T-loop elements meet at the tRNA elbow (Korostelev et al. 2006). Correspondingly, ASF remodeling was anticipated to contribute to the activation barrier for formation of the H1 hybrid state, wherein both the 3′-CCA terminus and body of A-site tRNA occupy hybrid configurations.
FIGURE 4.
Crystal structure of the ribosome and secondary structure of the A-site finger. (A) The crystal structure of the ribosome is illustrated as described in Figure 1 (Protein Data Bank accession codes: 1VSA, 2OW8) oriented 90° clockwise. Nucleotides of Helix 38 (H38) are highlighted in green. (B) Secondary structure of H38 (A-site finger) of 23S rRNA from Escherichia coli, highlighting the 26 residues (nt 876–901) and loop element replaced in ASF-truncated PRE complexes, as previously described (Komoda et al. 2006).
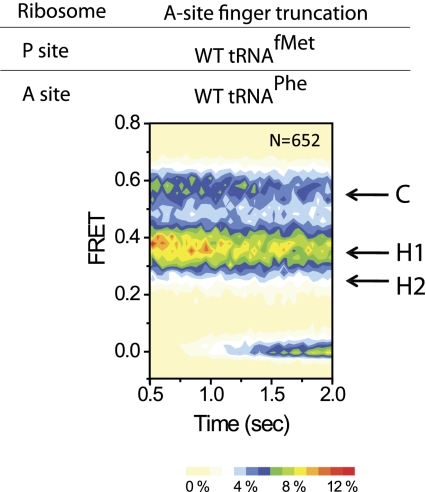
To test the hypothesis that the ASF attenuates the motion of the A-site tRNA body from its classical (A/A) to hybrid (A/P) position, and thus H1 state formation, smFRET experiments were performed on PRE complexes in which the ASF was truncated by deletion of residues 876–901 of 23S rRNA (Fig. 4B). This specific truncation was previously reported to accelerate the rate of translocation by ∼30% (Komoda et al. 2006). In line with the proposed model, the dominant FRET configuration observed corresponded to the H1 state (45 ± 1%) (Fig. 5; Supplemental Table S1). The net increase in H1 state occupancy (45% vs. 24%) resulted from a global reduction in the activation barriers for both A- and P-site tRNA hybrid state formation. However, enhanced H1 occupancy primarily resulted from an increase in the rate at which both tRNA bodies simultaneously transitioned to hybrid positions (kC→H1 = 4.9 sec−1 vs. 1.7 sec−1). The reverse transition (kH1→C) was reduced by twofold as well. Taken together, these findings provide strong supporting evidence for the hypothesis that the ASF attenuates motions of the A-site tRNA body toward the P site. Since the forward transition into the H1 state (kC→H1) is accelerated and the reverse transition back to the classical state (kH1→C) is decelerated by the ASF truncation, it seems unlikely that the ASF simply serves as a steric hindrance for motions of the peptidyl-tRNA body. Rather, it would appear from these data that the truncation alters PRE complex dynamics through a global destabilization of classical tRNA configurations. Consistent with this notion, the ASF deletion was also observed to increase the transition rate (kC→H2 = 2.4 sec−1 vs. 1.6 sec−1) between classical and H2 states (P/E, A/A), while decreasing the reverse rate more than threefold (kH2→C = 2.1 sec−1 vs. 7.1 sec−1). Collectively, such findings implicate the ASF as a key structural determinant of the ribosome energy landscape.
FIGURE 5.
A-site finger (ASF) truncation promotes the formation of the H1 hybrid state (P/E, A/P). Histograms are displayed as in Figure 2. ASF-truncated ribosomes with native deacylated WT tRNAfMet in the P site and WT fMet-Phe-tRNAPhe in the A site are dominated by the 0.35 H1 FRET state.
Stabilization of the H1 hybrid state accelerates puromycin reactivity
To probe the nature of the H1 hybrid state and its role in translocation, the rates of peptide bond formation and translocation were investigated for wild-type and ASF-truncated complexes. First, the rate of peptide bond formation in the PRE complex was probed using a previously described assay in which puromycin is used to release a Cy3-labeled nascent peptide from peptidyl-tRNA bound within surface-immobilized ribosome complexes (Munro et al. 2007; Feldman et al. 2010). Puromycin, an analog of the 3′ end of aminoacyl-tRNA, binds weakly to the large subunit A site via interactions with the A loop and reacts rapidly with the nascent chain when it resides in the P site (Semenkov et al. 2000). Due to steric occlusion of the puromycin binding site, wild-type PRE complexes, in which peptidyl-tRNA predominantly resides in the A site, react slowly with puromycin. Residual puromycin reactivity is reported to reflect spontaneous hybrid state excursions, wherein the peptide transiently occupies the P site (Blanchard et al. 2004; Sharma et al. 2004; Dorner et al. 2006). Accordingly, PRE complexes in the H1 hybrid state are expected to exhibit an enhancement in puromycin reactivity.
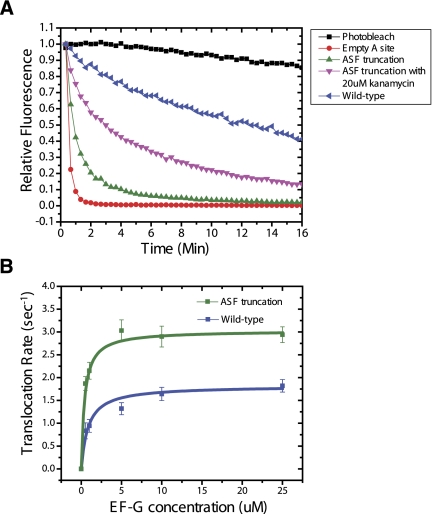
As shown in Figure 6A, wild-type ribosome complexes bearing Cy3-peptidyl-tRNA in the P site and lacking A-site tRNA react rapidly (4.3 min−1) with puromycin (2 mM) (Materials and Methods). By contrast, wild-type PRE complexes bearing Cy3-peptidyl-tRNA in the A site exhibited markedly slower rates (0.05 min−1), consistent with the previously reported rate (Munro et al. 2007). However, when analogous experiments were performed on PRE complexes bearing the ASF truncation, a dramatically enhanced rate of puromycin reactivity was exhibited (0.50 min−1) (Fig. 6A). Such results could not be explained by incomplete occupancy of the A site, as equivalent results were obtained when simultaneously tracking puromycin reactivity and A-site tRNA occupancy using Cy5(acp3U47)-labeled Phe-tRNAPhe (data not shown). Further supporting the proposed model, the aminoglycoside kanamycin, known to stabilize peptidyl-tRNA in the classical state (Feldman et al. 2010), markedly reduced puromycin reactivity of the ASF-truncated PRE complex (0.15 min−1) (Fig. 6A). These findings suggest that the puromycin binding site is nearly as accessible in the ASF-truncated PRE complex as when the A site is entirely vacant. These data provide compelling evidence in support of the H1 FRET state assignment and the notion that this PRE complex configuration closely resembles the intermediate state for translocation.
FIGURE 6.
A-site finger truncation increases the rate of puromycin reactivity and translocation. (A) Single-molecule puromycin assay reporting on the availability of large subunit A site within the peptidyl transferase center (PTC). The reactivities of three ribosome complexes are shown: the initiation complex lacking A-site tRNA (red circles), the wild-type PRE complex (blue triangles), the ASF-truncated PRE complex (green triangles), and the ASF-truncated PRE complex in the presence of 20 μM kanamycin (pink inverted triangles). (B) Single-molecule translocation assay reporting on the rate of translocation observed in wild-type and ASF-truncated PRE complexes (schematized in Supplemental Fig. S5). The translocation rate (mean ± SD) of the WT and the ASF-truncated PRE complexes is plotted against EF-G concentration (0.5–25 μM), fit using the Michaelis-Menton equation in Origin. ASF-truncated ribosome: Vmax = 3.03 ± 0.02 sec−1, Km = 0.41 ± 0.08 μM. WT ribosome: Vmax = 1.82 ± 0.01 sec−1, Km = 0.84 ± 0.05 μM. Errors given as standard error of the fit.
PRE complexes in which the peptidyl-tRNA body occupies a hybrid position are the preferred substrates for EF-G catalyzed translocation
Previous investigations have suggested that the “unlocked state” of the PRE complex represents a key intermediate during translocation (Valle et al. 2003; Agirrezabala et al. 2008; Julian et al. 2008). The unlocked state minimally entails P/E hybrid state formation, rotation of the small ribosomal subunit with respect to the large in the direction of translocation and closure of the L1 stalk (Helices 76–78 of 23S rRNA and the ribosomal protein L1). Using smFRET imaging methods, the unlocked state can be monitored in ribosome complexes site-specifically labeled at the L1 protein and P-site tRNA by formation of a high-FRET state that occurs when the L1 stalk contacts deacylated tRNA in the P/E hybrid state (Fei et al. 2008, 2009; Munro et al. 2010a). Consistent with this view, enhancements in the rate of unlocked state formation correlate with increased rates of translocation (Munro et al. 2010c).
Predicting that H1 state occupancy lies along the reaction coordinate for translocation, we set out to examine the rate of unlocked state formation in both wild-type and ASF-truncated PRE complexes (Supplemental Fig. S4). Here, the L1 protein was fluorescently labeled with Cy5 (Cy5-S55C), and deacylated P-site tRNAfMet was labeled with Cy3 (Cy3-s4U8). Consistent with the proposed model, transitions into the unlocked state occurred approximately fourfold more rapidly in ASF-truncated PRE complexes, while the rate leaving the unlocked state (kHigh→) decreased by ∼20% (Supplemental Table S2). As a result, unlocked state occupancy increased from 3 ± 1% (WT) to 9 ± 3% (ASF-truncated). Thus, consistent with the model that unlocked state formation is a key translocation intermediate, the enhanced A/P hybrid state occupancy observed in the context of the ASF truncation lowers the activation barrier for unlocked state formation.
To test the relationship between H1 FRET state occupancy and the rate of translocation, we next followed the reaction of EF-G with WT and ASF-truncated PRE complexes using a newly established single-molecule fluorescence-based translocation assay (Munro et al. 2010c). This assay is based on an increase in fluorescence intensity that occurs when Cy3-labeled peptidyl-tRNA (acp3U47) is translocated to the P site (Supplemental Fig. S5). When buffer containing no EF-G/GTP was delivered to surface-immobilized ribosomes, spontaneous translocation events were rarely observed (<1%) in the wild-type and ASF-truncated PRE complexes (Supplemental Table S3), consistent with the notion that the present experimental conditions (23° C, 15 mM Mg2+) generally preclude such processes (Blanchard et al. 2004). As anticipated from previous studies (Walker et al. 2008; Munro et al. 2010c), the rate of translocation observed for the WT PRE complex was EF-G concentration-dependent, where the Vmax for the WT complex was ∼1.6 sec−1 and the KM for EF-G was ∼0.9 μM. As expected from earlier studies (Komoda et al. 2006), ASF-truncated PRE complexes translocated approximately twofold more rapidly that the WT system (Vmax ∼ 3.1 sec−1) (Fig. 6B). For both WT and ASF-truncated PRE complexes, this step-wise increase in Cy3 fluorescence intensity was only observed in the presence of EF-G(GTP). Notably, the observed dependence of translocation rate on EF-G concentration also suggests that the ASF truncation decreased the KM for EF-G to the PRE complex by ∼50% (0.41 ± 0.08 μM). Correspondingly, this model predicts that EF-G engages the A site more rapidly in the ASF-truncated PRE complex than for the wild-type complex. This notion was directly tested by monitoring the rate at which EF-G engages the A site of the ribosome, marked by the appearance of FRET between Cy3-labeled tRNAPhe (Cy3- acp3U47) and Cy5-labeled EF-G (C terminus) (Munro et al. 2010c) (Supplemental Fig. S6A). As described previously (Munro et al. 2010c), the rate at which EF-G engages the A site reflects initial binding events as well as conformational processes in EF-G that require GTP hydrolysis and follows a hyperbolic saturation profile. The time interval between injection of EF-G/GTP and the first appearance of FRET was used to calculate keng, the rate at which EF-G productively engaged the A site (Supplemental Fig. S6A). As expected from the translocation data, this rate increased approximately twofold in the context of the ASF truncation (wild-type ∼ 1.3 sec−1 vs. ASF-truncated ∼ 2.6 sec−1) (Supplemental Fig. S6B). Taken together, these findings suggest that PRE complexes simultaneously occupying both the H1 and unlocked state closely resemble the preferred substrate for EF-G catalyzed translocation.
DISCUSSION
Structural features of the bacterial PRE complexes, revealed by cryo-EM (Frank and Agrawal 2000; Valle et al. 2003; Agirrezabala et al. 2008; Julian et al. 2008; Fischer et al. 2010; Ratje et al. 2010) and X-ray crystallographic methods (Selmer et al. 2006; Voorhees et al. 2009) provide a physical framework for understanding of the mechanism of translocation. However, despite the many breakthroughs in these areas, an understanding of how EF-G specifically recognizes and acts on the PRE complex to catalyze translocation has remained elusive. smFRET imaging and molecular dynamics simulations (Blanchard et al. 2004; Munro et al. 2007; Cornish et al. 2008; Fei et al. 2008, 2009; Reblova et al. 2009; Feldman et al. 2010; Munro et al. 2010a,b,c) have provided new insights into this aspect of the translation mechanism by revealing that the ribosome is intrinsically dynamic in nature and that such properties are a critical determinant of directional substrate movement.
Formation of the P/E hybrid state, subunit rotation, and L1 stalk closure are spontaneous, reversible processes that occur on distinct time scales (Munro et al. 2010a,b). Such findings help explain the strong temperature and condition dependence of the rate of translocation as well as the mechanisms of antibiotic action on the ribosome. When such processes correlate in time, the unlocked state of the PRE complex is achieved—a multistep process (Munro et al. 2010a) that reportedly limits the rate at which EF-G can productively engage the A site (Munro et al. 2010c).
A critical shortcoming of such investigations relates to the paucity of information regarding the positioning of peptidyl-tRNA when EF-G productively engages the A site. Prior investigations suggest that the position of peptidyl-tRNA in the A site plays a critical role in translocation (Semenkov et al. 2000; Dorner et al. 2006). Correspondingly, the precise nature and timing of peptidyl-tRNA motions within the PRE complex and their role in the mechanism of translocation has remained a matter of open debate (Agirrezabala et al. 2008; Julian et al. 2008; Walker et al. 2008; Fischer et al. 2010; Munro et al. 2010a,c; Ratje et al. 2010; Dunkle et al. 2011). Recent single-molecule investigations attempting to address this question suggest that EF-G can bind to the PRE complex with peptidyl-tRNA in both classical and hybrid configurations (Chen et al. 2011). Such findings are, indeed, consistent with earlier reports showing the rate of GTP hydrolysis is unaffected by tRNA positions within the PRE complex (Rodnina et al. 1997; Katunin et al. 2002; Seo et al. 2006; Walker et al. 2008). However, concrete conclusions regarding the conformation of the ribosome immediately prior to translocation were not obtained, in part due to the time resolution of the experiments performed.
Fast repositioning of the 3′ CCA end of peptidyl-tRNA within the A-site
Here, we have shown that the introduction of single point mutations in either the A or P loops of 23S rRNA, which markedly remodel the positions and dynamics of A- and P-site tRNAs within the PRE complex, can be efficiently restored to wild-type positions by compensatory mutations in the 3′-CCA acceptor stems of A- and P-site tRNA, respectively. These data corroborate the critical role that base-pairing interactions between tRNA and the large subunit of the ribosome are known to play in positioning of tRNA substrates within the PRE complex. They also provided an important validation of the use of site-directed mutagenesis to probe the ribosome energy landscape and the mechanism of translocation. Taken together, the structural and kinetic findings presented here, along with recent cryo-EM (Agirrezabala et al. 2008; Julian et al. 2008) data, suggest that contemporary models of PRE translocation dynamics and the classical-hybrid tRNA equilibrium must be revised.
The strategies used to enforce the base-pairing of the 3′-CCA end of peptidyl-tRNA in the A site with the large subunit P loop, and therefore the A/P hybrid state, stabilized the PRE complex in a configuration previously assigned as an H2 hybrid state, bearing deacylated tRNA in a P/E hybrid state and peptidyl-tRNA in a classical (A/A) position (Munro et al. 2007). These data are in global agreement with recent cryo-EM studies of the PRE complex (Agirrezabala et al. 2008; Julian et al. 2008; Fischer et al. 2010), arguing that the 3′-CCA end of peptidyl-tRNA can move independently of the tRNA body. Such findings support the speculation (Munro et al. 2009) that the H2 FRET state represents a mixture of hybrid PRE complex configurations (P/E; A/A and P/E; A/P), which are distinguished by the position of the 3′-CCA terminus of peptidyl-tRNA on the large subunit (Fig. 7). Hybrid and classical configurations of the A-site tRNA acceptor stem are likely to be in rapid, dynamic exchange and necessarily governed by the initial movement of P-site tRNA into its hybrid position. Dynamics of this nature likely occur without significant changes in the position of the peptidyl-tRNA body or substantial conformational changes within the PRE complex. Hence, the H2 hybrid state previously described (Munro et al. 2007) likely represents a mixture of peptidyl-tRNA configurations. In keeping with established nomenclature, PRE complexes in which the 3′-CCA end of peptidyl-tRNA pairs with the A loop are in an H2 configuration, while PRE complexes in which the 3′-CCA end of peptidyl-tRNA pairs with the P loop are in an H2* configuration. While this later state represents a PRE complex configuration in which both tRNAs occupy hybrid positions (P/E; A/P), the peptidyl-tRNA body remains classically configured in the A site.
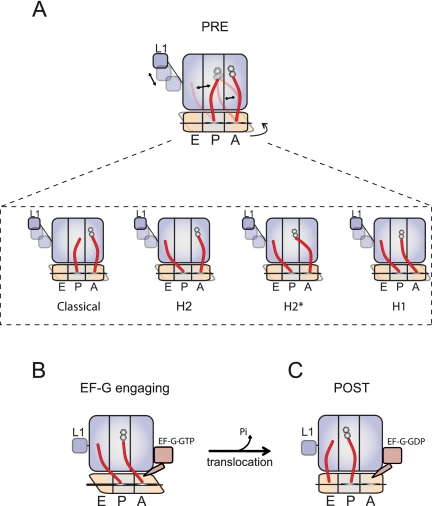
FIGURE 7.
Intrinsic motions of tRNA within the PRE complex and their relationship to EF-G catalyzed translocation. (A) Spontaneous conformational changes in the PRE complex result in motions of A- and P-site tRNAs with respect to each other and the ribosome. Together with previous investigations (Munro et al. 2007; Agirrezabala et al. 2008; Julian et al. 2008), the present findings suggest the existence of an additional hybrid state intermediate, the H2* state, in which the 3′-CCA end of peptidyl-tRNA occupies the large subunit P site, while the peptidyl-tRNA body remains classically configured within the A site. (B) The present findings suggest that EF-G preferentially engages the A site of the PRE complex once it has spontaneously achieved a configuration in which (1) the unlocked state has been achieved (minimally including small subunit rotation, P/E hybrid state formation and L1 stalk closure [Munro et al. 2010a]) and (2) the H1 state has been achieved wherein the body of peptidyl-tRNA in the A site has moved toward the P site. (C) After productively engaging the A site, EF-G facilitates the process of translocation.
Remodeling of the ASF regulates motion of the peptidyl-tRNA body and translocation
In contemporary models of the classical-hybrid tRNA equilibrium (Munro et al. 2007), formation of the H1 FRET state requires one or more large-scale remodeling events in the PRE complex that allow the peptidyl-tRNA body to move in the direction of translocation from its classical position (A/A) toward the P site. This transition was presumed to represent formation of the A/P hybrid state. Structural considerations suggested that such events may be related to remodeling of the inter-subunit bridge, B1a, which includes the ASF. However, as an A/P hybrid state can be achieved without bridge B1a remodeling (Agirrezabala et al. 2008; Julian et al. 2008), the nature and role of the H1 FRET state in the mechanism of translocation was largely unclear.
The smFRET experiments presented here reveal that the H1 FRET state is specifically stabilized in PRE complexes bearing truncations of the ASF previously shown to increase the rate of translocation (Komoda et al. 2006). Consistent with structural data showing that residues within the ASF (881–883 and 898–899; E.coli numbering) physically interact with A-site tRNA (Korostelev et al. 2006), these findings reveal that remodeling of the ASF regulates the motion of the peptidyl-tRNA body into, and out of, a hybrid position. The importance of such motions to ribosome function is highlighted first by the finding that ASF-truncated PRE complexes exhibit dramatically enhanced rates of puromycin reactivity. Furthermore, ASF-truncated complexes translocated more rapidly than wild-type PRE complexes and displayed a lower KM for EF-G catalyzed translocation. Together with the finding that the “unlocked state” is achieved more rapidly in the context of the ASF truncation, these data collectively suggest that the preferred substrate for EF-G catalyzed translocation is one in which both the H1 hybrid and unlocked states have been achieved. In line with previous findings (Munro et al. 2010c), the rate of unlocked state formation remained closely correlated with the observed rate of translocation. However, given that the unlocked state can be achieved in the absence of substrate translocation, conformational events downstream from unlocked state formation must be rate-determining for substrate movement. In light of previous biochemical (Peske et al. 2004) and structural (Zhang et al. 2009; Fischer et al. 2010; Ratje et al. 2010; Budkevich et al. 2011; Dunkle et al. 2011) data, the present findings implicate remodeling events within the PRE complex—related to conformational processes in the small subunit involving bridge B1a and the repositioning of peptidyl-tRNA body toward the P site—as playing a critical role in the translocation mechanism.
Structural remodeling of the ASF and the mechanism of translocation
While truncation of the ASF may simply relieve a steric hindrance that prevents passive diffusion of peptidyl-tRNA toward the P site, the observation that the ASF truncation also enhances the rate of P/E hybrid state formation suggests that it has a more global impact on conformational changes within the PRE complex. We speculate here that such motions likely entail remodeling events that influence the head domain of the 30S subunit. Swivel-like motions of the small subunit head in the direction of translocation have recently been implicated in controlling motions of the P-site tRNA toward the E site prior to translocation (Ratje et al. 2010). In addition, modeling efforts reported in this body of work suggested that 30S head swivel may enable EF-G to engage the small subunit A site within the PRE complex. Such efforts included the modeling of the peptidyl-tRNA body in an A/P hybrid configuration consistent with the FRET value assigned to the H1 hybrid state.
Swivel-like motions of the 30S head domain are posited to include a loss of interactions between ribosomal protein S13 and the tip of the ASF (Yusupov et al. 2001; Valle et al. 2003; Ratje et al. 2010). Correspondingly, the ASF's interactions with S13 in the classical state may attenuate motions of the head domain in a manner that sterically controls the motions of the peptidyl-tRNA body toward the P site. Thus, the remodeling of bridge B1a and head swivel appear to play an important role in regulating the coupling of A- and P-site tRNA motions toward their hybrid positions as well as the rate at which EF-G can engage the A site. Such observations suggest that spontaneous ASF remodeling likely contributes directly to the translocation reaction coordinate.
While shedding new light on the nature of hybrid tRNA configurations and spontaneous remodeling events in the PRE complex required for translocation, the present findings lead to further questions that will need to be addressed through future investigations. In particular, a direct means of ascertaining the order and timing of unlocked state formation, A- and P-site hybrid state formation, head swivel, and EF-G binding will be needed to more fully delineate the mechanism of translocation. In addition to providing critical insights into the molecular basis of translational fidelity, such endeavors may serve as a springboard for in-depth investigations of antibiotic action, programmed frameshifting, and other cellular mechanisms for the regulation of translation elongation.
MATERIALS AND METHODS
Ribosome expression and purification
All ribosomes used in the present experiments contain an MS2 tag in 23S rRNA expressed as previously described (Youngman and Green 2005). Preparation of MS2-tagged ribosomes was performed according to previously described procedures (Feldman et al. 2010). Truncation of the A-site finger (H38) was achieved by site-directed deletion of residues 876–901 of 23S rRNA and replacement of the closing loop sequence with a GAGA tetra loop, mutations previously shown to enhance the rate of translocation (Komoda et al. 2006).
Preparation of tRNAs with modified 3′ ends
Plasmids expressing tRNAfMet and tRNAPhe were kind gifts from Dr. Uttam L. RajBhandary and Dr. Knud Nierhaus, respectively (Seong and RajBhandary 1987; Junemann et al. 1996). Description of the tRNA purification protocols is detailed in Supplemental Material. To remove nucleotides from the 3′ end of tRNAfMet and tRNAPhe, tRNAs were incubated with snake venom phosphodiesterase I (SVP) from Worthington Biochemical Corporation in 50 mM Tris OAc pH 8.5, 50 mM KOAc, and 10 mM Mg(OAc)2 at a ratio of 8 nmoles tRNA/1 Unit SVP at 37°C for 30 min. The digested tRNAs were purified on a TSK Phenyl 5PW column as described in Supplemental Material. The ligation of specific RNA oligonucleotides (Dharmacon) onto the SVP-cleaved tRNAs was achieved by incubating them at a 10-fold molar excess over tRNA in the presence of 0.5 units of T4 RNA ligase I (New England BioLabs) on ice for 18–24 h. Ligation efficiency ranged from 40%–80% depending on the specific oligonucleotide sequence and tRNA identity. Ligated products were purified according to the procedures described for native tRNAs (Supplemental Material).
Preparation of ribosome complexes, ternary complex formation, and the site-specific labeling of tRNAs and the L1 protein
The preparation of pretranslocation ribosome complexes, ternary complex formation, and the labeling of tRNAs and the L1 (S55C) protein were achieved as previously described (Munro et al. 2007, 2010a,b).
Single-molecule fluorescence imaging
All single-molecule data were acquired using a lab-built prism-based TIR fluorescence microscope with a 1.2 NA 60× water-immersion objective (Nikon). All data were taken in Tris-polymix buffer, 15 mM Mg(OAc)2 using an oxygen-scavenging system and a cocktail of triplet state quenchers containing Trolox, cyclooctatetraene, and nitrobenzylalcohol, as previously described (Munro et al. 2007; Dave et al. 2009; Feldman et al. 2010). The Cy3 fluorophore within the PRE complex was directly excited using a single frequency diode laser (Ventus 532; Laser Quantum). Data were collected using a Photometrics Cascade 512 CCD (Roper Scientific) at a frame rate of 25 sec−1 and recorded using the MetaMorph imaging software package (Universal Imaging Corporation).
Analysis of FRET traces and hidden Markov modeling
The analysis of single-molecule fluorescence and FRET traces was performed using a suite of custom-made analytical software implemented in Matlab (The MathWorks) (Feldman et al. 2010). The segmental k-means algorithm (Qin 2004) was used to idealize each FRET trace. As previously described (Munro et al. 2010a), individual FRET trajectories recorded from PRE complexes containing Cy3- and Cy5-labeled tRNAs were idealized using a four state hidden Markov model; individual FRET trajectories recorded from PRE complexes containing Cy3- and Cy5-labeled P-site tRNA and L1 protein were idealized using a five state hidden Markov model. Time-averaged occupancies in each FRET state were calculated from the total dwell times observed divided by the total dwell time in all nonzero FRET states (Feldman et al. 2010). Kinetic parameters for conformational transitions were estimated from the dwell times observed in each state using the maximum interval likelihood algorithm implemented in QuB.
Puromycin reactivity assays
Single-molecule puromycin assays were performed as previously described in which a single, Cy3 fluorophore was covalently linked to the α-amino group of Met-tRNAfMet (Munro et al. 2007; Feldman et al. 2010). Puromycin (2 mM) was delivered to surface-immobilized complexes by stopped-flow, where the mixing time is ∼25–50 msec. The loss of fluorescence over time, reporting on peptide release, was followed by strobe illumination, taking a single, 40-msec frame every 20 sec over a period of 20 min. The fluorescence intensity for each experiment was normalized to the total image intensity of the first frame. The rate of puromycin reactivity can be approximated by fitting each fluorescence decay profile with a double exponential function (A1e-k1t+A2e-k2t) using the Origin software package (OriginLab). The weighted average of the two decay constants was used to estimate the rate of puromycin reactivity.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank Dr. Tsutomu Suzuki and members of his laboratory for helpful discussions as well as for the initial ASF-truncated ribosome constructs used to pilot the present investigations. We would also like to acknowledge helpful discussions and insights provided by all members of the Blanchard laboratory. This work was supported by the National Institute of Health (1R01GM079238), the Human Frontiers in Science Program (RGY0088), and the National Science Foundation (0644129).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.029033.111.
REFERENCES
- Agirrezabala X, Frank J 2009. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q Rev Biophys 42: 159–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirrezabala X, Lei J, Brunelle JL, Ortiz-Meoz RF, Green R, Frank J 2008. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol Cell 32: 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SC, Kim HD, Gonzalez RL Jr, Puglisi JD, Chu S 2004. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci 101: 12893–12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS 1968. Translocation in protein synthesis: A hybrid structure model. Nature 218: 675–677 [DOI] [PubMed] [Google Scholar]
- Brunelle JL, Youngman EM, Sharma D, Green R 2006. The interaction between C75 of tRNA and the A loop of the ribosome stimulates peptidyl transferase activity. RNA 12: 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budkevich T, Giesebrech J, Altman R, Munro J, Mielke T, Nierhaus KH, Blanchard S, Spahn C 2011. Structure and dynamics of the mammalian ribosomal pre-translocation complex. Mol Cell (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Stevens B, Kaur J, Cabral D, Liu H, Wang Y, Zhang H, Rosenblum G, Smilansky Z, Goldman YE, et al. 2011. Single-molecule fluorescence measurements of ribosomal translocation dynamics. Mol Cell 42: 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish PV, Ermolenko DN, Noller HF, Ha T 2008. Spontaneous intersubunit rotation in single ribosomes. Mol Cell 30: 578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave R, Terry DS, Munro JB, Blanchard SC 2009. Mitigating unwanted photophysical processes for improved single-molecule fluorescence imaging. Biophys J 96: 2371–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner S, Brunelle JL, Sharma D, Green R 2006. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat Struct Mol Biol 13: 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle JA, Wang L, Feldman MB, Pulk A, Chen VB, Kapral GJ, Noeske J, Richardson JS, Blanchard SC, Cate JH 2011. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332: 981–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J, Kosuri P, MacDougall DD, Gonzalez RL Jr 2008. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol Cell 30: 348–359 [DOI] [PubMed] [Google Scholar]
- Fei J, Bronson JE, Hofman JM, Srinivas RL, Wiggins CH, Gonzalez RL Jr 2009. Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation. Proc Natl Acad Sci 106: 15702–15707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg JS, Joseph S 2006. A conserved base-pair between tRNA and 23 S rRNA in the peptidyl transferase center is important for peptide release. J Mol Biol 364: 1010–1020 [DOI] [PubMed] [Google Scholar]
- Feldman MB, Terry DS, Altman RB, Blanchard SC 2010. Aminoglycoside activity observed on single pre-translocation ribosome complexes. Nat Chem Biol 6: 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H 2010. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature 466: 329–333 [DOI] [PubMed] [Google Scholar]
- Frank J, Agrawal RK 2000. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406: 318–322 [DOI] [PubMed] [Google Scholar]
- Fu J, Munro JB, Blanchard SC, Frank J 2011. Cryoelectron microscopy structures of the ribosome complex in intermediate states during tRNA translocation. Proc Natl Acad Sci 108: 4817–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian P, Konevega AL, Scheres SH, Lazaro M, Gil D, Wintermeyer W, Rodnina MV, Valle M 2008. Structure of ratcheted ribosomes with tRNAs in hybrid states. Proc Natl Acad Sci 105: 16924–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junemann R, Wadzack J, Triana-Alonso FJ, Bittner JU, Caillet J, Meinnel T, Vanatalu K, Nierhaus KH 1996. In vivo deuteration of transfer RNAs: Overexpression and large-scale purification of deuterated specific tRNAs. Nucleic Acids Res 24: 907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunin VI, Savelsbergh A, Rodnina MV, Wintermeyer W 2002. Coupling of GTP hydrolysis by elongation factor G to translocation and factor recycling on the ribosome. Biochemistry 41: 12806–12812 [DOI] [PubMed] [Google Scholar]
- Kim DF, Green R 1999. Base-pairing between 23S rRNA and tRNA in the ribosomal A site. Mol Cell 4: 859–864 [DOI] [PubMed] [Google Scholar]
- Komoda T, Sato NS, Phelps SS, Namba N, Joseph S, Suzuki T 2006. The A-site finger in 23 S rRNA acts as a functional attenuator for translocation. J Biol Chem 281: 32303–32309 [DOI] [PubMed] [Google Scholar]
- Korostelev A, Trakhanov S, Laurberg M, Noller HF 2006. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell 126: 1065–1077 [DOI] [PubMed] [Google Scholar]
- Korostelev A, Ermolenko DN, Noller HF 2008. Structural dynamics of the ribosome. Curr Opin Chem Biol 12: 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman KR, Dahlberg AE 1994. The importance of conserved nucleotides of 23 S ribosomal RNA and transfer RNA in ribosome catalyzed peptide bond formation. J Biol Chem 269: 16163–16169 [PubMed] [Google Scholar]
- Moazed D, Noller HF 1989. Intermediate states in the movement of transfer RNA in the ribosome. Nature 342: 142–148 [DOI] [PubMed] [Google Scholar]
- Munro JB, Altman RB, O'Connor N, Blanchard SC 2007. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell 25: 505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Sanbonmatsu KY, Spahn CM, Blanchard SC 2009. Navigating the ribosome's metastable energy landscape. Trends Biochem Sci 34: 390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Altman RB, Tung CS, Cate JH, Sanbonmatsu KY, Blanchard SC 2010a. Spontaneous formation of the unlocked state of the ribosome is a multistep process. Proc Natl Acad Sci 107: 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Altman RB, Tung CS, Sanbonmatsu KY, Blanchard SC 2010b. A fast dynamic mode of the EF-G-bound ribosome. EMBO J 29: 770–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Wasserman MR, Altman RB, Wang L, Blanchard SC 2010c. Correlated conformational events in EF-G and the ribosome regulate translocation. Nat Struct Mol Biol 17: 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle JM, Ramakrishnan V 2005. Structural insights into translational fidelity. Annu Rev Biochem 74: 129–177 [DOI] [PubMed] [Google Scholar]
- Pan D, Kirillov SV, Cooperman BS 2007. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell 25: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peske F, Savelsbergh A, Katunin VI, Rodnina MV, Wintermeyer W 2004. Conformational changes of the small ribosomal subunit during elongation factor G-dependent tRNA-mRNA translocation. J Mol Biol 343: 1183–1194 [DOI] [PubMed] [Google Scholar]
- Qin F 2004. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J 86: 1488–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratje AH, Loerke J, Mikolajka A, Brunner M, Hildebrand PW, Starosta AL, Donhofer A, Connell SR, Fucini P, Mielke T, et al. 2010. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 468: 713–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reblova K, Razga F, Li W, Gao H, Frank J, Sponer J 2009. Dynamics of the base of ribosomal A-site finger revealed by molecular dynamics simulations and Cryo-EM. Nucleic Acids Res 38: 1325–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV, Wintermeyer W 2001. Fidelity of aminoacyl-tRNA selection on the ribosome: Kinetic and structural mechanisms. Annu Rev Biochem 70: 415–435 [DOI] [PubMed] [Google Scholar]
- Rodnina MV, Savelsbergh A, Katunin VI, Wintermeyer W 1997. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature 385: 37–41 [DOI] [PubMed] [Google Scholar]
- Samaha RR, Green R, Noller HF 1995. A base pair between tRNA and 23S rRNA in the peptidyl transferase centre of the ribosome. Nature 377: 309–314 [DOI] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FV IV, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V 2006. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313: 1935–1942 [DOI] [PubMed] [Google Scholar]
- Semenkov YP, Rodnina MV, Wintermeyer W 2000. Energetic contribution of tRNA hybrid state formation to translocation catalysis on the ribosome. Nat Struct Biol 7: 1027–1031 [DOI] [PubMed] [Google Scholar]
- Seo HS, Abedin S, Kamp D, Wilson DN, Nierhaus KH, Cooperman BS 2006. EF-G-dependent GTPase on the ribosome. Conformational change and fusidic acid inhibition. Biochemistry 45: 2504–2514 [DOI] [PubMed] [Google Scholar]
- Seong BL, RajBhandary UL 1987. Escherichia coli formylmethionine tRNA: Mutations in GGGCCC sequence conserved in anticodon stem of initiator tRNAs affect initiation of protein synthesis and conformation of anticodon loop. Proc Natl Acad Sci 84: 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Southworth DR, Green R 2004. EF-G-independent reactivity of a pre-translocation-state ribosome complex with the aminoacyl tRNA substrate puromycin supports an intermediate (hybrid) state of tRNA binding. RNA 10: 102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin AS 1968. On the mechanism of ribosome function. The hypothesis of locking-unlocking of subparticles. Dokl Akad Nauk SSSR 179: 1467–1470 [PubMed] [Google Scholar]
- Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J 2003. Locking and unlocking of ribosomal motions. Cell 114: 123–134 [DOI] [PubMed] [Google Scholar]
- Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V 2009. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat Struct Mol Biol 16: 528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SE, Shoji S, Pan D, Cooperman BS, Fredrick K 2008. Role of hybrid tRNA-binding states in ribosomal translocation. Proc Natl Acad Sci 105: 9192–9197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JS, Parnell KM, Dorner S, Green R, Strobel SA 2004. Substrate-assisted catalysis of peptide bond formation by the ribosome. Nat Struct Mol Biol 11: 1101–1106 [DOI] [PubMed] [Google Scholar]
- Youngman EM, Green R 2005. Affinity purification of in vivo-assembled ribosomes for in vitro biochemical analysis. Methods 36: 305–312 [DOI] [PubMed] [Google Scholar]
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292: 883–896 [DOI] [PubMed] [Google Scholar]
- Zaher HS, Green R 2009. Fidelity at the molecular level: Lessons from protein synthesis. Cell 136: 746–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Dunkle JA, Cate JH 2009. Structures of the ribosome in intermediate states of ratcheting. Science 325: 1014–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]