Abstract
Kidney involvement is an under-recognized complication of both Hodgkin and non-Hodgkin lymphoma 1. The diversity of lymphoma-related renal manifestations makes diagnosis difficult: while abrupt worsening of kidney function may be the first sign of malignant disease, renal effects can be subtle or even silent. The etiologies of renal involvement are similarly varied. Here we discuss a case of non-Hodgkin lymphoma and associated kidney failure from several distinct malignancy-related mechanisms. We then take the opportunity to review the spectrum of lymphoma-related kidney involvement.
Keywords: renal failure, Hodgkin lymphoma, non-Hodgkin lymphoma, nephrotic
Introduction
Lymphoma-associated kidney impairment is a common reason for renal consultation in the oncology division of our hospital. The myriad of etiologies of renal injury due to hematologic tumors often present a diagnostic challenge, but most respond to effective treatment of the underlying malignancy. Below we present a patient who presented with acute kidney injury in the setting of newly-diagnosed non-Hodgkin lymphoma. The differential for his kidney injury is discussed, with an emphasis on mechanisms of disease. Treatment and prognosis are also reviewed.
Case Report
A 76 year-old man was transferred to our hospital for evaluation of kidney failure. Two months prior, he had developed progressive swelling of his extremities, abdomen and scrotum. Labs revealed a creatinine of 4.6 mg/dL (406.6 μmol/L) and nephrotic-range proteinuria. A faint IgG kappa monoclonal M spike was noted on SPEP, and a small quantity of free kappa light chains was found on UPEP and immunofixation. Subsequent bone marrow biopsy revealed a lymphoplasmacytic infiltrate with 4-5% plasma cells. Minimal change disease was diagnosed empirically, and methylprednisolone 250 mg daily was started. The patient’s kidney function continued to decline, and he was transferred for further workup.
The patient had a ten-year history of coronary artery disease, peripheral vascular disease, and diabetes mellitus. His creatinine had been 1.3 mg/dL (114.9 μmol/L) three months before admission. He had recently stopped smoking. He was retired, and lived with his wife in northern Massachusetts. Family history was negative for kidney disease or malignancy. He denied recent NSAID use.
On presentation, the patient’s blood pressure was 130/80, and he had an oxygen saturation of 95% on 3L oxygen. His exam was notable for expiratory wheezes and decreased bibasilar breath sounds. Cardiac exam was unremarkable. Ascites was present, and his scrotum was markedly swollen. He had 3+ pitting edema to the presacrum symmetrically. There were no palpable lymph nodes. Initial laboratory data revealed: serum Na 139 mg/dL, potassium 4.8 mg/dL, chloride 103 mg/dL, bicarbonate 25 mg/dL, urea nitrogen 92 mg/dL (32.8 mmol/L), creatinine 5.0 mg/dL (442 μmol/L), calcium 7.0 mg/dL, albumin 2.6 g/dL. Proteinuria was quantified at 19.5 g/day. Urine microscopy on a catheterized specimen was significant for numerous non-dysmorphic red blood cells and frequent pigmented granular casts. Serum leukocyte count was 7500 /uL, with normal differential. Hemoglobin was 9.8 g/dL, and platelet count was 95,000/uL. Bone marrow biopsy showed a variably cellular marrow, with approximately 15% lymphocyte and plasma cell infiltrates. On ultrasound, both kidneys appeared enlarged but structurally normal, measuring 14.2 and 14.5 cm in length. A CT-guided kidney biopsy was performed.
Kidney Biopsy
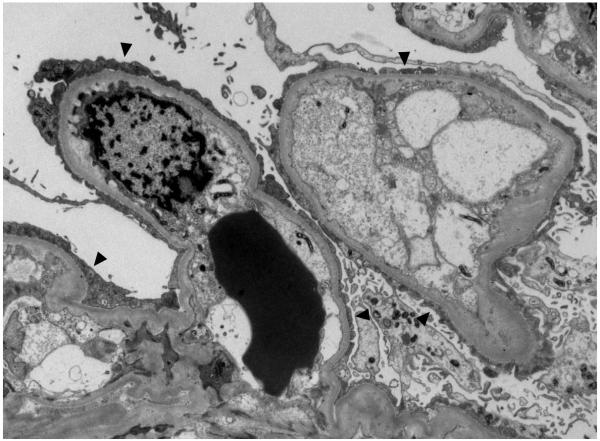
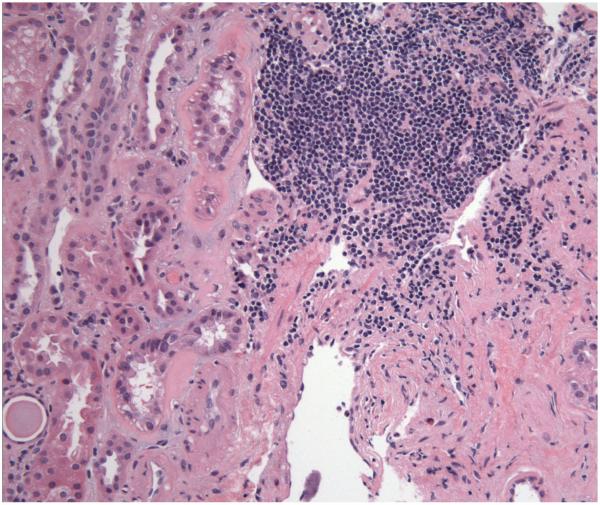
Kidney biopsy specimen revealed evidence of widespread acute and chronic changes. Severe glomerular visceral epithelial cell injury with diffuse foot process effacement was seen on electron microscopy (Figure 1), suggesting minimal change disease. Basement membrane thickness was normal, and electron dense deposits were absent. Evidence of acute tubular distension and epithelial lining attenuation consistent with acute tubular necrosis (ATN) was also present. There was prominent focal infiltration of the kidney parenchyma and subcapsular tissue with small, monomorphic lymphocytes, especially near arterioles (Figure 2). Although small sample size precluded further characterization of the infiltrate, the lack of prominent nucleoli and mitotic figures was most consistent with a low-grade lymphoma. Chronic changes were also observed: global glomerulosclerosis was seen in ~40% of the glomeruli (14 total in the sample) (Figure 3). There was also moderately severe arterial and arteriolar sclerosis, compatible with chronic vascular disease. Immunofluorescence microscopy was negative (not shown).
Figure 1.
Light microscopy of the cortex of the kidney. The glomerulus is well preserved and shows open capillaries, mild to moderate expansion of the mesangial matrix, and delicate glomerular basement membranes. Most tubules are unremarkable. One dilated distal tubule in the left lower corner contains a PAS-positive hyaline cast. The immunofluorescence microscopy did not reveal immune deposits or monoclonal paraproteins; casts were reactive for polyclonal IgA. PAS stain. Magnification: 40x objective; final magnification on the 10 inch image is 590x.
Figure 2.
Electron microscopy of the glomerular capillaries. Shown here are two glomerular capillaries with diffuse effacement of the foot processes of the visceral epithelial cells. The basement membranes are of normal thickness, and the endothelial cells appear swollen and occupy most of the capillary lumen. Electron dense deposits are not present along the peripheral capillary walls. Original magnification 5000x objective.
Figure 3.
Light microscopy of the outer medulla. There is focal infiltration of the parenchyma by small lymphoid cell aggregates, strongly suggestive of a lymphomatous involvement of the parenchyma; similar aggregates of CD20-positive B-cells were also seen in the bone marrow biopsy. Hematoxylin and eosin; final magnification 292x
Clinical Follow Up
Treatment with rituximab, dexamethasone and bortozemib was initiated. The patient responded rapidly: his proteinuria fell to 9.4 g daily, and creatinine improved to 1.2 mg/dL (203.3 μmol/L) on discharge one week later. Subsequently, he underwent two additional rounds of chemotherapy. Ten months after diagnosis, his lymphoma remains in remission. His serum creatinine is 1.2 mg/dL (106.1 μmol/L), and he has 1.3 g daily proteinuria.
Discussion
Our patient presented with kidney failure and the nephrotic syndrome concurrent with a diagnosis of lymphoplasmacytic lymphoma. His kidney biopsy showed two distinctive pathologic findings associated with lymphomatous disease: diffuse effacement of the visceral epithelial cell foot processes consistent with minimal change nephrotic syndrome (MCNS), and lymphocytic invasion of the parenchyma. He also presented with acute kidney injury and ATN, which have been associated with MCNS 2. Kidney involvement in lymphoma is a well-recognized phenomenon, but the presence of several distinct pathologic processes is rarely seen. Because biopsy is performed infrequently in lymphoma patients, however, it is likely that cases with multiple forms of kidney involvement are overlooked. Below we discuss the various disease states causing kidney dysfunction in systemic lymphomas (summarized in Table 1).
Table 1.
Prevalence and Presentation of Types of Renal Disease in Systemic Lymphomas
| Renal Lesion | Prevalence | Clinical Presentation |
|---|---|---|
| Lymphomatous infiltration | 34% of lymphomas (13% of Hodgkin’s pts) in autopsy series |
Often asymptomatic; can present with renal failure, proteinuria, flank pain |
| Minimal change nephrosis | 0.4% in Hodgkin’s; Few cases in NHL |
Nephrotic syndrome, sometimes with associated AKI |
| Monoclonal Ig deposition | rare | Proteinuria, renal failure |
| amyloidosis | rare | Nephrotic syndrome +/− renal failure |
| Immunotactoid glomerulopathy |
rare | Heavy proteinuria (60- 70% nephrotic range25), renal failure |
| Membranous glomerulopathy | rare | Proteinuria, renal failure |
Lymphocytic infiltration of kidney parenchyma (LIK) occurs commonly in lymphoma patients. In the largest case series of autopsies performed on lymphoma patients, 34% showed signs of parenchymal invasion, but only 14% had been diagnosed with LIK before time of death 1. Absence of clinical signs gives explanation for the under-diagnosis: kidney failure was present in only 0.5% of patients with LIK, and most lacked flank pain or features of volume overload. Other signs suggesting LIK include bilateral kidney enlargement and sub-nephrotic range proteinuria 3, 4. Biopsy typically shows diffuse infiltration of monomorphic lymphocytes and resultant tubule compression 3, 5. Tubules and glomeruli are usually morphologically normal. LIK has been associated with ATN in several case series, but kidney failure also occurs in patients with normal tubular morphology3. One explanation postulates that elevated pressure on the kidney parenchyma from the invading lymphocytes may be responsible for renal insufficiency, especially in light of concurrent rapid recovery and decrease in kidney size after starting chemotherapy 5. While improvement in LIK-induced kidney failure is typical after treatment of the underlying malignancy, complete recovery to baseline function is infrequent. Moreover, the presence of metastatic lymphoma in the kidney indicates advanced disease, and carries a poor overall prognosis. In Richmond et al’s analysis of 690 autopsies of patients with lymphoma, kidney involvement was associated with metastasis to other organs, including the heart, lungs, and GI tract 1.
Minimal change nephrotic syndrome (MCNS) represents about 40% of Hodgkin’s-associated glomerulopathies, and often presents early in the course of disease, preceding onset of lymphoma diagnosis in more than 30% of patients 6, 7. The overall prevalence of MCNS in Hodgkin disease is about 0.4% 8, and is even lower in non-Hodgkin lymphomas. Both idiopathic and malignancy-associated MCNS are thought to be mediated by a soluble permeability factor, as yet unidentified, which causes loss of selective capillary permeability and allows albumin and other negatively-charged molecules to cross the glomerular barrier 9, 10, 11. In lymphoma-associated MCNS, the permeability factor is supposed to be paraneoplastic in origin. While it is unclear whether idiopathic and lymphoma-associated MCNS develop in the same way, some pathogenetic similarities have been identified. It has been shown that T cells in both types of MCNS are driven to differentiate preferentially into Th2 rather than Th1 lymphocytes. Staining of T cells from Hodgkin patients shows upregulation of c-Maf, a transcription factor known to induce Th2 formation from naïve T-cells, as well as downregulation of the IL-12 receptor (IL-12R), whose stimulation is critical in Th1 T-cell differentiation 12. Studies using cDNA subtraction libraries in patients with idiopathic MCNS showed similar c-Maf upregulation and decreased expression of the IL-12R beta-2 chain 13. It has also been shown that c-mip (c-Maf-inducing protein) is increased in both idiopathic and lymphoma-associated MCNS 14. How dysregulation of normal T-cell development affects glomerular basement membrane permeability is unclear, but recent research suggests that impairment of T regulator cell function may lead to cytokine overproduction15. It should be noted that focal segmental glomerulosclerosis (FSGS) has been reported in seven Hodgkin lymphoma cases and four non-Hodgkin lymphoma patients, and is considered by some to represent a later stage of renal damage caused by the mechanism underlying MCD 16, 17. Its prevalence may be underestimated if renal biopsies are performed in the early stages of the nephrotic syndrome, or if there is biopsy sampling error.
As in multiple myeloma, the glomerular deposition of light chains, heavy chains or both in lymphoplasmacytic lymphomas can lead to proteinuria and kidney failure 18. Filtration of free light chains, which have a direct toxic effect on the tubules, can also cause progressive kidney dysfunction. The pathogenesis and presentation of immunoglobulin light chain kidney disease have been reviewed in detail elsewhere 19. Monoclonal immunoglobulin deposition disease (MIDD) differs from AL amyloid in that glomerular deposits lack a regular fibrillary structure, but similarly can cause heavy proteinuria and impaired glomerular filtration. In our patient, the small quantity of free light chains on SPEP and UPEP makes immune complex deposition an unlikely cause of his kidney failure.
Amyloidosis is a rare complication of Hodgkin lymphomas, and with advances in treatment has become even rarer 11. In the largest case series of 63 Hodgkin patients with the nephrotic syndrome, published in 1977, 18 had amyloidosis 20; only a few have been recognized since that time, in patients with advanced stages of lymphoma and presumed high levels of inflammation 6. It is presumed that most older cases of amyloid were of the AA subtype 6. Amyloid associated with NHL is also very unusual, though there have been no epidemiologic studies examining its prevalence. AL amyloid is more common than AA in NHL patients21. Renal manifestations include heavy proteinuria, anasarca, and filtration impairment from hypoalbuminemia-induced volume contraction and hypotension. Treatment focuses on the underlying lymphoma22.
Immunotactoid glomerulopathy (ITG) is an uncommon cause of proteinuria and renal failure in lymphoplasmacytic disorders, whose morphology provides an interesting link between MIDD and amyloidosis. It is characterized on renal biopsy by monomorphic microtubules larger than amyloid fibrils, which stain negative for Congo red, and colocalize with IgG and complement staining on immunofluorescence23. ITG has been associated with lymphoproliferative disorders in up to 33% of cases in one series24, although when paraproteinemia and monoclonal gammopathy were excluded, the prevalence fell to ≤7%. Prognosis is poor, with over 50% developing end-stage kidney disease by five years25.
Membranous glomerulopathy is seen less commonly in lymphomatous malignancies than in solid tumors, especially carcinomas. Both membranous and membranoproliferative glomerulonephritis have been reported in association with both Hodgkin and non-Hodgkin lymphoma 26, 27, 8, including several cases of crescentic GN. Given the rarity of cases, a true association between malignancy and kidney injury has been debated 18. More recent studies, however, document excess cancer risk both at the time of diagnosis of membranous nephropathy and in following years 28, 29. As in idiopathic membranous glomerulopathy, lymphoma-associated MGN is characterized by glomerular basement membrane antibody deposition, but the provoking antigen has yet to be determined. Treatment of lymphoma-related MGN focuses on the underlying malignancy. Response has been mixed in case reports: several patients achieved a recovery of kidney function with systemic chemotherapy, but no long-term followup was available 30.
Conclusion
Lymphoma-associated kidney involvement occurs by a variety of mechanisms, which differ widely in prevalence and clinical presentation. The multiple lesions seen on our patient’s biopsy present an excellent opportunity to review and the pathogenesis of lymphomatous renal disease. Treatment for all forms of lymphoma-related kidney injury focuses on therapy for the underlying malignancy. While kidney involvement portends a greater degree of metastatic disease, adequate data on prognosis are lacking.
References
- 1.Richmond J, Sherman RS, Diamond HD, Craver LF. Renal lesions associated with malignant lymphomas. Am J Med. 1962;32:184–207. doi: 10.1016/0002-9343(62)90289-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith JD, Hayslett JP. Reversible renal failure in the nephrotic syndrome. Am J Kidney Dis. 1992;19:201–13. doi: 10.1016/s0272-6386(13)80001-7. [DOI] [PubMed] [Google Scholar]
- 3.Obrador GT, Price B, O’Meara Y, Salant DJ. Acute renal failure due to lymphomatous infiltration of the kidneys. J Am Soc Nephrol. 1997;8:1348–54. doi: 10.1681/ASN.V881348. [DOI] [PubMed] [Google Scholar]
- 4.Glicklich D, Sung MW, Frey M. Renal failure due to lymphomatous infiltration of the kidneys. Report of three new cases and review of the literature. Cancer. 1986;58:748–53. doi: 10.1002/1097-0142(19860801)58:3<748::aid-cncr2820580323>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Tornroth T, Heiro M, Marcussen N, Franssila K. Lymphomas diagnosed by percutaneous kidney biopsy. Am J Kidney Dis. 2003;42:960–71. doi: 10.1016/j.ajkd.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355–77. doi: 10.1046/j.1523-1755.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 7.Audard V, Larousserie F, Grimbert P, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–60. doi: 10.1038/sj.ki.5000341. [DOI] [PubMed] [Google Scholar]
- 8.Rault R, Holley JL, Banner BF, el-Shahawy M. Glomerulonephritis and non-Hodgkin’s lymphoma: a report of two cases and review of the literature. Am J Kidney Dis. 1992;20:84–9. doi: 10.1016/s0272-6386(12)80323-4. [DOI] [PubMed] [Google Scholar]
- 9.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–60. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 10.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–60. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 11.Dabbs DJ, Striker LM, Mignon F, Striker G. Glomerular lesions in lymphomas and leukemias. Am J Med. 1986;80:63–70. doi: 10.1016/0002-9343(86)90049-5. [DOI] [PubMed] [Google Scholar]
- 12.Schreck S, Friebel D, Buettner M, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27:31–9. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 13.Sahali D, Pawlak A, Valanciute A, et al. A novel approach to investigation of the pathogenesis of active minimal-change nephrotic syndrome using subtracted cDNA library screening. J Am Soc Nephrol. 2002;13:1238–47. doi: 10.1681/ASN.V1351238. [DOI] [PubMed] [Google Scholar]
- 14.Audard V, Zhang SY, Copie-Bergman C, et al. Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin-Reed Sternberg cells and podocytes. Blood. 115:3756–62. doi: 10.1182/blood-2009-11-251132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araya C, Diaz L, Wasserfall C, et al. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2009;24:1691–8. doi: 10.1007/s00467-009-1214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallouk A, Pham PT, Pham PC. Concurrent FSGS and Hodgkin’s lymphoma: case report and literature review on the link between nephrotic glomerulopathies and hematological malignancies. Clin Exp Nephrol. 2006;10:284–9. doi: 10.1007/s10157-006-0437-4. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez Garcia E, Olea T, Hevia C, et al. Focal segmental glomerulosclerosis due to a relapsing non-Hodgkin’s lymphoma diagnosed by positron emission tomography. J Nephrol. 2007;20:626–8. [PubMed] [Google Scholar]
- 18.Alpers CE, Cotran RS. Neoplasia and glomerular injury. Kidney Int. 1986;30:465–73. doi: 10.1038/ki.1986.209. [DOI] [PubMed] [Google Scholar]
- 19.Ronco P, Plaisier E, Mougenot B, Aucouturier P. Immunoglobulin light (heavy)-chain deposition disease: from molecular medicine to pathophysiology-driven therapy. Clin J Am Soc Nephrol. 2006;1:1342–50. doi: 10.2215/CJN.01730506. [DOI] [PubMed] [Google Scholar]
- 20.Eagen JW. Glomerulopathies of neoplasia. Kidney Int. 1977;11:297–303. doi: 10.1038/ki.1977.47. [DOI] [PubMed] [Google Scholar]
- 21.Zhu LC, Sidhu GS, Yee HT, Cassai ND, Goldfarb DS, Wieczorek RL. AA-type amyloidosis associated with non-Hodgkin’s lymphoma: a case report. Hum Pathol. 2004;35:1041–4. doi: 10.1016/j.humpath.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331–41. doi: 10.2215/CJN.02740806. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MM, Korbet SM, Lewis EJ. Immunotactoid glomerulopathy. J Am Soc Nephrol. 2002;13:1390–7. doi: 10.1097/01.asn.0000013397.06964.19. [DOI] [PubMed] [Google Scholar]
- 24.Pronovost PH, Brady HR, Gunning ME, Espinoza O, Rennke HG. Clinical features, predictors of disease progression and results of renal transplantation in fibrillary/immunotactoid glomerulopathy. Nephrol Dial Transplant. 1996;11:837–42. doi: 10.1093/oxfordjournals.ndt.a027409. [DOI] [PubMed] [Google Scholar]
- 25.Korbet SM, Schwartz MM, Lewis EJ. Immuotactoid glomerulopathy (fibrillary glomerulonephritis) Clin J Am Soc Nephrol. 2006;1:1351–6. doi: 10.2215/CJN.01140406. [DOI] [PubMed] [Google Scholar]
- 26.Giron Gonzalez JA, Yebra Bango M, Merino Morales F, Menendez Caro JL, Diego Marin FJ, Durantez Martinez A. The association of non-Hodgkin’s lymphoma with glomerulonephritis. Postgrad Med J. 1986;62:1141–5. doi: 10.1136/pgmj.62.734.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powderly WG, Cantwell BM, Fennelly JJ, Warde P, McCabe MM, Towers RP. Renal glomerulopathies associated with Hodgkin’s disease. Cancer. 1985;56:874–5. doi: 10.1002/1097-0142(19850815)56:4<874::aid-cncr2820560428>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Lefaucheur C, Stengel B, Nochy D, et al. Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006;70:1510–7. doi: 10.1038/sj.ki.5001790. [DOI] [PubMed] [Google Scholar]
- 29.Bjorneklett R, Vikse BE, Svarstad E, et al. Long-term risk of cancer in membranous nephropathy patients. Am J Kidney Dis. 2007;50:396–403. doi: 10.1053/j.ajkd.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Moulin B, Ronco PM, Mougenot B, Francois A, Fillastre JP, Mignon F. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127–35. doi: 10.1038/ki.1992.270. [DOI] [PubMed] [Google Scholar]