Abstract
TGF-β is an important paracrine factor in tumorigenesis. Ligand binding of the type I and II TGF-β receptors initiate downstream signaling. The role of stromal TGF-β signaling in prostate cancer progression is unknown. In mice the conditional stromal knockout of the TGF-β receptor type II expression (Tgfbr2fspKO) resulted in the development of prostatic intraepithelial neoplasia and progression to adenocarcinoma within seven months. Clinically, we observed a loss of TGF-β receptor type II expression in 69% of human prostate cancer-associated stroma compared to 15% of stroma associated with benign tissues (n =140, p value < 0.0001). To investigate the mechanism of paracrine TGF-β signaling in prostate cancer progression, we compared the effect of the prostatic stromal cells from Tgfbr2fspKO and Tgfbr2floxE2/floxE2 mice on LNCaP human prostate cancer cells in vitro and tissue recombination xenografts. Induction of LNCaP cell proliferation and tumorigenesis was observed by Tgfbr2fspKO prostate stroma as a result of elevated Wnt3a expression. Neutralizing antibodies to Wnt3a reversed LNCaP tumorigenesis. The TGF-β inhibition of Wnt3a expression was in part through the suppression of Stat3 activity on the Wnt3a promoter. In conclusion, the frequent loss of stromal TβRII expression in human prostate cancer can relieve the paracrine suppression of Wnt3a expression.
INTRODUCTION
Prostate cancer is the second leading cause of cancer-related death in men in the United States. The understanding of stromal-epithelial interactions in the prostate will help determine the etiology of prostate cancer progression. Many factors including TGF-β and Wnt signaling molecules are found to be mutated or deregulated in prostate cancer. Conventionally, TGF-β plays a tumor suppressive role in normal and preneoplastic epithelia, yet paradoxically can promote motility and resistance to cell death in transformed epithelia (Bierie and Moses, 2006; Biswas et al., 2007; Roberts and Wakefield, 2003). Further data have emerged that TGF-β also has additional roles in the tumor microenvironment born out by mouse models with conditional ablation of TGF-β signaling in a subset of stromal fibroblasts (Bhowmick et al., 2004a; Bhowmick et al., 2004b). These studies suggest TGF-β can be a paracrine tumor suppressor as the stromal loss of TGF-β responsiveness initiated prostatic intraepithelial neoplasia (PIN) and squamous cell carcinoma in the forestomach (Bhowmick et al., 2004a). In contrast, Wnt ligands are long known oncogenes (Nusse et al., 1991; Nusse et al., 1984; Nusse and Varmus, 1982). Canonical Wnt signaling, through β-catenin stabilization, has been of specific interest in cancer biology as a regulator of cell proliferation, adhesion, survival, movement, and polarity. Together, the TGF-β and Wnt pathways have both opposite and synergistic roles in cancer progression with evidence of crosstalk in cytoplasmic signaling (Labbe et al., 2007; Nishita et al., 2000).
TGF-β signaling involves the binding of the ligand isoforms, TGF-β 1, 2, and 3 to the extracellular domain of the TGF-β receptor type II (TβRII), followed by the transphosphorylation of the TGF-β receptor type I (TβRI), and the subsequent activation of downstream signaling molecules including Smad2, Smad3, and Smad4 (Shi and Massague, 2003). TGF-β receptor type III (TβRIII) expression is specifically important for TGF-β2 signaling. Proposed mechanisms for resistance to TGF-β include decreased expression of TβRI, TβRII, or TβRIII on the cell surface of melanoma, pancreas, breast, and colon cancers (Buck et al., 2004; Calin et al., 2000; Goggins et al., 1998; Grady et al., 1999; Schmid et al., 1995). Most epithelial-derived tumors (> 85% of all human cancers) become resistant to the growth-inhibitory effect of TGF-β by different mechanisms (Elliott and Blobe, 2005; Pasche, 2001). However, both the loss and elevation of TβRII expressions are associated with prostate cancer (Gerdes et al., 1998; Kim et al., 1998; Kim et al., 1996; Royuela et al., 1998). The consequence of TGF-β signaling in the prostatic stromal cells in man has not been studied.
As many Wnt ligands are stromally secreted with cognate receptors expressed by the epithelia, Wnt proteins are good candidates for mediating stromal-epithelial interactions. Nineteen closely related but distinct Wnt ligands are reported to date. In vertebrates, Wnt signaling can involve canonical and/or non-canonical pathways (Clevers, 2006; Johnson and Rajamannan, 2006). The canonical or β-catenin-dependent pathway, rely on specific Wnt ligands to bind a transmembrane receptor complex for cytoplasmic accumulation of β-catenin, and eventual nuclear translocation with lymphoid enhancer binding factro1/T-cell-specific factor (LEF1/TCF) transcription factors. Mutations in the canonical Wnt signaling pathway components, such as APC and β-catenin, are common in carcinoma of the colon, but are relatively rare in prostate cancer (Morin, 1999; Voeller et al., 1998). Yet, nuclear β-catenin accumulation in prostate cancer specimens is comparatively frequent (Chen et al., 2004; Chesire et al., 2002; Morin, 1999; Verras and Sun, 2006; Yardy and Brewster, 2005). The report of epithelial expression of Wnt/β-catenin target gene, Twist, in 90% of human prostate cancer tissues further suggests the role of deregulated Wnt signaling pathway (Kwok et al., 2005; Kwok et al., 2007). Thus, other mechanisms, besides mutations, need to be considered to explain the pervasive β-catenin/TCF activity documented in human prostate cancer.
This study was designed to address the role of stromal TβRII in prostatic tomorigenesis. The mouse with conditional knockout for TβRII in the stromal compartment (Tgfbr2fspKO) was previously reported to develop PIN (Bhowmick et al., 2004a). Here, we report the Tgfbr2fspKO mouse prostates can in fact progress to adenocarcinoma. Stromal cells deficient in TGF-β responsiveness were further found to promote tumorigenesis in a Wnt3a dependent manner. Finally, the loss of TβRII expression in stromal cells was recapitulated in human prostate cancer, in contrast to stromal cells associated with benign prostate epithelia.
MATERIALS AND METHODS
Animals
Tgfbr2floxE2/floxE2 and Tgfbr2fspKO mice bred on the C57BL/6 background were generated and maintained as previously described (Bhowmick et al., 2004a). Adult male severe combined immunodeficient (SCID) mice and C57BL/6 mice were purchased from Harlan (Indianapolis, IN). Vanderbilt Institutional Animal Care and Use Committee approved the animal procedures.
Cell Culture and production of conditioned medium
Primary mouse prostate stromal cell cultures were generated from 6–8 week old Tgfbr2floxE2/floxE2 or Tgfbr2fspKO mice. Briefly, the prostates were dissected, cut into 2mm3 pieces, plated on 6-well plates in high glucose Dulbecco’s Modified Eagle’s Medium, supplemented with 10% fetal bovine serum, 10−8 M testosterone, and 5 μg/mL insulin (Sigma-Aldrich, St. Louis, MO). Once the cells reached confluence, they were subcultured up to five passages and then discarded. LNCaP cells were purchased from ATCC, routinely passaged in RPMI medium containing 10% fetal bovine serum and 0.1% penicillin/streptomycin. Conditioned Tgfbr2floxE2/floxE2 or Tgfbr2fspKO stromal media were collected after two passages, from approximately 1×106 cells plated on a 100mm dish for 3 days in culture media and centrifuged prior to application on LNCaP cells. The conditioned media was normalized by the cell numbers at the time of collection.
Tissue rescue and tissue recombination
Tissue rescues were performed by direct grafting of prostates dissected from 6 week old Tgfbr2floxE2/floxE2 or Tgfbr2fspKO mice into the renal capsule of SCID mice, as previously described (Wang et al., 2000). The rescued tissues were harvested after 7 months. Prostate tissue recombinants were generated by combining 250,000 Tgfbr2floxE2/floxE2 or Tgfbr2fspKO prostatic stromal cells with either prostate epithelial organoids from wild type mice, or 100,000 cultured LNCaP cells, in 50 μl of rat-tail collagen. The prostate epithelial organoids were obtained from C57BL/6 mice (8–12 weeks) by collagenase digestion as previously reported (Hayward et al., 1992; Turner et al., 1990). The tissue recombinants were grafted under the renal capsule of C57BL/6 mice (for recombinants of prostate organoids) or SCID mice (for LNCaP cell recombinants) (Cunha, 1994; Fujii et al., 1982; Hayward et al., 2001; Wong et al., 1992). Host mice were sacrificed 2 months or 7 weeks following grafting. The grafts were removed, photographed, and processed for histologic evaluation. The dimensions were measured, and the tumor volume was calculated by the formula: volume = width × length × 0.52 using Image J program. Note that the formula underestimates the volume of invasive tumors, and accordingly underestimate the significance of size differences between small noninvasive and larger invasive tumors.
Measurement of cell proliferation and luciferase assay
LNCaP cells were passaged at 105 cells per well in 24-well plates. After overnight growth and attachment, the culture media were changed to either Tgfbr2floxE2/floxE2 or Tgfbr2fspKO stromal conditioned media. The cells in Tgfbr2fspKO stromal condition medium were treated with or without Wnt3a neutralization antibody at 1 ng/ml to 15 ng/ml, final concentration. Cell numbers were counted by hemocytometer after 72 hours.
The cells passaged in the 24-well plates were otherwise transfected with c-myc luciferase construct (He et al., 1998) with Lipofectamine LTX (Invitrogen, Carlsbad, CA), according to the users’ manual. Next day the cells were treated with conditioned media with/without Wnt3a neutralizing antibody, or control rat IgG for 24hrs before the cells were harvested, analyzed using Luciferase assay system (Promega, Madison, WI), normalized by total protein content.
Antibodies and immunohistochemistry
Paraffin-embedded tissue sections (5 μm) were de-paraffinized and hydrated through xylene and graded alcohols using a standard protocol. 1% antigen unmasking solution (Vector laboratories, Burlington, CA) was used for antigen retrieval according to manufacturer instructions. Subsequent immunohistochemical staining used antibodies against phosphorylated-histone H3 (1:1000, Upstate), TGFβ type II receptor or p63 (1:1000, Santa Cruz Biotech, Santa Cruz, CA), and Twist (1:500, Santa Cruz). Appropriate HRP-conjugated secondary antibodies and DAB incubation (Dako North America, Carpinteria, CA) was used for visualization. The ApopTag peroxidase in situ apoptosis detection kit (Chemicon, Temecula, CA) was used to detect apoptotic cells, according to manufacturer’s instructions.
The host SCID mice grafted with prostate tissue recombinants were injected intraperitoneally with mouse monoclonal Wnt3a or isotype control IgG (R&D Systems), at 100 μg per mouse in a 0.2 ml volume. Injection was started two weeks after grafting and subsequently twice weekly until sacrifice on the fourth week post grafting.
SiRNA transfection and Western analysis
Primary cultured mouse prostate stromal cells were transfected with Stat3 siRNA or the control siRNA using siRNA transfection reagent (Santa Cruz Biotech), the cells were harvested after 48 hours. Samples were separated by 10% SDS-PAGE gel and electro-transferred to nitrocellulose membrane. Membranes were blocked and incubated with primary antibodies for Wnt3a (R&D systems) or anti-actin (Santa Cruz) overnight at 4°C. Respective horseradish peroxidase conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) were applied to the blots for 1 hour. Protein bands were visualized using ECL plus Western blotting detection system (GE Healthcare).
Real-time quantitative reverse transcription-PCR and ChIP analysis
RNA was extracted using the RNAeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Reverse transcription and quantitative real-time PCR used iScript and iQ SYBR Green supermix, respectively (Bio-Rad, Hercules, CA). Relative quantitation was calculated by ΔΔCt method normalized to 18s rRNA. See supplemental material for sequences of RT-PCR primers.
Tgfbr2floxE2/floxE2 and Tgfbr2fspKO prostate stromal cells were grown to confluence prior to cross-linked with 1% formaldehyde, lysed, and sonicated as described previously (Nissen and Yamamoto, 2000). Chromatin fragments were immunoprecipitated with Stat3 antibody and normal mouse IgG as negative control (Santa Cruz Biotech) overnight at 4°C. After immunoprecipitation, 60 μL protein G Agarose were added to pull down the chromatin/protein-antibody complex, and further steps following the protocol from the EZ Chip kit (Upstate, Temecula, CA). PCRs were performed with for 30 cycles with the primers: forward-TGGACTCAGGAGGAATCAATGTGG and reverse-CGAGGTAGTTGTTAGGGAGTCCAA for the -854 Stat3 binding site of Wnt3a promoter and forward-AGAGGGACCCATTCCTCTGTGAAA and reverse-TGGAGCTGGAACTCAAGGAACTGT for the -3542 Stat3 binding site of Wnt3a promoter. PCR products were resolved on a 1.5% agarose gel and visualized with ethidium bromide.
Human prostate tumor samples and tissue microarray
Human prostate samples were collected from radical prostatectomy tissue, with prostate cancer and adjacent normal areas indicated by a pathologist consistent with Institutional Review Board approval. The tissues were evaluated following 4% paraformaldehyde fixation, paraffin embedding, and hematoxylin and eosin staining of 5 μm sections. Fifteen such benign prostate samples in addition to the samples from tissue microarray (TMA, Imgenex, San Diego, CA) that included 125 tissue cores with benign and cancer histology were immunohistochemically stained. Information on all tissue samples including Gleason score, prostate specific antigen (PSA) expression, clinical stage, and H&E were available.
Statistical Analysis
The univariant associations between the clinical correlates and the stromal TGF-β were tested using Wilcoxon rank-sum tests. To express the relationship between the proportions of stromal TGF-β and Gleason scores, log-linear models were considered. Odds of TGF-β expression were computed for benign and malignant samples, and the results were reported in terms of the odds ratio. All reported P-values were two-sided.
RESULTS
The loss of TβRII expression in the prostate stroma leads to adenocarcinoma
The Tgfbr2fspKO mouse model was used to study the contribution of the loss of TβRII in the prostatic stroma to prostatic adenocarcinoma progression. The Tgfbr2fspKO mouse prostates develop PIN lesions by six weeks of age with 100% penetrance, as previously reported (Figure 1A) (Bhowmick et al., 2004a). Electron microscopy further supported the failure of epithelial differentiation, as there were no secretory vesicles in the six-seven week old Tgfbr2fspKO mouse prostate epithelial cells, compared their presence in Tgfbr2floxE2/floxE2 mouse prostates (Figure 1B). If PIN is assumed to be a precursor of prostate adenocarcinoma, the progression of the disease was predicted in under long-term observation (Davidson et al., 1995; Qian et al., 1995). Since the Tgfbr2fspKO mice die by seven weeks of age, the prostates were rescued at six weeks, and allografted to the renal capsule of immunocompromised male SCID mice. Twenty five percent of the Tgfbr2fspKO prostates developed into adenocarcinoma by seven months following grafting (Figure 1C). Tissue-rescued prostates from six-week old Tgfbr2floxE2/floxE2 mice under the same conditions were histologically normal by H&E staining. Immunohistochemistry for the expression of TβRII confirmed a significant decrease in TβRII expression in the stromal compartment of Tgfbr2fspKO prostates (Figure 1D).
Figure 1.
The loss of TβRII expression in the prostatic stroma of mice leads to transformation of adjacent epithelia. A. Histologic comparison of Tgfbr2floxE2/floxE2 (left) and Tgfbr2fspKO (right) mouse prostates by H&E staining suggest Tgfbr2fspKO mouse prostates develop PIN by 6 weeks of age. B. Electron microscopy indicates the absence of secretory vesicles in Tgfbr2fspKO mouse prostates compared to their presence in Tgfbr2floxE2/floxE2 prostates. Scale bar represents 2 μm. C. Following tissue rescue of Tgfbr2floxE2/floxE2 and Tgfbr2fspKO prostates for seven months, the histology of the Tgfbr2fspKO prostates progressed to adenocarcinoma while the Tgfbr2floxE2/floxE2 prostates maintained a wild type phenotype. D. Immunohistochemistry for TβRII expression of the rescued tissues showed positive stromal and epithelial staining in the Tgfbr2floxE2/floxE2 prostates, yet only epithelial staining in the Tgfbr2fspKO prostates. Scale bar represents 50 μm for panels A, C, and D; 25 μm for insets.
Epithelial proliferation and differentiation markers were used to evaluate development of prostate adenocarcinoma. The mitotic rate of the prostatic epithelium of Tgfbr2fspKO mice was four-fold greater than that from Tgfbr2floxE2/floxE2 mice in the seven-month progression model, as determined by quantitating phosphorylated-histone H3 expression (Figure 2A). The mouse dorsolateral prostate (mDLP) antibody was used to localize secretions found in differentiated prostatic epithelium, was present in the Tgfbr2floxE2/floxE2 tissues but was focally absent in Tgfbr2fspKO tissues (Figure 2B) (Donjacour et al., 1990). Then mDLP staining in both normal and malignant rescued tissues confirmed the prostatic origin of the tissues. P63 expression was basally localized in the Tgfbr2floxE2/floxE2 tissues, as expected (Figure 2C) (Kurita et al., 2004). In contrast, p63 positive cells were rare and scattered in the Tgfbr2fspKO prostate rescues, supportive of the progression of adenocarcinoma and indicating a disruption of the basal cell layer. Another reported marker for prostatic adenocarcinoma progression, Twist, was detected only in the Tgfbr2fspKO tissue rescues that progressed to adenocarcinoma (Figure 2D) (Hotz et al., 2007; Kwok et al., 2005; Zhang et al., 2007). The loss of TβRII expression in the stroma resulted in the progression of prostate tumorigenesis in mice.
Figure 2.
Tgfbr2fspKO prostates develop adenocarcinoma in seven months. A. Phosphorylated-histone H3 staining of the mitotic cells suggested higher proliferation rate in the Tgfbr2fspKO prostates rescues than the normal Tgfbr2floxE2/floxE2 prostates rescues. The mean ± standard deviation of positive staining is indicated in each panel (P < 0.01, n = 6 for both test and control). B. Immunohistochemistry for mouse dorsolateral prostate secretory proteins (mDLP) was detectible control Tgfbr2floxE2/floxE2 prostates, but often lost in the Tgfbr2fspKO tissue rescues. C. Immunohistochemistry for p63 revealed disorganized staining pattern in the prostates of Tgfbr2fspKO compared to Tgfbr2floxE2/floxE2. D. Immunohistochemistry for Twist expression was positive in the adenocarcinoma Tgfbr2fspKO tissues, but not expressed in the Tgfbr2floxE2/floxE2 prostates. The sections were nuclear counterstained with hematoxylin (blue). Scale bar represents 50 μm for panels and 25 μm for insets.
Wnt3a mediates the progression of prostate tumors by Tgfbr2fspKO prostate stroma
In light of the observed expression of Twist in Tgfbr2fspKO prostate adenocarcinoma, and recent publications on the importance of Wnt signaling in prostate cancer progression, we hypothesized that Wnt ligand isoforms are paracrine mediators of the loss TGF-β responsiveness in the stromal cells. Reverse transcription real-time PCR analysis of Tgfbr2floxE2/floxE2 and Tgfbr2fspKO prostatic stromal cells revealed that four out of nineteen Wnt ligands had elevated expression in Tgfbr2fspKO cells relative to Tgfbr2floxE2/floxE2 cells (Figure 3A). Among them, Tgfbr2fspKO stromal cells had a median Wnt3a elevation of 4-fold over Tgfbr2floxE2/floxE2 cells. Wnt 5a, Wnt 6, and Wnt 10b were also expressed higher by Tgfbr2fspKO cells, however, viable antibodies for these Wnt isoforms were not available to perform further confirmatory studies. Elevated Wnt3a expression was confirmed at the protein level by Western blot detection (Figure 3B). This indicated TGF-β signaling as a suppressor of Wnt3a expression in prostate stromal cells and potential paracrine mediator prostate epithelial Wnt signaling.
Figure 3.
Tgfbr2fspKO prostatic stromal cells have elevated Wnt3a expression. A. The screening of 19 Wnt isoforms by real-time PCR revealed specific Wnt isoforms to have greater mRNA expression by cultured Tgfbr2fspKO prostatic stromal cells relative to control, Tgfbr2floxE2/floxE2 stromal cells. Each dot represents a comparative expression level of a Tgfbr2fspKO sample relative to the average expression level of the Tgfbr2floxE2/floxE2 samples (baseline). The data were normalized to 18s ribosomal RNA expression. The dotted horizontal line is at the value of 1 representing no difference from the Tgfbr2floxE2/floxE2 average. The thick horizontal lines indicate the medians within each group. (There are 3 data (83.9, 88.0, 410.1) in Wnt3a and 1 (51.5) in Wnt10b that are out of the plot range.) B. Western blot confirmed specifically Wnt3a protein expression was greater in Tgfbr2fspKO prostatic stromal cells compared to that from Tgfbr2floxE2/floxE2 cells.
To test the specific role of Wnt3a on epithelial transformation and proliferation, LNCaP cells were incubated with conditioned media from either Tgfbr2floxE2/floxE2 or Tgfbr2fspKO mouse prostatic stromal cells in the presence or absence of a Wnt3a neutralizing antibody. The transcriptional activity of a canonical Wnt oncogenic target, c-myc, was measured by a luciferase reporter assay. The LNCaP cells incubated with Tgfbr2fspKO conditioned media consistently had three-fold greater c-myc reporter activity than those cells incubated with Tgfbr2floxE2/floxE2 conditioned media (Figure 4A). Under the same conditions, the addition of the Wnt3a neutralizing antibody down regulated reporter activity of the LNCaP cells incubated with Tgfbr2fspKO conditioned media to levels similar to that found when associated with Tgfbr2floxE2/floxE2 conditioned media alone. Further, Tgfbr2fspKO conditioned media induced 2.7-fold increase in proliferation of LNCaP cells relative to Tgfbr2floxE2/floxE2 conditioned media (Figure 4B). Wnt3a neutralizing antibody reduced the proliferative effect of Tgfbr2fspKO conditioned medium in a dose dependent manner. The presence of 10 ng/ml Wnt3a neutralizing antibody decreased LNCaP cell proliferation to comparable levels as cells grown in Tgfbr2floxE2/floxE2 conditioned medium.
Figure 4.
Wnt3a expression is down regulated by TGF-β in prostate stromal cells. A. Conditioned media from Tgfbr2floxE2/floxE2 or Tgfbr2fspKO stromal cells were incubated with LNCaP cells in the presence or absence of Wnt3a neutralizing antibody and isotype control IgG. Subsequently, luciferase reporter assay for the c-myc promoter was used as readout for canonical Wnt activity in LNCaP cells. B. Cell counting was used to measure LNCaP cell proliferation following incubation with Tgfbr2floxE2/floxE2 or Tgfbr2fspKO prostatic stromal conditioned media. The addition of Wnt3a neutralizing antibody inhibited LNCaP cell proliferation in a dose dependent manner. The graphs in panels A and B indicate mean ± standard deviation (P < 0.01, n = 12). C. Wnt3a promoter analysis suggested Stat transcription factor binding consensus sites. ChIP analysis suggested greater Stat3 binding to the Wnt3a -854 and -3542 promoter elements in Tgfbr2fspKO stromal cells compared to Tgfbr2floxE2/floxE2 stromal cells. D. Western blotting for Wnt3a following siRNA-knockdown of Stat3 in Tgfbr2fspKO stromal cells showed inhibition of Wnt3a expression. The scrambled (Scmb.) siRNA did not affect Wnt3a expression. Actin expression served as a loading control.
Next, the mechanism of transcriptional up regulation of Wnt3a in the Tgfbr2fspKO prostatic stromal cells was analyzed. A transcription factor binding site search 5 kb upstream of the Wnt3a transcription start site suggested putative Stat DNA binding elements at -863/-854 bp and -3551/-3542 bp (http://www.cbrc.jp/research/db/TFSEARCH.html, http://genome-www.stanford.edu/cgi-bin/genecards/carddisp.pl?gene=Wnt3a, http://www.cbil.upenn.edu/cgi-bin/tess/tess). ChIP analysis indicated that in vivo, the -854 bp consensus site had elevated Stat3 binding in Tgfbr2fspKO prostate stromal cells compared to Tgfbr2floxE2/floxE2 stroma. However, the -3542 bp consensus site was specifically occupied by Stat3 in Tgfbr2fspKO prostatic stromal cells but not detectible in the Tgfbr2floxE2/floxE2 prostatic stromal cells (n = 4, Figure 4C). Accordingly, we focused on the role of Stat3 on Wnt3a expression, in Tgfbr2fspKO prostate stromal cells. Western blotting resolved reduced Wnt3a expression when Stat3 was knocked-down by siRNA compared to scrambled siRNA control (Figure 4D). Together, the data indicate stromal TGF-β responsiveness regulates Wnt3a expression in a Stat3-dependant mechanism to induce Wnt activity in adjacent epithelial cells.
Tgfbr2fspKO prostatic stromal cells promote human prostate cancer progression
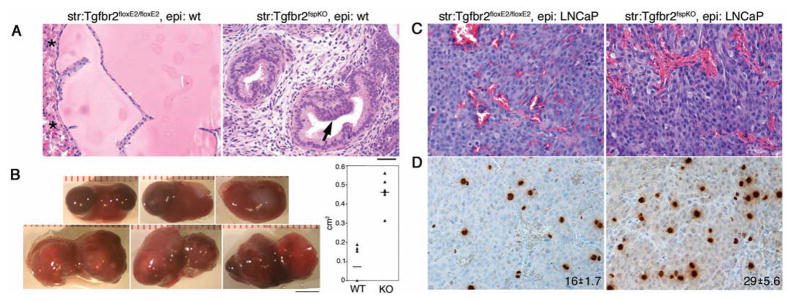
To determine if the tumorigenic effects of the prostatic stroma were a result of paracrine signaling, tissue recombination techniques were used to combine cultured Tgfbr2floxE2/floxE2 or Tgfbr2fspKO prostatic stromal cells with adult wild type mouse prostatic epithelial organoids. Following allografting the tissue recombinants into syngenic C57BL/6 male mice for eight weeks, we found that the Tgfbr2floxE2/floxE2 stroma-associated prostatic grafts were comparable to intact prostates of wild type mice. The Tgfbr2fspKO stroma-associated prostatic grafts developed PIN lesions, recapitulating the intact Tgfbr2fspKO prostates (Figure 5A). These results revealed that paracrine factors affected prostatic epithelial differentiation and established the tissue recombination methodology as a viable model for subsequent grafting experiments. To enable the study of stromal-epithelial signaling in adenocarcinoma, we developed a chimeric model of mouse prostatic stromal cells with LNCaP cells, an established human prostate cancer epithelial line. A five-fold increase in gross tumor volumes was observed in recombinants of LNCaP/Tgfbr2fspKO compared to tumors of LNCaP/Tgfbr2floxE2/floxE2 recombinants (Figure 5B). Although the histology of the two chimeric tissues was not appreciably different (Figure 5C), immunohistochemistry of phosphorylated-histone H3 revealed increased mitosis in LNCaP/Tgfbr2fspKO tissue recombinants compared to LNCaP/Tgfbr2floxE2/floxE2 recombinants (Figure 5D). Together, the loss of TβRII expression in the stroma initiated paracrine transformation of normal epithelia and supported tumor progression of prostate cancer.
Figure 5.
Tgfbr2fspKO prostatic stromal cells increase tumorigenicity of prostate epithelial cells. A. Tissue recombinant of Tgfbr2floxE2/floxE2 or Tgfbr2fspKO mouse prostate stromal cells with wild type mouse prostate organoids recapitulated the histology of the respective intact mice. Arrow indicates PIN lesion development in the tissue recombinant associated with Tgfbr2fspKO stromal cells. Asterisks indicate kidney parenchyma. B. The gross representations of the LNCaP/Tgfbr2fspKO tumors in renal xenografts were larger than control, LNCaP/Tgfbr2floxE2/floxE2 tumors. Tumor volumes calculated using Image J software were graphed as mean ± standard deviation (P < 0.01, n = 6). Scare bar represents 4 mm. C. H&E for the LNCaP/Tgfbr2floxE2/floxE2 and LNCaP/Tgfbr2fspKO recombinant tumors histology showed little difference. D. Immunohistochemistry for phosphorylated-histone H3, indicated the mitotic index of LNCaP/Tgfbr2fspKO tumors to be greater than LNCaP/Tgfbr2floxE2/floxE2 tumors. The mean positive staining is indicated in each panel ± standard deviation (P < 0.01, n = 6). The scale bar in panel A represents 50 μm for panels A, C, and D.
Next, host mice harboring the chimeric LNCaP/Tgfbr2fspKO tissue recombinants were treated with the Wnt3a neutralizing antibody or isotype IgG control. The antibodies were injected intra-peritoneally two weeks following xenografting. Although the gross tumor size between the isotype IgG control and the Wnt3a neutralizing antibody were not appreciably different, the histology of the tumors revealed areas of necrosis in the tumors when mice were treated with the neutralizing antibody compared to control (Figure 6A). Further immuno-localization for cells under going apoptosis confirmed 2.3-fold greater number in tumors treated with the Wnt3a neutralizing antibody compared to the control (p value = 0.001, Figure 6B). There was a lack of mitotic cells in the areas of necrosis, as determined by phosphorylated-histone 3 immuno-localization (Figure 6B). The mitotic rate in the surviving tumors from neutralizing antibody treated mice was significantly less than that in isotype control mice (p value = 0.002, Figure 6C). Thus, down regulation of TGF-β signaling in the prostatic stromal compartments is associated with canonical Wnt signaling in the adjacent epithelia to support tumor initiation and further tumor progression. Inhibiting paracrine Wnt3a activity was effective in reducing growth of tumors deficient in stromal TGF-β signaling.
Figure 6.
Wnt3a neutralizing antibody inhibits tumorigenic progression of LNCaP/Tgfbr2fspKO tissue recombinants. Wnt3a neutralizing antibody or isotype control IgG was i. p. injected to the hosted SCID mice twice a week for two weeks, started two weeks post-grafting. A. Histology by H&E showed increased necrotic areas in the tumors from Wnt3a antibody injected mice, compared to the ones from control mice (indicated by dashed line). B. Greater number of apoptotic cells was localized in the tumors treated with Wnt3a neutralizing antibody compared to control by Apop-tag immunohistochemistry (P < 0.01, n = 8). C. Immunohistochemistry of phosphorylated-histone H3 showed less mitotic cells in the tumors from Wnt3a treated mice compared to control (P < 0.01, n = 6). The mean ± standard deviation is indicated in each panel. The scale bar represents 50 μm for panels and 25 μm for insets.
TβRII expression is lost in stromal cells of human prostate adenocarcinomas
Finally, to explore the role of stromal TGF-β signaling in human prostate cancer progression, we localized TβRII expression by immunohistochemistry of 140 benign and malignant prostate tissues. Prostate samples from patients who underwent radical prostatectomy were obtained from Vanderbilt University and Imgenex Co. These patients received no documented treatment before surgery. The tissues were grouped based on Gleason score and compared based on the staining for TβRII in the prostatic stroma (Figure 7). The TβRII antibodies that tested in the respective Tgfbr2floxE2/floxE2 and Tgfbr2fspKO prostates in Figure 1D were used for these immunohistochemical studies. TβRII was highly expressed in epithelial cells of all prostate samples examined. The stromal TβRII staining pattern, albeit less intense, was representative of >95% of the tissue in each array spot based on blinded pathology scoring (Figure 7). Stromal TβRII was expressed in 85% of the tissues associated with benign epithelia. In contrast, an average 31% of the prostate cancer tissues with Gleason scores 6–10 maintained stromal TβRII staining. Further the clinical correlates, pre-surgical serum PSA expression and age (p value=0.97, 0.31, respectively), did not statistically distinguish between benign and prostate cancer in this population. There was no correlation of stromal TGF-β expression and a specific Gleason score. As the proportions of stromal TGF-β expression for malignant samples were relatively similar to each other than that of benign samples, counts in malignant samples were combined and compared to the benign group. Multivariant analysis suggested the odds of positive stromal TβRII is 11.5 times as high in the benign group compared to the malignant group (with 95% power; confidence interval: 4.2 to 31.3; p value < 0.0001).
Figure 7.
Immunohistochemistry for TGF-β type II receptor (TβRII) expression is not detectable in stromal cells of human prostate adenocarcinomas. The pathologic grade of the representative immunohistochemistry images is indicated as benign or Gleason score. Note TβRII was consistently expressed in epithelial cells, but often lost in stromal cells of neoplastic tissues. Scale bar represents 50 μm. The table indicates the distribution of tissue pathology with positive histochemical TβRII staining in the stromal compartment.
DISCUSSION
The progression of the grafted prostate tissue rescues from Tgfbr2fspKO mouse to adenocarcinoma led us focus on the consequence of the loss of TGF-β signaling in the stroma on malignant progression. Although PIN lesions spontaneously developed in the Tgfbr2fspKO mouse prostates by 5–7 weeks of age, it was not clear if the model supported further progression to adenocarcinoma (Bhowmick et al., 2004a). Due to early lethality of the Tgfbr2fspKO mice, we used tissue rescue and recombination grafting techniques to reveal the long-term role of stromal TGF-β signaling in tumor progression. As only 25% of the Tgfbr2fspKO mouse prostates progressed to adenocarcinoma, we chose to use an established human prostate cancer cell line, LNCaP, to further study the paracrine impact of the loss of TGF-β responsiveness in the stroma. LNCaP cells do not express functional TGF-β receptors (Guo and Kyprianou, 1999). Thus, the resulting differences in the tumor size between Tgfbr2floxE2/floxE2 and Tgfbr2fspKO prostatic stroma associated tissue recombinants (Figure 5) were due to TGF-β signaling differences in the tumor microenvironment. It is likely stromally derived factors normally suppressed by TGF-β signaling, accelerated LNCaP tumor progression. A candidate approach identified Wnt3a as one such TGF-β regulated cytokine, subsequently was shown to have an important role in tumor survival. Elevated Wnt signaling is attributed in the initiation and progression of prostate cancer with relatively infrequent mutations in the pathway (Yardy and Brewster, 2005). This study provides a mechanism for the elevated Wnt activity in prostate epithelia. The loss of TβRII expression in the stroma of 69% of human prostatic cancer tissues and the resulting signaling repercussions suggests the relatively frequent evidence of elevated Wnt signaling in prostate cancer can be a result of paracrine activity. The data further supports stromal TGF-β signaling to be a tumor suppressor in the prostate (Bhowmick and Moses, 2005; Bhowmick et al., 2004b).
The expression of TβRII in mature prostate epithelial cells is thought to contribute to the relatively low proliferative state. Accordingly, previous studies hypothesized that a loss of epithelial TβRII expression played a role in the process of high-grade prostate cancer progression. However, immunohistochemical staining for TβRII expression are ambiguous with reports of both decreased and elevated TβRII expression in prostate adenocarcinomas (Cardillo et al., 2000; Gerdes et al., 1998; Kim et al., 1998; Kim et al., 1996; Royuela et al., 1998). Our focus was on stromal TβRII expression in prostate cancer progression. We do not make any conclusions regarding the status of epithelial TβRII expression, as the immunohistochemical development was extended to enable visualization of stromal expression. The apparent histochemical staining for TβRII was significantly higher in epithelial cells than that found in the stromal compartment at any pathologic state (Figure 7). Stromal staining associated with PIN lesions in the human tissues were not examined, since it was impossible to determine which lesions would progress to cancer. The question of TβRII antibody specificity in our immunohistochemistry study was addressed by the positive and negative stromal staining pattern in the Tgfbr2floxE2/floxE2 and Tgfbr2fspKO prostatic tissues, respectively (Figure 1).
We report on TGF-β regulation of paracrine Wnt signaling, rather than the better-studied intracellular interaction between the TGF-β and Wnt signaling pathways. Incidentally, many Wnt ligands are stromally secreted with cognate receptors expressed by the epithelia, such as human prostate cancer tissues (Ellwood-Yen et al., 2003; Kwok et al., 2005). Wnt target genes, like Twist (Figure 2) and c-myc (Figure 4) were up regulated in the epithelium associated with Tgfbr2fspKO prostate stroma (Bhowmick et al., 2004a). In a comparison of primary cultured prostatic stromal cells from Tgfbr2floxE2/floxE2 and Tgfbr2fspKO mice, we found that the expression of Wnt ligands were up regulated in Tgfbr2fspKO stromal cells (Figure 3). There was a pronounced up regulation of Wnt3a at both RNA and protein levels. Ectopic expression has shown that Wnt3a to be a secreted form of Wnt ligand, unlike other Wnt proteins that associate with the cell membrane (Shibamoto et al., 1998). Although no Smad binding elements were found in the Wnt3a gene promoter, Stat binding sites were predicted. Interestingly, TGF-β signaling is reported to inhibit Stat3 in T lymphocytes (Bright and Sriram, 1998) and corroborated here in the prostate stroma. We showed that the knockout of TGF-β signaling in prostate stromal fibroblasts resulted in the up regulation of Stat3 binding of the Wnt3a promoter by ChIP analysis (Figure 4). The neutralization of Wnt3a in Tgfbr2fspKO stromal conditioned media reduced LNCaP proliferation in vitro. The neutralization of Wnt3a in mice with LNCaP tumor xenografts also resulted in greater areas of cell death due to necrosis and apoptosis in LNCaP tumors accompanied by lower rate of mitosis compared to those treated with the IgG isotype control (Figure 6). The mechanism for the TGF-β paracrine cross-talk with Wnt signaling seems to be mediated in part through Stat3 signaling.
Previously, we reported that paracrine HGF signaling is involved in the tumorigenesis observed in Tgfbr2fspKO mice. Here, the data suggests that disruption of TGF-β signaling in the prostatic stromal cells up regulates the expression of Wnt3a to promote tumorigenesis in a Stat3 dependent manner. Clearly, tumor initiation and progression is not a single signaling pathway event. Interestingly, the induction of HGF and Wnt3a, resulting from the disruption of stromal Tgfbr2, is tied by elevated activation of Stat3. We showed in this study increased Stat3 binding with Wnt3a promoter activity. Stat3 activation can also induce HGF expression by co-operation with Src (Qiao et al., 2002; Sam et al., 2007; Wojcik et al., 2006). In the context of a tumor many growth factors, including IL-6, HGF and IGF-1, are known stimulators of Stat proteins. Thus there are multiple potential mechanisms that would support paracrine Wnt3a signaling in prostate cancer. Like other targeted monoclonal antibody-based therapies in the clinic, the Wnt3a neutralizing antibody was well tolerated by the host mice. This is the first attempt of using Wnt3a neutralizing antibody to inhibit prostate tumor growth. The promising outcome suggests such Wnt antagonists can be effective for prostate cancer patients, specifically those with undetectable stromal TβRII expression.
Supplementary Material
Acknowledgments
Dr. Xiuping Yu (Vanderbilt University) generously provided primers for the Wnt isoforms. The work was supported by the DOD through DAMD (W81XWH-04-1-0046 to N.A.B) as well as through NIH grants (CA108646, CA126505 to N.A.B and FGM079879A to V.R.P).
Abbreviations
- TGF-β
Transforming growth factor beta
- TβRII
TGF-β type II receptor
- Tgfbr2floxE2/floxE2
floxed TGF-β type II receptor
- Tgfbr2fspKO
TGF-β type II receptor conditional fibroblast knockout
- PIN
Prostatic intraepithelial neoplasia
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004a;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004b;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–13. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Sriram S. TGF-beta inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J Immunol. 1998;161:1772–7. [PubMed] [Google Scholar]
- Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C. Prognostic significance of transforming growth factor beta receptor II in estrogen receptor-negative breast cancer patients. Clin Cancer Res. 2004;10:491–8. doi: 10.1158/1078-0432.ccr-0320-03. [DOI] [PubMed] [Google Scholar]
- Calin GA, Gafa R, Tibiletti MG, Herlea V, Becheanu G, Cavazzini L, et al. Genetic progression in microsatellite instability high (MSI-H) colon cancers correlates with clinico-pathological parameters: A study of the TGRbetaRII, BAX, hMSH3, hMSH6, IGFIIR and BLM genes. Int J Cancer. 2000;89:230–5. [PubMed] [Google Scholar]
- Cardillo MR, Petrangeli E, Perracchio L, Salvatori L, Ravenna L, Di Silverio F. Transforming growth factor-beta expression in prostate neoplasia. Anal Quant Cytol Histol. 2000;22:1–10. [PubMed] [Google Scholar]
- Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–56. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–94. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–44. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Davidson D, Bostwick DG, Qian J, Wollan PC, Oesterling JE, Rudders RA, et al. Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: predictive accuracy in needle biopsies. J Urol. 1995;154:1295–9. [PubMed] [Google Scholar]
- Donjacour AA, Rosales A, Higgins SJ, Cunha GR. Characterization of antibodies to androgen-dependent secretory proteins of the mouse dorsolateral prostate. Endocrinology. 1990;126:1343–54. doi: 10.1210/endo-126-3-1343. [DOI] [PubMed] [Google Scholar]
- Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–93. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–38. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Fujii H, Cunha GR, Norman JT. The induction of adenocarcinomatous differentiation in neoplastic bladder epithelium by an embryonic prostatic inductor. J Urol. 1982;128:858–61. doi: 10.1016/s0022-5347(17)53221-8. [DOI] [PubMed] [Google Scholar]
- Gerdes MJ, Larsen M, McBride L, Dang TD, Lu B, Rowley DR. Localization of transforming growth factor-beta1 and type II receptor in developing normal human prostate and carcinoma tissues. J Histochem Cytochem. 1998;46:379–88. doi: 10.1177/002215549804600312. [DOI] [PubMed] [Google Scholar]
- Goggins M, Shekher M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58:5329–32. [PubMed] [Google Scholar]
- Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–4. [PubMed] [Google Scholar]
- Guo Y, Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–71. [PubMed] [Google Scholar]
- Hayward SW, Del Buono R, Deshpande N, Hall PA. A functional model of adult human prostate epithelium. The role of androgens and stroma in architectural organisation and the maintenance of differentiated secretory function. J Cell Sci. 1992;102 (Pt 2):361–72. doi: 10.1242/jcs.102.2.361. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–42. [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–76. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–9. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Ahn HJ, Lang S, Oefelein MG, Oyasu R, Kozlowski JM, et al. Loss of expression of transforming growth factor-beta receptors is associated with poor prognosis in prostate cancer patients. Clin Cancer Res. 1998;4:1625–30. [PubMed] [Google Scholar]
- Kim IY, Ahn HJ, Zelner DJ, Shaw JW, Lang S, Kato M, et al. Loss of expression of transforming growth factor beta type I and type II receptors correlates with tumor grade in human prostate cancer tissues. Clin Cancer Res. 1996;2:1255–61. [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–64. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- Kwok WK, Ling MT, Yuen HF, Wong YC, Wang X. Role of p14ARF in TWIST-mediated senescence in prostate epithelial cells. Carcinogenesis. 2007 doi: 10.1093/carcin/bgm185. [DOI] [PubMed] [Google Scholar]
- Labbe E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, et al. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 2007;67:75–84. doi: 10.1158/0008-5472.CAN-06-2559. [DOI] [PubMed] [Google Scholar]
- Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999;21:1021–30. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, et al. Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann’s organizer. Nature. 2000;403:781–5. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–29. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, et al. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–6. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Pasche B. Role of transforming growth factor beta in cancer. J Cell Physiol. 2001;186:153–68. doi: 10.1002/1097-4652(200002)186:2<153::AID-JCP1016>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM, et al. Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res. 1995;55:5408–14. [PubMed] [Google Scholar]
- Qiao H, Hung W, Tremblay E, Wojcik J, Gui J, Ho J, et al. Constitutive activation of met kinase in non-small-cell lung carcinomas correlates with anchorage-independent cell survival. J Cell Biochem. 2002;86:665–77. doi: 10.1002/jcb.10239. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royuela M, De Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Paniagua R. Transforming growth factor beta 1 and its receptor types I and II. Comparison in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Growth Factors. 1998;16:101–10. doi: 10.3109/08977199809002121. [DOI] [PubMed] [Google Scholar]
- Sam MR, Elliott BE, Mueller CR. A novel activating role of SRC and STAT3 on HGF transcription in human breast cancer cells. Mol Cancer. 2007;6:69. doi: 10.1186/1476-4598-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P, Itin P, Rufli T. In situ analysis of transforming growth factor-beta s (TGF-beta 1, TGF-beta 2, TGF-beta 3), and TGF-beta type II receptor expression in malignant melanoma. Carcinogenesis. 1995;16:1499–503. doi: 10.1093/carcin/16.7.1499. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–70. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Turner T, Bern HA, Young P, Cunha GR. Serum-free culture of enriched mouse anterior and ventral prostatic epithelial cells in collagen gel. In Vitro Cell Dev Biol. 1990;26:722–30. doi: 10.1007/BF02624429. [DOI] [PubMed] [Google Scholar]
- Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–3. [PubMed] [Google Scholar]
- Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, et al. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–17. [PubMed] [Google Scholar]
- Wojcik EJ, Sharifpoor S, Miller NA, Wright TG, Watering R, Tremblay EA, et al. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene. 2006;25:2773–84. doi: 10.1038/sj.onc.1209306. [DOI] [PubMed] [Google Scholar]
- Wong YC, Cunha GR, Hayashi N. Effects of mesenchyme of the embryonic urogenital sinus and neonatal seminal vesicle on the cytodifferentiation of the Dunning tumor: ultrastructural study. Acta Anat (Basel) 1992;143:139–50. doi: 10.1159/000147240. [DOI] [PubMed] [Google Scholar]
- Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–26. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xie D, Li X, Wong YC, Xin D, Guan XY, et al. Significance of TWIST expression and its association with E-cadherin in bladder cancer. Hum Pathol. 2007;38:598–606. doi: 10.1016/j.humpath.2006.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.