Abstract
During development of the urogenital tract, fibroblast growth factor 8 (Fgf8) is expressed in mesonephric tubules, but its role in this tissue remains undefined. An evaluation of previously generated T-Cre-mediated Fgf8-deficient mice (T-Cre; Fgf8flox/Δ2,3 mice), which lack Fgf8 expression in the mesoderm, revealed that the cranial region of the Wolffian duct degenerated prematurely and the cranial mesonephric tubules were missing. As a result, the epididymis, vas deferens and efferent ductules were largely absent in mutant mice. Rarb2-Cre was used to eliminate FGF8 from the mesonephric tubules but to allow expression in the adjacent somites. These mutants retained the cranial end of the Wolffian duct and formed the epididymis and vas deferens, but failed to elaborate the efferent ductules, indicating that Fgf8 expression by the mesonephric tubules is required specifically for the formation of the ductules. Ret knockout mice do not form the ureteric bud, a caudal outgrowth of the Wolffian duct and progenitor for the collecting duct network in the kidney, but they do develop the cranial end normally. This indicates that Fgf8, but not Ret, expression is essential to the outgrowth of the cranial mesonephric tubules from the Wolffian duct and to the development of major portions of the sex accessory tissues in the male reproductive tract. Mechanistically, FGF8 functions upstream of Lhx1 expression in forming the nephron, and analysis of Fgf8 mutants similarly shows deficient Lhx1 expression in the mesonephric tubules. These results demonstrate a multifocal requirement for FGF8 in establishing the male reproductive tract ducts and implicate Lhx1 signaling in tubule elongation.
Keywords: FGF8, T-Cre, Mesonephric tubules, Wolffian duct, Müllerian duct, Efferent ductules, Epididymis, Mouse
INTRODUCTION
The mammalian male reproductive tract is composed of the testis and the sex accessory tissues, including the epididymis, vas deferens, efferent ductules and seminal vesicles. During development, the Wolffian duct (WD) plays a major role in the formation of the accessory tissues (Drawbridge et al., 2003; Hannema and Hughes, 2007; Wilhelm et al., 2007). At the earliest stage of urogenital differentiation (E8.5-9.0) in the mouse, only pronephric components, which originate in the intermediate mesoderm, are observed in the urogenital tract. By E9.5, the WD forms from the coalescence of mesenchymal cells in the intermediate mesoderm in craniocaudal succession and induces a progressive wave of mesonephric tubules in the surrounding mesonephric mesenchyme/nephrogenic cord. It is worth noting that the cranial mesonephric tubules consist of a short outgrowth from the WD that connects with elements derived from the nephrogenic cord, while the caudal mesonephric tubules are derived only from the nephrogenic cord and are not attached to the WD (Gumpel-Pinot et al., 1971; Mugford et al., 2008; Sainio et al., 1997). Development of the urogenital tract then continues from the caudal end of the WD, which forms the ureteric bud in response to GDNF expression by the metanephric mesenchyme around E10.5 (Sanchez et al., 1996). During E15.5-16.5, gender specification occurs. The Müllerian duct degenerates in the male and production of androgenic hormones and hormone-like factors by the developing testes maintain tissues in the WD and mesonephric tubules (Capel, 2000; Cate et al., 1986), which differentiate into the epididymis, vas deferens, efferent ductules and seminal vesicles by E17.5 (Drawbridge et al., 2003; Hannema and Hughes, 2007; Ilio and Hess, 1994; Sainio and Raatikainen-Ahokas, 1999; Wilhelm et al., 2007).
The mammalian female reproductive tract is composed of the ovary and the sex accessory tissues, including oviduct, uterus, cervix and vagina, which are primarily derivatives of the Müllerian duct (Kobayashi and Behringer, 2003). At E11.5, the Müllerian duct begins to develop and elongate in coordination with the WD, extending towards the cloaca. At E15.5-16.5, the WD largely degenerates in the female, and by E17.5, the Müllerian duct differentiates into the female reproductive organs (Kobayashi and Behringer, 2003; Yin and Ma, 2005).
Studies have implicated several genes in the formation of the WD, Müllerian duct and mesonephric tubules. The homeodomain transcriptional factor Lhx1, also known as Lim1, is expressed in epithelia of the mesonephric tubules, as well as the Wolffian and Müllerian ducts. In the absence of Lhx1 expression in the WD, the WD along with the Müllerian duct degenerate prematurely, resulting in the loss of both male and female reproductive tract tissues (Kobayashi et al., 2005; Kobayashi et al., 2004). Similarly, loss of Lhx1 expression in the Müllerian duct causes deficiencies in female reproductive tract development (Kobayashi et al., 2004). Wnt4 is strongly expressed in the Müllerian duct and ovary throughout development. In Wnt4-deficient female mice, the Müllerian duct is missing, whereas the WD is maintained (Vainio et al., 1999). Despite the clear involvement of several genes in the development of the WD, Müllerian duct and mesonephric tubules, the signaling that drives this development is not well understood.
Members of the FGF family of secreted signaling molecules play an important role in a diverse range of developmental events. Several FGFs or FGF receptors (FGFRs) have been reported to regulate the formation of the urogenital tract. Fgf10 is expressed caudally in mesoderm surrounding the WD (Thomson and Cunha, 1999). In Fgf10-deficient male mice, the caudal WD and subsequently the seminal vesicles are absent or develop abnormally (Donjacour et al., 2003). FGF7 increases the thickness and decreases apoptotic cell death in cultured WD (Maeshima et al., 2007). Furthermore, Fgfr1/2 conditional knockout mice fail to form mesonephric tubules, have massive cell apoptosis within the metanephric mesenchyme and have deficient renal branching of the WD derivative, the ureteric bud (Poladia et al., 2006; Zhao et al., 2004).
Our group has demonstrated that conditional loss of Fgf8 expression in mesoderm using T(Brachyury)-Cre causes aberrant cell death within the cortical metanephric mesenchyme and a block in nephron formation. Furthermore, Fgf8 and Wnt4 function cooperatively to regulate Lhx1 expression in kidney development (Perantoni et al., 2005). Other studies have demonstrated that Lhx1 and Wnt4 regulate the development of the mesonephric tubules, WD and Müllerian duct (see above). Therefore, we hypothesized that Fgf8 also functions in the development of mesodermally derived tissues in the urogenital tract, namely, the WD, Müllerian duct and mesonephric tubules.
Here, we examine Fgf8 function in the mesonephros using previously characterized T-Cre; Fgf8flox/Δ2,3 mice (Perantoni et al., 2005). We confirm that Fgf8 is expressed in mesonephric tubules as shown in our earlier study. Moreover, these mice do not form the cranial region of the WD or mesonephric tubules, which is consistent with the subsequent loss of the epididymis, vas deferens and efferent ductules in male mice, suggesting that Fgf8 plays a major role in forming the male reproductive tract.
MATERIALS AND METHODS
Animals
Generation, maintenance and genotyping of T-Cre; Fgf8flox/Δ2,3 mice (Perantoni et al., 2005), AP2-Cre (Nelson and Williams, 2004), Rarb2-Cre (Kobayashi et al., 2005) and the Ret knockout mice (Schuchardt et al., 1994; Schuchardt et al., 1996) have been described previously. Mouse lines with conditional floxed alleles of Fgfr1 and Fgfr2 were kind gifts from Chuxia Deng (NIDDK, Bethesda, MD, USA) and David Ornitz (Washington University School of Medicine, St Louis, MO, USA), respectively, and have been described previously (Xu et al., 2002; Yu et al., 2003). FGFR mutants were generated by crossing females that were homozygous for floxed alleles of Fgfr1 or Fgfr2, or both Fgfr1 and Fgfr2 to males that were homozygous for TCre and heterozygous for the corresponding Fgfr null allele(s). Hence, littermate controls were TCre/+; FgfrXflox/+ where X is 1 or 2 or 1 and 2. Generation of chimeric mice using Ret–/–; Hoxb7/GFP ES cells was performed as previously described (Chi et al., 2009), so that the wild-type cells in the Hoxb7 expression domain (including the nephric duct and mesonephric tubules) of the resulting chimeric embryos were labeled with ECFP, whereas the Ret–/– cells were labeled with EGFP. Mice were managed according to NIH Guidelines for the Care and Use of Laboratory Animals.
Organ culture
Cultured mesonephroi were used for studies of Ret–/– mice. Entire urogenital tracts, containing mesonephric and metanephric rudiments, were dissected in CO2-independent medium and were cultured on Transwell Clear filters (Costar, Milano, Italy), in 5% CO2 at 37°C, as described previously (Srinivas et al., 1999) in DMEM with 10% fetal calf serum (Hyclone, Logan, UT) and photographed by fluorescence microscopy (Zeiss AxioObserver, Chester, VA).
In situ hybridization
Embryos or dissected urogenital organs were fixed and processed for whole-mount in situ hybridization as described previously (Pizard et al., 2004).
Immunostaining
Anti-pan-cytokeratin staining of mesonephroi was performed as described previously (Ehrenfels et al., 1999) with some modifications. Briefly, dissected urogenital organs were fixed in ice-cold 100% methanol for 5 minutes followed by two brief washes in phosphate-buffered saline containing 0.1% Triton X-100 (PBST). All subsequent washes and incubations were carried out in PBST. The samples were blocked in 10% heat-inactivated sheep serum (Sigma, St Louis, MO) for 1 hour at room temperature, washed and incubated with a 1:100 dilution of pan-cytokeratin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After three washes, they were incubated with Cy3 or FITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratory, West Grove, PA) at 1:1000 dilution overnight at 4°C. After an extensive wash, the urogenital regions were photographed by fluorescence microscopy (Zeiss AxioObserver).
WT1 staining of paraffin-sectioned testis was achieved using a Bond System autostainer (Leica, Mount Waverley VIC, Australia). After pretreatment with citrate buffer (pH 6.0) for heat-induced epitope retrieval (HIER), the slides were incubated with 2% goat serum (Vector Laboratories, Burlingame, CA) for 20 minutes at room temperature. Following this incubation, the slides were incubated with WT1 antibody (Santa Cruz Biotechnology) at 1:500 dilution 30 minutes at room temperature and then with biotinylated 2Ab Goat a/Rabbit IgG (Vector Laboratories) at 1:100 dilution 30 minutes at room temperature. TUNEL staining for apoptotic cells was performed using an Apoptag kit (Intergen, Purchase, NY) according to manufacturer’s instructions. Sections were photographed using a Zeiss AxioCam mounted on an Olympus Provis AX70 microscope (Center Valley, PA).
RESULTS
Fgf8 is expressed in mesonephric tubules
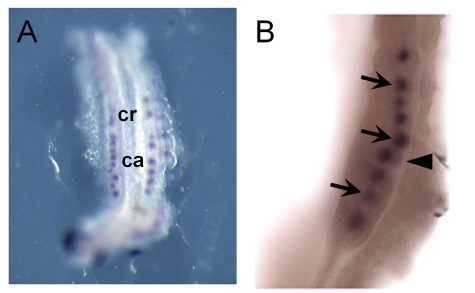
Previously, we demonstrated that Fgf8 is expressed in the primitive streak at E7.5 and in various mesodermal lineages, including somites, at E9.5 in normal embryos. Moreover, ablation of the expression of Fgf8 using T-Cre resulted in the complete loss of Fgf8 in all mesodermal lineages by E9.5 (Perantoni et al., 2005). To further characterize its expression in the mesonephros, we performed whole-mount in situ hybridization using slightly older embryos. In normal E10.5 embryos (T-Cre; Fgf8flox/+), Fgf8 was clearly expressed in tubules/vesicles in the mesonephros [Fig. 1A; cranial (cr) or caudal (ca) mesonephric tubule]. At higher magnification, Fgf8 expression was detected in the mesonephric tubules (Fig. 1B, black arrows) adjacent to the WD (black arrowhead) at E10.5. At E11.5, Fgf8 expression was no longer detected in the mesonephros (data not shown).
Fig. 1.
Fgf8 is expressed in mesonephric tubules. (A,B) Whole-mount in situ hybridization of normal embryos at E10.5 using an Fgf8 probe. The black arrows label the mesonephric tubules and the arrowhead indicates the Wolffian duct. ca, caudal mesonephric tubules; cr, cranial mesonephric tubules.
The cranial portion of the mesonephros is missing in T-Cre; Fgf8flox/Δ2,3 mice
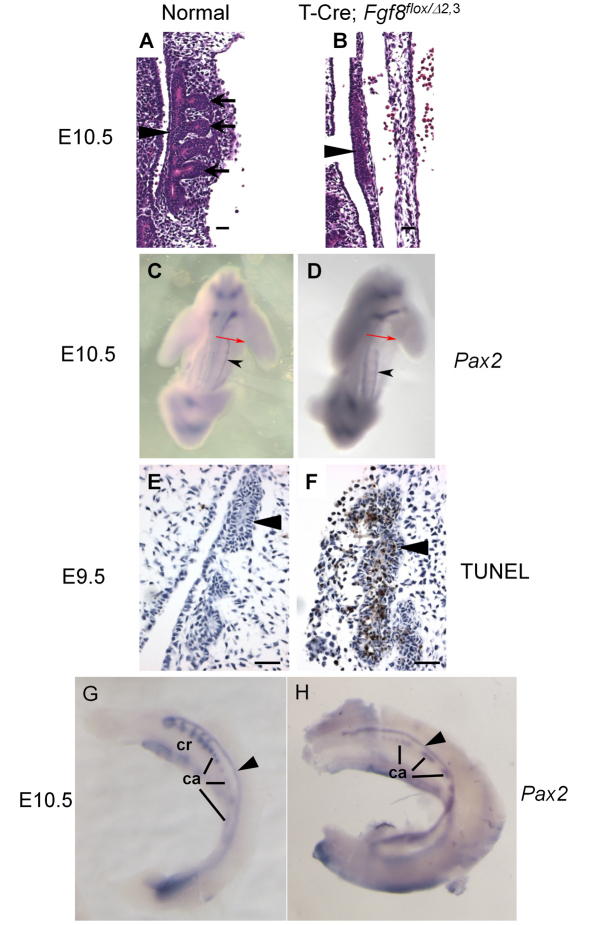
Next, we examined the development of mesonephric structures in T-Cre; Fgf8flox/Δ2,3 mice. Histologically, the cranial mesonephric tubules (Fig. 2A, black arrows) are arrayed along the WD (black arrowhead) in normal E10.5 mice. However, in T-Cre; Fgf8flox/Δ2,3 mice, no mesonephric tubules were demonstrable adjacent to the WD (Fig. 2B). To verify and extend this observation, we evaluated Pax2 expression by in situ hybridization in E10.5 embryos. Pax2 acts redundantly with Pax8 to induce the development of the WD from intermediate mesoderm (Bouchard et al., 2002) and thus serves as an early marker for this event. In the E10.5 mesonephros, the Pax2 expression domain, which corresponds with the WD, is truncated at the cranial end in mutant embryos, beginning about two somites lower (Fig. 2C,D). To determine whether the cranial end of the WD initially forms and then regresses, we assessed embryos for Pax2 expression at E9.5 when it is normally first observed in the developing urogenital tract. The expression domains appear comparable between mutant and normal embryos at this stage (beginning around somite 9 and extending over 12 somites, data not shown), suggesting that the tissue does form initially, so we also examined the mesonephros by TUNEL staining for apoptosis. Histologically, the cranial region of the WD is present, but massive cell death (brown-stained cells) is apparent in the duct at this stage of development (Fig. 2E,F), indicating that the WD initially forms but then undergoes premature tissue degeneration with complete loss of the cranial end by E10.5.
Fig. 2.
T-Cre; Fgf8flox/Δ2,3 mice fail to form mesonephric tubules. (A,B) Hematoxylin and Eosin stained sections (×400) of normal (A) and T-Cre; Fgf8flox/Δ2,3 (B) embryos at E10.5. Black arrows label the mesonephric tubules and black arrowheads indicate the WD. The mesonephric tubules were not formed in T-Cre; Fgf8flox/Δ2,3 embryos. (C,D) Abbreviated whole-mount in situ hybridization staining (overnight, 4°C) for Pax2 (in order to label the WD but not the mesonephric tubules) reveals that the WD (black arrowheads) normally extends to the junction of the forelimb and trunk (red arrows) in E10.5 controls but not in mutants (D) due to an anterior truncation. (E,F) TUNEL staining shows the premature degeneration of the Wolffian duct (arrowheads) in E9.5 mutants. (G,H) Lengthy whole-mount in situ hybridization staining (4 days, 4°C) for Pax2 in E10.5 embryos shows the presence of only the caudal mesonephric tubules in T-Cre; Fgf8flox/Δ2,3 embryos (black arrowhead, WD). Scale bars in A,B,E,F: 20 μm. ca, caudal mesonephric tubules; cr, cranial mesonephric tubules.
Normally, mesonephric tubules consist of 4-6 WD-attached cranial tubules and many unattached caudal tubules (Sainio et al., 1997). To determine which of these were missing in T-Cre; Fgf8flox/Δ2,3 mice, we performed whole-mount in situ hybridization using a Pax2 probe. In normal mice, Pax2, like Fgf8, is expressed in both the cranial and caudal mesonephric tubules, but, unlike Fgf8, it is also expressed in the WD, which in this case provides positional information (Fig. 2G) (Torres et al., 1995). In T-Cre; Fgf8flox/Δ2,3 mice, only some caudal mesonephric tubules were demonstrable. On the other hand, the cranial mesonephric tubules were completely absent (Fig. 2H). We therefore conclude that not only is Fgf8 expression lost from the cranial mesonephric tubules, but the tissue itself is also missing, suggesting that Fgf8 is necessary for tubule formation or maintenance.
As GATA3 is required for proper patterning of the Wolffian duct and because it acts downstream of PAX2 (Grote et al., 2006), we evaluated expression of Gata3 by whole-mount in situ hybridization in E10.5 normal and T-Cre; Fgf8flox/Δ2,3 embryos. Gata3 expression is normally detected throughout the WD, but, in mutant embryos, it was absent from the degenerating cranial end (data not shown). Expression was sustained, however, in the remaining caudal region of the WD. This observation is consistent with Gata3 functioning downstream of Pax2 and demonstrates that FGF8 is not required for expression of Gata3 in the WD.
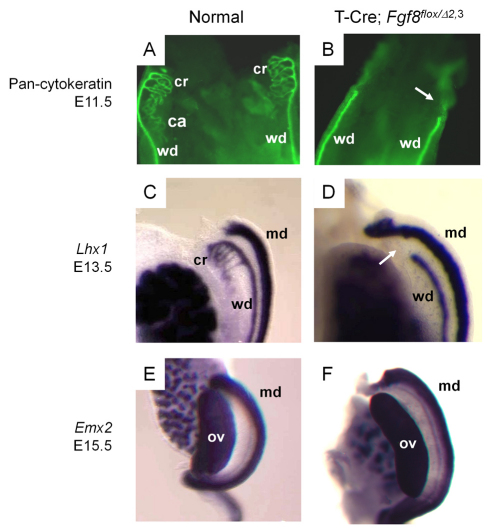
To further examine the development of mesonephric tissues in T-Cre; Fgf8flox/Δ2,3 mice, we performed immunohistochemistry using a pan-cytokeratin antibody, which labels both the mesonephric tubules and WD (Sainio et al., 1997). These tubules and the cranial aspect (white arrow) of the WD were both missing in mutant mice at E11.5 (Fig. 3A,B). Lhx1 is also normally expressed in these tissues and in the Müllerian duct (Fujii et al., 1994; Karavanov et al., 1998; Kobayashi et al., 2005). Using a Lhx1 probe at E13.5, we could clearly discern the truncation of the WD (white arrow) and loss of the cranial mesonephric tubules but normal formation of the Müllerian duct in mutant mice (Fig. 3C,D). To confirm that the Müllerian duct was preserved in T-Cre; Fgf8flox/Δ2,3 mice, Emx2 expression was evaluated (Miyamoto et al., 1997). There was no difference in Emx2 expression between normal and T-Cre; Fgf8flox/Δ2,3 female mice (Fig. 3E,F), indicating that Müllerian duct formation is not FGF8 dependent. Thus, the role of FGF8 in the mesonephros is probably limited to the male reproductive tract.
Fig. 3.
The mesonephric tubules and cranial Wolffian duct are missing in T-Cre; Fgf8flox/Δ2,3 mice, whereas the Müllerian duct is maintained. (A,B) Immunofluorescence using a Pan-cytokeratin antibody was performed in E11.5 embryos. The mesonephric tubules and cranial region of the Wolffian duct (white arrow in B) were lost in T-Cre; Fgf8flox/Δ2,3 embryos. (C-F) Whole-mount in situ hybridization for Lhx1 (C,D) or Emx2 (E,F) on normal (C,E) and T-Cre; Fgf8flox/Δ2,3 (D,F) embryos at E13.5 (C,D) or E15.5 (E,F). (C,D) The entire cranial region of the Wolffian duct and associated mesonephric tubules are missing in T-Cre; Fgf8flox/Δ2,3 mice (white arrow in D). (E,F) The Müllerian duct was maintained in T-Cre; Fgf8flox/Δ2,3 mice. ca, caudal mesonephric tubules; cr, cranial mesonephric tubules; wd, Wolffian duct; md, Müllerian duct; ov, ovary.
T-Cre; Fgf8flox/Δ2,3 mice develop abnormal male reproductive tract tissues
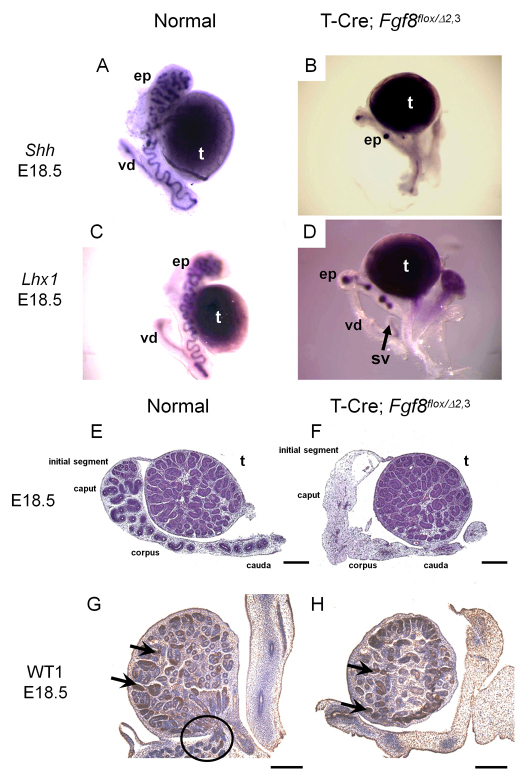
As the efferent ductules are thought to be derived from the mesonephric tubules and the epididymis and vas deferens from the WD (Hinton et al., 1998; Ilio and Hess, 1994), we examined the male reproductive tract in mutant mice for alterations, using Lhx1 and Shh probes, both of which are expressed in the epididymis and vas deferens (Bitgood and McMahon, 1995; Kobayashi et al., 2004; Turner et al., 2004; Vainio et al., 1999). In control male mice, Lhx1 and Shh were expressed continuously in the coiled duct of the epididymis and in the straight duct of the vas deferens at E18.5 (Fig. 4A,C). In T-Cre; Fgf8flox/Δ2,3 male mice, Lhx1 and Shh were detected only in limited regions of the corpus and cauda of the epididymis and in a short proximal duct of the vas deferens (Fig. 4B,D). Moreover, Lhx1 and Shh were not detected in the caput region of the epididymis (Fig. 4B,D). By histological analyses of E18.5 fetuses, the epididymal initial segment, caput, corpus and most of the cauda were missing in mutants (Fig. 5E,F). Furthermore, we were unable to detect any tubular connection between the epididymis and the testes, indicating that the efferent ductules (circled in Fig. 5G) were also absent. To confirm that the testes themselves developed normally in T-Cre; Fgf8flox/Δ2,3 mutants, we performed immunohistochemistry using an antibody for Sertoli cell marker WT1 (Chang et al., 2008). The expression of WT1 was consistently maintained in the seminiferous tubules, which harbor the Sertoli cells, of both normal and mutant mouse testes (Fig. 4G,H, black arrows). These studies in summary then indicate that most of the ductal system of the male reproductive tract is developmentally abnormal in the absence of Fgf8 expression, whereas the testes are unaffected.
Fig. 4.
The male reproductive tract ductal system is deficient in T-Cre; Fgf8flox/Δ2,3 mice. (A-D) Male reproductive tissues from E18.5 normal (A,C) and mutant T-Cre; Fgf8flox/Δ2,3 (B,D) mice show incomplete development of the epididymis and vas deferens. (A-D) Whole-mount in situ hybridization of normal (A,C) and T-Cre; Fgf8flox/Δ2,3 (B,D) male embryos at E18.5 using Shh (A,B) or Lhx1 (C,D) probes. (E,F) Hematoxylin and Eosin staining sections (×40) of normal (E) or T-Cre; Fgf8flox/Δ2,3 (F) male embryos at E18.5. (G,H) Immunohistochemistry targeting WT1 in Sertoli cells/seminiferous tubules (black arrows) was carried out in normal (G) or T-Cre; Fgf8flox/Δ2,3 (H) male embryos at E18.5. A black circle in G identifies the efferent ductules, which were not observed in mutants. Scale bars: 100 μm. ep, epididymis; t, testis; sv, seminal vesicle; vd, vas deferens.
Fig. 5.
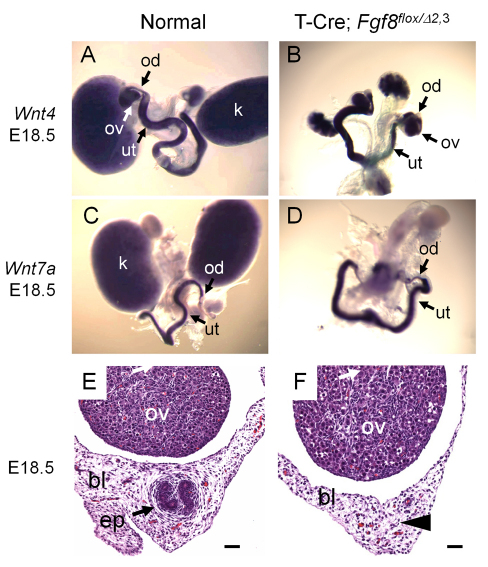
Female reproductive tract tissue, epoöphoron, is absent in mutant T-Cre; Fgf8flox/Δ2,3 mice. (A-D) Whole-mount in situ hybridization of female reproductive tissues from neonatal control (A,C) and T-Cre; Fgf8flox/Δ2,3 (B,D) mice showing normal development of ovary, oviduct and uterus using Wnt4 (A,B) or Wnt7a (C,D) probes. (E,F) Hematoxylin and Eosin stained sections of the broad ligament (×200) in normal (E) and T-Cre; Fgf8flox/Δ2,3 (F) female E18.5 embryos. An arrow labels the epoöphoron in the normal control (E). In T-Cre; Fgf8flox/Δ2,3 mice, the epoöphoron was absent (arrowhead in F). Scale bars: 20 μm. bl, broad ligament; ep, epoöphoron; k, kidney; ov, ovary; od, oviduct; ut, uterus.
Role of Fgf8 in the development of the female reproductive tract
We next examined the female reproductive tract tissues. Consistent with the result that the Müllerian duct had no gross malformation in T-Cre; Fgf8flox/Δ2,3 mice, the ovary, oviduct and uterus developed normally in these mice. Wnt4 and Wnt7a are expressed normally in the female reproductive tract (Heikkila et al., 2001; Vainio et al., 1999) in mutant mice (Fig. 5A-D). These ovaries also showed no abnormalities histologically (supplementary material Fig. S1). The only observed difference was the loss in mutant females of the vestigial and presumed nonfunctional epoöphoron (Fig. 5E,F), which is a remnant of the mesonephros and a homolog of the epididymis in males. This is consistent then with observations in mutant male mice.
Lhx1 functions downstream of Fgf8 in the mesonephros
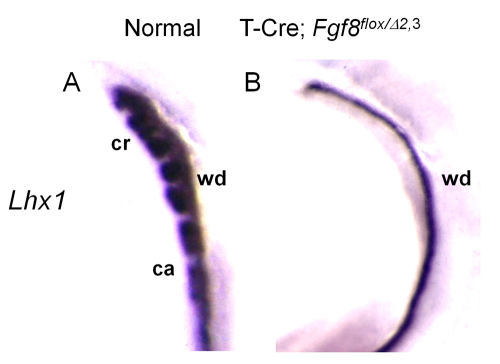
To assess the molecular effects of Fgf8 loss on mesonephric development, we evaluated the expression of genes that have been implicated in this process. Lhx1 has been shown to function downstream of Fgf8 in nephrogenesis (Perantoni et al., 2005). Moreover, the conditional loss of Lhx1 expression in the WD results in defects in male reproductive tract development, including the absence of major regions of the epididymis and vas deferens (Kobayashi et al., 2005). This phenotype is quite similar to that observed in T-Cre; Fgf8flox/Δ2,3 mice. Therefore, Lhx1 might function downstream of Fgf8 in the mesonephros as in the metanephros. To further examine this issue, we performed whole-mount in situ hybridization using a Lhx1 probe. Lhx1 was expressed in the WD in both normal and mutant E10.5 embryos, but expression was missing from the remaining caudal mesonephric tubules in mutants (Fig. 6A,B), suggesting that Lhx1 also functions downstream of Fgf8 in these structures. As the cranial mesonephric tubules are not formed in mutant mice, it is not possible to address this directly.
Fig. 6.
Lhx1 functions downstream of Fgf8 in the mesonephros. (A,B) Whole-mount in situ hybridization of normal (A) and T-Cre; Fgf8flox/Δ2,3 (B) embryos at E10.5 using a Lhx1 probe. Lhx1 expression is lost even from the caudal mesonephric tubules, which are retained in mutant embryos (see Fig. 2G,H). ca, caudal mesonephric tubules; cr, cranial mesonephric tubules; wd, Wolffian duct.
Both FGFR1 and FGFR2 mediate signaling in male reproductive tract development
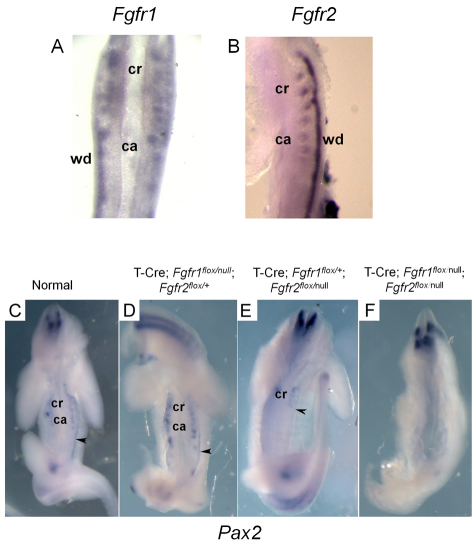
We next investigated the expression of FGF receptors (FGFRs) in the mesonephros by whole-mount in situ hybridization using probes for Fgfr1, Fgfr2, Fgfr3 and Fgfr4. Fgfr1 and Fgfr2 were expressed in both mesonephric tubules and WD (Fig. 7A,B); however, Fgfr1 was barely detectable in both structures by this method. Fgfr3 and Fgfr4 were not detected (data not shown). Ablation of Fgfr2 in the WD/ureteric bud via Hoxb7-Cre activity (Zhao et al., 2004) results in reduced branching morphogenesis of the bud in the metanephros. Moreover, mice with both Fgfr1 and Fgfr2 conditionally inactivated in the nephrogenic cord using Pax3-Cre lack mesonephric tubules (Poladia et al., 2006). As this phenotype is also observed in our T-Cre; Fgf8flox/Δ2,3 mice, FGFR1 and FGFR2 might serve as the mediators for FGF8 signaling in the mesonephros.
Fig. 7.
Fgfr1 and Fgfr2 are both involved in maintaining tissues of the urogenital tract. (A,B) Fgfr1 (A) or Fgfr2 (B) are expressed in the mesonephric tubules and Wolffian duct. (C-E) Normal (C), Fgfr1-ablated (D) and Fgfr2-ablated (E) tissues contain the Wolffian duct (black arrowhead) and mesonephric tubules, as demonstrated by whole-mount in situ hybridization with a Pax2 probe. Fgfr2-ablated (E) tissues maintain cranial but not caudal mesonephric tubules. Note that, although T-Cre; Fgfr1flox/null; Fgfr2flox/+ and T-Cre; Fgfr1flox/+; Fgfr2flox/null embryos are shown in D,E, identical phenotypes were observed for the simpler genotypes, i.e. T-Cre; Fgfr1flox/null and T-Cre; Fgfr2flox/null. (F) Ablation of both Fgfr1 and Fgfr2 results in loss of the Wolffian duct and all mesonephric tubules. ca, caudal mesonephric tubules; cr, cranial mesonephric tubules; wd, Wolffian duct.
To assess the functional involvement of Fgfr1 and Fgfr2 in male reproductive tract development, we used floxed conditional loss-of-function alleles for each receptor, and inactivated the gene(s) simultaneously in both the WD and mesonephric tubules with T-Cre. In normal and T-Cre; Fgfr1flox/null; Fgfr2flox/+ or T-Cre; Fgfr1flox/+; Fgfr2flox/null mutant E10.5 embryos, expression of either Pax2 (Fig. 7C-E) or Lhx1 (not shown) revealed that the WD forms normally and contains no cranial truncation. Furthermore, both cranial and caudal mesonephric tubules were evident in Fgfr1 mutants. However, in T-Cre; Fgfr1flox/+; Fgfr2flox/null (Fig. 7E) and T-Cre; Fgfr2flox/null (not shown) embryos, only the cranial mesonephric tubules were observed. In embryos in which both receptors had been ablated, the WD and the mesonephric tubules were not detected, as demonstrated by the total absence of Pax2 expression in the urogenital region (Fig. 7F). Our studies suggest that both Fgfr1 and Fgfr2 are involved in the development of the male reproductive tract ductal system. Moreover, the fact that the phenotype with ablation of both receptors is significantly worse for the WD than with the loss of Fgf8 may indicate that an additional FGF family member is required to maintain the caudal region of the WD.
Ret is not required for the cranial outgrowth of the Wolffian duct
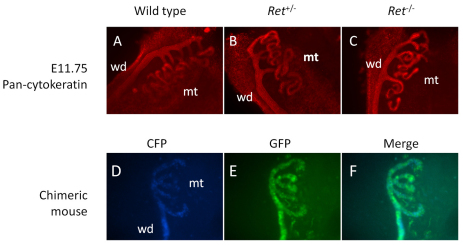
The GDNF-receptor Ret is detected in the WD (Costantini and Shakya, 2006; Schuchardt et al., 1996), and, in Ret–/– mice, outgrowth of the ureteric bud from the WD does not occur, resulting in renal agenesis (Schuchardt et al., 1996). Moreover, in Ret–/– ↔ wild-type chimeric embryos, the cells lacking Ret expression fail to contribute to ureteric bud morphogenesis (Chi et al., 2009). As cranial mesonephric tubules are also formed, in part, from outgrowths from the WD, we speculated that Ret might regulate the formation of these structures as in the metanephros. However, observation of the mesonephros in E11.75 Ret-null embryos by pan-cytokeratin immunohistochemistry indicated that there were negligible differences relative to normal embryos in the formation of the cranial mesonephros (Fig. 8A-C). To confirm this, chimeric mice were generated by injecting Ret–/– ES cells, bearing the WD marker Hoxb7-GFP, into wild-type embryos (Shakya et al., 2005). Both Ret–/– (green, GFP+) cells and wild-type cells (blue, CFP+) contributed equally to mesonephric tubule formation (Fig. 8D-F), unlike the ureteric bud where Ret–/– cells do not contribute to the bud tips (Chi et al., 2009; Shakya et al., 2005). These results indicate that Ret does not mediate cranial mesonephric tubule outgrowth.
Fig. 8.
Ret does not contribute to cranial mesonephric tubular outgrowths. (A-C) Immunofluorescence of wild-type (A), Ret+/– (B) or Ret–/– (C) mice at E11.75 using pan-cytokeratin antibody. (D-F) Mesonephros of chimeric mice shown using Ret–/–; Hoxb7/GFP ES cells in host embryos expressing CFP under Hoxb7 regulation. Wild-type CFP+ cells (D) and Ret–/– GFP+ cells (E) contribute equally to the mesonephric tubules (F, merged CFP and GFP). wd, Wolffian duct; mt, mesonephric tubules.
Fgf8 expression is independent of Wolffian duct-derived tubular contributions
It has already been demonstrated that T-Cre activity results in the recombination of floxed target sequences in the lineages of the mesonephric tubules and WD (Perantoni et al., 2005). As outgrowths from the duct contribute to a region of the cranial mesonephric tubules, we sought to exclude this tissue as a source of Fgf8 expression in the anterior mesonephros. To accomplish this, we examined Fgf8 expression and deletion using an AP2-Cre line, which is active in the WD lineage. AP2-Cre is expressed in the WD and tubular outgrowths from the WD but not in mesonephric tubular elements believed to originate from the nephrogenic cord (supplementary material Fig. S2A,B). In AP2-Cre; Fgf8flox/Δ2,3 mice, Fgf8 expression was preserved in the mesonephric tubules (supplementary material Fig. S2C,D). Moreover, the formation of the male reproductive tract ductal system was also normal in AP2-Cre; Fgf8flox/Δ2,3 mice (supplementary material Fig. S2E,F). These data indicate that Fgf8 expression in mesonephric tubules is not the result of contributions from WD progenitors.
Fgf8 expression in cranial mesonephric tubules is essential for formation of the efferent ductules but not for the cranial aspect of the Wolffian duct
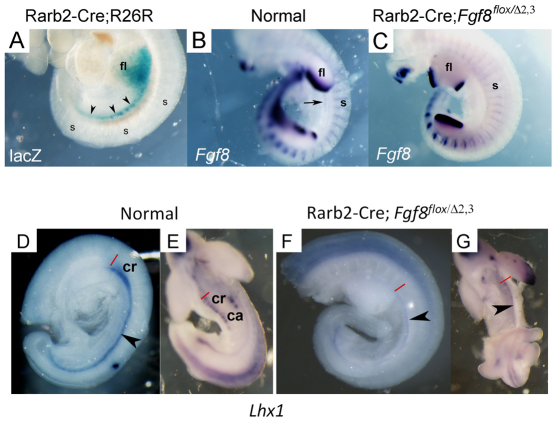
Although Fgf8 is expressed in the mesonephric tubules, the mesonephros itself is immediately juxtaposed to the somites, which also produce FGF8 and could contribute at this stage in development. To determine the relevant source(s), Fgf8 was ablated specifically in the mesonephric tubules using Rarb2-Cre, which targets the nephrogenic cord. In Fig. 9A, we confirm that Rarb2-Cre is active in the urogenital tract/mesonephros (lacZ+ staining) but not in the somites using the Rosa26R Cre reporter mouse (Soriano, 1999). Furthermore, in E10.5 Rarb2-Cre; Fgf8flox/Δ2,3 mutants, Fgf8 expression is lost in the urogenital tract (black arrow in B indicates position in normal embryo) but not in the somites (Fig. 9B,C). An assessment of these mutants by whole-mount in situ hybridization using a Lhx1 probe (Fig. 9D-G) demonstrated that, in contrast to defects caused by T-Cre-mediated Fgf8 inactivation, in Rarb2-Cre; Fgf8flox/Δ2,3 mutants, the cranial end of the WD (black arrowhead) formed normally; however, the mesonephric tubules were not detected. This early phenotype is consistent with the grossly normal epididymis observed in E18.5 mutant males (Fig. 10A,B); however, the mass of smaller tubules forming the efferent ductules is apparently missing from the mutants (Fig. 10C,D). Histological examination revealed the well formed epididymis but apparent complete loss of the efferent ductules in mutants (Fig. 10E-H). Serial sections of the reproductive tract seem to indicate that the small tubules that comprise the initial segment are formed normally in these mutants (supplementary material Fig. S3A,B). The rete testis also appears grossly normal in mutant testes, despite the deficiency, suggesting it forms independent of its interface, the efferent ductules. These findings demonstrate that FGF8 signaling from the mesonephric tubules as well as an additional tissue source, most likely the somites due to their proximity, are both required for proper patterning of the male reproductive tract ductal system.
Fig. 9.
E10.5 Rarb2-Cre; Fgf8flox/Δ2,3 embryos fail to develop mesonephric tubules. (A) β-Galactosidase staining of Rarb2-Cre; Rosa26R embryos at E10.5. (B,C) Rarb2-Cre; Fgf8flox/Δ2,3 mice lose expression of Fgf8 in the mesonephric tubules but not in the somites. An arrow labels the mesonephric tubules. (D-G) Whole-mount in situ hybridization of normal (D,E) and Rarb2-Cre; Fgf8flox/Δ2,3 (F,G) embryos at E10.5 using a Lhx1 probe. The cranial region (red line at rostral end) of the Wolffian duct (black arrowhead) forms normally in mutants but the mesonephric tubules are lost. ca, caudal mesonephric tubules; cr, cranial mesonephric tubules; fl, forelimb; s, somites.
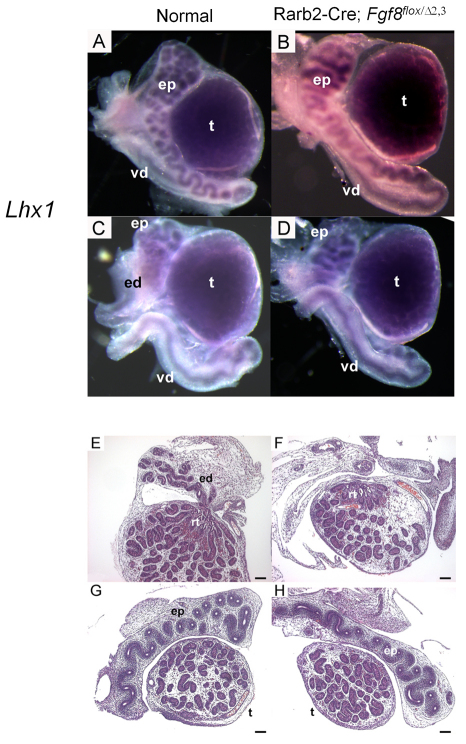
Fig. 10.
E18.5 Rarb2-Cre; Fgf8flox/Δ2,3 male reproductive tracts lack efferent ductules. (A-D) Whole-mount in situ hybridization of normal (A,C) and mutant (B,D) reproductive tracts using a Lhx1 probe shows no deficiencies in the development of the epididymis or vas deferens, but the small tubular network that constitutes the efferent ductules (C) is missing in the mutant (D). (E-H) This is confirmed by histology. ed, efferent ductules; ep, epididymis; rt, rete testis; t, testis; vd, vas deferens. Scale bars: 100 μm.
DISCUSSION
Our studies have determined that Fgf8 is necessary for proper patterning of the male reproductive tract, regulating the development of the efferent ductules, epididymis and vas deferens. It achieves this through the growth and maintenance of the cranial region of the WD along with the associated mesonephric tubules, both of which provide progenitors for these tissues. Furthermore, FGF8 signaling is provided from distinct embryonic tissues. Using Rarb2-Cre, we were able to demonstrate the essential nature of Fgf8 expression in the mesonephric tubules for forming the efferent ductules. By exclusion, our studies implicate the somites as the other FGF8 source required for male reproductive tract development, as it provides the only alternative site within the immediate proximity of the cranial region of the WD. In chick, undefined signals from the paraxial mesoderm/somites drive the expression of Pax2 and Lhx1 in the intermediate mesoderm, and also induce the formation of the pronephros (Mauch et al., 2000), so this is the likely source. Our studies indicate that the undefined signal in chick tissues is probably FGF8 or at least a member of the FGF family. Unfortunately, Cre lines developed to target the paraxial mesoderm are generally also active in the intermediate mesoderm, e.g. Dll1-Cre (Wehn et al., 2009), or are only active in limited populations within the paraxial mesoderm, e.g. Ripply2-Cre and the anterior presomitic mesoderm (Terry Yamaguchi, personal communication), so specifically ablating Fgf8 from the somites alone is not possible at this time.
It is clear from studies of lineage that are based upon gene expression/reporter patterns, including those for Hoxb7 (Kobayashi et al., 2005), Wt1 (Sainio et al., 1997), Wnt9b (Carroll et al., 2005), and now AP2-Cre, that the WD contributes to the formation of the cranial mesonephric tubules. However, the stubby cranial outgrowths from the WD must provide only a small part of the lengthy coiled cranial mesonephric tubule overall, with the remainder presumed to originate from the nephrogenic cord-derived mesonephric vesicles/tubules adjacent to the WD (Gumpel-Pinot et al., 1971; Mugford et al., 2008; Sainio et al., 1997). It is in these periodic tubular structures that line the WD in which FGF8 is produced in the mesonephros. Such a scheme is comparable with the development of the metanephros where the caudal end of the WD is stimulated to form an epithelial outgrowth, i.e. the ureteric bud, which eventually connects with nephrogenic cord-derived renal vesicles to produce nephrons and the collecting duct system. In the case of the male reproductive tract tissues, three to five efferent ductules interface with the rete testis in the mouse testes. A similar number of cranial mesonephric tubules are observed at E10.5, and it is therefore likely that these FGF8-induced cranial outgrowths from the WD are providing the interface between the WD-derived epididymis and nephrogenic cord-derived efferent ductules.
The expansion and coiling of the epididymis resembles that of the developing tubular epithelia of the nephron in that both processes require Fgf8 and involve growth/elongation; however, the mechanisms probably differ as FGF8 may not be continuously present in the mesonephros because it is in the metanephros. The expression of Fgf8 in the mesonephros is restricted to the mesonephric tubules and is relatively short lived, appearing in these structures when they arise at E9.5 and disappearing by E11.5. In the developing kidney, Fgf8 persists in newly formed nephronic epithelia and is expressed late in more mature cell populations such as the podocytes. Its targeted loss in mesoderm, however, halts development with formation of the renal vesicle (Perantoni et al., 2005), so it must function in both the expansion and convolution of the nephron. In the male reproductive tract, Fgf8 expression ceases long before initiation of coiling in the efferent ductules and epididymis, which begins around E14. Although testosterone stabilizes these structures in males, expression of androgen receptors is not observed in the efferent ductules in the mouse until E16 and even later in the epididymis (Cooke et al., 1991), so it also is unlikely to be involved directly. However, androgen receptors are expressed in the surrounding stroma as early as E13, so there could be indirect effects from androgen signaling that would help maintain male reproductive tract tissues, perhaps through expression of another FGF family member, such as Fgf2 (Lan et al., 1998) or Wnt9b, which is also required for development of these tissues and which functions upstream of Fgf8 expression (Carroll et al., 2005). Alternatively, T-Cre also eliminates expression of Fgf8 from the adjacent mesoderm-derived somites, which could provide a constitutive source of this factor throughout organogenesis.
It is widely believed that, while the efferent ductules develop from the mesonephric tubules, the epididymis, vas deferens and seminal vesicles are derived primarily from the WD (Ilio and Hess, 1994; Marshall et al., 1979; Takeuchi, 1992). Consistent with this, we showed that the epididymis, vas deferens and efferent ductules are deficient in T-Cre; Fgf8flox/Δ2,3 mice due to the malformation of the mesonephric tubules and cranial WD. However, the seminal vesicles, which are also believed to originate from the WD, developed normally in T-Cre; Fgf8flox/Δ2,3 mice (data not shown). Studies have demonstrated that, although the vas deferens develop from the middle segment of WD, the seminal vesicles originate from a more caudal segment (Ilio and Hess, 1994). Furthermore, it has been demonstrated that Fgf10 is essential to maintain the seminal vesicles during development (Donjacour et al., 2003; Thomson and Cunha, 1999), is expressed primarily around the caudal region of the WD in urogenital tissues (Thomson and Cunha, 1999) and requires FGFR1/2 for its signaling (Powers et al., 2000). Our results show that although the cranial region of the WD is absent, the caudal WD develops normally in T-Cre; Fgf8flox/Δ2,3 mice, which may explain why the seminal vesicles also develop normally in these mice.
Studies have implicated other genes in the development of mesonephric tubules. Targeted deletion of Fgfr1/2 in the nephrogenic cord using Pax3-Cre results in the loss of mesonephric tubules (Poladia et al., 2006). This is consistent with our current studies, which further demonstrate that FGF8 is a principal mediator of FGF signaling in the mesonephros and suggest that this growth factor is involved in an autocrine fashion to sustain the cells responsible for FGF8 production. As observed for T-Cre; Fgf8flox/Δ2,3 mice, Wnt9b-null mice also do not form mesonephric tubules (Carroll et al., 2005), nor do they develop a male reproductive tract ductal system. Interestingly, as these mice fail to express Fgf8 in metanephric mesenchyme, the authors conclude that Wnt9b, which is secreted by the ureteric bud, acts upstream of FGF8 signaling. This may indicate that Wnt9b also functions upstream of FGF8 signaling in the mesonephros, where it is expressed by the ureteric bud precursor – the WD. Pax2-deficient mice also lack mesonephric tubules (Torres et al., 1995). However, our studies show that Pax2 expression in T-Cre; Fgf8flox/Δ2,3 mice is preserved in some of these tubules (Fig. 2G,H), suggesting that Pax2 is expressed independently and possibly upstream of FGF8 signaling.
The mechanism by which FGF8 functions in the mesonephros may well entail the activation of homeodomain gene Lhx1. Deletion of this transcription factor in the WD yields a similar phenotype in the male reproductive tract as observed with the loss of Fgf8 in the mesonephros, suggesting that FGF8 signaling occurs upstream of Lhx1 expression (Kobayashi et al., 2005). Furthermore, Lhx1 expression was lost from mesonephric tubules with conditional deletion of Fgf8 expression in the mesonephros. This is also consistent with our prior findings in the developing nephron (Perantoni et al., 2005) for which we observed that Lhx1 is downregulated in renal progenitors deficient in FGF8 and that FGF8 induces Lhx1 expression in metanephric mesenchyme.
During development, the Müllerian duct is closely associated with the WD, i.e. the caudal tip of the Müllerian duct is physically in contact with the WD (Orvis and Behringer, 2007). As a result of this association, there is a possibility that the cells from the WD contribute to formation of the Müllerian duct. This belief was supported by studies demonstrating that experimental interruption of growth of the WD blocked the complete formation of the Müllerian duct at the point of the blockade in WD extension (Grünwald, 1937). More recent studies, however, suggest that the WD may not contribute cells to the Müllerian duct, but instead serves as a guide for its formation and extension (Dohr et al., 1987). Efforts to clarify the operative mechanism through lineage-tracing studies, using a WD-specific Cre reporter, demonstrate that the WD apparently does not provide progenitors for Müllerian duct formation (Orvis and Behringer, 2007). Instead, it is more likely to provide signals, such as secretion of WNT9b (Carroll et al., 2005), which sustain growth in and guide the extension of the Müllerian duct. In our studies, we show that Fgf8 regulates the formation of the WD but not the Müllerian duct. In spite of the defect in the WD, the Müllerian duct developed normally in T-Cre; Fgf8flox/Δ2,3 mice. This may indicate that, despite its close association, development of the Müllerian duct is independent of signals from the cranial region of the WD, but further study is needed to confirm this.
The Hoxb7-Cre line is commonly applied to studies of the urogenital tract as it is expressed in the WD but not the nephrogenic cord (Kobayashi et al., 2005; Yu et al., 2002). Our current results show that AP2-Cre may provide an alternative to the Hoxb7-Cre line in studies that require targeting of the WD, as there is strong activation of a Cre reporter via this transgene throughout the duct but not in the cord. Pax3-Cre may be useful for specifying the posterior end of the mesonephros, especially the caudal mesonephric tubules, because it is not active in the anterior region of the mesonephros (J.K., unpublished) (Jarad and Miner, 2009). On the other hand, the Rarb2-Cre line seems well suited to address research questions related to development at the cranial end of the mesonephros as a complement to Pax3-Cre. Because of their unique expression patterns, all three lines may be of value for further analyses of development in the mesonephros.
It is intriguing that expression of Fgf8 in the somites may regulate formation of structures in the intermediate mesoderm. Although only the cranial end of the WD is affected in the current studies, the fact that ablation of both Fgfr1 and Fgfr2 resulted in the complete loss of the WD suggests that another FGF family member may be essential in generating and maintaining that tissue. Recently, Fgf4 and Fgf8 were shown to be redundant in maintaining somitogenesis in the developing mouse embryo (Naiche et al., 2011). It is conceivable that both factors together also promote formation of the WD in the intermediate mesoderm. Alternatively, FGF8 may function in conjunction with FGF10, which maintains the caudal end of the WD. The overlapping expression domains would then preserve formation of the majority of the structure even with the loss of one of these factors. These speculative models are readily testable with existing genetic tools, and such studies are ongoing.
Supplementary Material
Acknowledgments
We thank Drs Seppo Vainio and Richard R. Behringer for providing plasmids and probes, and Gian L. Perantoni, Usama Qadri and Sergey Plisov for excellent technical assistance. We also thank Drs Norman Rosenblum and Richard Behringer for the Rarb2-Cre line, and Dr Trevor Williams for the Ap2-Cre line. We are grateful Dr Barry Hinton for the critical review of our manuscript and to the NIH Fellows Editorial Board for helpful discussions and revision.
Footnotes
Funding
This work was supported by the National Institutes of Health, National Cancer Institute and Center for Cancer Research. J.K. and Y.U. were supported in part by the Japan Society for the Promotion of the Science. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.051888/-/DC1
References
- Bitgood M. J., McMahon A. P. (1995). Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172, 126–138 [DOI] [PubMed] [Google Scholar]
- Bouchard M., Souabni A., Mandler M., Neubuser A., Busslinger M. (2002). Nephric lineage specification by Pax2 and Pax8. Genes Dev. 16, 2958–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B. (2000). The battle of the sexes. Mech. Dev. 92, 89–103 [DOI] [PubMed] [Google Scholar]
- Carroll T. J., Park J. S., Hayashi S., Majumdar A., McMahon A. P. (2005). Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 [DOI] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P., et al. (1986). Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell 45, 685–698 [DOI] [PubMed] [Google Scholar]
- Chang H., Gao F., Guillou F., Taketo M. M., Huff V., Behringer R. R. (2008). Wt1 negatively regulates beta-catenin signaling during testis development. Development 135, 1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Michos O., Shakya R., Riccio P., Enomoto H., Licht J. D., Asai N., Takahashi M., Ohgami N., Kato M., et al. (2009). Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. S., Young P., Cunha G. R. (1991). Androgen receptor expression in developing male reproductive organs. Endocrinology 128, 2867–2873 [DOI] [PubMed] [Google Scholar]
- Costantini F., Shakya R. (2006). GDNF/Ret signaling and the development of the kidney. BioEssays 28, 117–127 [DOI] [PubMed] [Google Scholar]
- Dohr G., Tarmann T., Schiechl H. (1987). Different antigen expression on Wolffian and Mullerian cells in rat embryos as detected by monoclonal antibodies. Anat. Embryol. 176, 239–242 [DOI] [PubMed] [Google Scholar]
- Donjacour A. A., Thomson A. A., Cunha G. R. (2003). FGF-10 plays an essential role in the growth of the fetal prostate. Dev. Biol. 261, 39–54 [DOI] [PubMed] [Google Scholar]
- Drawbridge J., Meighan C. M., Lumpkins R., Kite M. E. (2003). Pronephric duct extension in amphibian embryos: migration and other mechanisms. Dev. Dyn. 226, 1–11 [DOI] [PubMed] [Google Scholar]
- Ehrenfels C. W., Carmillo P. J., Orozco O., Cate R. L., Sanicola M. (1999). Perturbation of RET signaling in the embryonic kidney. Dev. Genet. 24, 263–272 [DOI] [PubMed] [Google Scholar]
- Fujii T., Pichel J. G., Taira M., Toyama R., Dawid I. B., Westphal H. (1994). Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev. Dyn. 199, 73–83 [DOI] [PubMed] [Google Scholar]
- Grote D., Souabni A., Busslinger M., Bouchard M. (2006). Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133, 53–61 [DOI] [PubMed] [Google Scholar]
- Grünwald P. (1937). Zur Entwicklungsmechanik des Urogenitalsystems beim Huhn. Arch. Entwicklungsmech. 136, 786–813 [DOI] [PubMed] [Google Scholar]
- Gumpel-Pinot M., Martin C., Croisille Y. (1971). Organogenesis of the mesonephros in birds. Realization in vitro of quail-chicken chimera mesonephros. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 272, 737–739 [PubMed] [Google Scholar]
- Hannema S. E., Hughes I. A. (2007). Regulation of Wolffian duct development. Horm. Res. 67, 142–151 [DOI] [PubMed] [Google Scholar]
- Heikkila M., Peltoketo H., Vainio S. (2001). Wnts and the female reproductive system. J. Exp. Zool. 290, 616–623 [DOI] [PubMed] [Google Scholar]
- Hinton B. T., Lan Z. J., Rudolph D. B., Labus J. C., Lye R. J. (1998). Testicular regulation of epididymal gene expression. J. Reprod. Fertil. 53 Suppl., 47–57 [PubMed] [Google Scholar]
- Ilio K. Y., Hess R. A. (1994). Structure and function of the ductuli efferentes: a review. Microsc. Res. Tech. 29, 432–467 [DOI] [PubMed] [Google Scholar]
- Jarad G., Miner J. H. (2009). The Pax3-Cre transgene exhibits a rostrocaudal gradient of expression in the skeletal muscle lineage. Genesis 47, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanov A. A., Karavanova I., Perantoni A., Dawid I. B. (1998). Expression pattern of the rat Lim-1 homeobox gene suggests a dual role during kidney development. Int. J. Dev. Biol. 42, 61–66 [PubMed] [Google Scholar]
- Kobayashi A., Behringer R. R. (2003). Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 4, 969–980 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Shawlot W., Kania A., Behringer R. R. (2004). Requirement of Lim1 for female reproductive tract development. Development 131, 539–549 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kwan K. M., Carroll T. J., McMahon A. P., Mendelsohn C. L., Behringer R. R. (2005). Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132, 2809–2823 [DOI] [PubMed] [Google Scholar]
- Lan Z. J., Labus J. C., Hinton B. T. (1998). Regulation of gamma-glutamyl transpeptidase catalytic activity and protein level in the initial segment of the rat epididymis by testicular factors: role of basic fibroblast growth factor. Biol. Reprod. 58, 197–206 [DOI] [PubMed] [Google Scholar]
- Maeshima A., Sakurai H., Choi Y., Kitamura S., Vaughn D. A., Tee J. B., Nigam S. K. (2007). Glial cell-derived neurotrophic factor independent ureteric bud outgrowth from the Wolffian duct. J. Am. Soc. Nephrol. 18, 3147–3155 [DOI] [PubMed] [Google Scholar]
- Marshall F. F., Reiner W. G., Goldberg B. S. (1979). The embryologic origin of the caput epididymidis in the rat. Invest. Urol. 17, 78–82 [PubMed] [Google Scholar]
- Mauch T. J., Yang G., Wright M., Smith D., Schoenwolf G. C. (2000). Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev. Biol. 220, 62–75 [DOI] [PubMed] [Google Scholar]
- Miyamoto N., Yoshida M., Kuratani S., Matsuo I., Aizawa S. (1997). Defects of urogenital development in mice lacking Emx2. Development 124, 1653–1664 [DOI] [PubMed] [Google Scholar]
- Mugford J. W., Sipila P., Kobayashi A., Behringer R. R., McMahon A. P. (2008). Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev. Biol. 319, 396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche L. A., Holder N., Lewandoski M. (2011). FGF4 and FGF8 comprise the wavefront activity that controls somitogenesis. Proc. Natl. Acad. Sci. USA 108, 4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. K., Williams T. (2004). Frontonasal process-specific disruption of AP-2alpha results in postnatal midfacial hypoplasia, vascular anomalies, and nasal cavity defects. Dev. Biol. 267, 72–92 [DOI] [PubMed] [Google Scholar]
- Orvis G. D., Behringer R. R. (2007). Cellular mechanisms of Mullerian duct formation in the mouse. Dev. Biol. 306, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perantoni A. O., Timofeeva O., Naillat F., Richman C., Pajni-Underwood S., Wilson C., Vainio S., Dove L. F., Lewandoski M. (2005). Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132, 3859–3871 [DOI] [PubMed] [Google Scholar]
- Pizard A., Haramis A., Carrasco A. E., Franco P., Lopez S., Paganelli A. (2004). Whole-mount in situ hybridization and detection of RNAs in vertebrate embryos and isolated organs. Curr. Protoc. Mol. Biol. 14, Unit 14–9 [DOI] [PubMed] [Google Scholar]
- Poladia D. P., Kish K., Kutay B., Hains D., Kegg H., Zhao H., Bates C. M. (2006). Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev. Biol. 291, 325–339 [DOI] [PubMed] [Google Scholar]
- Powers C. J., McLeskey S. W., Wellstein A. (2000). Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7, 165–197 [DOI] [PubMed] [Google Scholar]
- Sainio K., Raatikainen-Ahokas A. (1999). Mesonephric kidney – a stem cell factory? Int. J. Dev. Biol. 43, 435–439 [PubMed] [Google Scholar]
- Sainio K., Hellstedt P., Kreidberg J. A., Saxen L., Sariola H. (1997). Differential regulation of two sets of mesonephric tubules by WT-1. Development 124, 1293–1299 [DOI] [PubMed] [Google Scholar]
- Sanchez M. P., Silos-Santiago I., Frisen J., He B., Lira S. A., Barbacid M. (1996). Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382, 70–73 [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D’Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. (1994). Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D’Agati V., Pachnis V., Costantini F. (1996). Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development 122, 1919–1929 [DOI] [PubMed] [Google Scholar]
- Shakya R., Watanabe T., Costantini F. (2005). The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell 8, 65–74 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Goldberg M. R., Watanabe T., D’Agati V., al-Awqati Q., Costantini F. (1999). Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev. Genet. 24, 241–251 [DOI] [PubMed] [Google Scholar]
- Takeuchi S. (1992). Differentiation and maturation of excurrent duct system of rat testes. Nippon Hinyokika Gakkai Zasshi 83, 1043–1051 [DOI] [PubMed] [Google Scholar]
- Thomson A. A., Cunha G. R. (1999). Prostatic growth and development are regulated by FGF10. Development 126, 3693–3701 [DOI] [PubMed] [Google Scholar]
- Torres M., Gomez-Pardo E., Dressler G. R., Gruss P. (1995). Pax-2 controls multiple steps of urogenital development. Development 121, 4057–4065 [DOI] [PubMed] [Google Scholar]
- Turner T. T., Bomgardner D., Jacobs J. P. (2004). Sonic hedgehog pathway genes are expressed and transcribed in the adult mouse epididymis. J. Androl. 25, 514–522 [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P. (1999). Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405–409 [DOI] [PubMed] [Google Scholar]
- Wehn A. K., Gallo P. H., Chapman D. L. (2009). Generation of transgenic mice expressing Cre recombinase under the control of the Dll1 mesoderm enhancer element. Genesis 47, 309–313 [DOI] [PubMed] [Google Scholar]
- Wilhelm D., Palmer S., Koopman P. (2007). Sex determination and gonadal development in mammals. Physiol. Rev. 87, 1–28 [DOI] [PubMed] [Google Scholar]
- Xu X., Qiao W., Li C., Deng C. X. (2002). Generation of Fgfr1 conditional knockout mice. Genesis 32, 85–86 [DOI] [PubMed] [Google Scholar]
- Yin Y., Ma L. (2005). Development of the mammalian female reproductive tract. J. Biochem. 137, 677–683 [DOI] [PubMed] [Google Scholar]
- Yu J., Carroll T. J., McMahon A. P. (2002). Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301–5312 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A., Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063–3074 [DOI] [PubMed] [Google Scholar]
- Zhao H., Kegg H., Grady S., Truong H. T., Robinson M. L., Baum M., Bates C. M. (2004). Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 276, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.