Abstract
Consumption of seafood enriched in n-3 polyunsaturated fatty acids (PUFA) is associated with a decreased risk of cardiovascular disease. Several n-3 oxidation products from eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) have known protective effects in the vasculature. It is not known whether consumption of cooked seafood enriched in n-3 PUFA causes appreciable consumption of lipid oxidation products. We tested the hypothesis that baking Atlantic salmon (Salmo salar) increases the level of n-3 and n-6 PUFA oxidation products over raw salmon. We measured the content of several monohydroxy-fatty acids (MHFA), prostanoids, and resolvins. Our data demonstrate that baking did not change the overall total levels of MHFA. However, baking resulted in selective regio-isomeric loss of hydroxy fatty acids from arachidonic acid (20:4n-6), and EPA while significantly increasing hydroxyl-linoleic acid levels. The content of prostanoids and resolvins were reduced several-fold with baking. The inclusion of coating upon the salmon prior to baking reduced the loss of some MHFA but had no effect upon prostanoid losses incurred by baking. Baking did not decrease n-3 PUFA content indicating that baking of salmon is an acceptable means of preparation that does not alter the potential health benefits of high n-3 seafood consumption. The extent to which the levels of MHFA, prostanoids and resolvins in the raw or baked fish have physiologic consequence for humans needs to be determined.
Keywords: Salmon, lipid peroxidation, resolvins, prostaglandins
Introduction
Elevated consumption of n-3 polyunsaturated fatty acids (PUFA) is associated with reduced risk for cardiovascular disease (1, 2). Current data indicate that an intake of 250 mg/day of eicosapentaenoic acid (EPA 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), equivalent to less than 3 kcal of PUFA-derived energy, is sufficient to reduce cardiovascular disease risk (2). Increased consumption of n-3 PUFA easily occurs by including more seafood in the diet. In particular, Atlantic salmon (Salmo salar) is an excellent source of EPA and DHA.
The processing and cooking of seafood leads to the production of lipid peroxidation products such as oxy-cholesterols, hydro(pero)xy fatty acids, and aldehydes derived from cholesterol, n-3 PUFA and n-6 PUFA (3–6). Data by Al-Saghir demonstrates that frying and steaming of salmon minimally increases (< 15%) levels of conjugated dienes (a marker of hydro(pero)xy fatty acid content) over raw salmon (3). On the other hand, total peroxides are elevated with frying (mostly a result of added oil) but not with steaming (7). Oxidized cholesterols such as 7-ketocholesterol and 7-hydroxycholesterol are elevated with roasting or frying of salmon (3, 4). Unlike the analyses of cholesterol oxidation products, little data exist regarding speciation of fatty acid oxidation products in raw salmon versus cooked.
In vitro and in vivo studies demonstrate that n-3 PUFA oxidation (enzymatic and free-radical catalyzed) leads to the formation potentially cytotoxic products such as trans-4-hydroxy-2-hexenal and trans-4-oxo-2-hexenal and to the formation of cytoprotective and anti-inflammatory compounds such as F3-isoprostanes, F3-cyclopentenones, and resolvins and protectins (6, 8–16). Thus, the question arises as to whether relevant amounts of n-3 fatty acid oxidation products are consumed when eating salmon and how the content of oxidation products are altered by baking, a common method of salmon preparation.
In this work, we tested the hypothesis that baking of salmon fillets would result in the increase in n-3 and n-6 derived lipid oxidation products. As part of a controlled feeding study, we tested uncooked salmon fillets versus a variety of salmon fillets baked with different coatings. In contrast to our hypothesis, our data indicate that, except for hydroxy-linoleic acid derivatives, cooking leads to a decrease in most n-3 and n-6 lipid oxidation products in the finished salmon fillet compared to uncooked salmon.
Materials and Methods
Chemicals
HPLC and reagent grade n-hexane, 2-propanol, anhydrous methanol and other solvents were purchased from EM Science (Gibbstown, NJ). Sodium methoxide was purchased from Sigma Chemicals (St Louis, MO) and fatty acid methyl ester, phospholipid, and methyl triheptadecanoin standards were purchased from Nu-Chek Prep (Elysian, MN). All dueterated and non-deuterated prostanoids, mono-hydroxyfatty acids (MHFA), and resolvins were purchased from Cayman Chemical Co. (Ann Arbor, MI).
Salmon preparation
Atlantic salmon (Salmo salar) were raised by Cooke Aquaculture, Blacks Harbor New Brunswick, Canada. Salmon smolts were stocked into an industry sea cage. Fish were fed a commercial dry pellet based on size and adjusted based on water temperature. For the first two months following stocking, fish were fed 4 times per day with 3.0 mm feed. Fish were then fed a suitable sized commercial diet until harvest, but were not fed when water temperatures were less than 1.5° C. Salmon were processed to remove skin and a thin layer of external fat. Fillets were wrapped in polyethylene bags and immediately shipped on ice from Maine to the Grand Forks Human Nutrition Research Center, Grand Forks, ND. Upon arrival, salmon fillets were immediately transferred to frozen storage at −20° C until use.
Salmon fillets were prepared in the manner designed for service in an upcoming clinical trial. Salmon fillets were thawed at 4° C overnight in a commercial food refrigerator. Salmon was either left frozen (uncooked) or prepared in 90 g portions with each of six different recipes, as shown in Table 1. Salmon filets were placed atop 100 g portions of brown rice in a single portion bake and serve container (Pactiv Pressware, Columbus, OH) and covered with the remaining ingredients for each recipe. Each portion was baked in a preheated oven at 177° C (350° F) for 30 minutes, or until the fish reached an internal temperature of 63° C (145° F) according to food service guidelines (17), removed from the oven and sealed with film and immediately placed in an −20° C commercial food freezer until being sent to the analytic laboratory for processing. Upon arrival at the analytic laboratory, the samples were held at −80° C until analysis. In a separate determination, cooking reduced the original 100 g mass of the raw filets to a mean of 93.5 grams (n = 2), a loss of 6.5% in total weight.
Table 1.
Salmon recipes
| Recipe | Ingredients | Grams |
|---|---|---|
| Plain salmon | Salmon, raw | 90.0 |
|
| ||
| Butter | 10.0 | |
|
| ||
| Dill salmon | Salmon, raw | 90.0 |
| Dill, dry | 0.2 | |
| Onion powder | 0.5 | |
| Butter | 10.0 | |
|
| ||
| Barbecue salmon | Salmon, raw | 90.0 |
| Barbecue sauce | 10.0 | |
|
| ||
| Italian salmon | Salmon, raw | 90.0 |
| Italian seasoning | 2.0 | |
| Butter | 10.0 | |
|
| ||
| Lemon garlic salmon | Salmon, raw | 90.0 |
| Lemon juice | 5.0 | |
| Lemon pepper | 2.0 | |
| Garlic | 2.0 | |
| Butter | 10.0 | |
|
| ||
| Teriyaki salmon | Salmon, raw | 90.0 |
| Teriyaki sauce | 10.0 | |
Phospholipid Fatty Acid Analysis
Phospholipid fatty acid content of the salmon fillets was determined in raw and plain roasted salmon. Tissue samples (1.1–1.6 g) were isolated from ice cold raw and baked salmon filets using a 2.5 cm coring device. Total lipid from the samples were extracted with n-hexane/2-propanol (3:2, by Vol.) using a glass Tenbroeck homogenizer as described by Radin (18). Sample extracts were concentrated to zero under a steady-stream of N2 at 50° C then re-solvated in 4.0 mL n-hexane/2-propanol (3:2, by Vol.) and stored at −80° C until use. Phospholipid from extracted samples run in triplicate were isolated from a portion of the extracts by thin layer chromatography (TLC) (silica gel 60, EMD Chemicals, Darmstadt, Germany) using a solvent system of heptane/isopropyl ether/glacial acetic acid (60:40:4, by Vol.). Bands corresponding to phospholipid standards found at the origin were scrapped off the TLC plate and transferred to a glass test tube. Methyl triheptadecanoin (internal standard) was added to the test tube and the samples were allowed to dry at 110° C for 45 min. Esterified fatty acids in the sample were methylated in 2.5% sodium methoxide at 40° C for 60 min. The reaction was stopped with methyl formate and the fatty acid methyl esters were extracted with n-hexane.
Fatty acid methyl ester content was quantified using a Shimadzu 2010 gas chromatograph (Kyoto, Japan) equipped with a flame ionization detector and a capillary column (SP 2330; 30 m × 0.32 mm i.d., Supelco, Bellefonte, PA). Sample runs were initiated at 180° C followed by a temperature gradient to 200° C over 8 min starting at 2 min. The temperature was held at 200° C until the end of the run at 20 min. Fatty acid methyl ester standards were used to establish relative retention times and response factors. The internal standard, methyl heptadecanoate, and the individual fatty acids were quantified by peak area analysis (Shimadzu Class VP 7.2.1 Datasystem, Kyoto, Japan). The detector response was linear, with correlation coefficients of 0.998 or greater within the sample concentration range for all standards.
Analysis of lipid oxidation products
Prostanoids, resolvins, and MHFA were determined as previously described for prostanoids with modifications to allow for MHFA and resolvin determination (19, 20). Frozen raw and frozen prepared salmon samples were pulverized under liquid nitrogen conditions to a fine homogeneous powder. Fish samples (~50mg) were incubated in 200 μL of 80 mM Hepes buffer (pH 7.4) containing 300 mM sodium chloride, 20 mM CaCl2, 8 mM Triton X-100, 60% glycerol, 2 mg/ml BSA, 100 pg of PGE2-d4, 100pg of 5(S)-HETE-d8, and 1 ng of 20:4n6-d8 as internal standards with soluble phospholipaseA2 (sPLA2; ~ 0.9 μmole/min of total activity, Cayman Chemical Co, Ann Arbor, MI) to release esterified prostanoids and MHFA from phospholipids. This enzyme was tested for contamination with isoprostanes and no contamination was detected in the quantities of enzyme used in the experiments. To validate a completeness of prostanoid hydrolysis from phospholipids under these conditions, 20:4n-6 released from PL was quantified. After 1 h of incubation at ambient temperature, prostanoids and MHFA were extracted with acetone using liquid/liquid extraction as previously described (19, 20). This method allows for a ~90% of extraction efficiency for prostanoids and resolvins and increases sensitivity during MS analysis as the result of reduction of basal noise (20). After 10 min of centrifugation (2000 × g) at 4 °C, MHFA and free fatty acids were extracted from supernatant by using 3 × 2.0 ml of hexane with ~85% of efficiency as was determined by the recovery of 5(S)-HETE-d8, 9(S)-HODE-d4, (±)5-HEPE, and 20:4n6-d8 standards. Prostanoids were extracted from the same supernatant after MHFA extraction by acidification of supernatant with formic acid to pH = 3.5 (30 μL of 2M formic acid), and extraction with 2 mL of chloroform. The chloroform extract containing prostanoids was transferred to silanized with Sigmacote® (Sigma Chemical Co.,St. Louis, MO) tube, flushed with nitrogen, and cooled at −80°C for at least 15 min separate any residual upper phase, which is then removed and discarded before analysis. Prostanoid and MHFA extracts were dried down under a stream of nitrogen and transferred to 300 μL silanized microvial inserts (National Scientific, Rockwoods, TN; catalog No. C4010-S630) using 2 × 0.15 ml of hexane for prostanoids or chloroform/methanol (1:10) for MHFA. The solvent in microvial inserts was dried down under a stream of nitrogen, and 20 μL of acetonitrile was added for MHFA, or 30 μL of acetonitrile/water (1:2) for prostanoids, and vortexed for 30 s.
Reverse-phase HPLC electrospray ionization mass spectrometry for MHFA analysis
MHFA separation was carried out using a Luna C-18(2) column (3 μm, 100 A° pore diameter, 150 × 2.0 mm; Phenomenex, Torrance, CA) with a security guard cartridge system (C-18) (Phenomenex). The HPLC system consisted of an Agilent 1100 series LC pump equipped with a wellplate autosampler (Agilent Technologies, Santa Clara, CA). The autosampler was set at 4°C. Fifteen microliters out of a 20 μL sample was injected onto a chromatographic column. The solvent system was composed of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The flow rate was 0.2 ml/min, and the initial solvent conditions started with 10% solvent B. At 2 min, the percentage of B was increased to 65% over 8 min; at 15 min, the percentage of B was increased to 90% over 5 min, at 20 min it was increased to 98% over 15 min, and was kept at 98% for 20 min to wash hydrophobic substrates from the column; at 55 min, percentage of B was reduced to 10% over 5 min. Equilibration time between runs was 15 min.
MS analysis was performed using a quadrupole mass spectrometer (API3000; Applied Biosystems, Foster City, CA) equipped with a TurboIonSpray ionization source. Analyst software version 1.4.2 (Applied Biosystems) was used for instrument control, data acquisition, and data analysis. The mass spectrometer was optimized in the multiple reaction monitoring mode. The source was operated in negative ion electrospray mode at 450°C, electrospray voltage was −4,250 V, nebulizer gas was zero grade air at 8 L/min, and curtain gas was ultrapure nitrogen at 11 L/min. Precursor/product ion transitions, declustering potential, collision energy, focusing potential, entrance potential, and collision cell exit potential were optimized individually for each analyte as presented supplementary data (S1). Collision gas was 12 L/min for all analytes. The quadrupole mass spectrometer was operated at unit resolution. MHFA were quantified using 15-S-HETE-d8 as the internal standards.
Reverse-phase HPLC electrospray ionization mass spectrometry for prostanoid and resolvin analysis
Prostanoid and resolvin separation was performed using conditions as previously described (19) using the same column and instrumentation as described for MHFA. Twenty five μL out of a 30 μL sample was injected onto a chromatographic column. The solvent system was composed of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The flow rate was 0.2 ml/min, and the initial solvent conditions started with 20% solvent B. The solvent B was increased from 20% to 42.5% over 50 min, at 50 min was increased further to 90% over 10.5 min to wash the column, and at 65.5 min it was returned back to 20% over 1 min for column equilibration. Equilibration time between runs was 14 min.
MS analysis was performed using the same instrumentation as described for MHFA. The mass spectrometer was optimized in the multiple reaction monitoring mode. The source was operated in negative ion electrospray mode at 350°C, electrospray voltage was −4,250 V, nebulizer gas was zero grade air at 8 L/min, and curtain gas was ultrapure nitrogen at 11 L/min. Precursor/product ion transitions, declustering potential, collision energy, and collision cell exit potential were optimized individually for each analyte as presented in Table 3. Focusing potential was 2200 V, and entrance potential was 210 V for all analytes. Collision gas was 12 L/min for all analytes. The quadrupole mass spectrometer was operated at unit resolution. Prostanoids were quantified using PGE2-d4 as the internal standards.
Statistical Analysis
Data are normalized to 100 grams, representative of a serving size and are presented as the mean ± the S.D. Data were analyzed utilizing a one-way ANOVA with Dunnett's multiple comparison test or Student's t-test as appropriate using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). Statistical significance was taken as p ≤ 0.05.
Results
In this study, we measured the extent to which cooking altered the levels of lipid oxidation products in salmon to test the specific hypothesis that baking would elevate the level of lipid oxidation products. We measured numerous fatty acid oxidation products derived from enzymatic and non-enzymatic processes. Lipid oxidation products were determined using two separate chromatographic analyses. One analysis determined the regio-isomers and enantiomers of MHFA content for hydroxy-linoleic/octadecadienoic acid (HODE), hydroxy-EPA (HEPE), hydroxy-arachidonic/eicosatetraenoic acid (HETE). A second chromatographic analysis determined prostanoid and resolvin content.
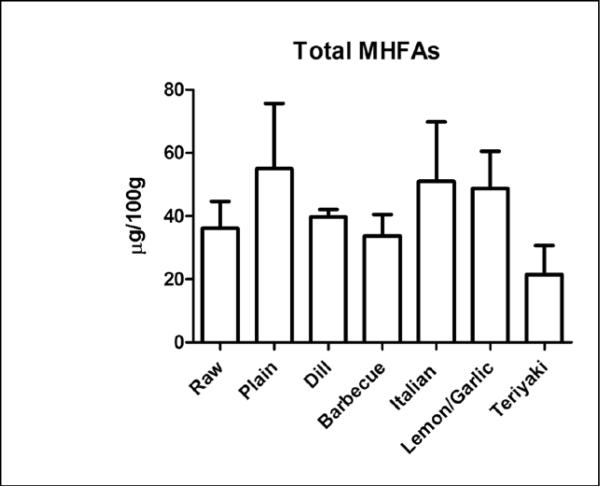
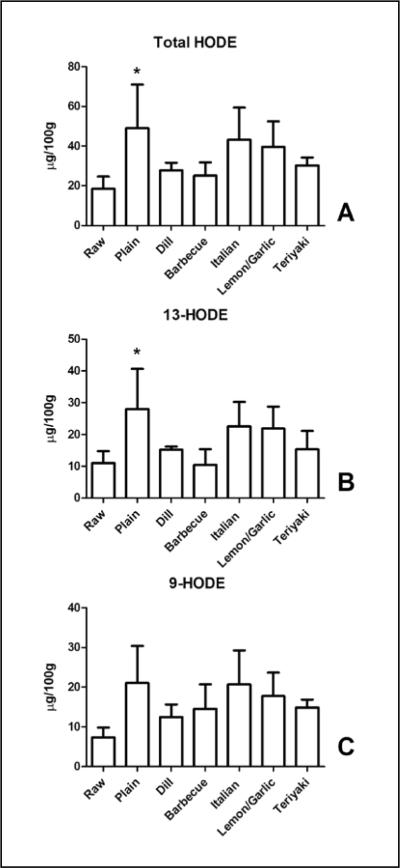
Total MHFA content (the sum of the MHFA we measured) was not significantly different between the groups compared to control with values ranging from 36.1 ± 8.5 μg/ 100g to 55.0 ± 20.8 μg/100g (Figure 1). There was a significant increase in total HODE content (18.5 ± 6.2 μg/100g to 49.1 ± 21.9 μg/100 g, Figure 2A), which was comprised of similar amounts of 9-HODE and 13-HODE, the predominant regio-isomers (Figure 2B,C) (21). The increase in HODE was suppressed by the inclusion of toppings on the salmon. The intersample variability in the MHFA content for the “plain” and “Italian” coatings is in part owing to the variability in the HODE content for those samples. The cause for this variability versus other MFHA determined in the same analytical run is not clear.
Figure 1.
Baking does not alter total mono-hydroxy fatty acids (MHFA) in salmon. Salmon samples (raw and baked) were analyzed for several MFHAs. The total amount of MHFA per 100 gram serving was obtained by summation of the content of 9- and 13-HODE, 5-,8-,12-, and 15-HETE, and 5-,12-, and 15-HEPE determined in each sample. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples.
Figure 2.
Baking increases linoleate-derived HODE levels in salmon. (A) The total content of HODE (summated 13- and 9-HODE content) was significantly elevated by baking. Note that some coatings clearly reduced the increase in total HODE content. 13-HODE (B) but not 9-HODE (C) content is elevated in salmon by baking. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples. * denotes p < 0.05 using a one-way ANOVA with Dunnett's multiple comparison test comparing samples to the control “Raw” sample.
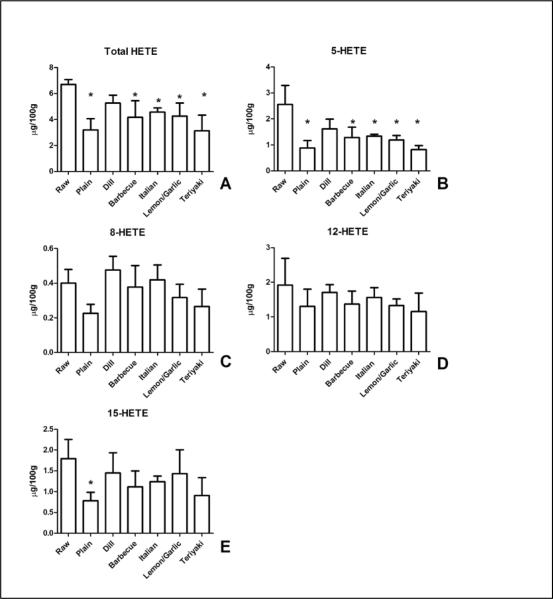
In contrast to HODE, cooking caused a reduction in the total levels of HETEs whose levels ranged from a mean of 6.7 ± 0.4 μg/100 to 3.1 ± 1.2 μg/100g (Figure 3). There was a significant loss of total 5-HETE content with cooking in most of the groups tested (Figure 3B). While the 8-HETE and 12-HETE regio-isomers (3C,D) were not affected by cooking compared to the raw salmon, baking reduced the content of 15-HETE (3E), an effect blocked by the inclusion of coatings.
Figure 3.
Baking selectively decreases 5-HETE levels in salmon. (A) Content of HETE (summated 5-, 8-, 12-, and 15-HETE) was significantly reduced by baking regardless of coatings. (B) 5-HETE content was reduced in most groups compared to control, raw, salmon. 8-HETE (C) and 12-HETE (D) content were not altered. 15-HETE content (E) was reduced by baking, a result inhibited by coating of the salmon. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples. * denotes p < 0.05 using a one-way ANOVA with Dunnett's multiple comparison test comparing samples to the control “Raw” sample.
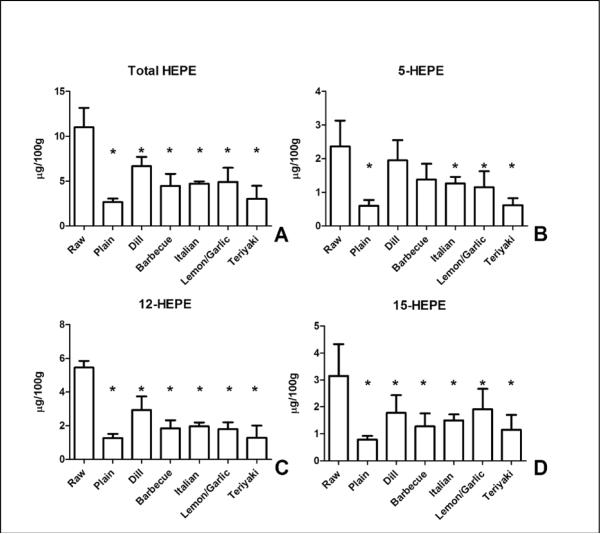
EPA-derived HEPE content was reduced significantly by cooking in all test groups compared to the uncooked fish (Figure 4). Total HEPE content ranged from 11.0 ± 2.2 μg/100g in the uncooked fish to 2.7 ± 0.4 μg/100g in plain, baked salmon. Baking significantly reduced the content of the 5, 12, and 15-HEPE regio-isomers, an effect that was not prevented by the coatings with the exception of 5-HEPE in samples coated with dill and barbecue.
Figure 4.
Baking reduces EPA-derived HEPE in salmon. (A) Content of HEPE (summated 5-, 12-, and 15-HEPE content) was significantly reduced by baking regardless of coatings compared to control, raw, salmon. (B) 5-HEPE content was reduced in most groups compared to control, raw, salmon. 12-HEPE (C) content and 15-HEPE content (D) were reduced by baking, an effect not blocked by the coatings. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples. * denotes p < 0.05 using a one-way ANOVA with Dunnett's multiple comparison test comparing samples to the control, “Raw” sample.
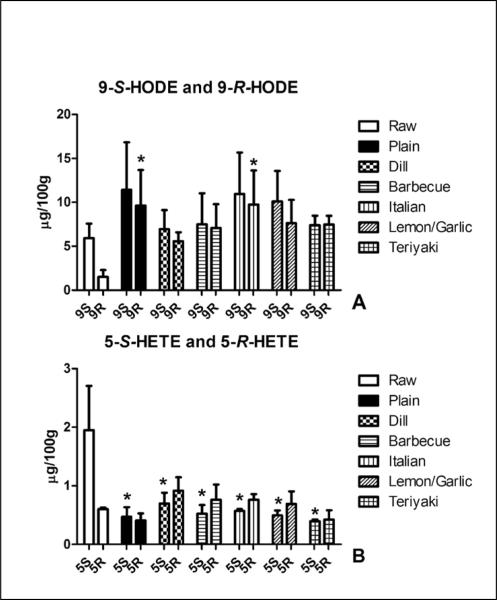
Analysis of MHFA chirality aids in determining enzymatic versus non-enzymatic lipid peroxidation. Lipoxygenase mediated lipid oxidation yields the formation of S-hydroperoxides and lipoxygenases are present in fish (21). On the other hand, free radical catalyzed lipid oxidation results in racemic levels of both R and S-hydroperoxides (21). We were able to separate the 9-S-HODE, 9-R-HODE, 5-S-HETE and 5-R-HETE enantiomers. The mean ratio of the 9-S enantiomer to the 9-R enantiomer was 4.2 and suggests an enzyme catalyzed formation of 9-HODE (Figure 5A). On the other hand, nearly racemic amounts of the 9-HODE enantiomers were present following cooking. Baking significantly increased the content of 9-R-HODE versus raw salmon. Similarly, 5-S-HETE was 3.3-fold greater than 5-R-HETE in the uncooked salmon, with racemic levels present in the cooked fish (Figure 5B). The content of 5-S-HETE was significantly reduced by cooking as opposed to 5-R-HETE.
Figure 5.
Baking alters the enantiomeric ratio of 5-S/R-HETE and 9-S/R-HODE. (A) An enantiomeric ratio of 5-S-HETE:5-R-HETE of 3.3 was observed in raw salmon samples versus the nearly racemic content found in baked salmon samples. Note that while the levels of 5-R-HETE was not altered, the content of 5-S-HETE was significantly reduced by baking in all samples. (B) An enantiomeric ratio of 9-S-HODE:9-R-HODE of 4.2 was observed in raw salmon samples versus the nearly racemic content found in baked salmon samples. Significantly elevated content of 9-R-HODE was observed in the plain cooked and lemon/ garlic coated salmon versus the control, raw salmon. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples. * denotes p < 0.05 using a one-way ANOVA with Dunnett's multiple comparison test comparing samples to the analogous enantiomer in the control, “Raw” sample.
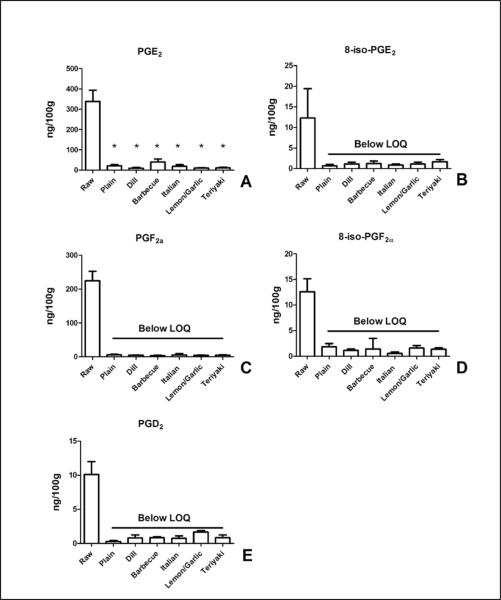
We then examined the content of more complex lipid oxidation products. The enzymatically derived prostanoids, PGE2, PGF2α, and PGD2 were all present in the uncooked salmon at levels much lower than MHFAs (Figure 6). Cooking reduced the levels of these eicosanoids at least 10-fold, in many cases to below the LOQ of our detection method (6ng/100g). In uncooked salmon, levels of PGE2 (339 ± 55 ng/ 100g, 6A) were greater than PGF2α (225 ± 28 ng/100g, 6C) or PGD2 (10 ± 2 ng/100g, 6E). Similar to the loss of enzymatically-derived prostaglandins, cooking reduced the levels of the isoprostanes, 8-iso-PGF2α, and 8-iso-PGE2 (Figure 6B,D).
Figure 6.
Baking reduces prostanoid content of salmon. The content of enzymatically- derived (PGE2, PGF2α, and PGD2) and non-enzymatically-derived (8-iso-PGE2 and 8-iso-PGF2α) prostanoids were determined in raw and baked salmon. PGE2 (A) and PGF2α (C) had the highest levels in raw salmon. The content of the isoprostanes 8-iso-PGE2 and 8-iso-PGF2α were both reduced by baking suggesting that de novo lipid peroxidation of ARA did not occur. Levels of all prostanoids (except PGE2) measured were decreased below the limit of quantitation (LOQ) by baking. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples. * denotes p < 0.05 using a one-way ANOVA with Dunnett's multiple comparison test comparing samples to the control, “Raw” sample.
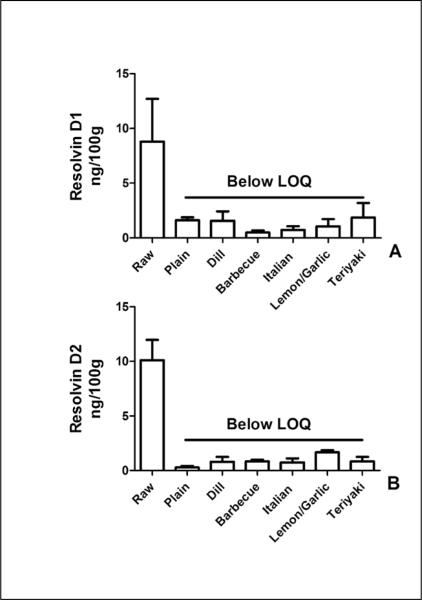
We examined the content of DHA-derived resolvins D1 and D2. Levels of both anti-inflammatory molecules were approximately 10 ng/100 gram of uncooked fish and were reduced to below our LOQ by cooking of the fish (Figure 6).
One potential explanation for loss of lipid oxidation products is the melting of lipids and lipid oxidation products from the salmon flesh during baking. In order to test this possibility, we determined phospholipid fatty acid content in the raw and plain-baked salmon. There was no change in total phospholipid fatty acid content as a result of baking (supplementary data, S2). There were losses in the very minor fatty acids, eicosenoic acid (20:1n-9) and the n-3 analog of eicosatetraenoic acid (20:4n-3); however these changes represented < 2% of the total n-9 and n-3 fatty acids. There were no other changes in n-3, n-6, or n-9 fatty acids.
Discussion
Consumption of fish high in n-3 fatty acids, specifically EPA and DHA, is recommended as a means to reduce risk for cardiovascular disease and reduce the risk for other inflammatory diseases. Concerns exist regarding the extent to which cooking of fish may lead to the formation of potentially deleterious lipid oxidation products. On the other hand, oxidation of n-3 fatty acids may lead to the formation of cytoprotective products. Little work has been performed examining the speciation of fatty acid lipid oxidation products in high, n-3 fish like salmon. Measurements of total peroxide or conjugated diene values (3, 7), while useful, overlook contributions of individual fatty acid species. In this work, we examined the extent to which baking alters the levels of specific fatty acid oxidation products and if salmon contained levels of fatty acid oxidation products of significance to human health. Surprisingly, our data demonstrated that baking selectively reduced the values of ARA, EPA, and DHA-derived oxidation products but not those from linoleic acid.
Total levels of MHFA (a summation of total HODE, HEPE, and HETE analyzed) were unchanged by baking of the salmon. Our data are similar to those obtained by Al-Shagir and colleagues in which frying alone minimally elevated (<15%) levels of conjugated dienes and peroxides in the fat extracted from the fish. It is surprising that HODE were found at greater levels than HETE or HEPE analyzed although levels of linoleic acid (18:2n-6) were less than those of ARA or EPA in the salmon. However, our assays only determined 4 out of the 8 HETE region-isomers and 3 out of the 10 possible HEPE region-isomers. Thus the contribution of HETE and HEPE to total MHFA is underestimated compared to HODE in which the dominant region-isomers (9-HODE and 13-HODE) were determined.
Our data indicate fatty acid specific losses of MFHA, specifically selective loss of MHFA regio-isomers. Because EPA and ARA differ with the addition of an ethenyl bond but not total carbons, we can assess similarities exist owing to the carbon position of the hydroxyl group. The losses of the 5-hydroxy MFHA, 5-HETE and 5-HEPE, suggest that the placement of hydroxyl group proximal to the glycerol backbone of the phospholipid or triacylglycerol molecule influence subsequent breakdown of the MHFA to other products. This may explain the lack of effects on HODE in which the hydroxyl group is further away from the glycerol backbone. On the other hand, there was no other correlation between the 12-HETE and 12-HEPE or 15-HETE and 15-HEPE. Given that losses in MHFA occurred in all EPA-derived products, but only one of the ARA-derived MFHA, suggest that the larger number of double bonds of EPA is allowing for subsequent oxidation reactions of HEPE to occur over HETE or HODE. Similar effects of n-3 PUFA upon n-6 PUFA have been observed (22, 23).
Raw salmon contained appreciable amounts of the enzymatically derived prostanoids, PGE2 and PGF2α while the levels of other prostanoids were minimal. We estimate that the PGE2 content to be approximately 10 nM concentration in the intact fish meat prior to digestion. The extent to which prostanoids derived from dietary sources are able activate cellular PGE2 receptors following passage from the lumen of the gut is not known. However, dietary derived PGE2, and potentially PGF2α, could have effects on the gut. A 10 nM concentration of PGE2 is able to activate PGE2 receptors which regulate water and chloride absorption (24–27). Indeed the Kd of PGE2 for the EP3 and EP4 isoforms of the PGE2 receptor is below 1 nM, and the EP3 and EP4 subclasses are both highly expressed in the gut (28, 29). The extent to which dietary-derived prostanoids could then reach effective concentrations in the blood for cardiovascular benefit is questionable.
The fact that isoprostanes were reduced, but not elevated, by baking indicates that elevating the internal temperature of the fish to 63° C (145° F) does not cause significant oxidative damage to the ARA present in the fish. While one possibility is that any isoprostanes formed during heating could have been subsequently degraded, we find this unlikely given numerous data showing increases in isoprostane/neuroprostane formation under highly oxidizing conditions such as iron/ascorbate incubation for several hours (30). Thus, even in the presence of prostanoid degradation, we would have expected to still see an increase in isoprostane content with baking. While we did not specifically measure EPA-derived F3-isoprostanes, we suspect that similar losses of F3-isoprostanes would occur with baking. Our data suggest that the basis for the reduction in content of prostanoids and resolvins is likely the result of thermal degradation of these hydroxylated molecules. Given that there was no loss of fatty acids from the salmon as a result of baking, it is unlikely that the losses of lipid oxidation products were the result of their melting from the salmon tissue.
We examined the extent to which inclusion of coatings may alter the formation or loss of lipid oxidation products as a result of baking. Inclusion of antioxidant food extracts has been used to limit lipid oxidation (31, 32). There was no consistent effect of the type of baked coatings upon fatty acid oxidation products. While the coatings inhibited the baking-induced increase in HODE content, the coatings were not able to block the decreases on multiple HEPE as well as the prostanoids and resolvins. Interestingly, the dill coating did appear to reduce the loss of 5-HETE and 5-HEPE; however, the mechanisms underlying this effect are not clear.
In summary, our data indicate that baking of farm-raised Atlantic salmon does not increase levels of oxidative damage to PUFA, but rather leads to the decrease of pre-existing oxidation products in a species-dependent manner, perhaps through thermal degradation. While detectable levels of prostanoids and resolvins were present in raw salmon, they were largely ablated by cooking. Furthermore, our data indicate that baking does not cause of loss of PUFA from the fish. Thus, baking of salmon is an acceptable means of preparation that does not alter the potential health benefits of high n-3 seafood consumption. Our analyses do not comprise a complete lipidomic characterization of the possible lipid oxidation products. However, we addressed several analytes of potential biological relevance. The extent to which the levels of MHFA, prostanoids and resolvins in the raw or baked fish have physiologic consequence for humans needs to be determined.
Supplementary Material
Figure 7.
Baking reduces resolving D1 and resolvin D2 content of salmon. (A) Resolvin D1 content in raw salmon samples was 8.8 ± 3.9ng per 100g. All baked samples had resolvin D1 content below the limit of quantitation (LOQ) of the assay. (B) Resolvin D2 content in raw salmon was 10.1 ± 1.9 ng per 100 g. All baked samples had resolvin D2 content below the limit of quantitation (LOQ) of the assay. Data presented are the mean ± S.D. of three, independently prepared and analyzed salmon samples.
Acknowledgment
We thank Cooke Aquaculture, Blacks Harbor New Brunswick, Canada for their kind donation of salmon fillets for the upcoming feeding trial, a portion of which was used for this project. The authors thank Brock Thuen for his excellent technical work.
Financial Support This work was funded by USDA 5450-51000-048-00D (MJP, SKR) and USDA 1915-31000-003-00D (WRW, GSB), NIH 2P20RR017699-09 (TAR), the NIH-funded Centers of Biomedical Research Excellence (COBRE) Mass Spectrometry Core Facility Grant 5P20RR017699 (MYG) and NIH/NINDS 1R21NS064480-02 (MYG).
Abbreviations Used
- (PUFA)
polyunsaturated fatty acids
- (ARA 20:4n-6)
arachidonic acid
- (EPA 20:5n-3)
eicosapentaenoic acid
- (DHA 22:6n-3)
docosahexaenoic acid
- (MHFA)
monohydroxy-fatty acids
- (HODE)
hydroxy-octadecadienoic acid
- (HEPE)
hydroxy-EPA
- (HETE)
hydroxy-eicosatetraenoic acid
Footnotes
Literature Cited
- 1.Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am J Clin Nutr. 2008;88(6):1618–25. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. Jama. 2006;296(15):1885–99. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 3.Al-Saghir S, Thurner K, Wagner KH, Frisch G, Luf W, Razzazi-Fazeli E, Elmadfa I. Effects of different cooking procedures on lipid quality and cholesterol oxidation of farmed salmon fish (Salmo salar) J Agric Food Chem. 2004;52(16):5290–6. doi: 10.1021/jf0495946. [DOI] [PubMed] [Google Scholar]
- 4.Echarte M, Zulet MA, Astiasaran I. Oxidation process affecting fatty acids and cholesterol in fried and roasted salmon. J Agric Food Chem. 2001;49(11):5662–7. doi: 10.1021/jf010199e. [DOI] [PubMed] [Google Scholar]
- 5.Refsgaard HH, Brockhoff PM, Jensen B. Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. J Agric Food Chem. 2000;48(8):3280–5. doi: 10.1021/jf000021c. [DOI] [PubMed] [Google Scholar]
- 6.Kawai K, Matsuno K, Kasai H. Detection of 4-oxo-2-hexenal, a novel mutagenic product of lipid peroxidation, in human diet and cooking vapor. Mutat Res. 2006;603(2):186–92. doi: 10.1016/j.mrgentox.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Elmadfa I, Al-Saghir S, Kanzler S, Frisch G, Majchrzak D, Wagner KH. Selected quality parameters of salmon and meat when fried with or without added fat. Int J Vitam Nutr Res. 2006;76(4):238–46. doi: 10.1024/0300-9831.76.4.238. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD. Resolvins and protectins: natural pharmacophores for resolution biology. Prostaglandins Leukot Essent Fatty Acids. 82(4–6):327–32. doi: 10.1016/j.plefa.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282(13):9323–34. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 10.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13(11):1193–202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Yin H, Liu W, Goleniewska K, Porter NA, Morrow JD, Peebles RS., Jr. Dietary supplementation of omega-3 fatty acid-containing fish oil suppresses F2-isoprostanes but enhances inflammatory cytokine response in a mouse model of ovalbumin-induced allergic lung inflammation. Free Radic Biol Med. 2009;47(5):622–8. doi: 10.1016/j.freeradbiomed.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008;283(18):12043–55. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem. 2007;282(4):2529–37. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Yin H, Milne GL, Porter NA, Morrow JD. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2006;281(20):14092–9. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 15.Song WL, Paschos G, Fries S, Reilly MP, Yu Y, Rokach J, Chang CT, Patel P, Lawson JA, Fitzgerald GA. Novel eicosapentaenoic acid-derived F3-isoprostanes as biomarkers of lipid peroxidation. J Biol Chem. 2009;284(35):23636–43. doi: 10.1074/jbc.M109.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long EK, Picklo MJ., Sr. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radic Biol Med. 2010;49(1):1–8. doi: 10.1016/j.freeradbiomed.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Association NR. ServSafe Essentials. 5th edition ed. 2010. [Google Scholar]
- 18.Radin NS. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- 19.Brose SA, Thuen BT, Golovko MY. LC/MS/MS method for analysis of E series prostaglandins and isoprostanes. J Lipid Res. 2011;52(4):850–9. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golovko MY, Murphy EJ. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J Lipid Res. 2008;49(4):893–902. doi: 10.1194/jlr.D700030-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Schneider C. An update on products and mechanisms of lipid peroxidation. Mol Nutr Food Res. 2009;53(3):315–21. doi: 10.1002/mnfr.200800131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis TA, Gao L, Yin H, Morrow JD, Porter NA. In vivo and in vitro lipid peroxidation of arachidonate esters: the effect of fish oil omega-3 lipids on product distribution. J Am Chem Soc. 2006;128(46):14897–904. doi: 10.1021/ja064399o. [DOI] [PubMed] [Google Scholar]
- 23.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273(22):13605–12. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 24.Stillman BA, Audoly L, Breyer RM. A conserved threonine in the second extracellular loop of the human EP2 and EP4 receptors is required for ligand binding. Eur J Pharmacol. 1998;357(1):73–82. doi: 10.1016/s0014-2999(98)00522-6. [DOI] [PubMed] [Google Scholar]
- 25.Bastien L, Sawyer N, Grygorczyk R, Metters KM, Adam M. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem. 1994;269(16):11873–7. [PubMed] [Google Scholar]
- 26.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 27.Kreydiyyeh SI, Markossian S, Hodeify RF. PGE2 exerts dose-dependent opposite effects on net water and chloride absorption from the rat colon. Prostaglandins Other Lipid Mediat. 2006;79(1–2):43–52. doi: 10.1016/j.prostaglandins.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149(6):611–23. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483(2):285–93. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 30.Long EK, Murphy TC, Leiphon LJ, Watt J, Morrow JD, Milne GL, Howard JR, Picklo MJ., Sr. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J Neurochem. 2008;105(3):714–24. doi: 10.1111/j.1471-4159.2007.05175.x. [DOI] [PubMed] [Google Scholar]
- 31.Min B, Chen MH, Green BW. Antioxidant activities of purple rice bran extract and its effect on the quality of low-NaCl, phosphate-free patties made from channel catfish (Ictalurus punctatus) belly flap meat. J Food Sci. 2009;74(3):C268–77. doi: 10.1111/j.1750-3841.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 32.Bao HN, Ushio H, Ohshima T. Antioxidative activities of mushroom (Flammulina velutipes) extract added to bigeye tuna meat: dose-dependent efficacy and comparison with other biological antioxidants. J Food Sci. 2009;74(2):C162–9. doi: 10.1111/j.1750-3841.2009.01069.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.