Abstract
During development and regeneration, directed migration of cells, including neural crest cells, endothelial cells, axonal growth cones and many types of adult stem cells, to specific areas distant from their origin is necessary for their function. We have recently shown that adult skeletal muscle stem cells (satellite cells), once activated by isolation or injury, are a highly motile population with the potential to respond to multiple guidance cues, based on their expression of classical guidance receptors. We show here that, in vivo, differentiated and regenerating myofibers dynamically express a subset of ephrin guidance ligands, as well as Eph receptors. This expression has previously only been examined in the context of muscle-nerve interactions; however, we propose that it might also play a role in satellite cell-mediated muscle repair. Therefore, we investigated whether Eph-ephrin signaling would produce changes in satellite cell directional motility. Using a classical ephrin ‘stripe’ assay, we found that satellite cells respond to a subset of ephrins with repulsive behavior in vitro; patterning of differentiating myotubes is also parallel to ephrin stripes. This behavior can be replicated in a heterologous in vivo system, the hindbrain of the developing quail, in which neural crest cells are directed in streams to the branchial arches and to the forelimb of the developing quail, where presumptive limb myoblasts emigrate from the somite. We hypothesize that guidance signaling might impact multiple steps in muscle regeneration, including escape from the niche, directed migration to sites of injury, cell-cell interactions among satellite cell progeny, and differentiation and patterning of regenerated muscle.
Keywords: Satellite cells, Muscle regeneration, Cell migration, Ephrin, Mouse, Quail
INTRODUCTION
Skeletal muscle regeneration after injury is both rapid and efficient: in the rodent, muscle patterning and contractile ability are regained within days of myonecrotic injury, with complete recovery of mass and strength within 21 days (Rosenblatt and Woods, 1992). This process is dependent on satellite cells, the resident stem cell of skeletal muscle. When stimulated by factors released by damaged muscle, satellite cells rapidly exit the quiescent state and proliferate extensively to build up a supply of adult myoblasts, which will subsequently differentiate and fuse either with each other or with existing myofibers to repair local damage (Hawke and Garry, 2001). Although these responses to nearby damage (activation, proliferation and commitment to differentiation) have been extensively studied, significantly less is understood about whether and how satellite cells would detect and respond to injuries to areas of the myofiber distant from them, and potentially be recruited to their repair.
In vitro motility of satellite cell-derived cell lines (Ocalan et al., 1988; Yao et al., 1996; Dedieu et al., 2003; Bae et al., 2008) and primary satellite cells (Bischoff, 1997; Jansen and Pavlath, 2006; Mylona et al., 2006) is regulated by both cell-surface receptors and extracellular environmental factors. Recently, we showed that activated primary satellite cells are highly motile in the context of their native substrate (the surface of the myofiber), moving up to 2.4 cm in 24 hours (Siegel et al., 2009). The same study found that activated satellite cells express mRNAs encoding multiple examples of classical guidance pathway ligands and receptors (Ephs, ephrins, semaphorins, plexins, neuropilins and Robos). A role in re-innervation of nascent myofibers during satellite cell differentiation in vivo has been proposed for one such ligand, Sema3A (Tatsumi et al., 2009); however, the function of the remaining ligands and receptors in satellite cell activity is unclear. In this work, we focused on Eph/ephrin signaling as the best understood of the classical cellular guidance pathways, and investigated whether such signaling could influence satellite cell activity.
Eph receptors and their ephrin ligands are guidance molecules usually associated with cell migration and axon guidance during development (Krull et al., 1997; Ciossek et al., 1998; Eberhart et al., 2000). More recently, roles for these proteins in regenerative and homeostatic processes in neurons (Goldshmit et al., 2006; Bolsover et al., 2008; Lai and Ip, 2009), vasculature (Wang et al., 2010), bone (Edwards and Mundy, 2008; Irie et al., 2009; Arthur et al., 2010), and stem cell niches (Stokowski et al., 2007; Arthur et al., 2010; Genander et al., 2010; Murai and Pasquale, 2010) have been established. Eph/ephrin involvement in tumor progression (Castano et al., 2008; Merlos-Suarez and Batlle, 2008; Noberini and Pasquale, 2009) has also been described in several organ systems. Eph receptors are the largest known family of receptor tyrosine kinases, with 16 homologs of Eph receptors divided into two classes based on their ligand specificity. Fourteen of them are present in mammals: EphA1-A8 and A10, and EphB1-B4 and B6 (Lai and Ip, 2009; Bolsover et al., 2008). The ligands are also divided into two classes: ephrins A1-A5 are anchored to the extracellular side of the plasma membrane by a glycosyl-phosphatidylinositol (GPI) linker, and ephrins B1-B3 are Type I transmembrane proteins with short cytoplasmic tails (Lai and Ip, 2009). It is typical for ephrin-As to bind to EphA receptors and ephrin-Bs to bind to EphB receptors; an exception is EphA4, which can bind to both classes of ephrin (for reviews, see Klein, 2009; Frisen et al., 1999). Most frequently, engagement of an ephrin ligand leads to changes in the adhesion and cytoskeletal architecture of the Eph-expressing cell, usually a rapid depolymerization of actin filaments that results in a retraction of cellular processes and repulsion from the source of the ephrin (Orioli and Klein, 1997; Wilkinson, 2000; Cowan and Henkemeyer, 2002; Gallo and Letourneau, 2004). Eph/ephrin signaling is further complicated in that ligand-receptor interaction can elicit a response in both the Eph-expressing and ephrin-expressing cells; signals transduced through Eph receptors are considered to be ‘forward’ and signals transduced through ephrins are ‘reverse’ (for a review, see Davy and Soriano, 2005). Thus, Eph/ephrin signaling is crucial for processes requiring accurate long-range guidance of migrating cells or axons, boundary formation, cell sorting and patterning.
In this study, we investigated whether activated primary mouse satellite cells are capable of responding to ephrin engagement by altering their motility or spatial alignment. We have taken advantage of the classical ‘stripe assay’ (Walter et al., 1987; Knoll et al., 2007) to test satellite cells’ response to bound ephrin in vitro. We show that multiple ephrins elicit a repulsive migratory response in satellite cells, and that these ephrins are differentially present on the surface of healthy and regenerating myofibers, suggesting the potential for ephrin-mediated guidance during muscle regeneration. We extended these results by grafting labeled satellite cells into developing quail embryos and show that satellite cells respect ephrin-defined boundaries that regulate migration of cranial neural crest cells into the hindbrain and somite-derived myoblasts into the limb bud. Both in vitro and in vivo, ephrin signaling also appears to affect the patterning of differentiating satellite cells. We propose a model in which Eph/ephrin signaling between differentiated myofibers and their associated muscle satellite cells regulates multiple aspects of satellite cell behavior during acute regeneration, including, but not limited to, pathfinding and patterning.
MATERIALS AND METHODS
Muscle satellite cell isolation and culture
Adult mouse myoblasts were isolated from wild-type (B6D2F1, Jackson Labs), CBAgfp (Jackson Labs), or ROSAmTmG (Jackson Labs) female mice by our published methods (Capkovic et al., 2008). Briefly, mice were euthanized, hindlimbs removed and skinned, and muscles removed in PBS. Following physical and enzymatic dissociation, cell slurries were filtered and pelleted then plated in Ham’s F12 (Invitrogen) supplemented with 15% horse serum (Equitech), 5 nM FGF2 and penicillin/streptomycin (Sigma) on gelatin-coated plates. Cells were maintained at 37°C and 5% CO2 in a humidified incubator.
Viable myofiber explants were isolated using our published methods (Cornelison and Wold, 1997; Cornelison et al., 2004). Muscles were dissected as above, but were not physically dissociated. After collagenase digestion, free-floating myofibers were picked with a glass pipette and transferred into growth medium for culture as above.
Immunohistochemistry and western blotting
For fluorescence immunocytochemistry of cultured cells, satellite cells prepared as above were re-plated onto glass coverslips coated with 20 μg/ml laminin (Sigma), allowed to adhere for a minimum of 2 hours and then fixed in 4% ice-cold paraformaldehyde (PFA). Cells were blocked for 1 hour at room temperature with 10% normal goat serum with 1% Nonidet-P40 (except in the case of primary antibodies raised in goat, which were blocked in 10% chicken albumin), then incubated with primary antibody overnight at 4°C. Cells were washed, incubated with secondary antibody for 1 hour at room temperature, washed again and mounted using Vectashield (Vector Labs) containing DAPI to visualize nuclei. All fluorescent images were acquired and processed on an Olympus BX61 upright microscope using μManager (www.micro-manager.org) software. Digital background subtraction was used to remove signal that was less than or equal to levels present in control samples (processed without primary antibody) and was applied equally to the entire field.
For immunohistochemistry of muscle sections, tibialis anterior muscles were dissected, then flash-frozen in liquid nitrogen-cooled isopentane. Blocks were cryosectioned at 20 μm and then postfixed in 4% PFA for 10 minutes. Antibody work was carried out as above; binding was detected with diaminobenzidine (DAB; Vector Labs) for 15 minutes in the dark.
For western blotting, whole muscle was dissected free of fat and connective tissues, then homogenized in Allen buffer (50 mM Tris pH 7.4, 10 mM EDTA pH 8, 5 mM EGTA pH 7.5, 0.5% Triton X-100, 1× Roche Complete Protease Inhibitors), electrophoresed, and transferred to polyvinylidene fluoride (PVDF) membrane. Membrane strips were blocked in Starting Block (Thermo Scientific), incubated overnight at 4°C with primary antibody, washed, incubated with secondary antibody for one hour at room temperature, washed, and reassembled for chemiluminescence detection (SuperSignal West Pico, Pierce).
Primary antibodies (Santa Cruz, unless noted otherwise) and dilutions were: EPHA1 1:200, EPHA2 1:100, EPHA3 1:200, EPHA4 1:200, EPHA5 1:200, EPHA6 1:200, EPHA7 1:200, EPHA8 1:200, EPHB1 1:200, EPHB2 1:200, EPHB3 1:200, EPHB4 1:200, EPHB6 1:500 (AbCam), ephrin-A1 1:100, ephrin-A2 1:200, ephrin-A3 1:200, ephrin-A5 1:500 (AbCam), ephrin-B1 1:200, ephrin-B2 1:200, ephrin-B3 1:200. Secondary antibodies used and dilutions were: goat anti-rabbit HRP 1:50,000 (Santa Cruz), anti-mouse HRP 1:10,000 (Pierce), anti-goat 1:10,000 (Santa Cruz). Chemiluminescence was detected with a Fuji Camera System.
Flow cytometry
Proliferating satellite cells (96 hours after harvest) were removed from the plate with collagenase and fixed in 4% PFA. Cell suspensions were blocked and stained using chicken anti-syndecan-4 (Cornelison et al., 2004) and primary antibodies to Eph/ephrin as above. Following secondary antibody staining, cell suspensions were analyzed on an Accuri C6 flow cytometer; the fraction of syndecan-4+ve cells also positive for each Eph or ephrin was calculated from three individually isolated and stained cell populations.
Stripe assays
Coverslips programmed with recombinant ephrin stripes were prepared following the method of Bonhoeffer and colleagues (Knoll et al., 2007). Acid-washed glass coverslips were pressed to a silicone matrix inlaid with 40 μm channels (purchased from M. Bastmeyer, Karlsruhe, Germany). To program the stripes, anti-human Alexa Fluor (Invitrogen) at 2 μg/μl was conjugated to human Fc:ephrin chimeras (R&D Systems) at 10 μg/ml for 1 hour at room temperature. Conjugated Fc:ephrin+antibody was pushed into the matrix with a Hamilton syringe then incubated at 37°C for 30 minutes; this was repeated three times. Hank’s balanced salt solution (500 μl) was flushed through the channels and the coverslip was removed and coated with 200 μl of laminin at 20 μg/ml (Sigma). Primary satellite cells at four days post-isolation were plated on the prepared coverslips in growth medium; 24-48 hours after plating, the coverslips were fixed in 4% PFA. Cellular response to ephrin was analyzed by calculating the area exclusive to either ephrin or laminin then counting the number of cells per area using ImageJ (NIH) software. For time-lapse analysis, coverslips were imaged every 5-10 minutes in a stagetop incubator (LiveCell Imaging) attached to a Leica DMI 5100 inverted microscope. Images were acquired using MetaMorph 7.6.1 (Molecular Devices) and processed with ImageJ. Significance of occupancy rates was determined by comparing ratios of cells ‘on’ versus ‘off’ the fluorescent stripes using a linear mixed model; P-values for pairwise comparisons of the control with each ephrin were adjusted for multiple comparisons using Dunnett’s correction. For differentiation studies, cells were allowed to adhere to the coverslips then the media was switched to Kaighn’s F-12 supplemented with 3% horse serum; differentiation was allowed to progress to 48 hours before fixation.
Japanese quail embryos
Japanese quail eggs (Ozark Egg Company, Stover, MO, USA) were incubated at 37°C to Hamburger and Hamilton (HH) stage 10 (for hindbrain grafts) or stage 16 (for limb bud grafts). The eggs were sprayed with 70% ETOH, 1.5 ml of albumin was removed, a hole was cut in the shell to expose the embryo and 5% India Ink solution in PBS/penicillin/streptomycin (Sigma) was injected under the blastodisc for visualization of the embryo. Satellite cells from ROSAmTmG or CBAgfp mice (Jackson Labs) were isolated and cultured as above. At 72-96 hours post-isolation, cells were moved to 25 μl hanging drops and incubated for 24 hours.
To prepare stage 10 quail embryos for satellite cell transplantation in the hindbrain, a tungsten needle was used to pull back the vitelline membrane above the hindbrain. A glass needle filled with a lipophilic membrane dye, DiI or DiD (Invitrogen), was used to pipette dye into the lumen of the hindbrain for visualization of the emerging neural crest, and the embryos were then incubated for 1 hour at 37°C. Using a glass needle, a satellite cell pellet was gently placed into the hindbrain of the embryo and incubated for 16-20 hours at 37°C.
To prepare stage 16 quail embryos for satellite cell transplantation in the limb bud, a tungsten needle was used to pull back the vitelline membrane above somite 17. Using the tungsten needle, an incision was made directly lateral to somite 17. A satellite cell pellet was gently placed into the incision using a glass needle and incubated for 36-48 hours at 37°C until the embryos reached stage 21. Embryo hydration was maintained with tape and PBS/penicillin/streptomycin (Sigma).
For ectopic overexpression of ephrin-A5, eggs were windowed and hydrated with PBS/penicillin/streptomycin, then the embryos were injected with 0.2 mg of either pCAX-eGFP or pMES-ephrinA5 plasmid into the lumen of the neural tube. Fast Green FCF (Sigma, F-7252) at 10 mg/ml was added to the needle for visualization of the injection site. Plasmids were electroporated using platinum electrodes and an electroporator (Gene Pulser Xcell Electroporations System, Bio-Rad). Eggs were sealed with cellophane tape and incubated for 1 hour at 37°C before engraftment of labeled satellite cells as previously described. Eggs were then resealed, and allowed to develop for an additional 24 hours.
RESULTS
Activated muscle satellite cells and regenerating myofibers upregulate Eph receptors and ephrin ligands
In order to plan initial experiments into whether satellite cells use ephrin/Eph signaling as a mechanism to interact with each other or the environment during homeostasis or muscle regeneration, we first determined which of each family of proteins are present in muscle tissue, either on satellite cells or differentiated myofibers, or both. Previously, we showed that activated primary satellite cells express mRNAs encoding multiple Eph receptors and ephrin ligands (Siegel et al., 2009). Although we did not initially expect that satellite cells quiescent beneath the exterior lamina would express Ephs or ephrins, we found when we examined frozen sections from uninjured muscle using commercially available antibodies for all known mouse Ephs and ephrins that EphB1 and EphB2 staining corresponded to profiles beneath the lamina and possessing a nucleus (Fig. 1A). We also noted positive Eph and ephrin staining in other cell types present in the muscle, such as neuronal and vascular cells, as well as strong and specific EphA5 expression in mononuclear interstitial cells (Fig. 1A). When we repeated the survey on sections from muscle three days following injury by barium chloride injection, we observed upregulation of multiple Ephs and ephrins in mononuclear cells associated with injured and regenerating myofibers (supplementary material Fig. S1) as well as on nascent myofibers.
Fig. 1.
Activated muscle satellite cells and regenerating myofibers upregulate Eph receptors and ephrin ligands. (A) Sections of tibialis anterior (TA) muscle from a wild-type mouse were analyzed for expression of all known murine Ephs and ephrins by indirect immunohistochemistry. Of these, EphB1 and EphB2 identified profiles (red, arrows) consistent with what would be expected for quiescent satellite cells when co-stained for laminin (green) and DAPI (blue.) EphA5 expression could be robustly detected on mononuclear cells that were never found beneath a myofiber lamina (arrowhead). In addition, non-muscle cell types such as vascular cells and neurons that stain positively for Eph/ephrin were also noted in section (see example of EphA4). (B) Activated muscle satellite cells express multiple Eph receptors as well as ephrins. Primary adult satellite cells were cultured for four days and then fixed and immunostained for cell-surface expression of Ephs and ephrins (Alexa 488, green) and nuclei (DAPI, blue). Equivalent cell populations co-stained with syndecan-4 to definitively identify satellite cells were analyzed using the same immunohistochemical reagents by flow cytometry, to provide a quantitative measure of expression prevalence; the fraction of syndecan-4-positive cells co-expressing each Eph or ephrin is noted in each panel (based on data in supplementary material Table S1). Asterisks indicate samples for which the standard deviation was more than 20%. (C) Transverse cryosections of adult muscle, uninjured and 72 hours post injury, show dynamic expression of Ephs and ephrins. Scale bars: 100 μm.
To determine more specifically which Eph and ephrin proteins are expressed by activated satellite cells, we examined monocultures of satellite cells under conditions in which >95% of cells present can be identified as satellite cells by syndecan-4 expression (unpublished data) and >90% of them would be expected to be proliferating (Capkovic et al., 2008). By immunocytochemistry, Eph and ephrin staining (when present) was localized to the cell membrane (Fig. 1B). To quantify the fraction of activated satellite cells expressing each protein, we repeated the staining on cell suspensions and analyzed them by flow cytometry in conjunction with staining for syndecan-4 to mark satellite cells. The percentages shown in each panel of Fig. 1B represent the average fraction of cells expressing each protein, compared with the total syndecan-4+ve satellite cell population.
To survey ephrin and Eph expression in adult mouse muscle, we carried out western blotting analysis on whole muscle lysate from uninjured mice as well as immunohistochemistry on uninjured and regenerating muscle sections. By western blot, four ephrins and nine Ephs were detected, with high expression of ephrin-B1, ephrin-A2, EphA2 and EphB6 (not shown). To determine where the ephrin and Eph expression is localized, we stained frozen sections of uninjured mouse tibialis anterior (TA) muscle as well as TA muscle three days after injury by injection of barium chloride (Caldwell et al., 1990). We found that ephrin-A2 and ephrin-A3 are localized at the periphery of individual muscle fibers in experimentally undamaged muscle (Fig. 1C; supplementary material Fig. S2). We saw more punctate peripheral localization for EphA2, EphB1 and EphB2 in uninjured muscle (Fig. 1C; supplementary material Fig. S2).
To assess potential changes in myofiber ephrin and/or Eph expression after muscle injury, we repeated the survey on TA muscle three days after injury. After staining and imaging under the same conditions, we found that ephrin-A3, EphA2 and EphB2 expression is maintained whereas ephrin-A2, ephrin-B1, EphA5 and EphB1 expression is increased (Fig. 1C; supplementary material Fig. S2.) Some staining appeared to be associated specifically with nascent myofibers, such as EphA7 (supplementary material Fig. S1).
Muscle satellite cells respond specifically and repulsively to Eph/ephrin signaling in vitro
Classical ephrin/Eph signaling directs migration and segregation and, typically, will elicit a repulsive response (Tessier-Lavigne, 1995). To test the response of satellite cells to ephrin, we used the established ‘stripe assay’ protocol (Knoll et al., 2007) to investigate whether satellite cells in vitro would respond to individual immobilized ephrins. Primary satellite cell cultures were challenged with stripes of immobilized Fc:ephrin fusion proteins from both classes of ephrin (ephrins A1-A5, ephrins B1-B3). After 24 hours, several Fc:ephrin stripes had elicited a repulsive response from the satellite cells compared with control stripes containing Fc alone (Fig. 2A,E,F). In particular, ephrins showing dynamic and/or increased expression on regenerating or nascent myofibers (such as ephrin-A2, ephrin-A3 and ephrin-B1) showed a very strong repulsive effect, whereas those ephrins not significantly expressed in muscle (such as ephrin-A1 and ephrin-B3) were less effective (Fig. 2A). An exception was ephrin-A5, which elicited a very strong response but has only minimal expression on injured muscle fibers; we hypothesize that this might be due to potential functional redundancy of ephrin-A5 with ephrin-A2 (Haustead et al., 2008).
Fig. 2.
Satellite cells respond repulsively to Eph/ephrin signaling in vitro. (A) Relative affinity of satellite cells four days after isolation for control stripes versus stripes programmed with Fc-chimerae of eight different recombinant ephrins. Percent total indicates the average number of nuclei over the area of substrate for each data set (n≥3). *P<0.05, **P<0.005. (B) Static images from a time-lapse series showing a single GFP-labeled satellite cell as it moves between ephrin-B1 programmed stripes; red outlines the cell membrane. White arrows indicate the next directional change, which is usually in the opposite direction of the filopodia contacting ephrin-B1 (t24=2.8 hours). The cyan track in panel C represents the entire path of this cell. (C,D) Cell-tracking analysis of images taken by time-lapse microscopy of cells plated on ephrin-B1 programmed stripes (C) and control (unprogrammed) stripes (D) for ∼13 hours. Each track represents the path taken by an individual cell over the course of the time-lapse movie (see also supplementary material Movies 1 and 2). (E,F) Images taken with 4×, 10× and 20× magnification objectives 24 hours after 4-day-old satellite cells (red) were plated over control (E) or ephrin-B1 programmed (F) stripes (blue). The white boxes represent the location of the 20× image. Scale bars: 40 μm in D; 20 μm in F.
Because the cellular response to ephrin stimulation is generally characterized by repulsion and retraction of cytoplasmic processes, we used fluorescence time-lapse microscopy to analyze morphological changes in satellite cells on ephrin-B1 stripes versus control stripes of Alexa Fluor (Fig. 2B-D; supplementary material Movies 1-3). Cells were imaged for 24 hours at 7-minute intervals, and then individual cells were tracked through the field of view (Fig. 2C,D; supplementary material Movies 2, 3). A strong avoidance response to the ephrin-B1 stripes can be observed compared with satellite cells on the control stripes. Note the satellite cell responding to ephrin-B1 (Fig. 2C, cyan track) which moved a distance of ∼90 μm in 2 hours (Fig. 2B; supplementary material Movie 1). This satellite cell can be observed making multiple contacts with the ephrin-B1 stripes, which then cause an immediate change in polarity as multiple filopodia are then extended in the opposite direction. Typically, two to four filopodia extend for directional opportunities for movement and the satellite cell chooses the filopodia with directionality farthest from the filopodia last to contact ephrin-B1 (Fig. 2B, arrows). These results demonstrate that ephrin signaling can modify primary satellite cell motility in vitro in a repulsive manner.
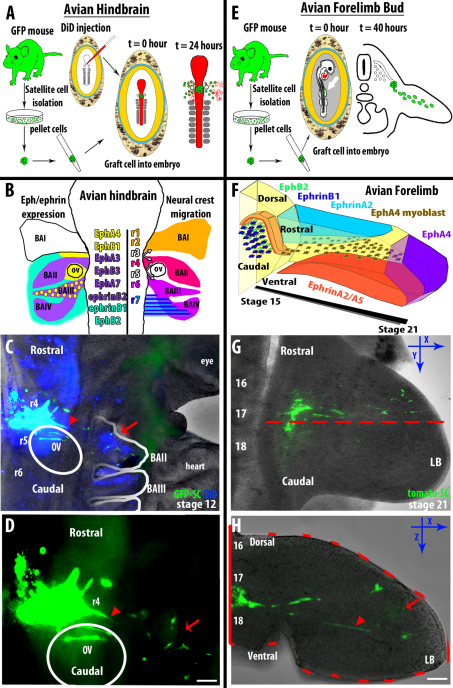
Muscle satellite cells respect presumptive ephrin-defined migration boundaries in vivo
To test whether the in vitro activity described above accurately predicted the satellite cell response to physiological levels of native ephrins, we capitalized on two ephrin-mediated cell migration events in the developing quail embryo: emigration of cranial neural crest cells from the hindbrain to the branchial arches, and emigration of embryonic myoblasts from the somite to the limb bud. Neural crest cells are the largest known migratory population in the developing vertebrate, a population that spans the length of the embryo. They undergo an epithelial-to-mesenchymal transition (EMT) at the dorsal neural tube then individual migrating cells respond to localized migration cues to locate target sites prior to differentiating into one of many mature cell types (i.e. neurons, glia, melanocytes, etc.) (for a review, see Dupin et al., 2010). Well-characterized neural crest streams are located in rhombomeres (r) 4 and 6, which are patterned through multiple ephrin/Eph signaling events. In particular, ephrin-B1 and EphB2 are expressed in the outlying areas that prevent neural crest cells from escaping the branchial arches (Fig. 3B) (Mellott and Burke, 2008). We therefore investigated whether satellite cells would conform to the same spatial boundaries as the neural crest cells. Fluorescently labeled satellite cells were pelleted in hanging drop culture, then grafted into the neural tube proximal to r4 of a 6-8 somite quail embryo; endogenous neural crest cells were labeled with a lipophilic membrane dye (DiD) (Fig. 3A). After 24 hours, we observed that some satellite cells were able to migrate away from the pellet and exit the neural tube along with the migrating neural crest, traveling out into the r4 and r6 streams and respecting the ephrin-mediated boundaries (n=15; Fig. 3C,D, arrows; supplementary material Movie 4). Importantly, this suggests that satellite cells are subject to positive guidance cues as well, demonstrating the same unidirectional migration seen in neural crest cells as they populate the branchial arches (supplementary material Movie 4). In addition, we detected what appeared to be differentiated satellite cells (elongated, fluorescent cell profiles) near the neural tube (Fig. 3C,D, arrowheads), which suggests that satellite cells might maintain their commitment to become myocytes even in a non-myogenic environment.
Fig. 3.
Satellite cells respect ephrin-defined migration boundaries in vivo. (A) Schematic showing the experimental process of isolation, culture and pelleting of GFP-expressing primary satellite cells. The satellite cell pellet was then grafted into the DiD-labeled rhombomere 4 of a developing quail embryo (HH stage 10) and incubated for 24 hours during which time satellite cells (green) and the endogenous DiD-labeled neural crest cells (red) emigrate within ephrin-defined streams. (B) On the left, a summary of documented ephrin and Eph expression within the developing avian hindbrain, which, in part, govern the formation of the neural crest cell streams. On the right, color-coded rhombomeres indicating the neural crest origin from the neural tube and their conformed migration streams. As indicated with white arrows, neural crest cells from rhombomeres 3 and 5 do not create streams but join in neighboring streams. (C) A fixed quail embryo 24 hours after a primary satellite cell engraftment (green) shows a mix of neural crest cells (blue) and satellite cells (green) within the migration stream into branchial arch II (red arrow). Labeled, elongated cells that might be differentiated progeny of engrafted satellite cells can be seen within the neural tube (red arrowhead). (D) The magnified image of panel C reveals all of the satellite cells (green) respecting the ephrin-defined boundaries of branchial arch II (red arrow). See supplementary material Movie 3 for a time-lapse representation of engrafted mouse satellite cell migration with endogenous neural crest. When the embryo was electroporated with a plasmid expressing ephrin-A5 prior to cell grafts, the labeled satellite cells did not enter the streams (supplementary material Fig. S4). (E) Representation of the isolation, culture and pelleting of fluorescent primary satellite cells. The satellite cell pellets were grafted lateral to somite 17 at HH stage 16 of a developing quail embryo and incubated for 40 hours. At HH stage 21, the embryos were fixed and the limb buds were examined. (F) A summary of documented ephrin and Eph expression in the developing limb bud starting at HH stage 15 up to HH stage 21, depicting EphA4-expressing myogenic precursor cells delaminating from the dermomyotome and emigrating into the forelimb, avoiding areas of ephrin expression. (G) Membrane-labeled satellite cells (green) emigrate from a position lateral to somite 17 out into the forelimb of a stage 21 quail embryo 40 hours after engraftment. The red dashed line indicates level of the transverse section shown in H. (H) Transverse section of the forelimb reveals examples of both compact (red arrow) and elongated (red arrowhead) satellite cells that have emigrated from the grafted pellet lateral to somite 17 and out into the limb while maintaining ephrin-defined boundaries. C, G and H brightfield images were filtered with an unsharpened mask. BA, branchial arch; LB, limb bud; OV, otic vesicle; r, rhombomere. Scale bars: 100 μm in D; 200 μm in H.
Although it has not been as extensively described, myoblast migration into the forelimb of an avian embryo is also regulated by Eph/ephrin signaling. Pax3- and EphA4-expressing cells will undergo EMT and delaminate from the dermomytome and migrate out into the forelimb, avoiding areas of ephrin-A5 expression (Swartz et al., 2001). To determine whether satellite cells will migrate out into the limb bud and maintain locally defined boundaries, we grafted a labeled satellite cell pellet lateral to somite 17 in the quail embryo at stage 16 (Fig. 3E). Mirroring our observations in the hindbrain, satellite cells migrated within the same area as that predicted for embryonic myogenic precursor cells in the forelimb (n=17; Fig. 3G). In transverse sections, we found that satellite cells (Fig. 3H, arrows) as well as elongated figures that might be differentiated descendants of labeled satellite cells (Fig. 3H, arrowheads) maintained the spatial distribution that would be predicted if they were responding to the known ephrin-mediated boundaries illustrated in Fig. 3F. Combined, these data suggest that satellite cells are capable of migration in vivo and that endogenous levels of guidance cues, including ephrins, will modify that migration consistent with their effects on endogenous target cells.
In vitro, differentiating muscle satellite cells orient according to ephrin patterning
Ephrin/Eph signaling is largely dependent on cell-cell contacts in order to create change in the directionality of a migrating cell; however, ephrin/Eph signaling also plays an important role in cellular segregation and patterning, as seen in the developing somite (Durbin et al., 1998; Watanabe et al., 2009) or the late embryonic forelimb (Swartz et al., 2001; Wada et al., 2003). We observed that elongated, possibly differentiating, satellite cells in the forelimb were uniformly oriented with respect to the axis of the stream exiting the limb bud (Fig. 3H, arrowhead). In addition, cells grafted directly into the developing somite, rather than lateral to the somite, also appeared to differentiate parallel to endogenous primary myotome cells (supplementary material Fig. S3).
Interestingly, we also observed oriented differentiation in vitro in response to ephrin stripes. Labeled satellite cells plated on Alexa fluor control stripes or ephrin-B1 stripes were incubated for 24 hours then switched into low serum media without FGF2 and incubated for an additional 48 hours to promote satellite cell differentiation. Cells plated on control stripes differentiated in small radial clusters, as is usually seen in vitro, whereas on ephrin-B1-programmed coverslips, the satellite cells were primarily located on the ephrin-free areas (as would be expected from the motility studies) and were aligned and differentiated in parallel to the Fc:ephrin stripes (Fig. 4A). However, when plated on stripes of ephrin-A1 (which did not affect satellite cell motility in vitro), no parallel alignment of differentiated cells was observed (Fig. 4A). Differentiating satellite cells did not express EphA1, the only Eph receptor specific for ephrin-A1 (Fig. 4B), but they robustly expressed EphA2 (Fig. 4B), which is the primary signaling receptor for ephrin-A1 in many systems (for a review, see Pasquale, 2010). EphA2 binds and signals in response to ephrin-A2, ephrin-A3 and ephrin-A5, and oriented, parallel myotube patterning was observed in response to stripes programmed with Fc:ephrin chimeras of each (Fig. 4B,C). These data suggest that although patterning of nascent myotubes is affected by specific Eph/ephrin interactions, the actual signaling complex might be more complicated than a simple one-to-one correlation of an Eph and an ephrin. In particular, crosstalk from other adhesion receptors, which has been observed in many other systems (for a review, see Arvanitis and Davy, 2008), might influence satellite cell responses to ephrin stimulation. Future work will focus on defining potential Eph-ephrin pair(s) involved in myotube patterning, the stage(s) of differentiation at which Eph/ephrin signaling impinges on myotube patterning, and other signaling pathways that might also be acting to mediate myotube alignment in response to ephrin.
Fig. 4.
Differentiating muscle satellite cells orient according to ephrin patterning in vitro. Activated satellite cells four days post-isolation (red) were plated over ephrin-programmed stripes or control stripes for 24 hours before switching to low serum media to promote differentiation. (A) Control stripes do not affect myotube alignment, nor do stripes programmed with ephrin-A1. However, ephrin-B1 stripes promote parallel alignment of differentiating muscle satellite cells, as would be expected for regenerating myotubes in vivo. (B) Differentiating satellite cells do not express EphA1 but do express EphA2, a receptor for all ephrin-As, including ephrin-A1. The boxed image in the upper right corner is lacking the primary antibody for EphA1 or EphA2 but was developed under identical conditions and shows minimal reactivity as the negative control. (C) Parallel alignment to other ephrin-As, including ephrin-A3 and ephrin-A5, was also observed. The fading green stripes at the bottom of each image indicate the position of the ephrin or control stripes that continue to the top of each image. Gamma correction=1.45 in A,C. Scale bars: 200 μm in A,B; 100 μm in C.
DISCUSSION
Eph receptors are the largest family of mammalian receptor tyrosine kinases; both Eph receptors and their ligands (ephrins) are expressed by almost all tissues in the developing embryo (Baker and Antin, 2003). Eph receptors have also been shown to interact functionally with multiple other transmembrane receptors including Fgfrs, Cxcr4, integrins and cadherins (reviewed by Arvanitis and Davy, 2008). Via bidirectional regulation of cell adhesion, Eph/ephrin signaling mediates axon guidance, cell migration, cell sorting, boundary formation and cell fusion (reviewed in Pasquale, 2008). In mammalian development, Eph/ephrin interactions are best studied as mediators of motor axon guidance (Tessier-Lavigne, 1995; Drescher et al., 1997; Orioli and Klein, 1997; Gallo and Letourneau, 2004; Bashaw and Klein, 2010) and neural crest migration (Krull et al., 1997; Wang and Anderson, 1997; McLennan and Krull, 2002). They are also instrumental in directing and maintaining boundary formation in the somite (Durbin et al., 1998; Watanabe et al., 2009), hindbrain (Cooke et al., 2001; Cooke et al., 2005; Kemp et al., 2009) and skull (Twigg et al., 2004; Merrill et al., 2006).
More recently, roles have been demonstrated for Eph/ephrin signaling during adult tissue homeostasis and repair. Eph/ephrin interactions are implicated in mediating neuronal plasticity (reviewed by Yamaguchi and Pasquale, 2004; Dalva et al., 2007) and nerve re-growth after injury (Goldshmit et al., 2006; Liu et al., 2006; Du et al., 2007). In non-neuronal tissues, ephrins are required for such diverse processes as T-cell maturation and function (Stimamiglio et al., 2010), vascular growth and patterning (reviewed in Kuijper et al., 2007), bone homeostasis (reviewed by Edwards and Mundy, 2008), glucose metabolism and insulin secretion (Konstantinova et al., 2007; Kulkarni and Kahn, 2007). In the context of adult stem cells, ephrins mediate key activities in stem/progenitor cells of bone (Arthur et al., 2010), intestine (reviewed by Pitulescu and Adams, 2010), dental pulp (Stokowski et al., 2007) and hair follicles (Genander et al., 2010).
During vertebrate muscle development, Eph signaling is necessary not only to direct innervation of specific muscular domains (Araujo et al., 1998; Eberhart et al., 2000; Feng et al., 2000; Lai et al., 2001) but also for emigration of presumptive limb myoblasts into the dorsal and ventral muscle masses of the limb (Tickle and Altabef, 1999; Swartz et al., 2001). A role for guidance signaling in adult myogenesis has not previously been suggested, possibly because motility of adult myoblasts is not, as yet, as thoroughly examined a component of muscle regeneration as it is in the context of limb muscle development. Although other classical guidance molecules, particularly members of the semaphorin family, have previously been implicated in late stages of muscle regeneration (Ko et al., 2005; Tatsumi et al., 2009) and our RT-PCR data suggests the potential for guidance signaling via multiple classes of receptors, we chose to focus on Eph/ephrin signaling initially owing to the body of literature discussed above.
Cellular responses to Eph/ephrin signaling include reorganization of the cytoskeleton (frequently retraction of processes or filopodia by collapse of F-actin) via modulation of RhoA activity (Gallo and Letourneau, 2004; Sahin et al., 2005). During axon outgrowth or cell migration, this repulsive response guides growth cones or cells to their final destination, where frequently a different ephrin or ephrins is expressed and promotes formation of a neuromuscular junction (Lai et al., 2001) or cessation of migration and adhesion (Halloran and Wolman, 2006; Glazier et al., 2008; Wimmer-Kleikamp et al., 2008; Lee and Daar, 2009). Other changes downstream of ephrin signaling, such as adhesion receptor clustering and changes in substrate affinity, are required for cell sorting during rhombomere and somite segmentation (Cooke et al., 2001; Tanaka et al., 2003; Glazier et al., 2008; Kemp et al., 2009; Julich et al., 2009). Finally, in the case of interactions between stem cells and their niche, Eph/ephrin signaling has been shown to direct both homing to and activities within the niche via modifications in adhesion receptor activity and modulation of downstream signaling pathways (Stokowski et al., 2007; Arthur et al., 2010; Ting et al., 2010).
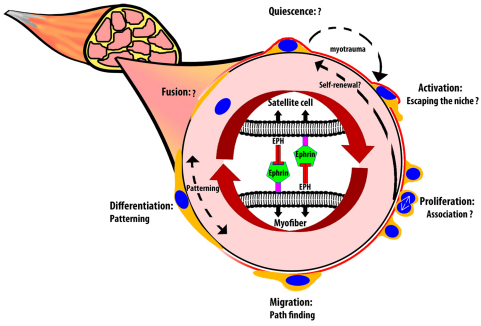
Given the previously described roles of ephrin signaling in other adult organ systems and our results described here, we propose that Eph/ephrin interactions might be involved in multiple aspects of the activated satellite cell response following muscle injury (Fig. 5). Although the extracellular signals impinging on quiescent satellite cells, which could potentially include Eph/ephrin signaling, is currently an area of keen interest, we limited these studies to activated cells based on both the comparatively limited expression of these molecules during quiescence and the general expectation that quiescent cells are not highly motile. However, a role for signaling between activated satellite cells and damaged or undamaged myofibers is suggested by, not only the expression of individual Ephs and ephrins on each cell type, but our in vitro motility assays; future work will focus on demonstrating an in vivo role as well. The expression not only of Eph receptors but also of ephrin ligands by activated satellite cells provides the possibility for Eph-mediated signaling between activated satellite cells also: our recent observations that cell-cell affinity between daughter cells is heterogeneous based on the plane of cell division with respect to the myofiber (Siegel et al., 2011) might be the result of such signaling. Finally, the continued expression of a subset of Ephs and ephrins after myogenic differentiation, as well as the more ordered and parallel myofibers that form during in vitro differentiation in the presence of specific ephrins, suggests a potential role in regenerated muscle patterning, for which there have to date been few candidate mediators. In addition to these functions, we leave open the possibility that guidance signaling through ephrins might impact other aspects of the satellite cell cycle not tested here, including initial exit from the sublaminar niche, cell sorting between either proliferating satellite cells or satellite cells and differentiated myofibers, and cessation of migration and fusion after differentiation.
Fig. 5.
Model of ephrin/Eph involvement in skeletal muscle regeneration. Consistent with their role in other systems, multiple opportunities exist in vivo for Eph/ephrin modulation of satellite cell adhesion, motility, association and patterning. Our evidence supports roles in migration within the tissue and longitudinal patterning of differentiating myofibers, and we hypothesize that Eph/ephrin signaling might affect additional aspects of satellite cell activity as well, such as interaction with the niche and/or sorting within the proliferating satellite cell population. In this figure, the myofiber basal lamina is shown in red and satellite cells in orange with blue nuclei.
Multiple examples of secreted growth factors, cytokines and chemokines that modify satellite cell motility in vitro have been described in the literature. Even so, it remains unclear whether satellite cell motility is necessary or beneficial to muscle repair and regeneration in vivo. The data discussed here represent the first observations of the potential for contact-dependent modification of satellite cell motility via Eph/ephrin signaling, supporting the hypothesis that directed relocation of activated satellite cells within the injured tissue might contribute to the efficiency of regeneration. Although repulsive interactions between activated, motile satellite cells and existing myofibers might at first seem counter-intuitive, it is important to bear in mind that these interactions probably do not lead to significant repulsion of the migrating cells away from the myofibers, but instead might serve to sustain ongoing cell motility through successive adhesion and release cycles (Rohani et al., 2011). In vivo, ephrin-expressing myofibers could be considered to be the equivalent of the in vitro stripes and could act to promote more linear migration paths (parallel to the myofibers).
In addition, our data also suggest a potential role for Eph/ephrin signaling in re-establishing myofiber patterning during or after satellite cell differentiation. Although myocyte alignment in vitro has previously been described with immortalized myoblasts adhered to micro-etched coverslips with or without associated stripes of laminin (Clark et al., 1997; Clark et al., 2002), this new data points to a potential cell-cell signaling mechanism actively mediating recapitulation of myofiber patterning during satellite cell-mediated muscle regeneration.
Eph/ephrin signaling between two distinct muscle cell types (muscle stem cells and differentiated myofibers) is a novel phenomenon and suggests exciting new avenues of inquiry in the area of myogenic regeneration in vivo. In addition, although it was not addressed at length here, given the expression we observe of both Ephs and ephrins on both activated satellite cells and differentiated myofibers, the potential also exists for Eph/ephrin signaling between activated satellite cells or between differentiated myofibers. Future work will concentrate on defining which Eph/ephrin pairs on which cells produce quantifiable changes in satellite cell motility and/or patterning in vivo as well as the downstream effects of such signaling on rapid and effective muscle regeneration. However, given the heterogeneous and dynamic expression we observe for multiple different receptors and ligands, including both A and B-type ephrins and their receptors, and the well-established promiscuity between ligand-receptor pairs (Pasquale, 2004) and their interactions with other cell-surface signaling receptors (Arvanitis and Davy, 2008), establishing a role for specific Eph/ephrin interactions either among activated satellite cells or between satellite cells and differentiated myofibers will not be trivial.
Supplementary Material
Acknowledgments
We thank Dr Leonard Hearne for assistance with statistical analysis.
Footnotes
Funding
This work was supported by the National Institutes of Health [AR056814 to D.D.W.C.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.068411/-/DC1
References
- Araujo M., Piedra M. E., Herrera M. T., Ros M. A., Nieto M. A. (1998). The expression and regulation of chick EphA7 suggests roles in limb patterning and innervation. Development 125, 4195–4204 [DOI] [PubMed] [Google Scholar]
- Arthur A., Zannettino A., Panagopoulos R., Koblar S. A., Sims N. A., Stylianou C., Matsuo K., Gronthos S. (2010). EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone 48, 533–542 [DOI] [PubMed] [Google Scholar]
- Arvanitis D., Davy A. (2008). Eph/ephrin signaling: networks. Genes Dev. 22, 416–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae G. U., Gaio U., Yang Y. J., Lee H. J., Kang J. S., Krauss R. S. (2008). Regulation of myoblast motility and fusion by the CXCR4-associated sialomucin, CD164. J. Biol. Chem. 283, 8301–8309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. K., Antin P. B. (2003). Ephs and ephrins during early stages of chick embryogenesis.. Dev. Dyn. 228, 128–142 [DOI] [PubMed] [Google Scholar]
- Bashaw G. J., Klein R. (2010). Signaling from axon guidance receptors. Cold Spring Harb. Perspect. Biol. 2, a001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. (1997). Chemotaxis of skeletal muscle satellite cells. Dev. Dyn. 208, 505–515 [DOI] [PubMed] [Google Scholar]
- Bolsover S., Fabes J., Anderson P. N. (2008). Axonal guidance molecules and the failure of axonal regeneration in the adult mammalian spinal cord. Restor. Neurol. Neurosci. 26, 117–130 [PubMed] [Google Scholar]
- Caldwell C. J., Mattey D. L., Weller R. O. (1990). Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 16, 225–238 [DOI] [PubMed] [Google Scholar]
- Capkovic K. L., Stevenson S., Johnson M. C., Thelen J. J., Cornelison D. D. W. (2008). Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp. Cell Res. 314, 1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano J., Davalos V., Schwartz S. J., Arango D. (2008). EPH receptors in cancer. Histol. Histopathol. 23, 1011–1023 [DOI] [PubMed] [Google Scholar]
- Ciossek T., Monschau B., Kremoser C., Loschinger J., Lang S., Muller B. K., Bonhoeffer F., Drescher U. (1998). Eph receptor-ligand interactions are necessary for guidance of retinal ganglion cell axons in vitro. Eur. J. Neurosci. 10, 1574–1580 [DOI] [PubMed] [Google Scholar]
- Clark P., Coles D., Peckham M. (1997). Preferential adhesion to and survival on patterned laminin organizes myogenesis in vitro. Exp. Cell Res. 230, 275–283 [DOI] [PubMed] [Google Scholar]
- Clark P., Dunn G. A., Knibbs A., Peckham M. (2002). Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int. J. Biochem. Cell Biol. 34, 816–825 [DOI] [PubMed] [Google Scholar]
- Cooke J., Moens C., Roth L., Durbin L., Shiomi K., Brennan C., Kimmel C., Wilson S., Holder N. (2001). Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development 128, 571–580 [DOI] [PubMed] [Google Scholar]
- Cooke J. E., Kemp H. A., Moens C. B. (2005). EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 15, 536–542 [DOI] [PubMed] [Google Scholar]
- Cornelison D. D. W., Wold B. J. (1997). Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191, 270–283 [DOI] [PubMed] [Google Scholar]
- Cornelison D. D. W., Wilcox-Adelman S. A., Goetinck P. F., Rauvala H., Rapraeger A. C., Olwin B. B. (2004). Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev. 18, 2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. A., Henkemeyer M. (2002). Ephrins in reverse, park and drive. Trends Cell. Biol. 12, 339–346 [DOI] [PubMed] [Google Scholar]
- Dalva M. B., McClelland A. C., Kayser M. S. (2007). Cell adhesion molecules: signalling functions at the synapse. Nat.. Rev. Neurosci. 8, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A., Soriano P. (2005). Ephrin signaling in vivo: look both ways. Dev.Dyn. 232, 1–10 [DOI] [PubMed] [Google Scholar]
- Dedieu S., Mazeres G., Poussard S., Brustis J. J., Cottin P. (2003). Myoblast migration is prevented by a calpain-dependent accumulation of MARCKS. Biol. Cell 95, 615–623 [DOI] [PubMed] [Google Scholar]
- Drescher U., Bonhoeffer F., Muller B. K. (1997). The Eph family in retinal axon guidance. Curr. Opin. Neurobiol. 7, 75–80 [DOI] [PubMed] [Google Scholar]
- Du J., Fu C., Sretavan D. W. (2007). Eph/ephrin signaling as a potential therapeutic target after central nervous system injury. Curr. Pharm. Des. 13, 2507–2518 [DOI] [PubMed] [Google Scholar]
- Dupin E., Calloni G. W., Le Douarin N. M. (2010). The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities. Cell Cycle 9, 238–249 [DOI] [PubMed] [Google Scholar]
- Durbin L., Brennan C., Shiomi K., Cooke J., Barrios A., Shanmugalingam S., Guthrie B., Lindberg R., Holder N. (1998). Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 12, 3096–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart J., Swartz M., Koblar S. A., Pasquale E. B., Tanaka H., Krull C. E. (2000). Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev. Neurosci. 22, 237–250 [DOI] [PubMed] [Google Scholar]
- Edwards C. M., Mundy G. R. (2008). Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int. J. Med. Sci. 5, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Laskowski M. B., Feldheim D. A., Wang H., Lewis R., Frisen J., Flanagan J. G., Sanes J. R. (2000). Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers. Neuron 25, 295–306 [DOI] [PubMed] [Google Scholar]
- Frisen J., Holmberg J., Barbacid M. (1999). Ephrins and their Eph receptors: multitalented directors of embryonic development. EMBO J. 18, 5159–5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G., Letourneau P. C. (2004). Regulation of growth cone actin filaments by guidance cues. J. Neurobiol. 58, 92–102 [DOI] [PubMed] [Google Scholar]
- Genander M., Holmberg J., Frisen J. (2010). Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells 28, 1196–1205 [DOI] [PubMed] [Google Scholar]
- Glazier J. A., Zhang Y., Swat M., Zaitlen B., Schnell S. (2008). Coordinated action of N-CAM, N-cadherin, EphA4, and ephrinB2 translates genetic prepatterns into structure during somitogenesis in chick. Curr. Top. Dev. Biol. 81, 205–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y., McLenachan S., Turnley A. (2006). Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res. Rev. 52, 327–345 [DOI] [PubMed] [Google Scholar]
- Halloran M. C., Wolman M. A. (2006). Repulsion or adhesion: receptors make the call. Curr. Opin. Cell Biol. 18, 533–540 [DOI] [PubMed] [Google Scholar]
- Haustead D. J., Lukehurst S. S., Clutton G. T., Bartlett C. A., Dunlop S. A., Arrese C. A., Sherrard R. M., Rodger J. (2008). Functional topography and integration of the contralateral and ipsilateral retinocollicular projections of ephrin-A–/– mice. J. Neurosci. 28, 7376–7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke T. J., Garry D. J. (2001). Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91, 534–551 [DOI] [PubMed] [Google Scholar]
- Irie N., Takada Y., Watanabe Y., Matsuzaki Y., Naruse C., Asano M., Iwakura Y., Suda T., Matsuo K. (2009). Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J. Biol. Chem. 284, 14637–14644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen K. M., Pavlath G. K. (2006). Mannose receptor regulates myoblast motility and muscle growth. J. Cell Biol. 174, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julich D., Mould A. P., Koper E., Holley S. A. (2009). Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development 136, 2913–2921 [DOI] [PubMed] [Google Scholar]
- Kemp H. A., Cooke J. E., Moens C. B. (2009). EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev. Biol. 327, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. (2009). Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat. Neurosci. 12, 15–20 [DOI] [PubMed] [Google Scholar]
- Knoll B., Weinl C., Nordheim A., Bonhoeffer F. (2007). Stripe assay to examine axonal guidance and cell migration. Nat. Protoc. 2, 1216–1224 [DOI] [PubMed] [Google Scholar]
- Ko J. A., Gondo T., Inagaki S., Inui M. (2005). Requirement of the transmembrane semaphorin Sema4C for myogenic differentiation. FEBS Lett. 579, 2236–2242 [DOI] [PubMed] [Google Scholar]
- Konstantinova I., Nikolova G., Ohara-Imaizumi M., Meda P., Kucera T., Zarbalis K., Wurst W., Nagamatsu S., Lammert E. (2007). EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359–370 [DOI] [PubMed] [Google Scholar]
- Krull C. E., Lansford R., Gale N. W., Collazo A., Marcelle C., Yancopoulos G. D., Fraser S. E., Bronner-Fraser M. (1997). Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. 7, 571–580 [DOI] [PubMed] [Google Scholar]
- Kuijper S., Turner C. J., Adams R. H. (2007). Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc. Med. 17, 145–151 [DOI] [PubMed] [Google Scholar]
- Kulkarni R. N., Kahn C. R. (2007). Ephs and ephrins keep pancreatic Beta cells connected. Cell 129, 241–243 [DOI] [PubMed] [Google Scholar]
- Lai K. O., Ip N. Y. (2009). Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr. Opin. Neurobiol. 19, 275–283 [DOI] [PubMed] [Google Scholar]
- Lai K. O., Ip F. C., Cheung J., Fu A. K., Ip N. Y. (2001). Expression of Eph receptors in skeletal muscle and their localization at the neuromuscular junction. Mol. Cell Neurosci. 17, 1034–1047 [DOI] [PubMed] [Google Scholar]
- Lee H. S., Daar I. O. (2009). EphrinB reverse signaling in cell-cell adhesion: is it just par for the course? Cell Adh. Migr. 3, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. P., Cafferty W. B., Budel S. O., Strittmatter S. M. (2006). Extracellular regulators of axonal growth in the adult central nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1593–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan R., Krull C. E. (2002). Ephrin-as cooperate with EphA4 to promote trunk neural crest migration. Gene Expr. 10, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott D. O., Burke R. D. (2008). Divergent roles for Eph and ephrin in avian cranial neural crest. BMC Dev. Biol. 8, 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suarez A., Batlle E. (2008). Eph-ephrin signalling in adult tissues and cancer. Curr. Opin. Cell Biol. 20, 194–200 [DOI] [PubMed] [Google Scholar]
- Merrill A. E., Bochukova E. G., Brugger S. M., Ishii M., Pilz D. T., Wall S. A., Lyons K. M., Wilkie A. O., Maxson R. E. J. (2006). Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Hum. Mol. Genet. 15, 1319–1328 [DOI] [PubMed] [Google Scholar]
- Murai K. K., Pasquale E. B. (2010). Restraining stem cell niche plasticity: a new talent of Eph receptors. Cell Stem Cell 7, 647–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylona E., Jones K. A., Mills S. T., Pavlath G. K. (2006). CD44 regulates myoblast migration and differentiation. J. Cell. Physiol. 209, 314–321 [DOI] [PubMed] [Google Scholar]
- Noberini R., Pasquale E. B. (2009). Proliferation and tumor suppression: not mutually exclusive for Eph receptors. Cancer Cell 16, 452–454 [DOI] [PubMed] [Google Scholar]
- Ocalan M., Goodman S. L., Kuhl U., Hauschka S. D., von der Mark K. (1988). Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev. Biol. 125, 158–167 [DOI] [PubMed] [Google Scholar]
- Orioli D., Klein R. (1997). The Eph receptor family: axonal guidance by contact repulsion. Trends Genet. 13, 354–359 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2004). Eph-ephrin promiscuity is now crystal clear. Nat. Neurosci. 7, 417–418 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2008). Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2010). Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer 10, 165–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu M. E., Adams R. H. (2010). Eph/ephrin molecules-a hub for signaling and endocytosis. Genes Dev. 24, 2480–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N., Canty L., Luu O., Fagotto F., Winklbauer R. (2011). EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 9, e1000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. D., Woods R. I. (1992). Hypertrophy of rat extensor digitorum longus muscle injected with bupivacaine. A sequential histochemical, immunohistochemical, histological and morphometric study. J. Anat. 181, 11–27 [PMC free article] [PubMed] [Google Scholar]
- Sahin M., Greer P. L., Lin M. Z., Poucher H., Eberhart J., Schmidt S., Wright T. M., Shamah S. M., O’Connell S., Cowan C. W., et al. (2005). Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46, 191–204 [DOI] [PubMed] [Google Scholar]
- Siegel A. L., Atchison K., Fisher K. E., Davis G. E., Cornelison D. D. (2009). 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27, 2527–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A. L., Kuhlmann P. K., Cornelison D. D. W. (2011). Muscle satellite cell proliferation and association: new insights from myofiber time-lapse imaging. Skeletal Muscle 1, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimamiglio M. A., Jimenez E., Silva-Barbosa S. D., Alfaro D., Garcia-Ceca J. J., Munoz J. J., Cejalvo T., Savino W., Zapata A. (2010). EphB2-mediated interactions are essential for proper migration of T cell progenitors during fetal thymus colonization. J. Leukoc. Biol. 88, 483–494 [DOI] [PubMed] [Google Scholar]
- Stokowski A., Shi S., Sun T., Bartold P. M., Koblar S. A., Gronthos S. (2007). EphB/ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem Cells 25, 156–164 [DOI] [PubMed] [Google Scholar]
- Swartz M. E., Eberhart J., Pasquale E. B., Krull C. E. (2001). EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development 128, 4669–4680 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamo T., Ota S., Sugimura H. (2003). Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 22, 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R., Sankoda Y., Anderson J. E., Sato Y., Mizunoya W., Shimizu N., Suzuki T., Yamada M., Rhoads R. P. J., Ikeuchi Y., et al. (2009). Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am. J. Physiol. Cell Physiol. 297, C238–C252 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M. (1995). Eph receptor tyrosine kinases, axon repulsion, and the development of topographic maps. Cell 82, 345–348 [DOI] [PubMed] [Google Scholar]
- Tickle C., Altabef M. (1999). Epithelial cell movements and interactions in limb, neural crest and vasculature. Curr. Opin. Genet. Dev. 9, 455–460 [DOI] [PubMed] [Google Scholar]
- Ting M. J., Day B. W., Spanevello M. D., Boyd A. W. (2010). Activation of ephrin A proteins influences hematopoietic stem cell adhesion and trafficking patterns. Exp. Hematol. 38, 1087–1098 [DOI] [PubMed] [Google Scholar]
- Twigg S. R., Kan R., Babbs C., Bochukova E. G., Robertson S. P., Wall S. A., Morriss-Kay G. M., Wilkie A. O. (2004). Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc. Natl. Acad. Sci. USA 101, 8652–8657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N., Tanaka H., Ide H., Nohno T. (2003). Ephrin-A2 regulates position-specific cell affinity and is involved in cartilage morphogenesis in the chick limb bud. Dev. Biol. 264, 550–563 [DOI] [PubMed] [Google Scholar]
- Walter J., Kern-Veits B., Huf J., Stolze B., Bonhoeffer F. (1987). Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development 101, 685–696 [DOI] [PubMed] [Google Scholar]
- Wang H. U., Anderson D. J. (1997). Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18, 383–396 [DOI] [PubMed] [Google Scholar]
- Wang Y., Nakayama M., Pitulescu M. E., Schmidt T. S., Bochenek M. L., Sakakibara A., Adams S., Davy A., Deutsch U., Luthi U., et al. (2010). Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sato Y., Saito D., Tadokoro R., Takahashi Y. (2009). EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc. Natl. Acad. Sci. USA 106, 7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G. (2000). Eph receptors and ephrins: regulators of guidance and assembly. Int. Rev. Cytol. 196, 177–244 [DOI] [PubMed] [Google Scholar]
- Wimmer-Kleikamp S. H., Nievergall E., Gegenbauer K., Adikari S., Mansour M., Yeadon T., Boyd A. W., Patani N. R., Lackmann M. (2008). Elevated protein tyrosine phosphatase activity provokes Eph/ephrin-facilitated adhesion of pre-B leukemia cells. Blood 112, 721–732 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Pasquale E. B. (2004). Eph receptors in the adult brain. Curr. Opin. Neurobiol. 14, 288–296 [DOI] [PubMed] [Google Scholar]
- Yao C. C., Ziober B. L., Sutherland A. E., Mendrick D. L., Kramer R. H. (1996). Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J. Cell. Sci. 109, 3139–3150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.