Abstract
Neural crest (NC) cells are multipotent progenitors that form at the neural plate border, undergo epithelial-mesenchymal transition and migrate to diverse locations in vertebrate embryos to give rise to many cell types. Multiple signaling factors, including Wnt proteins, operate during early embryonic development to induce the NC cell fate. Whereas the requirement for the Wnt/β-catenin pathway in NC specification has been well established, a similar role for Wnt proteins that do not stabilize β-catenin has remained unclear. Our gain- and loss-of-function experiments implicate Wnt11-like proteins in NC specification in Xenopus embryos. In support of this conclusion, modulation of β-catenin-independent signaling through Dishevelled and Ror2 causes predictable changes in premigratory NC. Morpholino-mediated depletion experiments suggest that Wnt11R, a Wnt protein that is expressed in neuroectoderm adjacent to the NC territory, is required for NC formation. Wnt11-like signals might specify NC by altering the localization and activity of the serine/threonine polarity kinase PAR-1 (also known as microtubule-associated regulatory kinase or MARK), which itself plays an essential role in NC formation. Consistent with this model, PAR-1 RNA rescues NC markers in embryos in which noncanonical Wnt signaling has been blocked. These experiments identify novel roles for Wnt11R and PAR-1 in NC specification and reveal an unexpected connection between morphogenesis and cell fate.
Keywords: Noncanonical Wnt signaling, Neural crest, Dishevelled, Xenopus, PAR-1, Microtubule-associated regulatory kinase, Cell polarity
INTRODUCTION
The neural crest (NC) comprises stem-cell-like cells that form in vertebrate embryos at the neural plate border, migrate to diverse locations in the body and differentiate into multiple cell types (Anderson, 1997; Crane and Trainor, 2006; Knight and Schilling, 2006; Le Douarin and Dupin, 2003; Sauka-Spengler and Bronner-Fraser, 2008). NC is specified by the combined action of several embryonic signaling pathways, including the Wnt, FGF, BMP and Notch pathways, and NC fates are maintained by a network of specific transcription factors. Once formed, NC cells undergo epithelial-mesenchymal transition (EMT) and migrate to many destinations in the body to contribute to diverse cell types, including face cartilage, melanocytes and the peripheral nervous system (Acloque et al., 2009; Heeg-Truesdell and LaBonne, 2004; Kuriyama and Mayor, 2008; Thiery et al., 2009; Yang and Weinberg, 2008). The large number of human diseases that are associated with NC abnormalities, including craniosynostosis, Waardenburg and Hirschsprung’s syndromes and cancers, draw considerable attention to studies of the mechanisms of NC development (Crane and Trainor, 2006; Heeg-Truesdell and LaBonne, 2004).
One pathway that is essential for NC specification in all vertebrate models examined is the Wnt pathway. Canonical Wnt signaling triggers β-catenin/TCF-dependent gene transcription and regulates cell proliferation and cell fate (Cadigan and Peifer, 2009; Clevers, 2006). The involvement of this pathway in NC formation was first established by genetic studies of Wnt1/Wnt3a double-knockout mice and in gain-of-function experiments in Xenopus (Ikeya et al., 1997; Saint-Jeannet et al., 1997), and was subsequently extended to other models (Dorsky et al., 1998; Garcia-Castro et al., 2002; Hari et al., 2002; Lewis et al., 2004; Wu et al., 2003). The transcription of many NC-specific genes, including Snail2, Snail and Twist, has been shown to depend on β-catenin/TCF (Garcia-Castro et al., 2002; Howe et al., 2003; LaBonne, 2002; Sauka-Spengler and Bronner-Fraser, 2008; Vallin et al., 2001; Wu et al., 2003), further supporting the model that NC formation involves the Wnt/β-catenin pathway.
Noncanonical Wnt ligands, such as Wnt5a and Wnt11 (Angers and Moon, 2009; van Amerongen and Nusse, 2009), do not stabilize β-catenin or activate TCF-dependent transcription, but regulate morphogenetic processes that involve changes in cell shape and motility, which are sometimes referred to as planar cell polarity (PCP) (Ciani and Salinas, 2005; Komiya and Habas, 2008; Saneyoshi et al., 2002; van Amerongen et al., 2008; Winklbauer et al., 2001). The signaling from Wnt5 or Wnt11 is thought to involve Ror and Ryk receptors (Grumolato et al., 2010; Hikasa et al., 2002a; Lin et al., 2010; Lu et al., 2004; Mikels et al., 2009; Minami et al., 2010), small Rho GTPases (Habas et al., 2003; Habas et al., 2001), Rho-associated kinase (Marlow et al., 2002; Winter et al., 2001), c-Jun N-terminal kinases (Boutros et al., 1998; Lisovsky et al., 2002; Pandur et al., 2002) and intracellular calcium (Sheldahl et al., 2003; Slusarski et al., 1997; Witze et al., 2008). Although noncanonical Wnt pathways have been shown to function in NC cell migration (Carmona-Fontaine et al., 2008; De Calisto et al., 2005; Matthews et al., 2008b), their importance for NC specification has remained unclear.
Craniofacial defects in Wnt5a knockout mice (Yamaguchi et al., 1999), and in wnt11 (silberblick) (Heisenberg et al., 2000; Heisenberg et al., 1996) and wnt5 (pipetail) (Piotrowski et al., 1996) zebrafish mutant embryos suggest possible roles for noncanonical Wnt signaling in NC development. The results of our study support the view that noncanonical signaling from Wnt11R is essential for NC specification in Xenopus embryos and that it might act by changing the localization and activity of the polarity kinase PAR-1.
PAR proteins are conserved regulators of cell polarity that interact with several embryonic signaling pathways, including the Wnt pathway (Doe and Bowerman, 2001; Goldstein and Macara, 2007; Knoblich, 2008; Ohno, 2001). PAR-1 associates with Dishevelled (Dvl, or Dsh) and participates in Frizzled-dependent Dvl recruitment (Ossipova et al., 2005; Sun et al., 2001). We show that PAR-1 is itself required for NC specification and can rescue NC defects in embryos with inhibited Wnt5 and Wnt11 signaling. These findings identify PAR-1 as a molecular target for noncanonical Wnt signaling and reveal an unexpected causal connection between cell polarization and the NC cell fate.
MATERIALS AND METHODS
DNA constructs and RNA synthesis
pCS2-Myc-PAR-1A, pCS2-Myc-PAR-1KD and GFP-PAR-1A in pXT7 have been described (Ossipova et al., 2005; Ossipova et al., 2007). Flag-PAR-1A and Flag-PAR-1A-KD have been generated by subcloning the PAR-1 coding region into the XhoI and NotI sites of pCS2-Flag (Hikasa and Sokol, 2011). Capped synthetic RNA for microinjection was generated using the mMessage mMachine Kit (Ambion) from the following DNA templates: pCS2-Myc-PAR-1A, pCS2-nucβGal (Ossipova et al., 2005), pCS2-Ror2 and pCS2-Ror2ΔC (Hikasa et al., 2002b), pSP64-XWnt11, pSP64-dnWnt11, pCS2-ΔN-Dsh, pCS2-DshDEP+ (Tada and Smith, 2000), pXT7-dnWnt5/11 (Choi and Sokol, 2009), pSP64T-XWnt5a (Moon et al., 1993), pSP64T-Xfz3 (Shi et al., 1998), pSP64T-Xwnt3a (Wolda et al., 1993) and Noggin (Lamb et al., 1993).
Embryo culture and microinjections, morpholino oligonucleotides and RT-PCR analysis
Fertilization and embryo culture were performed as described (Itoh et al., 2005). Embryos were microinjected in 1/3 × MMR containing 2% Ficoll 400 (Pharmacia) in the animal pole with 5 nl solution per blastomere at the four- to eight-cell stage, and cultured in 0.1 × MMR until the desired stages. PAR-1 morpholino (MO) (5′-TCGGCAGCGGTGTCCTGGTGGTCAT-3′), referred to as PAR-1BY MO (Ossipova et al., 2005), or control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′) was injected at 5-10 ng per blastomere. Further MOs were: PAR-1 MO2, 5′-TCATCCCGATACTGAAATTACCAAC-3′; Wnt11R MO1, 5′-CTTCATCTTCAAAACCCAATAACAA-3′; Wnt11R MO2, 5′-ACTGTATCCAAAGAGAGTTCCGAGG-3′; and Ror2 MO, 5′-ATTCTGGCTCCTGGTCCTGGACATC-3′. Wnt11R MOs were injected at 5-30 ng, whereas Ror2 MO was used at 20 ng per injection. The wnt11r:GFP construct containing the Wnt11R MO1 target sequence upstream of the GFP coding sequence was created by PCR-based mutagenesis in the pXT7 vector as described (Itoh et al., 2005).
For RT-PCR, total RNA was extracted from whole embryos and animal cap explants using RNeasy columns (Qiagen) and treated with RNase-free DNase I to remove genomic DNA. cDNAs were prepared using the Superscript First-Strand Synthesis System (Invitrogen). PCR primers for Twist, Snail2 (Mayor et al., 1995), MyoD, EF1α (Kibardin et al., 2006), Sox3 (Chalmers et al., 2002), Pax3 and Sox8 (de Croze et al., 2011) have been described. FoxD3 primers were: forward, 5′-TCCATCATCAAGTCTGAGCC-3′; reverse, 5′-ATAGTTGACGTGTTACCTGC-3′. The number of PCR cycles for each primer pair was determined empirically to maintain amplification in the linear range.
In situ hybridization, lineage tracing and immunocytochemistry
In situ hybridization and X-Gal staining were carried out using standard techniques (Harland, 1991) with the following antisense probes: FoxD3 (Sasai et al., 2001), Sox2 (Mizuseki et al., 1998), Slug/Snail2 (Mayor et al., 1995), Sox8 (O’Donnell et al., 2006), Sox9 (Cheung and Briscoe, 2003), MyoD (Hopwood et al., 1989), AP2 (Luo et al., 2002) and epidermal type I keratin XK70 (Winkles et al., 1985). Pax3 and Hairy2a antisense probes were generated with T7 RNA polymerase from pXT7-Xpax3 (GenBank accession number BC108573) and pBluescript-Xhairy2a (Shibata et al., 2005). Results have been quantified as a percentage of embryos with the described phenotypic change.
For cryosections, RNAs encoding Myc- or GFP-tagged proteins were injected into the animal region of four-cell albino embryos. Embryos were manually devitellinized at stage 10.5 and fixed in Dent’s solution for 2 hours. Indirect immunofluorescence on cryosections was performed essentially as described (Fagotto and Gumbiner, 1994). Embryos were cryosectioned using a Leica CM3050 cryostat. Images were digitally acquired on a Zeiss Axiophot microscope. The following antibodies were used: anti-GFP (B2, Santa-Cruz, 1:200), rabbit anti-occludin [a gift of S. Citi (Cordennonsi et al., 1997)] and anti-Myc (9E10) monoclonal antibodies as hybridoma supernatants (Roche, 1:50). Secondary antibodies were conjugated with Alexa Fluor 488 (Molecular Probes, 1:200). Imaging was performed on a Zeiss Axiophot microscope with the Apotome attachment at 400× magnification. A representative section of an experimental group containing 10-15 embryos is shown.
Immunoprecipitation, western analysis and protein purification
Immunoprecipitation (IP) and western blotting were carried out with embryo lysates essentially as described (Gloy et al., 2002; Itoh et al., 1998). The following antibodies and reagents were used: anti-Flag (M2) agarose beads (Sigma), anti-Myc monoclonal antibodies as hybridoma supernatants of 9E10 cells (Roche), anti-β-catenin 8E7 (Millipore), anti-Flag M2 (Sigma), anti-XTCF3N (Zhang et al., 2003), anti-hDvl2 (Itoh et al., 2005), anti-Tau-pS262/S356 (Seubert et al., 1995), anti-GFP (B2, Santa Cruz) and anti-tubulin (B512, Sigma).
Immune complex kinase assays of M2 agarose-precipitated Flag-PAR-1 from stage 10-11 embryo lysates are described in (Ossipova et al., 2005). Each reaction contained 1-3 μg of recombinant human Tau protein as a substrate. Human Tau in pET29b vector [a gift of P. Klein, (Hong et al., 1997)] was produced in E. coli BL21* and purified using benzonase endonuclease (Sigma) as described (Lindwall and Cole, 1984).
RESULTS
Modulation of neural crest markers by noncanonical Wnt signaling
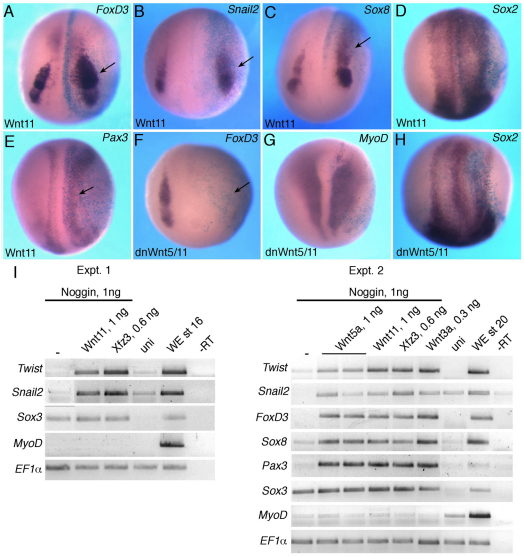
To evaluate the effects of noncanonical Wnt signaling on premigratory NC, two animal blastomeres of the four- to eight-cell embryos were injected with different doses of Wnt11 RNA. These injections were targeted to presumptive ectoderm and did not have major effects on gastrulation. The analysis of NC-specific markers at premigratory stages (stage 15/17) was carried out by in situ hybridization. Wnt11 expanded FoxD3 (61%, n=101) (Sasai et al., 2001), Snail2 (formerly Slug) (50%, n=57) (Mayor et al., 1995) and Sox8 (76%, n=13) (O’Donnell et al., 2006) in the NC territory, adjacent to the neural plate border (Fig. 1A-C). No significant decrease of the pan-neural marker Sox2 was observed (n=53), although some embryos showed slightly expanded neural plate at the injected side (Fig. 1D and supplementary material Fig. S1A), as evidenced by the shifted expression of Hairy2 (Glavic et al., 2004). Wnt11 also expanded Pax3 (57%, n=96) and AP2 (30%, n=33), which are early regulators of NC development that define the neural plate border (Bang et al., 1999; Luo et al., 2002; Monsoro-Burq et al., 2005; Sato et al., 2005) (Fig. 1E and supplementary material Fig. S1B), and reduced epidermal keratin XK70 in non-neural ectoderm (supplementary material Fig. S1C; 30%, n=12). These effects were largely restricted to the neural plate border, i.e. the prospective NC territory, as ectopic marker expression has not been detected in the neural plate or the epidermis.
Fig. 1.
Modulation of neural crest markers by noncanonical Wnt signaling. (A-H) Albino Xenopus embryos were injected animally with 0.5-1 ng Wnt11 (A-E) or dnWnt5/11 (F-H) mRNA at the four-cell stage, cultured until stage 14/16 and the expression of FoxD3 (A,F), Snail2 (B), Sox8 (C), Sox2 (D,H), Pax3 (E) and MyoD (G) analyzed by in situ hybridization. β-galactosidase is a lineage tracer (light blue) marking the injected side. Arrows point to altered marker expression. Dorso-anterior view is shown. (I) RT-PCR analysis of gene expression in neuralized animal caps. Animal caps were isolated from embryos at stage 9, injected with RNA as indicated, and cultured until stage 16/20 for RT-PCR analysis. Snail2, Twist, FoxD3, Sox8 and Pax3 are induced by Wnt signaling in the absence of significant changes in the pan-neural marker Sox3. –, no Wnt or Frizzled stimulation; uni, uninjected animal caps; WE, whole embryos; –RT, no reverse transcriptase.
We next examined NC markers in embryos in which Wnt11 function has been blocked by dominant-negative (dn) constructs. C-terminally truncated dnWnt5/11 and dnWnt11 proteins, which behave as dominant-negative mutants blocking Wnt5 and Wnt11 signaling (Choi and Sokol, 2009; Tada and Smith, 2000), strongly reduced the NC-specific marker FoxD3 (94%, n=53 for dnWnt5/11; 45%, n=33 for dnWnt11) in ectoderm (Fig. 1F and supplementary material Fig. S1D). Importantly, the pan-neural marker Sox2 (n=53) and somite-specific MyoD (n=37) did not decrease, indicating that neural induction and mesoderm formation remain unaffected (Fig. 1G,H and supplementary material Fig. S1E).
We next studied NC markers in ectoderm explants that have been neuralized by the presence of Noggin, a BMP inhibitor, essentially as described (LaBonne and Bronner-Fraser, 1998). RT-PCR analysis revealed that both Wnt11 and Wnt5a upregulated Twist and Snail2 expression at the relevant developmental stages (Fig. 1I), similar to the effect of Wnt3a and Fz3 (Deardorff et al., 2001; Saint-Jeannet et al., 1997). Wnt11 and Wnt5a injections also activated other NC markers, including FoxD3, Sox8 and Pax3. Neither Wnt protein significantly altered MyoD or the pan-neural marker Sox3, confirming our conclusion that noncanonical Wnt proteins do not affect mesoderm and neural tissue formation in these experiments (Fig. 1I). We did not observe any effects of Wnt11 on ectodermal cell proliferation at stage 12-14 using antibodies against phospho-histone H3 (data not shown). These observations reveal an essential role for Wnt5/11-like proteins in NC specification.
Lack of effect of Wnt5a and Wnt11 on canonical Wnt signaling
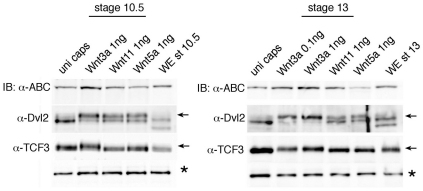
Although Wnt5 and Wnt11 proteins are usually considered β-catenin independent (Du et al., 1995), they have been reported to stabilize β-catenin under certain experimental conditions (Cha et al., 2009). We therefore assessed whether Wnt5a and Wnt11 stimulate β-catenin-dependent processes in gastrula ectoderm (Fig. 2). Consistent with published data, Wnt3a increased β-catenin levels and triggered TCF3 phosphorylation (Hikasa et al., 2010), but neither Wnt5a nor Wnt11 had this activity in ectodermal cells at stage 10/10.5 or 13/14, even at high doses of injected RNA (Fig. 2; data not shown). By contrast, all Wnt ligands were able to promote Dvl phosphorylation (Yanagawa et al., 1995), as measured by the appearance of a double band corresponding to Xenopus Dvl2. Thus, Wnt5a and Wnt11 do not stimulate canonical β-catenin-dependent signaling in gastrula ectoderm.
Fig. 2.
Wnt5a and Wnt11 do not stabilize β-catenin or trigger TCF3 phosphorylation in ectodermal cells. Xenopus embryos were injected with the indicated RNAs into animal pole blastomeres at the two-cell stage. Animal caps were isolated at stage 9 and cultured until stage 10/10.5 or stage 13/14 for western analysis (IB) with the indicated antibodies. Asterisk indicates a non-specific band that serves as a loading control. Arrows point to the position of phosphorylated TCF3 and phosphorylated Dvl. Uni, uninjected; WE, whole embryo; ABC, unphosphorylated (‘activated’) β-catenin.
Neural crest specification in embryos with manipulated Ror2 and Dvl function
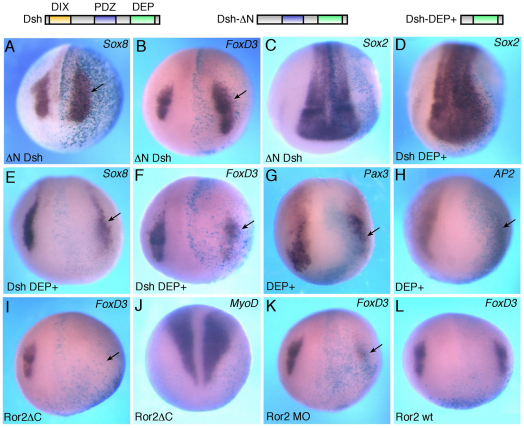
Different domains of Dvl are responsible for signaling specificity of the two main branches of the Wnt pathway (Axelrod et al., 1998; Boutros et al., 1998; Sokol, 2000; Tada and Smith, 2000). ΔN-Dsh rescues the loss of Wnt11 function in Xenopus and zebrafish embryos without affecting β-catenin signaling, whereas Dsh-DEP+ specifically inhibits the PCP-like pathway (Heisenberg et al., 2000; Tada and Smith, 2000). To examine Dvl involvement in premigratory stages of NC development, ΔN-Dsh and Dsh-DEP+ RNAs were targeted by microinjection into prospective ectoderm and their effects on NC induction assessed by in situ hybridization. ΔN-Dsh expanded the expression of FoxD3 (60%, n=48) and Sox8 (40%, n=37) at the injected side (Fig. 3A,B). By contrast, Dsh-DEP+ decreased the expression of FoxD3 (42%, n=64), Sox8 (67%, n=37) and Snail2 (58%, n=24) (Fig. 3E,F; data not shown). Dsh-DEP+ also reduced the early markers Pax3 (44%, n=45) and AP2 (66%, n=18) (Fig. 3G,H). The size of the neural plate (marked by Sox2) was not significantly affected, indicating that neural induction was normal (Fig. 3C,D). Thus, these Dvl constructs alter NC markers in a manner that is consistent with their ability to regulate noncanonical signaling.
Fig. 3.
Neural crest markers in embryos with modulated Dvl and Ror2 function. (A-H) Xenopus embryos were injected with the indicated Dvl (Dsh construct) RNAs (1 ng each) and processed for in situ hybridization as described in Fig. 1. Schematic representations of the Dvl mutant constructs are shown at the top. Dsh-ΔN activates Sox8 (A) and FoxD3 (B) but not Sox2 (C). Dsh-DEP+ slightly expands Sox2 (D) but inhibits Sox8 (E), FoxD3 (F), Pax3 (G) and AP2 (H). (I-L) The effects of Ror2 interference on NC marker expression. Ror2ΔC and Ror2MO, but not wild-type Ror2, inhibit FoxD3 (I,K,L). MyoD is not affected by Ror2ΔC (J). Arrows point to altered marker expression. Dorso-anterior view is shown.
We next aimed to elucidate which Wnt receptor mediates the observed effects and tested the involvement of Ror2, which has been implicated in Wnt5a signaling and is expressed in the NC (Grumolato et al., 2010; Hikasa et al., 2002a; Matsuda et al., 2001; Mikels and Nusse, 2006; Nishita et al., 2006; Schambony and Wedlich, 2007). We found that Ror2ΔC, a dominant-negative form of Ror2 that lacks the intracellular domain (Hikasa et al., 2002a; Mikels and Nusse, 2006), strongly inhibits FoxD3 (69%, n=106; Fig. 3I) and Sox8 (41%, n=12; supplementary material Fig. S2A). Consistent with a previous report (Hikasa et al., 2002a), Ror2ΔC did not affect MyoD or Sox2 gene expression (Fig. 3J and supplementary material Fig. S2C). Similarly to the dominant-negative construct, a morpholino oligonucleotide (MO) complementary to the 5′ untranslated region of Xenopus Ror2, but not a control MO, blocked FoxD3 expression (70%, n=50; Fig. 3K; data not shown). By contrast, wild-type Ror2 did not significantly affect the NC markers (Fig. 3L and supplementary material Fig. S2B). The lack of NC expansion in response to Ror2 possibly indicates limiting levels of endogenous Wnt ligands. Together, these observations suggest that Wnt5 and Wnt11 might act through Ror2 during NC specification in the early embryo.
Wnt11R is required for neural crest specification
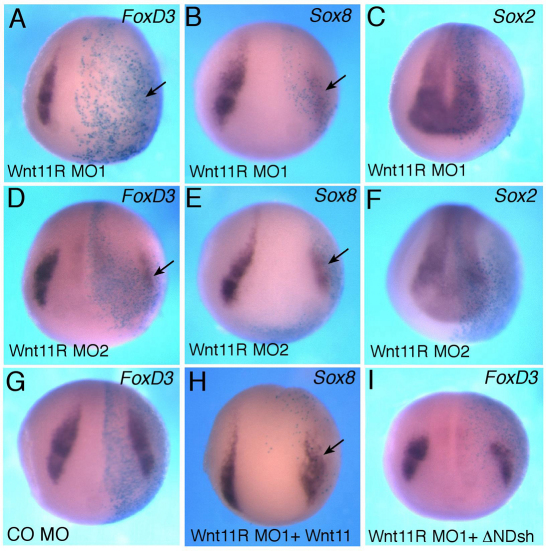
To identify a potential noncanonical Wnt ligand responsible for NC specification, we searched for Wnt proteins that are expressed in close proximity to the NC territory. One of the Wnt genes that is expressed in neuroectoderm, adjacent to the NC, is Wnt11R, which is closely related to Wnt11 (Garriock et al., 2005; Matthews et al., 2008a). Previous studies implicated Wnt11R in the formation of tail fin, a NC derivative, and in NC migration (Garriock et al., 2005; Garriock and Krieg, 2007; Matthews et al., 2008a). Since the previously used Wnt11R MO (Garriock et al., 2005) has three mismatches with the Wnt11R cDNA sequence from public databases (GenBank accession number AY695415) and would be predicted to confer only a partial phenotype, we designed two new MOs that are expected to block the translation of Wnt11R RNA and assessed their effects on the premigratory NC population (Fig. 4 and supplementary material Fig. S1F, Figs S3, S4). Wnt11R MO1 efficiently inhibited the in vivo translation of an mRNA containing the 5′UTR sequence of Wnt11R fused to the GFP coding sequence (supplementary material Fig. S3). Wnt11R MO1, but not the control MO, interfered with FoxD3 and Sox8 expression at the injected side in a dose-dependent manner with high penetrance (>75%; n=90 for FoxD3, n=30 for Sox8; Fig. 4A,B). Wnt11R MO2, with non-overlapping sequence, had similar effects on FoxD3 (61%, n=39) and Sox8 (92%, n=25) (Fig. 4D,E). The Wnt11R MOs did not inhibit Sox2, but we have observed some expansion of Sox2 on the injected side (Fig. 4C,F; data not shown). We also noticed that Wnt11R depletion resulted in the partial inhibition of Pax3 and AP2 (supplementary material Figs S1, S4), whereas a control MO injected at the same dose did not affect these markers. The partial effect suggests that Pax3 and AP2 might be under the control of multiple Wnt ligands or other signaling pathways.
Fig. 4.
Wnt11R is essential for neural crest specification. (A-F) Xenopus embryos were injected with the indicated MOs or RNAs and processed for in situ hybridization as described in Fig. 1. Wnt11R MO1 (A-C) and MO2 (D-F) inhibit FoxD3 (A,D) and Sox8 (B,E) but do not significantly affect Sox2 (C,F). (G) Control MO (CO MO) does not alter FoxD3. (H,I) Wnt11 and ΔN-Dsh RNAs partially suppress the inhibitory effect of Wnt11R MO1 on Sox8 (H) and FoxD3 (I).
As an additional control for specificity, Wnt11R MO1 was co-injected with Wnt11 or ΔN-Dsh RNAs. We observed that both RNAs were able to partially rescue the NC defect. About 50% of embryos co-injected with 10 ng of Wnt11R MO1 and 1 ng of Wnt11 RNA expressed Sox8 (n=98), whereas approximately one-third of embryos co-injected with Wnt11R MO1 and ΔN-Dsh expressed FoxD3 (n=122) (Fig. 4H,I). These experiments suggest that Wnt11R functions at the neural plate border to induce the NC fate.
Wnt11 signaling influences PAR-1 localization and activity
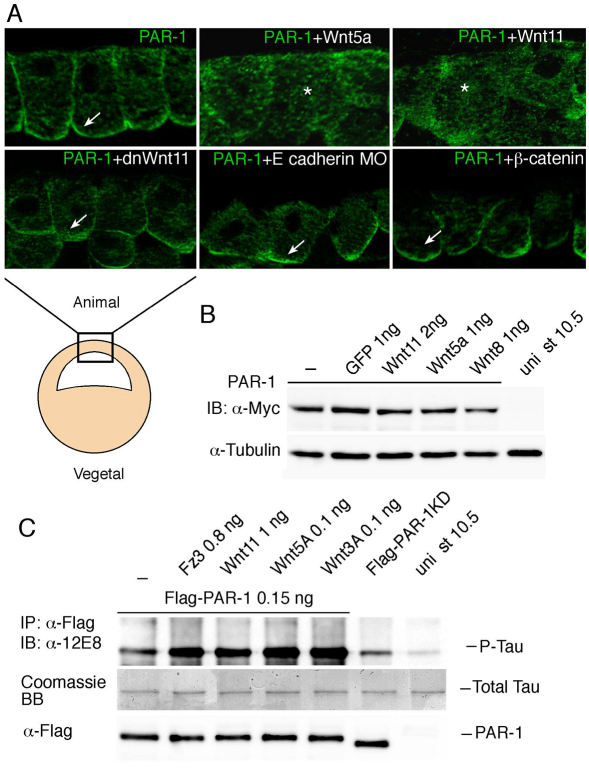
Noncanonical Wnt signaling has been proposed to involve PAR-1/MARK, a serine/threonine protein kinase that binds Dvl (Ossipova et al., 2005; Sun et al., 2001). Consistent with this role, PAR-1 is required for convergent extension movements in Xenopus embryos (Kusakabe and Nishida, 2004; Ossipova et al., 2005). To further assess the involvement of PAR-1 in Wnt signaling, we studied whether Wnt ligands can alter PAR-1 enzymatic activity or subcellular localization. Both Wnt5a and Wnt11 caused the redistribution of PAR-1 from the basolateral cortex to the cytoplasm without significant changes in protein levels (Fig. 5A,B and supplementary material Fig. S5A). This effect was accompanied by the alteration of epithelial cell morphology. Similar to the noncanonical Wnts, Wnt3a also affected PAR-1 distribution (data not shown). By contrast, dnWnt5/11, dnWnt11 and overexpressed β-catenin did not significantly alter the localization of PAR-1 at the basolateral cortex (Fig. 5A; data not shown). A previously characterized E-cadherin MO (Nandadasa et al., 2009) used at phenotypically active doses did not alter PAR-1 localization either, despite a pronounced effect on cell adhesion and epithelial morphology (Fig. 5A). Moreover, despite a pronounced effect on PAR-1, Wnt11 did not change the basolateral localization of occludin in double-immunostaining experiments (supplementary material Fig. S5B). These observations suggest that Wnt proteins have a specific effect on PAR-1 localization.
Fig. 5.
Wnt5 and Wnt 11 influence PAR-1 localization and activity. (A) Wnt5a or Wnt11 RNAs (1 ng each) trigger the relocalization of Myc-PAR-1 (co-injected as 0.3 ng of RNA) from the basolateral cortex to the cytoplasm in embryonic ectoderm (arrow, anti-Myc staining). The asterisk indicates cytoplasmic staining. By contrast, PAR-1 distribution is not significantly affected by dnWnt11, E-cadherin depletion or β-catenin overexpression (lower panels). The location of the superficial ectoderm cells used for this analysis is illustrated beneath. (B) Western analysis reveals comparable Myc-PAR-1 levels in the presence of Wnt or control GFP RNAs in stage 10.5-11 embryonic lysates. α-tubulin is a loading control. (C) Wnt signaling increases Flag-PAR-1 protein kinase activity as assessed by hTau phosphorylation at Ser262/356 detected by a phospho-specific antibody. Recombinant hTau protein was added at 1 μg per sample. Amounts of precipitated PAR-1 were assessed by anti-Flag antibody.
We next assessed how Wnt ligands modulate PAR-1 enzymatic activity in vivo. PAR-1 activation by Wnt proteins and Fz3 was assessed in Flag-PAR-1-expressing embryos in an immune complex kinase assay with human (h) Tau as a phosphorylation substrate (Nishimura et al., 2004). Co-expression of Wnt5a, Wnt11 or Wnt3a increased the ability of PAR-1 to phosphorylate hTau when measured using a phosphopeptide-specific antibody (Fig. 5C). We conclude that Wnt signaling dissociates PAR-1 from the cell cortex and upregulates its enzymatic activity.
PAR-1 is involved in neural crest specification
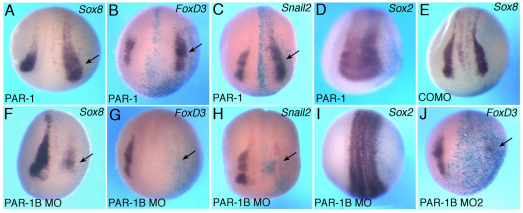
Since PAR-1 binds Dvl and PAR-1 activity and localization are regulated by noncanonical Wnt ligands, we hypothesized that PAR-1 is a mediator of noncanonical signaling during NC specification. This hypothesis was tested in gain- and loss-of-function experiments. PAR-1 RNA enhanced FoxD3 (54%, n=95), Snail2 (65%, n=26) and Sox8 (54%, n=61) (Fig. 6A-C), whereas Sox2 was largely unaltered (Fig. 6D). We next used a PAR-1 MO that was previously characterized and shown not to affect β-catenin signaling (Kusakabe and Nishida, 2004; Ossipova et al., 2005), suggesting that the canonical pathway is not involved. This PAR-1 MO, but not the control MO, suppressed Sox8 (90%, n=44), FoxD3 (82%, n=86) and Snail2 (83%, n=36) with high efficiency (Fig. 6E-H), without an effect on Sox2 (Fig. 6I). A second, non-overlapping, PAR-1 MO2 also inhibited FoxD3 (73%, n=15) but did not change Sox2 (Fig. 6J; data not shown). PAR-1 RNA and MOs also triggered the expected changes in Pax3 (Fig. 7A,B) and AP2 (supplementary material Fig. S6), supporting an essential role of PAR-1 in the early stages of premigratory NC development. No changes in ectodermal cell proliferation have been detected in cells with manipulated PAR-1 function (Ossipova et al., 2009), suggesting that PAR-1 has a primary effect on cell fate.
Fig. 6.
PAR-1 plays an essential role in neural crest specification. Xenopus embryos were injected with RNAs or MO and in situ hybridization analysis was carried out as described in Fig. 1. (A-D) PAR-1 RNA (0.3 ng) upregulates Sox8 (A), FoxD3 (B) and Snail2 (C) but does not affect Sox2 (D). (E-I) Effects of PAR-1 depletion. (E) Control MO (COMO) does not change Sox8. (J) PAR-1B MO2, which has a different sequence, has a similar effect to PAR-1B MO (G). Arrows indicate a change in marker gene expression. Dorso-anterior view is shown.
Fig. 7.
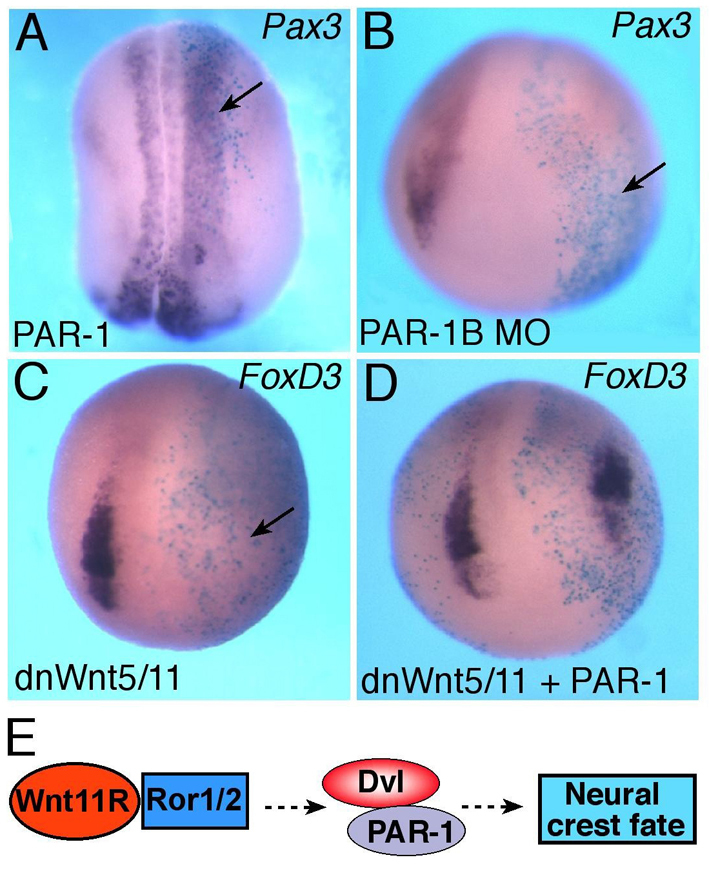
PAR-1 functions in the Wnt5/11 pathway upstream of Pax3. (A) PAR-1 RNA promotes Pax3 expression (stage 17). (B) PAR-1 is required for Pax3 expression (stage 14/15). (C,D) PAR-1 rescues the FoxD3 defect in embryos that were injected with dnWnt5/11 RNA. Dorso-anterior view, except A (dorsal view). Arrows indicate altered gene expression. (E) Model for Wnt11R signaling during NC specification. Dvl and the associated PAR-1 protein kinase are essential regulators of NC-specific transcription in response to Wnt11R/Ror2 signaling. Dashed lines represent indirect effects via unknown intermediates.
To evaluate whether PAR-1 is a downstream mediator of noncanonical Wnt signaling, we tested whether PAR-1 is able to rescue NC marker defects in embryos injected with dnWnt5/11 RNA (Fig. 7C,D). Importantly, PAR-1 overexpression was sufficient to partially restore FoxD3 expression in dnWnt5/11 RNA-injected embryos (45%, n=18), consistent with the view that PAR-1 functions downstream of Wnt5/11 signaling (Fig. 7E). Together, these experiments indicate that PAR-1 might mediate noncanonical Wnt signaling during NC specification.
DISCUSSION
In this study we show that noncanonical Wnt signaling, and specifically Wnt11R, is essential for NC-specific gene expression at premigratory stages of NC development. We find that Wnt proteins regulate the localization and the activity of the Dvl-associated protein kinase PAR-1, which itself plays a key role in regulating NC specification (Fig. 7E). This involvement of PAR-1, a cell polarity determinant, in NC formation reveals an unexpected connection between morphogenetic events and transcriptional control.
Our observations contrast with previous studies that have implicated noncanonical Wnt signaling in NC cell migration but not specification (Berndt et al., 2008; Carmona-Fontaine et al., 2008; De Calisto et al., 2005; Kuriyama and Mayor, 2008; Matthews et al., 2008a). Although we do not know why our results are different, the effect of Wnt11 on NC gene expression might have previously been overlooked because it likely requires higher levels of the interfering Wnt and Dvl (Dsh) constructs than are needed for highly sensitive cell migration assays. In a search for a specific Wnt ligand responsible for this signaling, we have shown that Wnt11R is required for NC specification. Of interest, Wnt11R is a true ortholog of other vertebrate Wnt11 genes (Garriock et al., 2007). Genetic analyses have demonstrated a role for Wnt11 in the ureteric branching of the mouse kidney (Majumdar et al., 2003) and in heart morphogenesis (Nagy et al., 2010), but its role in NC development has not been examined. Consistent with the role in NC specification, Xenopus Wnt11R is expressed in the neural plate, adjacent to the NC territory (Matthews et al., 2008a). Additionally, vertebrate Ror2 and PAR-1/MARK homologs are also expressed in early neuroectoderm, at the neural plate border, and later in migrating NC populations, as revealed in previous studies of Xenopus and mouse embryos (Hikasa et al., 2002a; Matsuda et al., 2001; Ossipova et al., 2002).
The Wnt11R pathway leading to NC specification appears to involve Dvl and could be similar to the PCP pathway identified in Drosophila studies (Simons and Mlodzik, 2008), although the involvement of a Wnt ligand, a Ror receptor or a PAR-1 homolog in fly PCP has not been conclusively demonstrated. When the vertebrate homologs of the ‘core’ PCP components, such as Prickle, Dishevelled, Frizzled and Strabismus/Van Gogh-like, are modulated, gain- and loss-of-function effects are often indistinguishable. For example, the embryonic body axis is shortened due to defective convergent extension when Strabismus or Prickle proteins are either overexpressed or depleted (Darken et al., 2002; Park and Moon, 2002; Takeuchi et al., 2003; Veeman et al., 2003), interfering with the subsequent analysis of the pathway. Our observations establish a new molecular assay in which the gain- and loss-of-function effects of noncanonical signaling are easily distinguished, thus eliminating the complication of morphology-based assays. This assay provides a straightforward means to identify additional pathway components by assessing a role in NC formation for other candidate proteins that affect PCP in Drosophila or convergent extension in vertebrates.
Our findings reveal an essential role for the epithelial polarity protein PAR-1 in NC development. Although the involvement of polarity proteins in cell migration is highly predictable (Imai et al., 2006; McCaffrey and Macara, 2009), their function at the premigratory stage was unexpected. Based on the effect of noncanonical Wnt proteins on PAR-1 localization and activity, and the ability of PAR-1 to rescue the NC defect in embryos in which noncanonical Wnt signaling has been inhibited, we propose that PAR-1 mediates Wnt11R signaling during NC specification (Fig. 7E). The direct interaction of PAR-1 and Dvl implies a direct mechanism, which remains to be investigated. The hypothesis that PAR-1 functions downstream of Wnt11R is also supported by the ability of PAR-1 MO to block FoxD3 induction by Fz3, which affects PAR-1 localization (data not shown) and has been implicated in noncanonical signaling (Witze et al., 2008) (supplementary material Fig. S6D-F). It is currently unknown how PAR-1 is regulated by Wnts and which proteins mediate its effects on NC marker expression, but possible mechanisms might involve the segregation of specific fate determinants after asymmetric mitosis or the polarization of the cytoskeleton, leading to mechanotransduction. These mechanisms are likely to involve small Rho GTPases, which have been reported to function in NC specification (Broders-Bondon et al., 2007; Guemar et al., 2007) and can be regulated by both Wnt and PAR signaling (Habas et al., 2001; Schlessinger et al., 2009). Recently, RhoGAP has been shown to regulate transcriptional events by binding to specific transcription factors and controlling tissue-specific transcription (Mammoto et al., 2009; Mammoto and Ingber, 2010).
Combined with previous studies (Ikeya et al., 1997; Saint-Jeannet et al., 1997), our observations support the view that vertebrate NC forms in response to signaling by both canonical and noncanonical Wnt ligands, although the details of these processes remain to be determined. Of interest, both noncanonical Wnt ligands and polarity proteins can regulate EMT and cell migration (Imai et al., 2006; Minichiello et al., 1999; Nishita et al., 2006; Witze et al., 2008), two processes that are characteristic for NC cell behavior. Since NC cells form at the boundary of neural and non-neural ectoderm, it is possible that the regulation of cadherin-mediated cell adhesion contributes to the process of NC fate specification, in addition to its known function in NC migration (Kashef et al., 2009; Park and Gumbiner, 2010; Taneyhill, 2008). Of note, both Wnt11 signaling and the RhoV/Chp GTPase, which is required for NC formation, have been implicated in the maintenance of adherens junctions in zebrafish embryos (Tay et al., 2010; Ulrich et al., 2005). Further studies are needed to examine the functions of cadherins and other cell junction components in NC specification.
Supplementary Material
Acknowledgments
We thank P. Krieg, R. Mayor, A. Muesch, M. Taira, D. Kessler, J.-P. Saint-Jeannet, S. Citi and P. Klein for plasmids and reagents, K. Itoh for comments on the manuscript and members of the S.Y.S. laboratory for discussions.
Footnotes
Funding
This work was supported by the National Institute of Health [grants NS040972 and GM077592 to S.Y.S.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.067280/-/DC1
References
- Acloque H., Adams M. S., Fishwick K., Bronner-Fraser M., Nieto M. A. (2009). Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 119, 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J. (1997). Cellular and molecular biology of neural crest cell lineage determination. Trends Genet. 13, 276–280 [DOI] [PubMed] [Google Scholar]
- Angers S., Moon R. T. (2009). Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- Axelrod J. D., Miller J. R., Shulman J. M., Moon R. T., Perrimon N. (1998). Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12, 2610–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang A. G., Papalopulu N., Goulding M. D., Kinter C. (1999). Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev. Biol. 212, 366–380 [DOI] [PubMed] [Google Scholar]
- Berndt J. D., Clay M. R., Langenberg T., Halloran M. C. (2008). Rho-kinase and myosin II affect dynamic neural crest cell behaviors during epithelial to mesenchymal transition in vivo. Dev. Biol. 324, 236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M., Paricio N., Strutt D. I., Mlodzik M. (1998). Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94, 109–118 [DOI] [PubMed] [Google Scholar]
- Broders-Bondon F., Chesneau A., Romero-Oliva F., Mazabraud A., Mayor R., Thiery J. P. (2007). Regulation of XSnail2 expression by Rho GTPases. Dev. Dyn. 236, 2555–2566 [DOI] [PubMed] [Google Scholar]
- Cadigan K. M., Peifer M. (2009). Wnt signaling from development to disease: insights from model systems. Cold Spring Harbor Perspect. Biol. 1, a002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Matthews H. K., Kuriyama S., Moreno M., Dunn G. A., Parsons M., Stern C. D., Mayor R. (2008). Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456, 957–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. W., Tadjuidje E., White J., Wells J., Mayhew C., Wylie C., Heasman J. (2009). Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr. Biol. 19, 1573–1580 [DOI] [PubMed] [Google Scholar]
- Chalmers A. D., Welchman D., Papalopulu N. (2002). Intrinsic differences between the superficial and deep layers of the Xenopus ectoderm control primary neuronal differentiation. Dev. Cell 2, 171–182 [DOI] [PubMed] [Google Scholar]
- Cheung M., Briscoe J. (2003). Neural crest development is regulated by the transcription factor Sox9. Development 130, 5681–5693 [DOI] [PubMed] [Google Scholar]
- Choi S. C., Sokol S. Y. (2009). The involvement of lethal giant larvae and Wnt signaling in bottle cell formation in Xenopus embryos. Dev. Biol. 336, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L., Salinas P. C. (2005). WNTS in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 [DOI] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., Mazzon E., De Rigo L., Baraldo S., Meggio F., Citi S. (1997). Occludin dephosphorylation in early development of Xenopus laevis. J. Cell Sci. 110, 3131–3139 [DOI] [PubMed] [Google Scholar]
- Crane J. F., Trainor P. A. (2006). Neural crest stem and progenitor cells. Annu. Rev. Cell Dev. Biol. 22, 267–286 [DOI] [PubMed] [Google Scholar]
- Darken R. S., Scola A. M., Rakeman A. S., Das G., Mlodzik M., Wilson P. A. (2002). The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 21, 976–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Calisto J., Araya C., Marchant L., Riaz C. F., Mayor R. (2005). Essential role of non-canonical Wnt signalling in neural crest migration. Development 132, 2587–2597 [DOI] [PubMed] [Google Scholar]
- de Croze N., Maczkowiak F., Monsoro-Burq A. H. (2011). Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc. Natl. Acad. Sci. USA 108, 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff M. A., Tan C., Saint-Jeannet J. P., Klein P. S. (2001). A role for frizzled 3 in neural crest development. Development 128, 3655–3663 [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Bowerman B. (2001). Asymmetric cell division: fly neuroblast meets worm zygote. Curr. Opin. Cell Biol. 13, 68–75 [DOI] [PubMed] [Google Scholar]
- Dorsky R. I., Moon R. T., Raible D. W. (1998). Control of neural crest cell fate by the Wnt signalling pathway. Nature 396, 370–373 [DOI] [PubMed] [Google Scholar]
- Du S. J., Purcell S. M., Christian J. L., McGrew L. L., Moon R. T. (1995). Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 15, 2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F., Gumbiner B. M. (1994). Beta-catenin localization during Xenopus embryogenesis: accumulation at tissue and somite boundaries. Development 120, 3667–3679 [DOI] [PubMed] [Google Scholar]
- Garcia-Castro M. I., Marcelle C., Bronner-Fraser M. (2002). Ectodermal Wnt function as a neural crest inducer. Science 297, 848–851 [DOI] [PubMed] [Google Scholar]
- Garriock R. J., Krieg P. A. (2007). Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Dev. Biol. 304, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock R. J., D’Agostino S. L., Pilcher K. C., Krieg P. A. (2005). Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev. Biol. 279, 179–192 [DOI] [PubMed] [Google Scholar]
- Garriock R. J., Warkman A. S., Meadows S. M., D’Agostino S., Krieg P. A. (2007). Census of vertebrate Wnt genes: isolation and developmental expression of Xenopus Wnt2, Wnt3, Wnt9a, Wnt9b, Wnt10a, and Wnt16. Dev. Dyn. 236, 1249–1258 [DOI] [PubMed] [Google Scholar]
- Glavic A., Silva F., Aybar M. J., Bastidas F., Mayor R. (2004). Interplay between Notch signaling and the homeoprotein Xiro1 is required for neural crest induction in Xenopus embryos. Development 131, 347–359 [DOI] [PubMed] [Google Scholar]
- Gloy J., Hikasa H., Sokol S. Y. (2002). Frodo interacts with Dishevelled to transduce Wnt signals. Nat. Cell Biol. 4, 351–357 [DOI] [PubMed] [Google Scholar]
- Goldstein B., Macara I. G. (2007). The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L., Liu G., Mong P., Mudbhary R., Biswas R., Arroyave R., Vijayakumar S., Economides A. N., Aaronson S. A. (2010). Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 24, 2517–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guemar L., de Santa Barbara P., Vignal E., Maurel B., Fort P., Faure S. (2007). The small GTPase RhoV is an essential regulator of neural crest induction in Xenopus. Dev. Biol. 310, 113–128 [DOI] [PubMed] [Google Scholar]
- Habas R., Kato Y., He X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854 [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid I. B., He X. (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L., Brault V., Kleber M., Lee H. Y., Ille F., Leimeroth R., Paratore C., Suter U., Kemler R., Sommer L. (2002). Lineage-specific requirements of beta-catenin in neural crest development. J. Cell Biol. 159, 867–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R. M. (1991). In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36, 685–695 [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E., LaBonne C. (2004). A slug, a fox, a pair of sox: transcriptional responses to neural crest inducing signals. Birth Defects Res. C Embryo Today 72, 124–139 [DOI] [PubMed] [Google Scholar]
- Heisenberg C. P., Brand M., Jiang Y. J., Warga R. M., Beuchle D., van Eeden F. J., Furutani-Seiki M., Granato M., Haffter P., Hammerschmidt M., et al. (1996). Genes involved in forebrain development in the zebrafish, Danio rerio. Development 123, 191–203 [DOI] [PubMed] [Google Scholar]
- Heisenberg C. P., Tada M., Rauch G.-J., Saúde L., Concha M. L., Geisler R., Stemple D. L., Smith J. C., Wilson S. W. (2000). Siberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81 [DOI] [PubMed] [Google Scholar]
- Hikasa H., Sokol S. Y. (2011). Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J. Biol. Chem. 286, 12093–12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H., Shibata M., Hiratani I., Taira M. (2002a). The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129, 5227–5239 [DOI] [PubMed] [Google Scholar]
- Hikasa H., Shibata M., Hiratani I., Taira M. (2002b). The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129, 5227–5239 [DOI] [PubMed] [Google Scholar]
- Hikasa H., Ezan J., Itoh K., Li X., Klymkowsky M. W., Sokol S. Y. (2010). Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev. Cell 19, 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M., Chen D. C., Klein P. S., Lee V. M. (1997). Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 272, 25326–25332 [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. (1989). MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 8, 3409–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L. R., Watanabe O., Leonard J., Brown A. M. (2003). Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 63, 1906–1913 [PubMed] [Google Scholar]
- Ikeya M., Lee S. M., Johnson J. E., McMahon A. P., Takada S. (1997). Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389, 966–970 [DOI] [PubMed] [Google Scholar]
- Imai F., Hirai S., Akimoto K., Koyama H., Miyata T., Ogawa M., Noguchi S., Sasaoka T., Noda T., Ohno S. (2006). Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development 133, 1735–1744 [DOI] [PubMed] [Google Scholar]
- Itoh K., Krupnik V. E., Sokol S. Y. (1998). Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr. Biol. 8, 591–594 [DOI] [PubMed] [Google Scholar]
- Itoh K., Brott B. K., Bae G. U., Ratcliffe M. J., Sokol S. Y. (2005). Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 4, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashef J., Kohler A., Kuriyama S., Alfandari D., Mayor R., Wedlich D. (2009). Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev. 23, 1393–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibardin A., Ossipova O., Sokol S. Y. (2006). Metastasis-associated kinase modulates Wnt signaling to regulate brain patterning and morphogenesis. Development 133, 2845–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. D., Schilling T. F. (2006). Cranial neural crest and development of the head skeleton. Adv. Exp. Med. Biol. 589, 120–133 [DOI] [PubMed] [Google Scholar]
- Knoblich J. A. (2008). Mechanisms of asymmetric stem cell division. Cell 132, 583–597 [DOI] [PubMed] [Google Scholar]
- Komiya Y., Habas R. (2008). Wnt signal transduction pathways. Organogenesis 4, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S., Mayor R. (2008). Molecular analysis of neural crest migration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1349–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe M., Nishida E. (2004). The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 23, 4190–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C. (2002). Vertebrate development: wnt signals at the crest. Curr. Biol. 12, R743–R744 [DOI] [PubMed] [Google Scholar]
- LaBonne C., Bronner-Fraser M. (1998). Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403–2414 [DOI] [PubMed] [Google Scholar]
- Lamb T. M., Knecht A. K., Smith W. C., Stachel S. E., Economides A. N., Stahl N., Yancopolous G. D., Harland R. M. (1993). Neural induction by the secreted polypeptide noggin. Science 262, 713–718 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Dupin E. (2003). Multipotentiality of the neural crest. Curr. Opin. Genet. Dev. 13, 529–536 [DOI] [PubMed] [Google Scholar]
- Lewis J. L., Bonner J., Modrell M., Ragland J. W., Moon R. T., Dorsky R. I., Raible D. W. (2004). Reiterated Wnt signaling during zebrafish neural crest development. Development 131, 1299–1308 [DOI] [PubMed] [Google Scholar]
- Lin S., Baye L. M., Westfall T. A., Slusarski D. C. (2010). Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J. Cell Biol. 190, 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. (1984). Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259, 5301–5305 [PubMed] [Google Scholar]
- Lisovsky M., Itoh K., Sokol S. Y. (2002). Frizzled receptors activate a novel JNK-dependent pathway that may lead to apoptosis. Curr. Biol. 12, 53–58 [DOI] [PubMed] [Google Scholar]
- Lu W., Yamamoto V., Ortega B., Baltimore D. (2004). Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119, 97–108 [DOI] [PubMed] [Google Scholar]
- Luo T., Matsuo-Takasaki M., Thomas M. L., Weeks D. L., Sargent T. D. (2002). Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Dev. Biol. 245, 136–144 [DOI] [PubMed] [Google Scholar]
- Majumdar A., Vainio S., Kispert A., McMahon J., McMahon A. P. (2003). Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Connor K. M., Mammoto T., Yung C. W., Huh D., Aderman C. M., Mostoslavsky G., Smith L. E., Ingber D. E. (2009). A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T., Ingber D. E. (2010). Mechanical control of tissue and organ development. Development 137, 1407–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F., Topczewski J., Sepich D., Solnica-Krezel L. (2002). Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 12, 876–884 [DOI] [PubMed] [Google Scholar]
- Matsuda T., Nomi M., Ikeya M., Kani S., Oishi I., Terashima T., Takada S., Minami Y. (2001). Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech. Dev. 105, 153–156 [DOI] [PubMed] [Google Scholar]
- Matthews H. K., Broders-Bondon F., Thiery J. P., Mayor R. (2008a). Wnt11r is required for cranial neural crest migration. Dev. Dyn. 237, 3404–3409 [DOI] [PubMed] [Google Scholar]
- Matthews H. K., Marchant L., Carmona-Fontaine C., Kuriyama S., Larrain J., Holt M. R., Parsons M., Mayor R. (2008b). Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 135, 1771–1780 [DOI] [PubMed] [Google Scholar]
- Mayor R., Morgan R., Sargent M. G. (1995). Induction of the prospective neural crest of Xenopus. Development 121, 767–777 [DOI] [PubMed] [Google Scholar]
- McCaffrey L. M., Macara I. G. (2009). Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harbor Perspect. Biol. 1, a001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A. J., Nusse R. (2006). Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A., Minami Y., Nusse R. (2009). Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 284, 30167–30176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y., Oishi I., Endo M., Nishita M. (2010). Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev. Dyn. 239, 1–15 [DOI] [PubMed] [Google Scholar]
- Minichiello J., Ben-Ya’acov A., Hearn C. J., Needham B., Newgreen D. F. (1999). Induction of epithelio-mesenchymal transformation of quail embryonic neural cells by inhibition of atypical protein kinase-C. Cell Tissue Res. 295, 195–206 [DOI] [PubMed] [Google Scholar]
- Mizuseki K., Kishi M., Matsui M., Nakanishi S., Sasai Y. (1998). Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125, 579–587 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Wang E., Harland R. (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8, 167–178 [DOI] [PubMed] [Google Scholar]
- Moon R. T., Campbell R. M., Christian J. L., McGrew L. L., Shih J., Fraser S. (1993). Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119, 97–111 [DOI] [PubMed] [Google Scholar]
- Nagy I. I., Railo A., Rapila R., Hast T., Sormunen R., Tavi P., Rasanen J., Vainio S. J. (2010). Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc. Res. 85, 100–109 [DOI] [PubMed] [Google Scholar]
- Nandadasa S., Tao Q., Menon N. R., Heasman J., Wylie C. (2009). N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development 136, 1327–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura I., Yang Y., Lu B. (2004). PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116, 671–682 [DOI] [PubMed] [Google Scholar]
- Nishita M., Yoo S. K., Nomachi A., Kani S., Sougawa N., Ohta Y., Takada S., Kikuchi A., Minami Y. (2006). Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J. Cell Biol. 175, 555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M., Hong C. S., Huang X., Delnicki R. J., Saint-Jeannet J. P. (2006). Functional analysis of Sox8 during neural crest development in Xenopus. Development 133, 3817–3826 [DOI] [PubMed] [Google Scholar]
- Ohno S. (2001). Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13, 641–648 [DOI] [PubMed] [Google Scholar]
- Ossipova O., He X., Green J. (2002). Molecular cloning and developmental expression of Par-1/MARK homologues XPar-1A and XPar-1B from Xenopus laevis. Mech. Dev. 119 Suppl. 1, S143–S148 [DOI] [PubMed] [Google Scholar]
- Ossipova O., Dhawan S., Sokol S., Green J. B. (2005). Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev. Cell 8, 829–841 [DOI] [PubMed] [Google Scholar]
- Ossipova O., Tabler J., Green J. B., Sokol S. Y. (2007). PAR1 specifies ciliated cells in vertebrate ectoderm downstream of aPKC. Development 134, 4297–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O., Ezan J., Sokol S. Y. (2009). PAR-1 phosphorylates Mind bomb to promote vertebrate neurogenesis. Dev. Cell 17, 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur P., Lasche M., Eisenberg L. M., Kuhl M. (2002). Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418, 636–641 [DOI] [PubMed] [Google Scholar]
- Park K. S., Gumbiner B. M. (2010). Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development 137, 2691–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Moon R. T. (2002). The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 4, 20–25 [DOI] [PubMed] [Google Scholar]
- Piotrowski T., Schilling T. F., Brand M., Jiang Y. J., Heisenberg C. P., Beuchle D., Grandel H., van Eeden F. J., Furutani-Seiki M., Granato M., et al. (1996). Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development 123, 345–356 [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J. P., He X., Varmus H. E., Dawid I. B. (1997). Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. USA 94, 13713–13718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T., Kume S., Amasaki Y., Mikoshiba K. (2002). The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417, 295–299 [DOI] [PubMed] [Google Scholar]
- Sasai N., Mizuseki K., Sasai Y. (2001). Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development 128, 2525–2536 [DOI] [PubMed] [Google Scholar]
- Sato T., Sasai N., Sasai Y. (2005). Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development 132, 2355–2363 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. (2008). A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557–568 [DOI] [PubMed] [Google Scholar]
- Schambony A., Wedlich D. (2007). Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell 12, 779–792 [DOI] [PubMed] [Google Scholar]
- Schlessinger K., Hall A., Tolwinski N. (2009). Wnt signaling pathways meet Rho GTPases. Genes Dev. 23, 265–277 [DOI] [PubMed] [Google Scholar]
- Seubert P., Mawal-Dewan M., Barbour R., Jakes R., Goedert M., Johnson G. V., Litersky J. M., Schenk D., Lieberburg I., Trojanowski J. Q., et al. (1995). Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J. Biol. Chem. 270, 18917–18922 [DOI] [PubMed] [Google Scholar]
- Sheldahl L. C., Slusarski D. C., Pandur P., Miller J. R., Kuhl M., Moon R. T. (2003). Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 161, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D. L., Goisset C., Boucaut J. C. (1998). Expression of Xfz3, a Xenopus frizzled family member, is restricted to the early nervous system. Mech. Dev. 70, 35–47 [DOI] [PubMed] [Google Scholar]
- Shibata M., Itoh M., Hikasa H., Taira S., Taira M. (2005). Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech. Dev. 122, 1322–1339 [DOI] [PubMed] [Google Scholar]
- Simons M., Mlodzik M. (2008). Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 42, 517–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski D. C., Corces V. G., Moon R. T. (1997). Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390, 410–413 [DOI] [PubMed] [Google Scholar]
- Sokol S. (2000). A role for Wnts in morpho-genesis and tissue polarity. Nat. Cell Biol. 2, E124–E125 [DOI] [PubMed] [Google Scholar]
- Sun T. Q., Lu B., Feng J. J., Reinhard C., Jan Y. N., Fantl W. J., Williams L. T. (2001). PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3, 628–636 [DOI] [PubMed] [Google Scholar]
- Tada M., Smith J. C. (2000). Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127, 2227–2238 [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Nakabayashi J., Sakaguchi T., Yamamoto T. S., Takahashi H., Takeda H., Ueno N. (2003). The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 13, 674–679 [DOI] [PubMed] [Google Scholar]
- Taneyhill L. A. (2008). To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh. Migr. 2, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay H. G., Ng Y. W., Manser E. (2010). A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly. PLoS ONE 5, e10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- Ulrich F., Krieg M., Schotz E. M., Link V., Castanon I., Schnabel V., Taubenberger A., Mueller D., Puech P. H., Heisenberg C. P. (2005). Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell 9, 555–564 [DOI] [PubMed] [Google Scholar]
- Vallin J., Thuret R., Giacomello E., Faraldo M. M., Thiery J. P., Broders F. (2001). Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J. Biol. Chem. 276, 30350–30358 [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Nusse R. (2009). Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Mikels A., Nusse R. (2008). Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 1, re9 [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. (2003). Zebrafish prickle, a modulator of noncanonical wnt/fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685 [DOI] [PubMed] [Google Scholar]
- Winklbauer R., Medina A., Swain R. K., Steinbeisser H. (2001). Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413, 856–860 [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Sargent T. D., Parry D. A., Jonas E., Dawid I. B. (1985). Developmentally regulated cytokeratin gene in Xenopus laevis. Mol. Cell. Biol. 5, 2575–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. G., Wang B., Ballew A., Royou A., Karess R., Axelrod J. D., Luo L. (2001). Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 [DOI] [PubMed] [Google Scholar]
- Witze E. S., Litman E. S., Argast G. M., Moon R. T., Ahn N. G. (2008). Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science 320, 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda S. L., Moody C. J., Moon R. T. (1993). Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev. Biol. 155, 46–57 [DOI] [PubMed] [Google Scholar]
- Wu J., Saint-Jeannet J. P., Klein P. S. (2003). Wnt-frizzled signaling in neural crest formation. Trends Neurosci. 26, 40–45 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. P., Bradley A., McMahon A. P., Jones S. (1999). A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1123 [DOI] [PubMed] [Google Scholar]
- Yanagawa S., van Leeuwen F., Wodarz A., Klingensmith J., Nusse R. (1995). The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9, 1087–1097 [DOI] [PubMed] [Google Scholar]
- Yang J., Weinberg R. A. (2008). Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 [DOI] [PubMed] [Google Scholar]
- Zhang C., Basta T., Jensen E. D., Klymkowsky M. W. (2003). The beta-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development 130, 5609–5624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.