Abstract
Deepwater rice (Oryza sativa) is adapted to survive conditions of severe flooding over extended periods of time. During such periods adventitious roots develop to provide water, nutrients, and anchorage. In the present study the growth of adventitious roots was induced by treatment with ethylene but not auxin, cytokinin, or gibberellin. Root elongation was enhanced between 8 and 10 h after submergence. The population of cells in the S phase and expression of the S-phase-specific histone H3 gene increased within 4 to 6 h. Within 6 to 8 h the G2-phase population increased. Cell-cycle activation was accompanied by sequential induction of a cdc2-activating kinase homolog, R2, of two cdc2 genes, cdc2Os-1 and cdc2Os-2, and of three cyclin genes, cycA1;3, cycB2;1, and cycB2;2, but only induction of the R2 gene expression preceded the induction of the S phase, possibly contributing to cell-cycle regulation in the G1 phase. Both cdc2 genes were expressed at slightly higher levels during DNA replication. Transcripts of the A-type cyclin accumulated during the S and G2 phases, and transcripts of the B-type cyclins accumulated during the G2 phase. Cyclin expression was induced at all nodes with a similar time course, suggesting that ethylene acts systemically and that root primordia respond to ethylene at an early developmental stage.
Deepwater rice (Oryza sativa) plants are particularly well adapted for surviving conditions of partial submergence. When flooded, growth of the youngest internode accelerates to keep the uppermost leaves above the rising water level, and adventitious roots grow from the nodes to provide water and nutrients to the newly developing upper parts of the plant.
Internodal growth has been characterized in detail in the past (Kende, 1987; Kende et al., 1993; Lorbiecke and Sauter, 1998). Induction of adventitious root growth is less well understood. Both processes are hormonally regulated. Submergence as the primary signal leads to an accumulation of ethylene in the plant, through both increased ethylene biosynthesis and physical entrapment as a result of the low rate of diffusion of ethylene in water. In the internode, ACC synthase activity, the rate-limiting step in ethylene biosynthesis, is increased approximately 8-fold upon submergence (Cohen and Kende, 1987). One member of the multigene family of ACC synthases in rice was expressed at higher levels in the internode after 12 h of submergence, possibly contributing to long-term ethylene biosynthesis (Zarembinsky and Theologis, 1997). ACC synthase activity in the nodal tissue is already high in air-grown plants and remains high after submergence (Cohen and Kende, 1987).
ACC oxidase activity and gene expression are induced approximately 2-fold in the nodal tissue after 4 h of submergence and eventually reach higher levels (Mekhedov and Kende, 1996), which probably contributes to long-term ethylene synthesis. Ethylene is only an intermediary player in the signal transduction pathway leading to internodal growth. The immediate growth-promoting hormone is GA (Raskin and Kende, 1984; Kende, 1987). Ethylene leads to an increased GA concentration in the tissue and to an increased responsiveness of the tissue toward GA, making lower GA concentrations more effective (Raskin and Kende, 1984). It has been suggested that a decrease in ABA levels in the internode is responsible for this change of sensitivity (Hoffmann-Benning and Kende, 1992). In general, ABA is known to counteract GA responses.
Adventitious roots are shoot-borne roots that are initiated as part of normal plant development in deepwater rice. As the plant develops the root initials mature to root primordia bearing all of the characteristic features found in primary or lateral roots, including the connection to the vasculature. However, unless treated with an appropriate stimulus, such as submergence or ethylene, the root primordia will not emerge through the nodal epidermis (see Fig. 9; Suge, 1985; Bleecker et al., 1987). Involvement of hormones other than ethylene in the induction of root primordia growth was not analyzed in great detail and thus cannot be ruled out. One difference between the induction of internodal and adventitious root growth by ethylene has become obvious from the studies of Bleecker et al. (1986). Whereas stem growth is saturated at 10 ppm ethylene, induction of adventitious root growth is much more sensitive to ethylene, with a saturating concentration at approximately 0.3 ppm. This suggests that signal transduction in the internode and in the node involves at least partly different mechanisms.
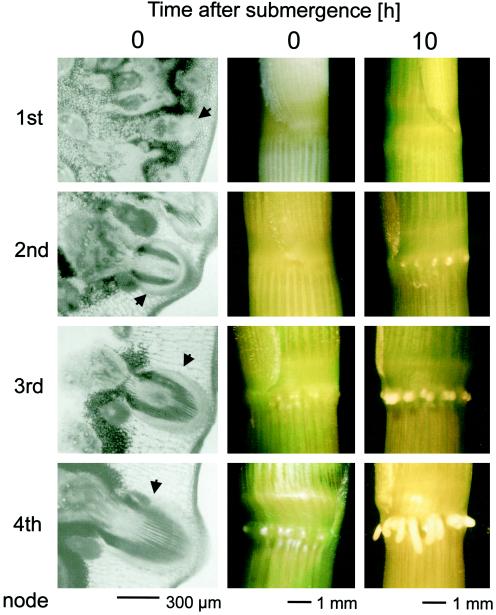
Figure 9.
Development and submergence-induced growth of adventitious roots from the youngest (1st) to the oldest (4th) node of adult plants. The first column shows cross-sections through the four different nodes of air-grown plants. The arrowheads indicate the positions of root initials. The second column shows front views of the four different nodes of air-grown plants. The third column shows front views of the four different nodes 10 h after submergence treatment. The adventitious roots at the second, third, and fourth nodes have emerged through the epidermis.
During formation of adventitious roots distinct developmental stages can be distinguished: (a) initiation, (b) early development, (c) growth arrest, and (d) emergence of the root primordium (which can also be characterized at the molecular level on the basis of gene expression or mutant phenotypes). Initiation of adventitious roots from differentiated cells in tobacco is marked by HRGPnt3 gene expression induced before the first primordial cell division (Vera et al., 1994). Early lateral and adventitious root primordium development in Arabidopsis is characterized by the expression of the LRP1 gene, which in lateral roots was shown to be turned off before the emergence of the primordium (Smith and Feodoroff, 1995). The rml (root meristemless) mutants of Arabidopsis (Cheng et al., 1995) are characterized by growth arrest of lateral and adventitious roots at a size of less than 2 mm, and the RML genes may thus be considered as markers for late lateral and adventitious root development, including emergence of the root primordium. The paucity of such data indicates that regulation of secondary root development, including adventitious root formation, is far from understood.
It is at this later stage, when adventitious root primordia have matured but not yet emerged through the nodal epidermal layer, when growth induction by submergence occurs in deepwater rice. To understand the regulation of this induction we analyzed the role of ethylene and other plant hormones as signaling components in roots. To understand the different mechanisms of growth regulation in adventitious root primordia compared with growth induction in the internode, we analyzed the regulation of cell growth and division in root primordia in response to the inductive signals.
Known cell-cycle regulatory genes from rice (Sauter, 1997) shown to be involved in regulating cell division in the internode (Sauter et al., 1995; Lorbiecke and Sauter, 1998) were analyzed with respect to a potential involvement in root meristem induction. Our results indicate that the cell-division cycle is activated in the intercalary meristem of the internode and in the apical adventitious root meristem with very similar kinetics. Despite these similarities the divergent expression patterns of cdc2 genes suggest different mechanisms of cell-cycle induction for roots and stems. In addition to a detailed analysis of root-growth induction and regulation, our work also provides the basis to identify as-yet-unrecognized genes involved in regulating or sustaining growth of adventitious root primordia in rice. These genes will be described elsewhere.
MATERIALS AND METHODS
Plant Material and Incubation Conditions
Seeds of deepwater rice (Oryza sativa L. cv Pin Gaew 56) were obtained from the International Rice Research Institute (Los Baños, The Philippines). Plants were grown essentially as described previously (Sauter, 1997) for 12 to 14 weeks. Whole plants were submerged in 600-L plastic tanks filled with tap water at 25°C with approximately 30 cm of the leaf tips remaining above water. Incubation was in an environmental growth chamber under continuous light (200 μE m−2 s−1). Control plants were kept in the same growth chamber. Isolated stem sections were used for the analysis of hormonal and inhibitor effects on adventitious root growth at the third node. The sections were cut from 2 cm below the third-highest node extending upward, with a total length of 20 cm. The sections contained the third and second nodes and the second-youngest internode between them. The sections were incubated in 150-mL beakers containing 25 mL of an aqueous solution of the appropriate hormones: IAA, GA3, 6-BA, hormone precursors, ethephon (2-chlorethanephosphoric acid), or ACC at the concentrations indicated.
The beakers with the stem sections were placed in plastic cylinders to ensure high humidity. Incubation was at 25°C under continuous light. An inhibitor of ethylene action, NBD (bicyclo[2.2.1]hepta-2,5-diene), was added to yield 50 μL/L in the gas phase. For submergence of excised stem sections, a 2-L glass cylinder was filled with distilled water and the stem sections were completely submerged. Growth of root initials was scored when the root had emerged through the epidermis of the node. Root growth is given as the percentage of roots emerged compared with the total number of root initials present. To determine length, roots were isolated from the node and photographed through binoculars. The photographed roots were then measured with a ruler and length was calculated to scale.
Flow-Cytometric Analysis
Roots were isolated from the third node and the nuclei were released according to the method of Sgorbati et al. (1986) with modifications. Roots were fixed in ice-cold 4% (v/v) formaldehyde in Tris buffer (10 mm Tris-HCl, 10 mm EDTA, 100 mm NaCl, pH 7.4, 0.1% [v/v] Triton X-100) for 15 min, and then washed twice for 10 min each in Tris buffer. The nuclei were isolated by crushing the roots with a glass rod in 500 μL of buffer. After the addition of 1 mL of DNA-staining solution (Partec, Münster, Germany) containing the fluorescent dye 4′,6-diamidino-2-phenylindole, the nuclei were filtered through a 22-μm nylon mesh and incubated for 60 min at room temperature. The stained nuclei were subjected to flow-cytometric analysis as described previously (Sauter, 1997). At least 10,000 nuclei were measured per time point.
Northern-Blot Analysis
Total RNA was isolated from excised roots with the TRIzol reagent (GIBCO-BRL) according to the manufacturer's instructions. In addition, RNA was precipitated with 4 m LiCl as described previously (Puissant and Houdebine, 1990). RNA separation, northern-blot hybridization, and quantification of relative mRNA abundance were carried out as described previously (Sauter, 1997; Lorbiecke and Sauter, 1998).
Estimation of Relative Transcript Levels of cycB2;2 with RT-PCR
RT-PCR was performed according to the method of Sauter et al. (1995) with modifications. Total RNA was isolated from 2-mm nodal sections as described above. First-strand cDNA synthesis was performed using 100 ng of DNase I-treated total RNA in a final volume of 10 μL with 2.5 μm of a primer specific for the 3′ end of cycB2;2 (5′-GCAAATTGCATGTGCCACA-3′), 100 μm deoxyribonucleotide triphosphate, 20 units of RNA-Guard (Pharmacia), 50 mm Tris-HCl, pH 8.3, 75 mm KCl, 3 mm MgCl2, 10 mm DTT, and 40 units of reverse transcriptase (SuperScript RNase H−, GIBCO-BRL). The reaction was carried out at 50°C for 75 min in a thermocycler. PCR reactions were performed in a final volume of 50 μL, including the 10 μL from the RT reaction and 200 μm deoxyribonucleotide triphosphate, 0.5 μm of the 3′-specific primer (see above), 0.5 μm of a cycB2;2 5′-specific primer (5′-GCTCAAGAGCGTGGCACTGT-3′), 20 mm Tris-HCl, pH 8.4, 50 mm KCl, 1.5 mm MgCl2, and 2.5 units of Taq DNA polymerase (GIBCO-BRL).
After a first denaturation step of 1 min at 94°C, the reaction cycles were: 1 min at 94°C, 2 min at 56°C, 2 min at 72°C, and a final 7 min at 72°C. The number of cycles was 35 for RNA from the first node, 25 for RNA from the second and third nodes, and 24 for RNA from the fourth node. In each case, the signal intensities were between the signals obtained from 50 or 200 ng of RNA (data not shown), and were therefore considered to be in the linear range of PCR amplification. PCRs with 100 ng of RNA but without RT were performed as a control for DNA contamination (data not shown). To ensure that the transcribed product was of the expected size, control assays with plasmid DNA containing the cycB2;2 cDNA were also carried out. The products were separated on a 2% (w/v) agarose gel, hybridized to a digoxigenin-labeled cDNA probe, and visualized by chemiluminescence according to the manufacturer's instructions (Boehringer Mannheim).
RESULTS
Time Course of Submergence-Induced Adventitious Root Growth
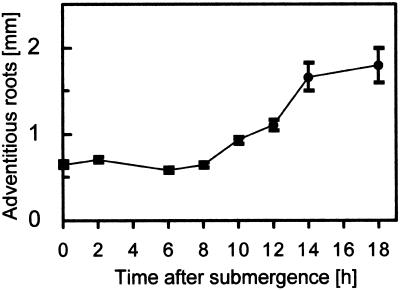
We analyzed submergence-induced growth of adventitious roots at the third node of adult deepwater rice plants. Root growth set in between 8 and 10 h after submergence (Fig. 1). The lag phase of 8 h for induction of adventitious root growth was twice as long as the lag phase for internodal growth, which was determined to be 4 h (Rose-John and Kende, 1985; Lorbiecke and Sauter, 1998). At the end of the experiment (after 18 h) the roots had approximately tripled in length.
Figure 1.
Submergence-induced growth of adventitious roots. Adventitious root lengths at the third node of intact plants were measured at various times after submergence. Average root lengths (±se) were determined in two independent experiments from isolated roots.
Submergence and Ethylene Induce the Cell-Division Cycle with Similar Kinetics
Induction of cell-division activity was analyzed in roots isolated from the third node of intact, submerged plants or in roots isolated from the third node of excised stem sections treated with ethephon. The ethephon concentration giving maximal induction of root growth (150 μm) was used in this study (see Fig. 5A). At various times after the onset of the growth-promoting treatment, flow-cytometric analysis was performed to measure the distribution of cells in the G1, S, and G2 phases (Fig. 2A). Growth induction resulted in an accumulation of cells first in the S phase and then in the G2 phase, suggesting that a subpopulation of cells in the G1 phase was induced to enter a new cell cycle in a synchronous manner.
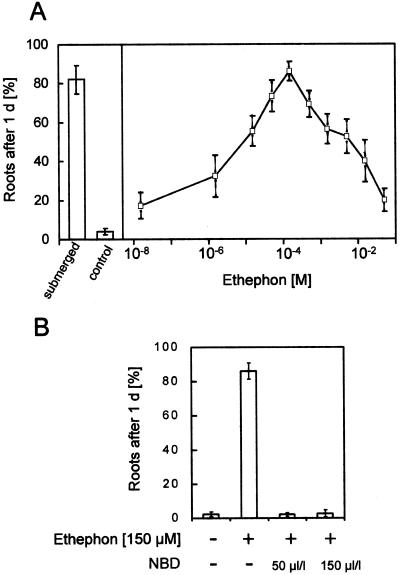
Figure 5.
Growth of adventitious roots at the third node of stem sections treated with ethephon as indicated for 24 h. A, Effect of ethephon treatment at various concentrations compared with untreated sections (control) or with sections submerged for the same time (submerged). B, Effect of ethephon treatment in combination with NBD, an inhibitor of ethylene action, at the concentrations indicated. Controls were incubated without the addition of hormone or inhibitor. Results are averages ± se of at least seven stem sections.
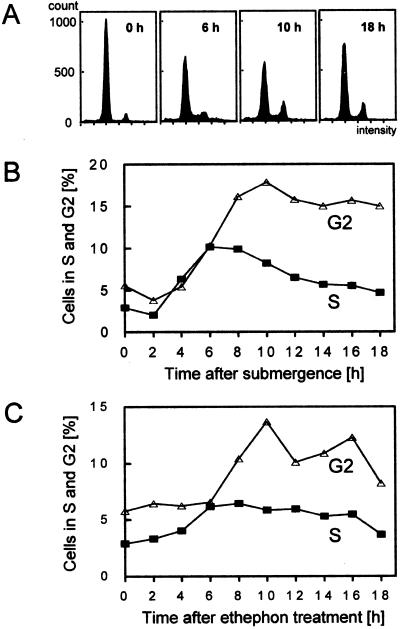
Figure 2.
Flow-cytometric analysis of adventitious roots at the third node. A, Histogram showing the distribution of cells in the G1 (large peak) and G2 (small peak) phases of the cell-division cycle 0, 6, 10, and 18 h after submergence. S-phase cells are represented between the two gap-phase peaks. B, Relative number of cells in the S (▪) and G2 (▵) phases in adventitious roots isolated from plants submerged for the times indicated. C, Relative number of cells in the S (▪) and G2 (▵) phases in adventitious roots isolated from stem sections treated with 150 μm ethephon for the times indicated.
In submerged plants entry of activated cells into the S phase occurred between 2 and 6 h after submergence, with an increase from 2% to 10.1% of S-phase cells compared with the total cell population (Fig. 2B). Entry of the induced cells into the G2 phase, with a 3-fold increase in the G2-phase population, was measured between 4 and 8 h after submergence. In ethephon-treated, isolated stem sections, induced cells accumulated in the S phase between 4 and 6 h of ethephon treatment and in the G2 phase between 6 and 10 h after induction (Fig. 2C). Therefore, both treatments induced cell-division activity in adventitious roots in a similar manner with a similar time course. As judged from the time between entry into the S phase and entry into the G2 phase, the cells required approximately 2 h to replicate their genome.
Submergence-Induced Cell-Division Activity Is Accompanied by Root-Specific Sequential Induction of Cell-Cycle Regulatory Genes
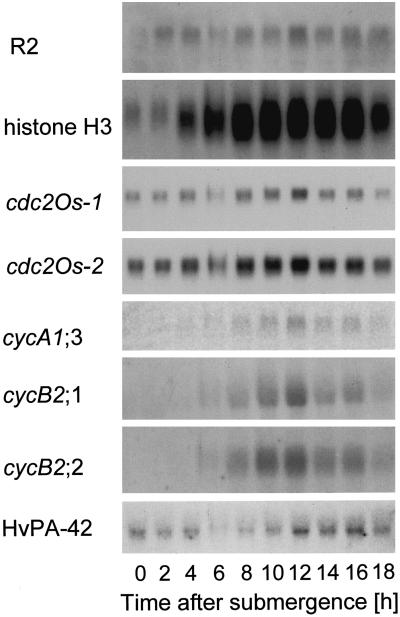
We previously analyzed the regulation by GA of cell division in the intercalary meristem of deepwater rice internodes (Sauter et al., 1995; Lorbiecke and Sauter, 1998), so we were interested in determining whether cell-cycle induction in adventitious roots by ethylene would require activation of the same genes that we found to be involved in the intercalary meristem. In this region, growth induction by GA was accompanied by increased expression of the CAK homolog R2, of one of the two known cdc2 genes from rice, cdc2Os-2, and of two mitotic B2-type cyclin genes, cycB2;1 (formerly cycOs1) and cycB2;2 (formerly cycOs2; Renaudin et al., 1996). Northern-blot analysis of these genes was performed on RNA obtained from adventitious roots isolated from plants after various times of submergence (Fig. 3). The same blot was also hybridized to a probe from a newly identified A-type cyclin, cycA1;3, to a histone H3 probe as a molecular marker for the S phase, and to HvPA-42 as a loading control (Sauter, 1997).
Figure 3.
Northern blots of adventitious roots at the third node of intact plants submerged for the times indicated. The same blot with 20 μg of RNA per lane was successively hybridized to gene-specific probes to determine expression of the CAK homolog R2, of two cdc2 genes, cdc2OS-1 and cdc2Os-2, of three mitotic cyclin genes, cycA1;3, cycB2;1, and cycB2;2, and of a histone H3 gene. Expression of HvPA-42 was used as a loading control. Expression of cycA1;3 was detected with a phosphor imager.
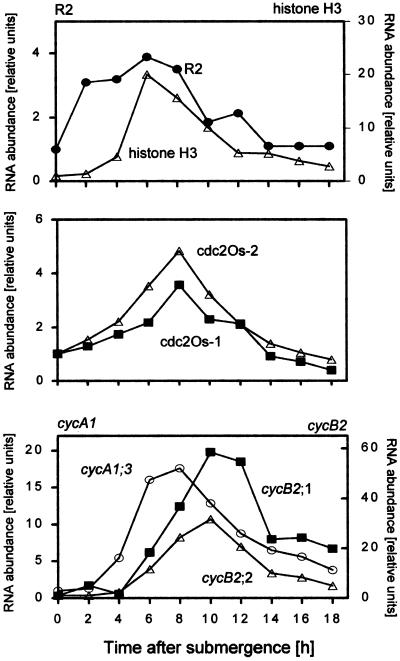
The northern blots (Fig. 3) and the quantified results obtained from them (Fig. 4) indicated that all of the genes analyzed, including cdc2Os-1, were induced by the growth-promoting treatment in roots, albeit at different times after the onset of that treatment. Within 2 h of submergence, while cells were still in the G1 phase, expression of the CAK gene homolog R2 increased. Two to four hours later, histone transcripts accumulated and cells began DNA synthesis (Fig. 2B). At about the same time, the levels of mRNA in cdc2Os-1 and cdc2Os-2 increased, with maximal expression levels after 8 h of submergence coinciding more or less with the S phase (Figs. 2B, 3, and 4).
Figure 4.
Expression levels of cell-cycle genes in adventitious roots at the third node of intact plants submerged for the times indicated. Results were obtained by quantifying the northern-blot data shown in Figure 3. mRNA levels at time 0 were arbitrarily set to 1; all other values were calculated as multiples of that and were corrected for loading differences using HvPA-42 as a control.
Transcripts of the A-type cyclin cycA1;3 accumulated in the roots of S-phase cells, clearly preceding the increase in the G2-phase population (Fig. 2B) or transcript accumulation of the mitotic B-type cyclins cycB2;1 and cycB2;2, which reached their highest levels 10 h after submergence (Figs. 3 and 4). A similar G2-phase-related expression pattern for these B-type cyclins was shown previously in partially synchronized suspension cells (Sauter, 1997) and in meristematic cells from the internode of rice (Sauter et al., 1995; Lorbiecke and Sauter, 1998). This finding agrees well with a predicted function of the gene products in regulating the onset of mitosis. Based on our expression data in planta, the A-type cyclin is likely to function earlier in the cell cycle, possibly at the transition from the S to the G2 phase.
Adventitious Root Growth Is Induced by Submergence or Ethylene, but Not by Added Auxin, Cytokinin, or GA
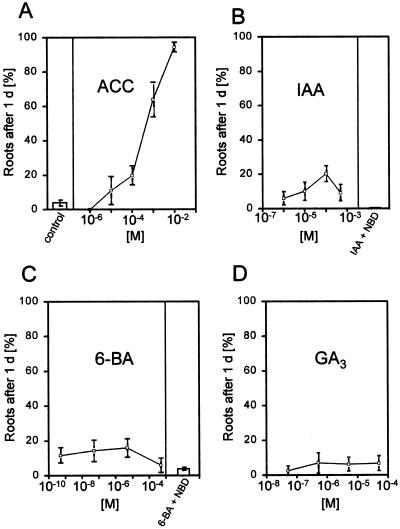
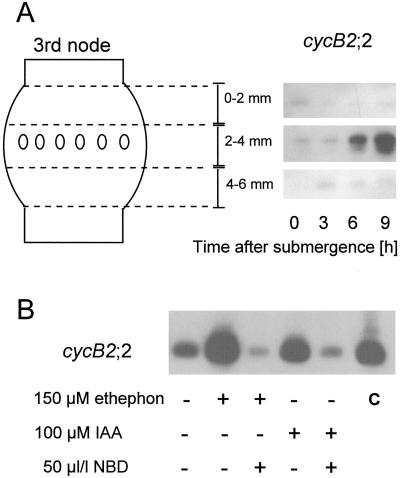
It has been determined that the growth of adventitious roots of deepwater rice can be induced by submergence or by treatment with ethylene (Vergara et al., 1976; Suge, 1985; Bleecker et al., 1986, 1987). We verified these results and extended them to test the effects of other plant hormones and of NBD, an inhibitor of ethylene action. These experiments were carried out with excised stem sections. We used either ethephon or ACC as precursors for ethylene evolution. Ethephon in the presence of water and at a pH greater than 3.5 releases ethylene. ACC is the direct natural precursor of ethylene and is converted to ethylene by endogenous ACC oxidase activity. At optimal concentrations both compounds induced root extension to a degree comparable with that induced by submergence and that described for direct ethylene application, suggesting that these precursors were converted to ethylene without obvious side effects up to the optimal concentrations (Figs. 5A and 6A).
The optimal root-growth-promoting concentration of ethephon was 150 μm, with both lower and higher concentrations leading to fewer roots emerging. None of the other hormones tested gave comparable root growth at any concentration (Fig. 6, B–D). IAA, the most common naturally occurring auxin, induced growth by about 20% in all root initials at its most effective concentration of 100 μm, far less then what was observed with submergence or ethylene treatment (Fig. 6B). These roots were also shorter than those of plants treated with ethylene (data not shown). Application of IAA to the hollow interior cylinder of the internode to allow uptake through the inner, thin epidermis to ensure basipetal transport to the node below also had no increased effect on root induction (data not shown).
Figure 6.
Growth of adventitious roots at the third node of stem sections treated for 24 h with a growth promoter or with a growth promoter plus NBD, an inhibitor of ethylene action, at the concentrations indicated. A, Effect of ACC; B, effect of IAA or IAA at its optimal concentration of 100 μm with NBD at 50 μL/L; C, effect of 6-BA or 6-BA at its optimal concentration of 5 μm with NBD at 50 μL/L; and D, effect of GA3 at the concentrations indicated. Controls were incubated without the addition of hormone. Results are averages ± se of at least seven stem sections per concentration.
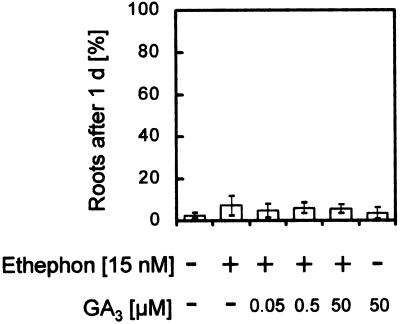
The cytokinin 6-BA was less effective than IAA at inducing root growth, with 15% induction at its most effective concentration (Fig. 6C), and GA3 had no significant effect at all on root growth (Fig. 6D). Using the same rice cultivar, Pin Gaew 56, Suge (1985) demonstrated that a combination of 10 ppm ethylene and 0.3 μm GA3, each at suboptimal concentrations, had a synergistic effect on the number of adventitious roots formed per node and on the length of the roots that developed. We were not able to repeat these results (Fig. 7); using a suboptimal ethephon concentration of 15 nm and increasing concentrations of GA3, we did not detect any root induction beyond that seen with ethephon alone (Fig. 7).
Figure 7.
Growth of adventitious roots at the third node of stem sections treated for 24 h with a suboptimal concentration of ethephon (15 nm) and increasing concentrations of GA3.
Ethylene Action Is Necessary for Adventitious Root Growth
Auxin and cytokinin have been shown to induce ethylene formation (Mattoo and White, 1991, and refs. therein). To determine whether the minor induction of root growth by auxin and cytokinin was a direct result of auxin or cytokinin action, or if it was a result of auxin- or cytokinin-induced ethylene formation and was therefore an effect of ethylene action, we used NBD as an inhibitor of ethylene action together with the growth-promoting hormone. Growth of roots was induced by applying ethephon, IAA, or 6-BA at their optimal concentrations (Figs. 5 and 6, B and C). When NBD was added with each hormone, growth induction was fully inhibited (Figs. 5B and 6, B and C). The effective concentration of NBD in inhibiting growth was 50 μL/L.
Ethylene Action Is Necessary for Mitotic Cyclin Induction
We analyzed mitotic cyclin gene expression as a molecular marker for cell-division activity using RT-PCR. Mitotic cyclins have been shown to be expressed only when needed, i.e. in dividing cells. For our assays we isolated RNA from tissue sections containing the root initials (Fig. 8). To ensure that we measured gene expression in the roots and not in the surrounding nodal tissue, we cut similar sections above and below the roots and included them in our preliminary assays (Fig. 8A). These controls showed that cycB2;2 gene expression and especially induction of gene expression was limited to the tissue section at 2 to 4 mm of the third node that contained adventitious roots. Induction of root growth with ethephon resulted in increased expression of cycB2;2, as already shown by northern-blot analysis (Figs. 3, 4, and 8B). This induction was completely inhibited when NBD was added together with ethephon. IAA, which induced some adventitious root growth (Fig. 6B), also induced some cycB2;2 expression, but, as with root growth, much less than ethylene did (Fig. 8B). The ethylene inhibitor NBD added together with IAA resulted in complete inhibition of IAA-induced cyclin gene expression. Our results suggest that IAA induces cell division and root growth through ethylene action.
Figure 8.
Gene expression of the mitotic cyclin cycB2;2 in adventitious roots at the third node as determined with RT-PCR. A, Gene expression in three consecutive tissue sections 2 mm in width as measured in plants submerged for the times indicated. This control experiment showed that significant cycB2;2 expression and induction was limited to the tissue section containing the roots. The time course of transcript accumulation was comparable to that found using northern-blot analysis (Figs. 3 and 4). B, Gene expression in the middle, root-containing tissue as a result of treatment for 12 h with ethephon, IAA, or NBD in combination with ethephon or IAA at the concentrations indicated. As a control (c), PCR was performed using the plasmid containing the cycB2;2 cDNA as a template source.
Root Primordia Are Responsive to Ethylene at an Early Developmental Stage
When deepwater rice plants are 12 to 14 weeks old, they have usually developed four nodes. Adventitious roots develop very early during node formation, and root initials can therefore already be found in the youngest or first node of the stem (Fig. 9). Development and growth of the root initials then continue, with plant development resulting in larger root initials in the oldest node. However, during normal development the roots will remain under the epidermal layer of the node, and outgrowth of the root will not commence until a proper signal is perceived (Fig. 9). To determine whether root growth depends not only on an inductive signal, but also on a proper developmental stage, we looked at root growth and induction of cycOs2 expression as a molecular marker for cell-division activity in all four nodes of submergence-induced rice plants.
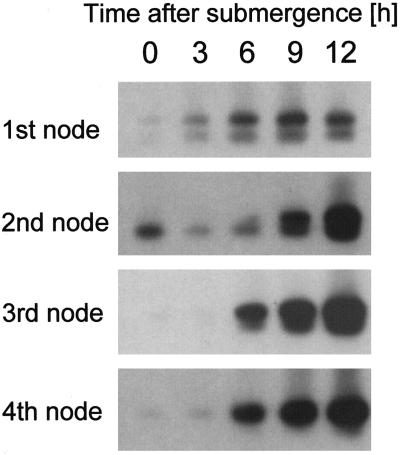
Root growth was visibly induced within 10 h at all nodes except the youngest. The difference in root length was most likely attributable to the fact that the size of the root primordia at the onset of growth differs considerably depending on age. Nonetheless, at the molecular level, the time course of cyclin gene induction was very similar at the second, third, and fourth nodes, irrespective of the difference in size or age of the root primordia (Fig. 10). Gene induction at the youngest node was not as strong (Fig. 10). Overall transcript levels were low in this tissue, and 35 cycles were used in PCR for RNA from the first node, whereas 25 and 24 cycles were sufficient for the second and third nodes and the fourth node, respectively. However, higher transcript levels were observed after 6 h of submergence in the youngest node. Even though the degree of induction seems to be lower, the kinetics of gene induction were comparable with those of the older nodes.
Figure 10.
Expression of cycB2;2 as determined with RT-PCR at the first, second, third, and fourth nodes of deepwater rice plants submerged for the times indicated.
As a control we compared our RT-PCR results from the third node with our northern-blot analysis results from the same node (Fig. 3), and found that the time course of cycB2;2 gene induction was very similar. We can conclude, therefore, that ethylene as the growth-promoting hormonal signal acts in a systemic fashion on all nodes of the rice stem, and that adventitious root initials are responsive to the hormone from a very early developmental stage.
DISCUSSION
Growth of adventitious roots in deepwater rice is induced by ethylene (Suge, 1985; Bleecker et al., 1987). When plants are submerged, the ethylene concentration increases (Métraux and Kende; 1983; Stünzi and Kende, 1989), leading to growth induction of root primordia. In the present study the addition of auxin, cytokinin, or GA did not result in significant root growth. At a suboptimal concentration of ethylene, GA did not enhance growth in a synergistic manner, as was previously shown by Suge (1985) for the same rice cultivar. It has been suggested that GA plays a role in determining the shape of root cells (Barlow et al., 1991; Traas et al., 1995; Tadeo et al., 1997). We cannot exclude that GA is required at a basal level in the tissue for proper root-cell development; however, the addition of GA by itself or in combination with ethylene did not induce root growth.
Auxin is the major growth-promoting hormone for the initiation of lateral and adventitious root growth. It induces cells in the pericycle and parenchyma to dedifferentiate and enter initial cell divisions. Formation of the root primordium itself is no longer dependent on auxin (Celenza et al., 1995; De Klerk et al., 1995; Pelosi et al., 1995). Initiation of adventitious root primordia in deepwater rice is part of the normal developmental program. The involvement of auxin at this step has not been analyzed. Our results show that auxin is ineffective in inducing growth of the mature root primordium. What little growth occurs after auxin treatment most likely results from auxin-induced ethylene synthesis, as suggested by the fact that it can be blocked by NBD, an inhibitor of ethylene action. The inhibitory effect of NBD can be observed not only at the physiological level, but also at the molecular level. Auxin-induced cyclin expression was also suppressed by the addition of NBD. Regulation of ethylene biosynthesis by IAA is a well-known phenomenon that has been studied in some detail (Peck and Kende, 1995). Treatment with the IAA-transport-inhibitor NPA did not inhibit ethylene-induced adventitious root growth (data not shown), supporting our conclusion that ethylene is the crucial growth-promoting hormone.
The minor root induction observed after cytokinin application also may have been a result of cytokinin-induced ethylene formation, as is most likely the case for auxin. Cytokinin-induced root growth was abolished in the presence of NBD. Therefore, the growth of the root primordia and the emergence of the node through the cuticle were dependent on and mediated by ethylene action.
When deepwater rice plants are submerged, the apical meristems in adventitious root primordia are activated before cell growth sets in. Between 4 and 6 h after submergence a subpopulation of cells in the G1 phase duplicate their genome in a synchronous manner and then move to the G2 phase, entering mitosis probably around 10 h after submergence. It is unlikely that the subpopulation of cells induced by ethylene is in a true resting state (G0), because the time needed for cell-cycle induction by submergence in roots is about the same as in the intercalary meristem of the internode, where cells are known to divide continually but are induced to divide faster upon submergence (Lorbiecke and Sauter, 1998).
All cell-cycle regulators analyzed are induced during cell-cycle activation, albeit in some cases only to a minor degree. Induction of the cdc2Os-1 and cdc2Os-2 genes occurs too late (in the S phase) to contribute to the initial meristem activation. This is in contrast to what happens in the intercalary meristem of the internode, where increased expression of the cdc2Os-2 gene is a very early, G1-phase-specific event possibly responsible for the G1- to S-phase induction. In roots only transcriptional activation of the R2 gene, which, based on sequence homology, is a putative CAK, could possibly play a role in regulating the G1 to S checkpoint. If so, regulation at the protein level through an activating phosphorylation of a G1-specific cyclin-dependent kinase may be important in driving cells into the S phase in the root apical meristem.
The A-type cyclin gene is expressed at higher levels during the S phase and the early G2 phase, clearly before the B-type cyclins. This expression pattern is in agreement with the proposed function of A-type cyclins in regulating S-/G2-phase progression. The B-type cyclins accumulate in the G2 phase and reach maximal transcript levels in the late G2 and M phases. These results coincide with our previous findings from suspension-cultured cells (Sauter, 1997) and from meristematic cells of the internode (Sauter et al., 1995; Lorbiecke and Sauter, 1998), and suggest that cycB2;1 and cycB2;2 are universal mitotic regulators in rice without tissue specificity.
When we compared cell-cycle induction in the internode with that in adventitious roots, we found great similarities with respect to the time course of induction and the cell-cycle regulatory genes involved. The only obvious difference was with respect to the cdc2 genes, both of which were induced in roots during the S phase, whereas only cdc2Os-2 was regulated in the internode where transcripts accumulated in the G1 phase, as was also found in partially synchronized suspension-cultured cells. The two meristems are triggered by two different hormonal signals: by GA in the internode and by ethylene in the root. In the internode ethylene is perceived but is not directly responsible for cell-cycle induction; rather, it acts on the homeostasis of two other plant hormones, ABA and GA. GA finally activates the cell cycle. In adventitious roots ethylene is the hormonal signal that leads directly to meristem activation. Therefore, specific transductory pathways must exist in the two organs with respect to the ethylene response. The pathway that is established in the internode leads to a decline in ABA and an increase in GA, whereas the pathway established in adventitious root initials results directly in growth induction. We are currently in the process of identifying components of signal transduction that are involved in only one of these pathways.
ACKNOWLEDGMENTS
This publication is based on a doctoral study by R.L. at the Faculty of Biology, University of Hamburg. The authors thank H. Mergemann for help with root-growth analysis. We are further indebted to Drs. E. van der Knaap and H. Kende (Michigan State University-Department of Energy Plant Research Laboratory, East Lansing) for providing the histone H3 PCR clone 9B; to Drs. S. Quast and K. Krupinska (Botanisches Institut, Universität Kiel, Germany) for providing the HvPA-42 cDNA; and to Drs. S. Hata and J. Hashimoto (National Institute of Agrobiological Resources, Ibaraki, Japan) for supplying the R2, cdc2Os-1, and cdc2Os-2 cDNAs.
Abbreviations:
- CAK
cdc2-activating kinase
- NBD
2,5-norbornadiene
- RT
reverse transcription
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Arthur und Aenne Feindt-Stiftung, and the Graduiertenkolleg Biotechnologie at the University of Hamburg and at the Technical University Hamburg-Harburg (to R.L.).
LITERATURE CITED
- Barlow PW, Brain P, Parker JS. Cellular growth in roots of a gibberellin-deficient mutant of tomato (Lycopersicon esculentum Mill.) and its wild-type. J Exp Bot. 1991;42:339–351. [Google Scholar]
- Bleecker A, Rose-John S, Kende H. Evaluation of 2,5-norbornadiene as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiol. 1987;84:395–398. doi: 10.1104/pp.84.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A, Schuette JL, Kende H. Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta. 1986;169:490–497. doi: 10.1007/BF00392097. [DOI] [PubMed] [Google Scholar]
- Celenza J, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Cheng J-C, Seeley KA, Sung Z. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Kende H. In vivo 1-aminocyclopropane-1-carboxylate synthase activity in internodes of deepwater rice. Plant Physiol. 1987;84:282–286. doi: 10.1104/pp.84.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Klerk G-J, Keppel M, Ter Brugge J, Meekes H. Timing of the phases in adventitious root formation in apple microcuttings. J Exp Bot. 1995;46:965–972. [Google Scholar]
- Hoffmann-Benning S, Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H (1987) Studies on internodal growth using deepwater rice. In DJ Cosgrove, DP Knievel, eds, Physiology of Cell Expansion during Plant Growth. American Society of Plant Physiologists, Rockville, MD, pp 227–238
- Kende H, Hoffmann-Benning S, Sauter M. The role of ethylene in regulating growth of deepwater rice. In: Pech J-C, Latché A, Balagné C, editors. Cellular and Molecular Aspects of Biosynthesis of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 329–334. [Google Scholar]
- Lorbiecke R, Sauter M. Induction of cell growth and cell division in the intercalary meristem of submerged deepwater rice (Oryza sativa L.) Planta. 1998;204:140–145. [Google Scholar]
- Mattoo AK, White WB. Regulation of ethylene biosynthesis. In: Mattoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 21–42. [Google Scholar]
- Mekhedov SL, Kende H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 1996;37:531–537. doi: 10.1093/oxfordjournals.pcp.a028976. [DOI] [PubMed] [Google Scholar]
- Métraux J-P, Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SC, Kende H. Sequential induction of the ethylene biosynthetic enzymes by indole-3-acetic acid in etiolated peas. Plant Mol Biol. 1995;28:293–301. doi: 10.1007/BF00020248. [DOI] [PubMed] [Google Scholar]
- Pelosi A, Lee MCS, Chandler SF, Hamill JD. Hormonal control of root primordia differentiation and root formation in cultured explants of Eucalyptus globulus seedlings. Aust J Plant Physiol. 1995;22:409–415. [Google Scholar]
- Puissant C, Houdebine L-M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. BioTechniques. 1990;8:148–149. [PubMed] [Google Scholar]
- Raskin I, Kende H. Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol. 1984;76:947–950. doi: 10.1104/pp.76.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin J-P, Doonan JH, Freeman D, Hashimoto J, Hirt H, Inzé D, Jacobs T, Kouchi H, Rouzé P, Sauter M and others. Plant cyclins: a unified nomenclature for plant A-, B- and D-type cyclins based on sequence organisation. Plant Mol Biol. 1996;32:1003–1018. doi: 10.1007/BF00041384. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Kende H. Short-term response of deep-water rice to submergence and ethylene. Plant Sci. 1985;38:129–134. [Google Scholar]
- Sauter M. Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J. 1997;11:181–190. doi: 10.1046/j.1365-313x.1997.11020181.x. [DOI] [PubMed] [Google Scholar]
- Sauter M, Mekhedov SL, Kende H. Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J. 1995;7:623–632. doi: 10.1046/j.1365-313x.1995.7040623.x. [DOI] [PubMed] [Google Scholar]
- Sgorbati S, Levi M, Sparvoli E, Trezzi F, Lucchini G. Cytometry and flow cytometry of 4′,6-diamidino-2-phenylindole (DAPI) stained suspensions of nuclei released from fresh and fixed tissues of plants. Physiol Plant. 1986;68:471–476. [Google Scholar]
- Smith DL, Feodoroff NV. LRP1, a gene expressed in lateral and adventitious root primordia of Arabidopsis. Plant Cell. 1995;7:735–745. doi: 10.1105/tpc.7.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stünzi JT, Kende H. Gas composition in the internal air spaces of deepwater rice in relation to growth induced by submergence. Plant Cell Physiol. 1989;30:49–56. [Google Scholar]
- Suge H. Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant Cell Physiol. 1985;26:607–614. [Google Scholar]
- Tadeo FR, Gómez-Cadenas A, Ben-Cheikh W, Primo-Millo E, Talón M. Gibberellin-ethylene interaction controls radial expansion in citrus roots. Planta. 1997;202:370–378. [Google Scholar]
- Traas J, Laufs P, Jullien M, Caboche M. A mutation affecting etiolation and cell elongation in Nicotiana plumbaginifolia causes abnormal division plane alignment and pattern formation in the root meristem. Plant J. 1995;7:785–796. [Google Scholar]
- Vera P, Lamb C, Doerner PW. Cell-cycle regulation of hydroxyproline-rich glycoprotein HRGPnt3 gene expression during the initiation of lateral root meristems. Plant J. 1994;6:717–727. [Google Scholar]
- Vergara BS, Jackson B, Datta SK (1976) Deep water rice and its response to deep water stress. In Climate and Rice. International Rice Research Institute, Los Baños, The Philippines, pp 301–319
- Zarembinsky TI, Theologis A. Expression characteristics of OS-ACS1 and OS-ACS2, two members of the 1-aminocyclopropane-1-carboxylate synthase gene family in rice (Oryza sativa L. cv. Habiganj Aman II) during partial submergence. Plant Mol Biol. 1997;33:71–77. doi: 10.1023/b:plan.0000009693.26740.c3. [DOI] [PubMed] [Google Scholar]