Abstract
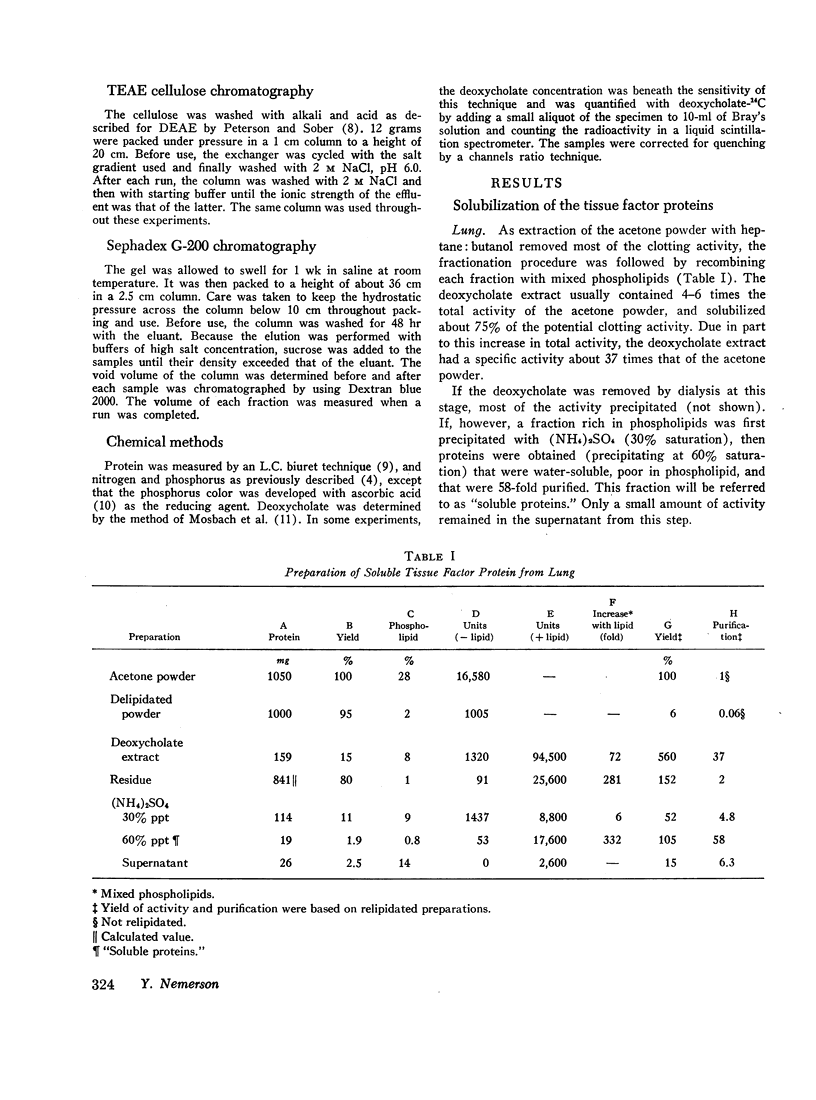
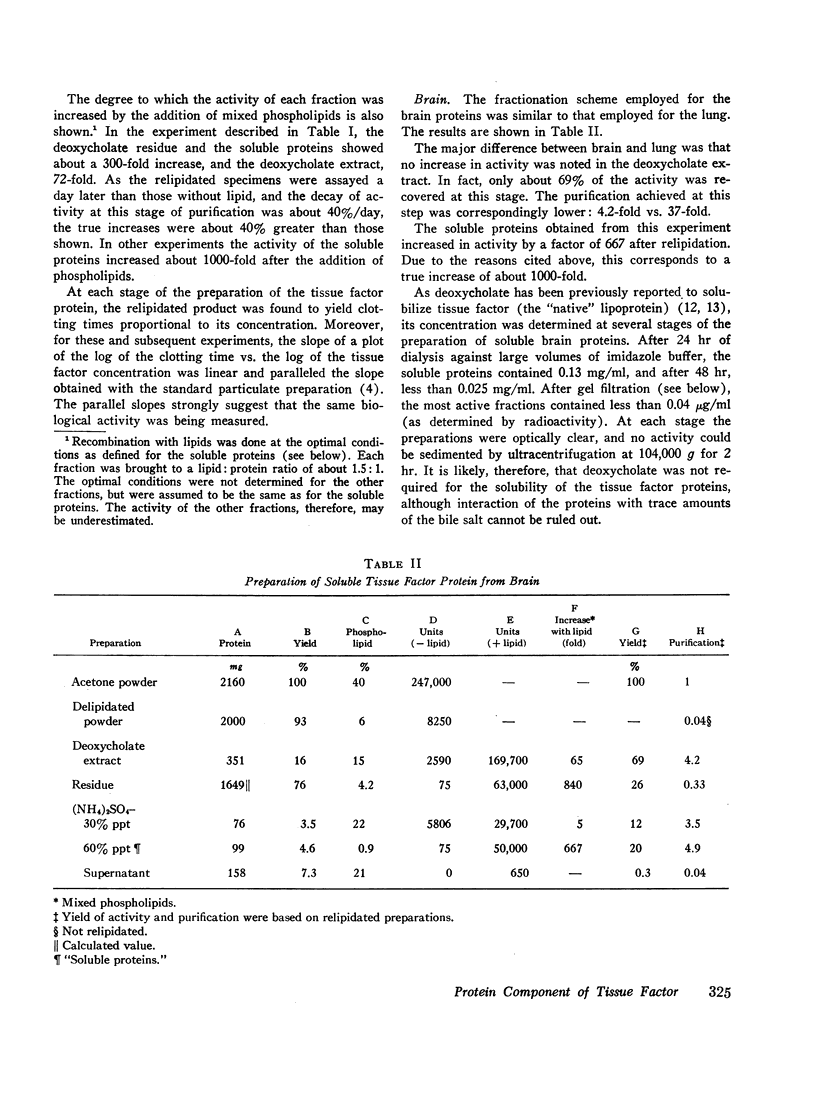
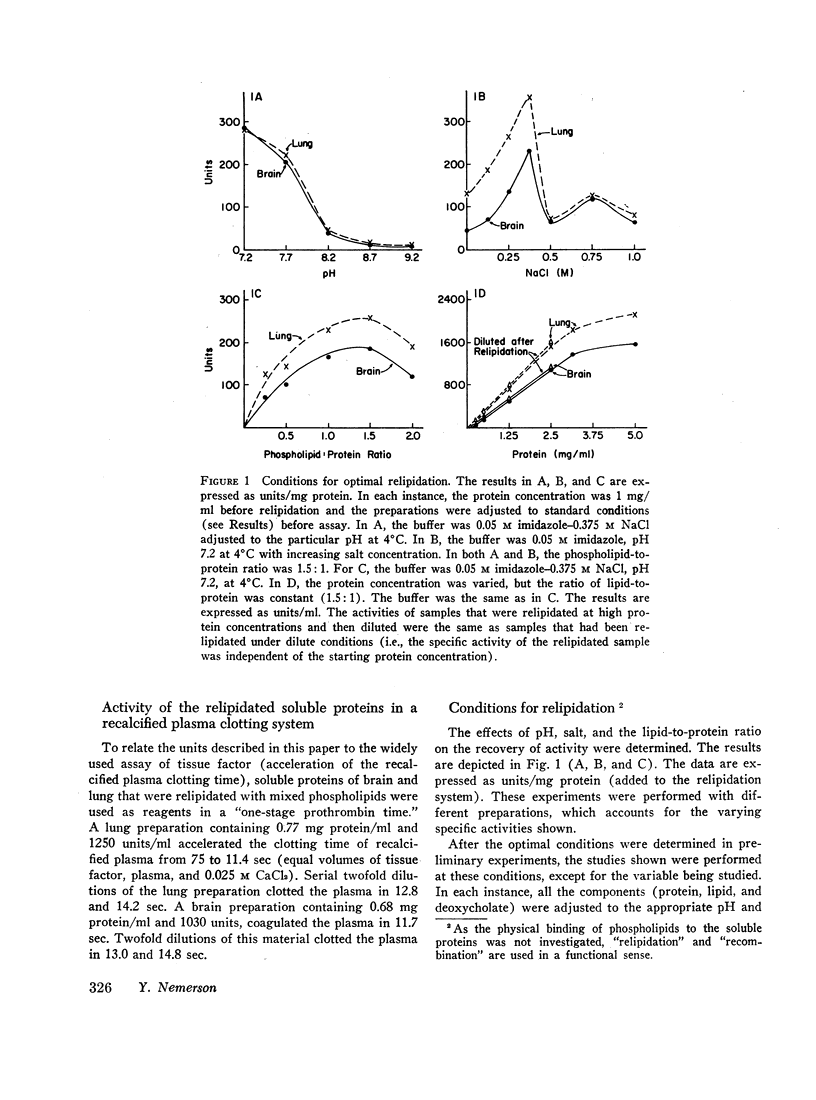
Soluble proteins were prepared from bovine lung and brain which, when combined with phospholipids, had the biological characteristics of tissue factor. The proteins were solubilized from delipidated (heptane; butanol-extracted) acetone powders with deoxycholate, and precipitated by (NH4)2SO4 (30-60% saturation). These soluble proteins, which contained less than 1% phospholipid, were essentially inert in an assay for tissue factor. When they were recombined with phospholipids, however, activity increased by a factor of 500-1000. Phosphatidylethanolamine was the most active specific phospholipid, followed by phosphatidylcholine. Phosphatidylserine was inert. Mixed phospholipids were from two to four times more active than phosphatidylethanolamine. Maximum activity was obtained at phospholipid to protein ratios of 1.5:1 (wt/wt), and when relipidation was performed in deoxycholate followed by dialysis against 0.05 M imidazole-0.375 M NaCl, pH 7.2.
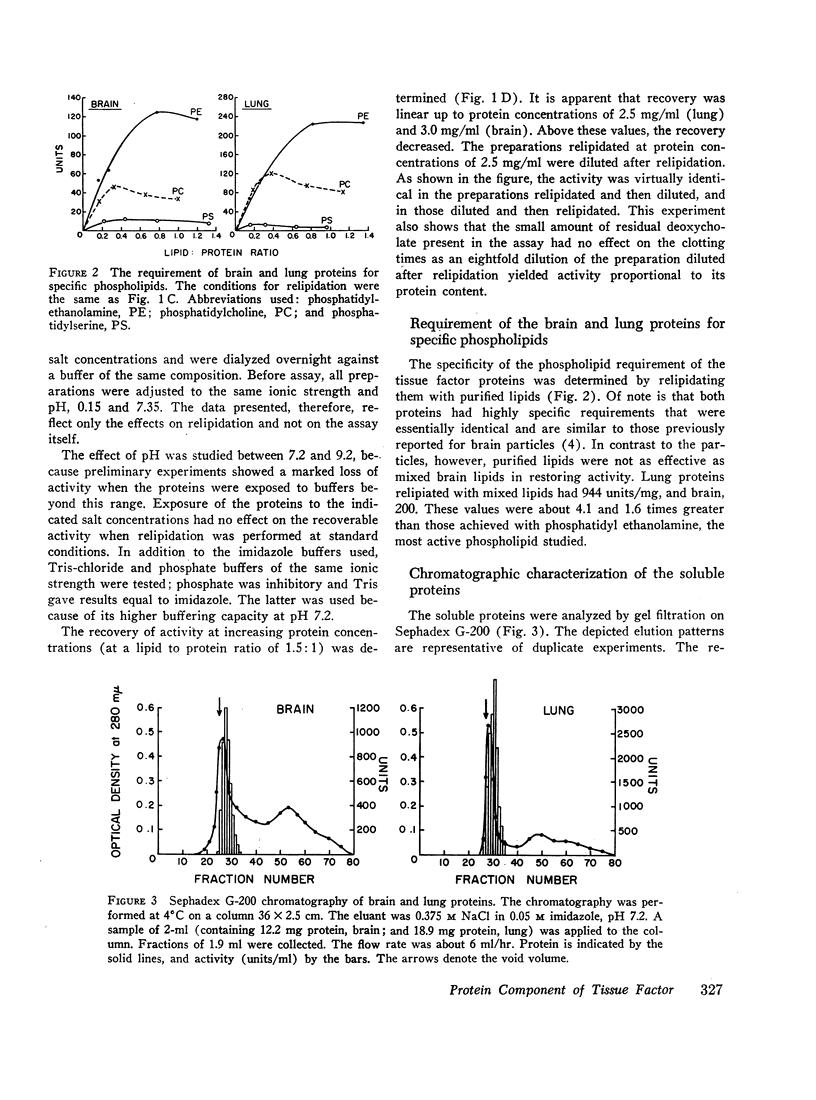
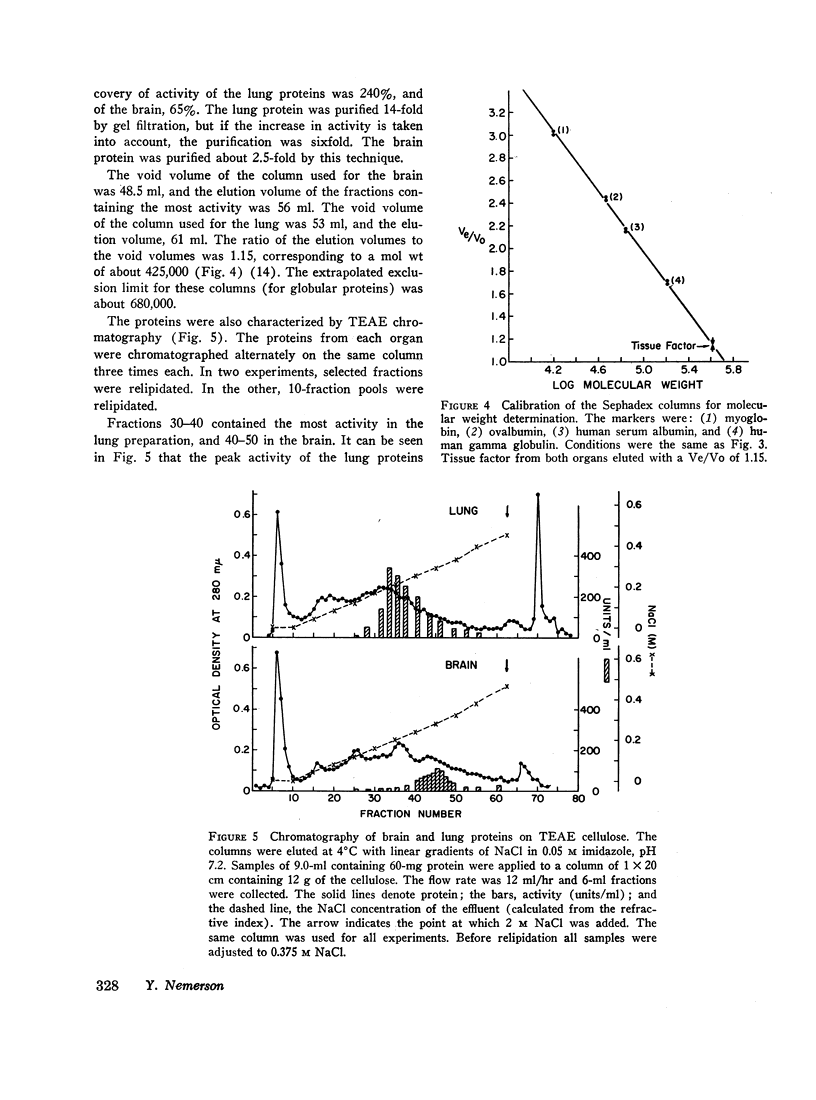
The proteins were characterized by gel filtration on Sephadex G-200; the activity eluted as a single peak with an apparent mol wt of 425,000. The brain and lung activities were recovered from chromatography on triethyl-aminoethyl-cellulose as single peaks, although the salt concentration required for elution was different for each protein. This suggests that within each organ there is one tissue factor-protein, but there may be molecular differences between brain and lung tissue factor. Alternatively, the chromatographic differences may simply reflect charge differences imparted to the proteins by residual lipids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duttera S. M., Byrne W. L., Ganoza M. C. Studies on the phospholipid requirement of glucose 6-phosphatase. J Biol Chem. 1968 May 10;243(9):2216–2228. [PubMed] [Google Scholar]
- FLEISCHER S., KLOUWEN H., BRIERLEY G. Studies of the electron transfer system. 38. Lipid composition of purified enzyme preparations derived from beef heart mitochondria. J Biol Chem. 1961 Nov;236:2936–2941. [PubMed] [Google Scholar]
- HECHT E. R., CHO M. H., SEEGERS W. H. Thromboplastin: nomenclature and preparation of protein-free material different from platelet factor 3 or lipid activator. Am J Physiol. 1958 Jun;193(3):584–592. doi: 10.1152/ajplegacy.1958.193.3.584. [DOI] [PubMed] [Google Scholar]
- Hecht E. Chemical nature of human brain thromboplastin. Nature. 1967 Apr 8;214(5084):197–198. doi: 10.1038/214197a0. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Cheever E. V., Seavey B. K. Influence of fatty acids on the electrophoretic behavior of proteins with special reference to pituitary hormone and thyroglobulin. J Biol Chem. 1968 Jan 25;243(2):260–267. [PubMed] [Google Scholar]
- MOSBACH E. H., KALINSKY H. J., HALPERN E., KENDALL F. E. Determination of deoxycholic and cholic acids in bile. Arch Biochem Biophys. 1954 Aug;51(2):402–410. doi: 10.1016/0003-9861(54)90495-6. [DOI] [PubMed] [Google Scholar]
- NEMERSON Y., SPAET T. H. THE ACTIVATION OF FACTOR X BY EXTRACTS OF RABBIT BRAIN. Blood. 1964 May;23:657–668. [PubMed] [Google Scholar]
- Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J Clin Invest. 1968 Jan;47(1):72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y. The reaction between bovine brain tissue factor and factors VII and X. Biochemistry. 1966 Feb;5(2):601–608. doi: 10.1021/bi00866a029. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide. Structural requirements of the phospholipid molecule. J Biol Chem. 1966 Mar 25;241(6):1386–1392. [PubMed] [Google Scholar]
- WEISS H. J., EICHELBERGER J. W., Jr, CROSBY W. H. Studies on the differences in activity of lung and brain thromboplastin. J Clin Invest. 1961 Feb;40:205–214. doi: 10.1172/JCI104246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS W. J. THE ACTIVITY OF LUNG MICROSOMES IN BLOOD COAGULATION. J Biol Chem. 1964 Mar;239:933–942. [PubMed] [Google Scholar]
- Williams W. J., Norris D. G. Purification of a bovine plasma protein (factor VII) which is required for the activity of lung microsomes in blood coagulation. J Biol Chem. 1966 Apr 25;241(8):1847–1856. [PubMed] [Google Scholar]
- Williams W. J. The activity of human placenta microsomes and brain particles in blood coagulation. J Biol Chem. 1966 Apr 25;241(8):1840–1846. [PubMed] [Google Scholar]



