Abstract
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to these guidelines and recommendations to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed or endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) is defined by the International League of Associations for Rheumatology (ILAR) as arthritis of unknown etiology that begins before the sixteenth birthday and persists for at least 6 weeks with other known conditions excluded (1). JIA is one of the more common chronic diseases of childhood, with a prevalence of approximately 1 per 1,000 (2,3). JIA often persists into adulthood and can result in significant long-term morbidity, including physical disability (4---9). Recent major advances in treatment have greatly improved short- and medium-term outcomes for children with JIA (10---17), yet no validated guidelines offer recommendations for the treatment of JIA.
To develop recommendations for the safest and most effective treatment of JIA on behalf of the American College of Rheumatology (ACR), we applied the established Research and Development /University of California at Los Angeles (RAND/UCLA) Appropriateness Method (18) to derive recommendations that are as evidence based as possible. Similar methods were used recently in the development of the ACR recommendations for the use of disease-modifying antirheumatic drugs (DMARDs) for rheumatoid arthritis (19) and the management of glucocorticoid-induced osteoporosis (20). We sought to give our recommendations additional strength by following the principles of the Appraisal of Guidelines for Research and Evaluation instrument (21), a framework designed specifically to assess the quality of clinical practice guidelines, including the methods used for their development and the content of the final recommendations.
Our effort focused on the initiation and safety monitoring of therapeutic agents in the treatment of JIA, including nonsteroidal antiinflammatory drugs (NSAIDs), intraarticular glucocorticoid injections, nonbiologic DMARDs, biologic DMARDs, and systemic glucocorticoids for the treatment of the systemic features of systemic arthritis. The indications for systemic glucocorticoids for the treatment of synovitis were not considered, owing to a lack of published evidence. We did not consider all ILAR categories of JIA individually and instead grouped children with JIA into distinct “treatment groups” (see Materials and Methods). We did not consider the economic costs of JIA or its treatment for two reasons: first, too few economic analyses of JIA exist to permit conclusions; second, the RAND/UCLA Appropriateness Method specifically does not consider cost implications (18). These recommendations were developed with international input and are intended to inform and benefit health care providers caring for children with JIA throughout the world. Many recommendations fall outside the present bounds of regulatory agency---approved labeling, but reflect common and widely accepted practices in the field.
The products of this project are termed “recommendations” rather than guidelines in order to reflect their nonprescriptive nature. They are meant to function as a reference and do not serve as a substitute for individualized patient assessment and clinical decision making, especially when conducted by specialist clinicians familiar with the treatment of JIA. Importantly, these recommendations are not intended to limit health care coverage for children with JIA.
MATERIALS AND METHODS
RAND/UCLA Appropriateness Method overview
The RAND/UCLA Appropriateness Method was originally developed to help determine when the benefits of a medical intervention outweigh the risks (18) with the understanding that the published literature often does not provide evidence at the level of detail required to guide decisions in everyday clinical practice. This method relies upon the efforts of two distinct groups of participants: the Core Expert Panel (CEP) and the Task Force Panel (TFP). Our CEP was composed of experienced pediatric rheumatologists from the US, Canada, and Europe who are among the world’s leaders in the investigation of the treatment of JIA. The TFP contained internationally recognized pediatric rheumatology clinicians and researchers from the US, Canada, and Europe; an advanced practice pediatric rheumatology nurse; a general pediatrician with expertise in evidence-based medicine; and a patient representative, i.e., a parent of a child with JIA who has broad experience in JIA and family treatment preferences.
The initial step was a systematic review of the literature, which was then used by the CEP to prepare a summary report of the latest scientific evidence. Concurrently, the CEP prepared a comprehensive list of clinical scenarios (or potential indications) for each medical intervention of interest to be addressed by the recommendations. The scenarios categorized hypothetical patients using all possible combinations of key clinical parameters, such as disease activity and prognostic features, relevant to the decision process. The evidence report and the clinical scenarios were presented to the TFP for their review.
Evaluation of the scenarios by the TFP led directly to the recommendations for each medical intervention. In the first round of voting, the TFP anonymously and independently rated the appropriateness of the medical interventions in the clinical scenarios based on the scientific evidence and their best clinical judgment. Differences in opinion from the first round of voting were discussed at a face-to-face meeting, followed by a second rating of the clinical scenarios. The final ratings were compiled and assessed by the CEP and no significant discrepancies were identified. Finally, the ratings were used by the CEP to create recommendations from the clinical scenarios.
Scope of recommendations
These recommendations cover the indications and safety monitoring for the use of NSAIDs, intraarticular glucocorticoid injections, nonbiologic DMARDs, biologic DMARDs, and systemic glucocorticoids for the treatment of the systemic features of systemic arthritis. In contrast to prior ACR recommendations, the scope of this project was not explicitly established by the ACR, but rather by the CEP members. An exhaustive list of broad potential topics for inclusion was constructed by the principal investigator and reviewed and revised by 3 other CEP members. The resultant list of 23 potential topics for consideration was distributed to all CEP members. Using a modified Delphi process via electronic mail, the list of topics was prioritized and gradually shortened until it was deemed feasible in the time allotted to the project. As a result, several relevant topics were necessarily omitted. Medication contraindications and intolerance were not considered. We suggest referring to the 2008 ACR recommendations for the use of DMARDs in rheumatoid arthritis (19) for general guidance about medication contraindications. Tapering or discontinuation of medications for patients with inactive disease was also not considered. Only the most relevant and frequently used agents within each medication class were included, as determined using a structured iterative process via electronic mail with repeated revisions to the proposed list until it was accepted by all CEP members. Agents not widely commercially available for the treatment of JIA at the time of the literature search (e.g., canakinumab, rilonacept, tocilizumab) were excluded. The indications for systemic glucocorticoids for the treatment of synovitis were not considered, owing to a lack of published evidence. The treatment of uveitis, enthesitis, and macrophage activation syndrome was not considered.
Systematic literature review
The systematic literature review was restricted to publications available in Medline. Briefly, we searched using PubMed for articles in English, with abstracts, and published from 1966 to the present. The final search strategy, developed in consultation with a virtual reference librarian, is shown in Supplementary Appendix A (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658). We performed the final search on March 3, 2009, and identified 756 articles for further consideration.
We next performed title and abstract review of the identified articles. In keeping with the scope of the recommendations, all studies that did not explicitly address any pharmacotherapeutic clinical outcomes of the medications of interest were excluded (e.g., radiographic descriptive studies, pharmacokinetics, genomics, etc.). Because of the relatively small number of identified articles, we included uncontrolled studies, regardless of the number of patients reported. Review articles were excluded. Two reviewers (KR, RF) reviewed all of the abstracts for inclusion, blinded to each other’s determinations. Disagreement was resolved by a third reviewer (ST-R) and confirmed by a fourth reviewer (TB), when necessary. Following title and abstract review, 239 articles remained for consideration.
Each of the full-length articles was abstracted by one of a team of reviewers (SC, RF, AJ, PN, NMP, KR, ST-R, or TB), with key elements from each article entered into an electronic database. Articles were excluded during the full-length article review when no pharmacotherapeutic clinical outcomes of medications of interest were identified; all of these exclusions were verified by a second reviewer (TB). As a result, 214 articles were included in the final comprehensive evidence report that was presented to the TFP prior to the first round of voting.
An identical literature search was repeated on October 5, 2009, to identify any new publications since the first systematic review. Thirty new articles were identified and subjected to the same review process as the initial search. Seven fully abstracted articles were added to the updated evidence report and presented to the TFP prior to the face-to-face meeting.
Scenario definitions
In order to develop clinical scenarios in accordance with the RAND/UCLA Appropriateness Method (18), several key clinical decision parameters were deemed necessary by the CEP: disease phenotype, prognostic features, disease activity, and current therapy. Definitions and values for these key parameters were as evidence based as possible and were determined using a structured iterative process via electronic mail with repeated revisions to the proposed parameters until they were accepted by all CEP members.
JIA treatment groups
Chronic childhood arthritis is a heterogeneous condition. The most recent ILAR disease classification criteria (1) divide JIA into 6 distinct categories. However, this disease classification system was not strictly applied to the development of these recommendations for two reasons: currently, there is minimal evidence to support the differential treatment of children with JIA for many of the category distinctions, and the inclusion of all 6 categories would unnecessarily increase the total number of scenarios to an unmanageable number for consideration by the TFP (18).
In place of the ILAR JIA classification, the CEP developed “treatment groups” for these recommendations with the goal of succinctly representing clinical decision making in the treatment of JIA. The JIA category of systemic arthritis proved to be especially challenging to evaluate. Attempts to exhaustively depict the myriad possible clinical presentations of systemic arthritis were impractical. Therefore, recommendations for the treatment of significant active systemic features (e.g., fever) and active arthritis for patients with systemic arthritis were considered separately and independently by the TFP. Other authors have suggested this dichotomy when considering therapeutic choices for systemic arthritis (22). The appropriate treatment of systemic arthritis patients with concurrently active systemic features and active arthritis may be expected to incorporate elements of both sets of recommendations, but this was not explicitly considered by the TFP. The 5 JIA treatment groups used in these recommendations are described below.
History of arthritis of 4 or fewer joints
This group includes patients with the ILAR categories of persistent oligoarthritis, as well as patients with psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis who have developed active arthritis in only 4 or fewer joints in total throughout the history of their disease course. Patients who currently have 4 or fewer active joints, but who have a history of 5 or more active joints in total, are considered in the “history of arthritis of 5 or more joints” treatment group. Patients with systemic arthritis or active sacroiliac arthritis are considered in separate treatment groups.
History of arthritis of 5 or more joints
This group includes patients with the ILAR categories of extended oligoarthritis, rheumatoid factor (RF)---negative polyarthritis, RF-positive polyarthritis, as well as patients with psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis who have developed active arthritis in 5 or more joints in total throughout the history of their disease. Patients in this group need not currently have 5 or more active joints. Patients with systemic arthritis or active sacroiliac arthritis are considered in separate treatment groups.
Active sacroiliac arthritis
This group includes all patients with clinical and imaging evidence of active sacroiliac arthritis. This group is anticipated to include primarily patients with the ILAR categories of enthesitis-related arthritis and psoriatic arthritis, but may include patients from any of the ILAR JIA categories.
Systemic arthritis with active systemic features (and without active arthritis)
This group includes all patients who fulfill the ILAR criteria for systemic arthritis and who have active fever of systemic JIA with or without other systemic features, but without active arthritis. An example of this clinical phenotype would be a patient whose arthritis resolved spontaneously or rapidly upon initiation of NSAIDs but who had persistent fever.
Systemic arthritis with active arthritis (and without active systemic features)
This category includes all patients who fulfill the ILAR criteria for systemic arthritis and who have active arthritis, but without active systemic features. An example of this clinical phenotype would be a patient whose systemic features resolved spontaneously or rapidly upon initiation of NSAIDs but whose arthritis remained active.
Features of poor prognosis
Risk stratification is crucial for guiding optimal treatment. The prognosis for patients with JIA is variable, and some clinical factors have been shown to predict worse outcomes. The CEP considered the published literature and their personal clinical experience in developing the features of poor prognosis for these recommendations.
Each JIA treatment group has its own respective features of poor prognosis. In each case, the presence of one listed feature is sufficient to classify the patient as having a poor prognosis for the purposes of these recommendations. A more complex approach to features of poor prognosis would have increased the number of scenarios to an unmanageable number for consideration by the TFP (18). The features of poor prognosis (and their corresponding literature references) are listed according to each JIA treatment group in Tables 1---5. Of note, the CEP believed there was insufficient published evidence available to include damage detected by imaging other than radiographs as a feature of poor prognosis in JIA.
Table 1.
Features of poor prognosis and disease activity for a history of arthritis of 4 or fewer joints
| Features of poor prognosis (must satisfy 1) |
| Arthritis of the hip (23---25) or cervical spine |
| Arthritis of the ankle (25---27) or wrist (26,28) AND marked (29) or prolonged (23,25,26,29,30) inflammatory marker elevation |
| Radiographic damage (erosions or joint space narrowing by radiograph) (31) |
| Disease activity levels |
| Low disease activity (must satisfy all) |
| 1 or fewer active joints |
| Erythrocyte sedimentation rate or C-reactive protein level normal |
| Physician global assessment of overall disease activity <3 of 10 |
| Patient/parent global assessment of overall well-being <2 of 10 |
| Moderate disease activity (does not satisfy criteria for low or high activity) |
| 1 or more features greater than low disease activity level AND fewer than 3 features of high disease activity |
| High disease activity (must satisfy at least 3) |
| 2 or more active joints |
| Erythrocyte sedimentation rate or C-reactive protein level greater than twice upper limit of normal |
| Physician global assessment of overall disease activity ≥7 of 10 |
| Patient/parent global assessment of overall well-being ≥4 of 10 |
Table 5.
Features of poor prognosis and disease activity for systemic arthritis with active arthritis (and without active systemic features)
| Features of poor prognosis (must satisfy 1) |
| Arthritis of the hip (38) |
| Radiographic damage (erosions or joint space narrowing by radiograph) (31) |
| Disease activity levels |
| Low disease activity (must satisfy all) |
| 4 or fewer active joints |
| Erythrocyte sedimentation rate or C- reactive protein level normal |
| Physician global assessment of overall disease activity <4 of 10 |
| Patient/parent global assessment of overall well-being <2 of 10 |
| Moderate disease activity (does not satisfy criteria for low or high activity) |
| 1 or more features greater than low disease activity level AND fewer than 3 features of high disease activity |
| High disease activity (must satisfy at least 3) |
| 8 or more active joints |
| Erythrocyte sedimentation rate or C- reactive protein level greater than twice upper limit of normal |
| Physician global assessment of overall disease activity ≥7 of 10 |
| Patient/parent global assessment of overall well-being ≥5 of 10 |
JIA disease activity
Recommendations require clear definitions of disease activity to make rational therapeutic choices. In these recommendations, “active joints” and “active arthritis” are defined by joints with swelling not due to deformity or joints with limitation of motion and with pain or tenderness (10). “Active fever” means current fever that is attributable to systemic arthritis disease activity. At least one continuous measure of JIA disease activity has been recently developed (39). Nevertheless, currently no continuous disease activity measures have been extensively validated or widely applied to daily clinical practice. Accordingly, the CEP developed levels of disease activity for these recommendations that are specific to each treatment group and with the goal of succinctly capturing clinical decision making. It should be noted that these disease activity levels are subjective and not strictly evidence based. However, the creation of discrete disease activity levels was requisite for the effective use of the RAND/UCLA Appropriateness Method.
These recommendations are based on 3 disease activity levels: low, moderate, and high. Patients with inactive disease were not considered. In a broad sense, the low disease activity level is meant to represent patients at the lowest disease activity level for which a majority of clinicians may consider altering the current medication regimen, and the high disease activity level is meant to represent patients with disease activity that is equivalent to or higher than the “average” subject that may have been enrolled in a clinical trial of the medications under consideration. The CEP considered baseline patient data from recently published clinical trials (10,15,17,40---42), data from a recent attempt to define minimal disease activity in JIA (43), and their personal clinical experience in developing the disease activity levels for these recommendations. The disease activity levels are listed according to each JIA treatment group in Tables 1---5.
During the evaluation of the clinical scenarios, TFP members were allowed to recommend continuation of current therapy (not initiate a new therapeutic agent), regardless of the current disease activity level. In addition to a patient’s current disease activity level, clinicians in practice may consider a patient’s disease activity level prior to initiating the current treatment regimen when evaluating the effectiveness of the current treatment. That is, a current state of low disease activity may represent a recent major improvement for one patient and no improvement for another. Due to the complexity and number of clinical scenarios, previous disease activity levels were not included in the recommendation process and TFP members considered the appropriateness of initiating new therapeutic agents based on a patient’s current disease activity level only.
Therapeutic agent definitions
Medication classes used in the recommendation scenarios are defined as follows: NSAID refers to all nonsteroidal antiinflammatory drugs used commonly in clinical practice in the US and includes selective cyclooxygenase 2 inhibitors but not aspirin. Calcineurin inhibitors refer to cyclosporine and tacrolimus. Tumor necrosis factor α (TNFα) inhibitors refer to adalimumab, etanercept, and infliximab.
Clinical evaluation of the effectiveness of a given therapeutic agent may depend on the dose received. For all recommendations, it is assumed that patients have received the maximum tolerated typical dose of prior medications, as listed in Supplementary Appendix B (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658). Of note, the dose of methotrexate was assumed to be 15 mg/m2 (0.6 mg/kg) and administered via the parenteral route. These specific doses were used as a guide for TFP members when considering the scenarios; higher doses of these therapeutic agents may be appropriate in some clinical situations.
Clinical evaluation of the effectiveness of a given therapeutic agent may depend on the duration of therapy. Some scenarios explicitly stated the duration of the current treatment regimen. In scenarios where duration of therapy was not defined, it was assumed that the duration of therapy was sufficiently long to assess the response to therapy.
The recommendations assume that all medication regimens may contain a single NSAID as adjunct therapy when considering the appropriateness of initiating new therapies. For example, a patient described as currently receiving methotrexate is assumed to be taking methotrexate with concurrent use of a single NSAID, as appropriate.
Many of the recommendations refer to “initiating” a new therapy for patients presently undergoing treatment for JIA. In these cases, “initiating” is defined as either adding the new therapy while continuing current therapy or switching to the new therapy while discontinuing the current therapy. To limit the total number of scenarios, we made no distinction between adding and switching for some of the recommendations. Of note, combination biologic DMARD therapy was not considered by these recommendations, owing to concern from the reported increased incidence of infections in studies of rheumatoid arthritis in adult populations (44,45). Accordingly, initiation of a new biologic DMARD is always intended to be accompanied by discontinuation of any current biologic DMARD.
Safety monitoring
Scenarios were created to evaluate the appropriateness of several safety monitoring interventions for selected therapeutic agents and were voted upon by the TFP in the same manner as the treatment scenarios.
RAND/UCLA Appropriateness Method voting and scoring
A teleconference was conducted to orient all TFP members to the voting task. Individual scenarios contained an explicit question that incorporated the key clinical parameters described above (e.g., “Rate the appropriateness of initiating TNFα inhibitor in a patient with a history of arthritis of 5 or more joints who has received methotrexate for 6 months and has moderate disease activity and features of poor prognosis”). There were 1,539 such individual scenarios and each TFP member evaluated all of them.
Each scenario was evaluated for “appropriateness,” which is defined as “the health benefits exceed the health risks by a sufficiently wide margin that the intervention is worth doing” (18). In this evaluation, the risks of initiating a particular therapeutic agent include the risk of not initiating an alternative agent (e.g., the risk of initiating NSAID monotherapy includes the risk of not initiating methotrexate with its greater potential for therapeutic benefit). The TFP scored each scenario on a 9-point scale, with scores of 7---9 denoting “appropriate,” 1---3 denoting “inappropriate,” and 4---6 denoting “uncertain,” i.e., either the risks and benefits are approximately equal or not enough information is available to make a meaningful evaluation. TFP members cast their votes while taking into account the currently available published literature (including but not limited to that provided to them in the evidence report) and their own personal clinical experience. Votes were cast independently by entering them into a formatted electronic spread sheet. The voting sheets were then electronically collected and combined to analyze the results.
The votes were analyzed in accordance with the RAND/UCLA Appropriateness Method (18), taking into account both the distribution and the median value of the votes. The scenario votes were first evaluated for disagreement (wide dispersion of votes), defined as one-third or more of the TFP assigning a score of inappropriate (1, 2, or 3), while one-third or more of the TFP assigned the same scenario a score of appropriate (7, 8, or 9). In the absence of disagreement, a median score of 3 or less classified a scenario as “inappropriate,” and a median rating of 7 or greater classified a scenario as “appropriate.” Those scenarios with median ratings in the 3.5 to 6.5 range, together with those where disagreement occurred, were classified as “uncertain.”
Following analysis of the first round of voting, a subsequent face-to-face meeting of the TFP was conducted and discussion was focused on scenarios where the voting outcome was “uncertain.” Following sufficient discussion on each group of scenarios, the TFP members voted for the final time. No attempts were made to “force” consensus among the TFP. Rather, the RAND/UCLA Appropriateness Method largely seeks to identify existing consensus (18).
Developing recommendations from votes
Statements listed as recommendations met the RAND/UCLA Appropriateness Method criteria for “appropriate” in the final TFP votes with no wide dispersion of votes, and the median score was in the appropriate range (7 to 9). In some instances, more than one individual therapeutic agent was recommended for a given combination of clinical parameters; these are noted as being “recommended as one treatment approach.” Indications for initiating therapeutic agents that did not meet the criteria for appropriate were summarized as either uncertain or inappropriate. In these summary statements, uncertain means that some or all of the scenarios met the definition of “uncertain” and some or none of the scenarios met the definition of “inappropriate.” Inappropriate means that all of the scenarios met the definition of “inappropriate” and none met the definition of “uncertain.” The recommendations were reviewed in detail by all CEP members to ensure proper and clear translation of the TFP votes into text.
During the face-to-face discussion of the scenarios, several points were raised that the TFP members believed should be mentioned in the recommendations, even though they were not addressed in the voting scenarios. Following discussion, consensus on these points was reached through a simple show of hands vote. These statements are clearly noted in the results, are not considered formal recommendations, and were not assigned a level of evidence.
Rating evidence for recommendations
For each final recommendation of appropriate or inappropriate, a level of evidence was assigned based on the methods of the University of Oxford Centre for Evidence-Based Medicine, UK (46). This categorization of evidence was used in the recently published European League Against Rheumatism recommendations for the management of rheumatoid arthritis (47) and incorporates the concept of “extrapolations,” where published data are used in a situation that has potentially clinically important differences from the original study situation. Such situations are frequently encountered when the patient’s clinical factors in a recommendation do not match exactly with those of the supporting studies (19). The Oxford system also distinguishes between recommendations for which there is low-grade evidence and those for which there is an absence of published evidence.
Level of evidence “A” was assigned when the recommendation was supported by randomized clinical trials (or consistent inception cohort studies for questions of prognosis). Level of evidence “B” was assigned when the recommendation was supported by nonrandomized controlled studies (e.g., cohort and case---control studies) or extrapolations from randomized clinical trials. Level of evidence “C” was assigned when the recommendation was supported by uncontrolled studies (case series), extrapolations from nonrandomized controlled studies, or marked extrapolations from randomized clinical trials (e.g., studies of adult arthritis patients applied to juvenile arthritis or studies of polyarthritis phenotype applied to oligoarthritis). Level of evidence “D” was assigned when the recommendation was based on expert opinion without supporting published evidence.
Managing perceived potential conflicts of interest
Perceived potential conflicts of interest were managed in a prospective and structured manner. All members of the CEP and TFP completed and submitted the ACR disclosure of interest form prior to participation in the project. The forms were updated prior to the face-to-face meeting. A summary listing of all perceived potential conflicts of interest was distributed to all project participants and was submitted for publication along with these recommendations.
Peer review of recommendations
Following submission of the draft recommendations, the ACR invited peer review by members of the ACR Practice Guidelines Subcommittee, the ACR Quality of Care Committee, and the ACR Board of Directors. The recommendations were ultimately subject to the regular review process of this journal.
Updates to ACR recommendations
We expect that knowledge of the appropriate therapeutic management of JIA will continue to advance. Clearly, these ACR recommendations will require updating to remain accurate and relevant. We suggest that approximately 3 years after the initiation of this current project (i.e., in 2012), the ACR Practice Guidelines Subcommittee and the ACR Quality of Care Committee evaluate newly published evidence and solicit expert opinion on the need for revision of these recommendations. Targeted updates focused only on areas of new published evidence may be appropriate.
Future research in the treatment of JIA may reasonably be directed by knowledge deficiencies identified by these recommendations. Of particular note to the CEP was the need for comparative long-term studies of the benefits and risks of early initiation of biologic therapies, development and implementation of continuous disease activity scores in clinical practice, studies of the evaluation and management of enthesitis, studies of the management of systemic arthritis and macrophage activation syndrome, and studies of the appropriate management of patients with inactive disease receiving therapy.
RESULTS
Each ACR recommendation for the treatment of JIA is followed by the assigned level of evidence and the corresponding publication citations.
General medication usage
The TFP considered the general usage of intraarticular glucocorticoid injections and the use of methotrexate when initiating TNFα inhibitors. These recommendations apply broadly across treatment groups.
Glucocorticoid joint injections
The use of glucocorticoid joint injections for active arthritis was recommended, regardless of concurrent therapy (no DMARD, nonbiologic DMARD, or biologic DMARD) or JIA treatment group (level C) (48---65). Glucocorticoid joint injections should be performed with triamcinolone hexacetonide, owing to its demonstrated superior efficacy (level A) (52,53). Intraarticular glucocorticoid injections are expected to result in clinical improvement of arthritis for at least 4 months (level A) (49---66). A shorter duration of clinical response may imply a need for escalation of systemic therapy. Intraarticular glucocorticoid injections that result in clinical improvement of arthritis for at least 4 months may be repeated as needed (level B) (50,53,57,64,65).
Methotrexate with TNFα inhibitors
Continuing methotrexate when initiating a TNFα inhibitor (etanercept or adalimumab) was recommended for patients who had a partial previous clinical response to methotrexate (level B) (67). The TFP did not reach agreement on continuing or discontinuing methotrexate when initiating a TNFα inhibitor (etanercept or adalimumab) for patients who had a poor previous clinical response to methotrexate. The appropriateness of continuing methotrexate when initiating infliximab was assumed and was not evaluated by the TFP, owing to the recognized potential for methotrexate to reduce the incidence of neutralizing antibodies to infliximab (68) and consistent with the labeling of infliximab (69).
Initiation of therapeutic agents
The appropriateness of initiating various therapeutic agents in the treatment of JIA is organized by treatment group. Each recommendation is characterized by the patient’s clinical factors: treatment group, current medication, disease activity, and features of poor prognosis. Individual therapeutic agents are listed in the order of escalation of therapy as determined by the TFP. In situations where two or more agents were recommended for similar patient clinical factors, the agents are listed alphabetically. The recommendations are not mutually exclusive, i.e., the initiation of more than one therapeutic agent may be appropriate for a given set of patient clinical factors due to overlapping indications. Therapeutic agents that were considered by the TFP but not recommended for initiation are listed at the end of each treatment group in alphabetical order.
History of arthritis of 4 or fewer joints
The definitions of disease activity and features of poor prognosis for this treatment group are listed in Table 1. A diagram of the overall treatment strategy is shown in Figure 1.
Figure 1.
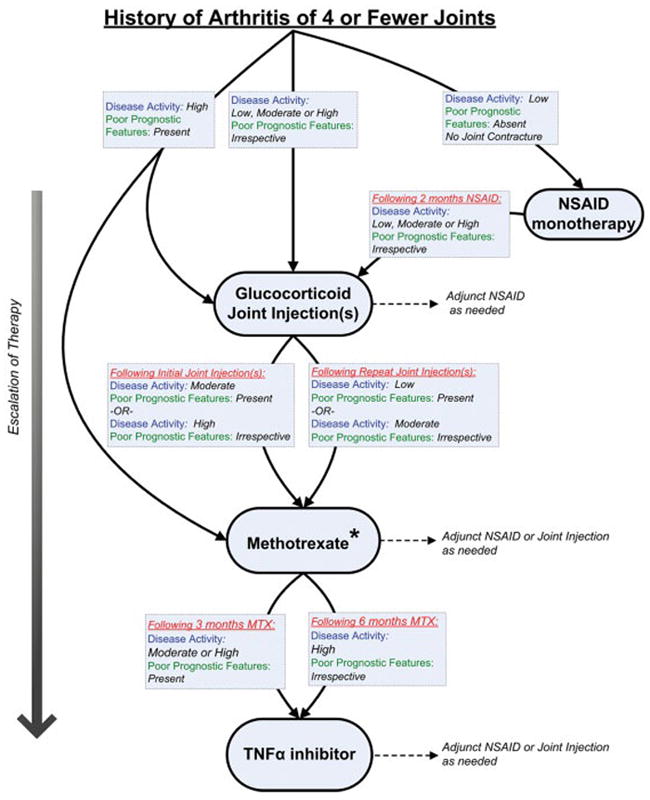
Treatment recommendations for patients with a history of arthritis of 4 or fewer joints. These recommendations are intended for patients with juvenile idiopathic arthritis (JIA) who have only developed active arthritis in 4 or fewer joints in total throughout the history of their disease course and are based upon duration of current therapy, disease activity, and features of poor prognosis. If criteria for escalation of therapy are not met, then continue current therapy along with adjunct nonsteroidal antiinflammatory drugs (NSAIDs) or glucocorticoid joint injections, as needed. Recommendations for reduction of therapy are not addressed. See Table 1 for definitions of disease activity and features of poor prognosis. * = sulfasalazine may be an appropriate treatment for patients with the enthesitis-related arthritis category of JIA (see text for details); MTX = methotrexate; TNFα = tumor necrosis factor α.
NSAID monotherapy
Initiation of NSAID monotherapy (without glucocorticoid joint injection) was recommended as one treatment approach for patients with low disease activity, without joint contracture, and without features of poor prognosis (level B) (70---76). Continuation of NSAID monotherapy (without additional therapy) for longer than 2 months was inappropriate for patients with active arthritis, irrespective of poor prognostic features.
Intraarticular glucocorticoid injections
Intraarticular glucocorticoid injections (with or without additional therapy) were recommended for all patients with active arthritis, irrespective of disease activity level, prognostic features, or joint contracture (level C) (48---66). As stated above, glucocorticoid joint injections should be performed with triamcinolone hexacetonide (level A) (52) and are expected to result in clinical improvement of arthritis for at least 4 months (level A) (49---66). A shorter duration of clinical improvement may imply a need for escalation of systemic therapy. Glucocorticoid injections that result in clinical improvement of arthritis for at least 4 months may be repeated as needed (level B) (50,53,57,64,65).
Methotrexate
Initiation of methotrexate was recommended as initial treatment (without prior therapy) for patients with high disease activity and features of poor prognosis (level C) (40,77---83). Following initial glucocorticoid joint injection(s), initiation of methotrexate was recommended for patients with high disease activity without features of poor prognosis and for patients with moderate disease activity and features of poor prognosis (level C) (40,77---83). Following repeated glucocorticoid injections, initiation of methotrexate was recommended for patients with moderate disease activity without features of poor prognosis and for patients with low disease activity and features of poor prognosis (level C) (40,77---83).
Sulfasalazine
Initiation of sulfasalazine was recommended following glucocorticoid joint injection or an adequate trial of NSAIDs for patients with the enthesitis-related arthritis category of JIA with moderate or high disease activity, irrespective of features of poor prognosis (level B) (84). Initiation of sulfasalazine was uncertain for patients who are not diagnosed with the enthesitis-related arthritis category of JIA.
TNFα inhibitors
Initiation of a TNFα inhibitor was recommended for patients who have received glucocorticoid joint injections and 3 months of methotrexate at the maximum tolerated typical dose and have moderate or high disease activity and features of poor prognosis (level C) (10,15). Initiation of a TNFα inhibitor was also recommended for patients who have received glucocorticoid joint injections and 6 months of methotrexate and have high disease activity without features of poor prognosis (level C) (10,15).
Additionally, initiation of a TNFα inhibitor was recommended for patients specifically with the enthesitis-related arthritis category of JIA who have received glucocorticoid joint injections and an adequate trial of sulfasalazine (without prior methotrexate) and have moderate or high disease activity, irrespective of prognostic features (level C) (11,85).
Abatacept
Initiation of abatacept was uncertain prior to initiation of a TNFα inhibitor.
Hydroxychloroquine
Initiation of hydroxychloroquine monotherapy (with or without concurrent NSAIDs) was inappropriate for patients with active arthritis (level C) (86).
Leflunomide
Initiation of leflunomide was uncertain.
Nonbiologic DMARD combinations
Initiation of nonbiologic DMARD combinations (methotrexate plus sulfasalazine and/or hydroxychloroquine) was uncertain.
History of arthritis of 5 or more joints
The definitions of disease activity and features of poor prognosis for this treatment group are listed in Table 2. A diagram of the overall treatment strategy is shown in Figure 2.
Table 2.
Features of poor prognosis and disease activity for a history of arthritis of 5 or more joints
| Features of poor prognosis (must satisfy 1) |
| Arthritis of the hip (23---25) or cervical spine |
| Positive rheumatoid factor (5,23,28,30,32,33) OR anti---cyclic citrullinated peptide (33,34) antibodies |
| Radiographic damage (erosions or joint space narrowing by radiograph) (31) |
| Disease activity levels |
| Low disease activity (must satisfy all) |
| 4 or fewer active joints |
| Erythrocyte sedimentation rate or C-reactive protein level normal |
| Physician global assessment of overall disease activity <4 of 10 |
| Patient/parent global assessment of overall well-being <2 of 10 |
| Moderate disease activity (does not satisfy criteria for low or high activity) |
| 1 or more features greater than low disease activity level AND fewer than 3 features of high disease activity |
| High disease activity (must satisfy at least 3) |
| 8 or more active joints |
| Erythrocyte sedimentation rate or C-reactive protein level greater than twice upper limit of normal |
| Physician global assessment of overall disease activity ≥7 of 10 |
| Patient/parent global assessment of overall well-being ≥5 of 10 |
Figure 2.
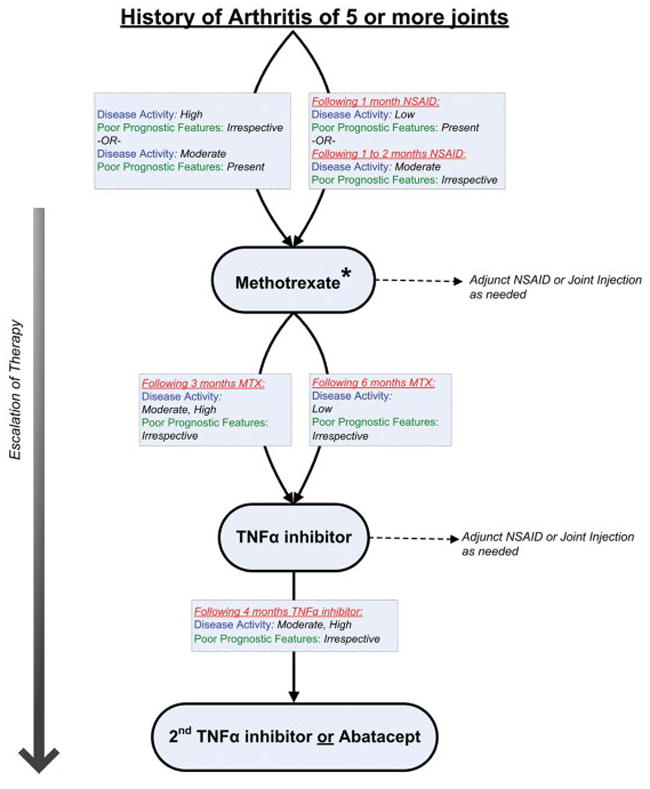
Treatment recommendations for patients with a history of arthritis of 5 or more joints. These recommendations are intended for patients with juvenile idiopathic arthritis who have developed active arthritis in 5 or more joints in total throughout the history of their disease and are based upon duration of current therapy, disease activity, and features of poor prognosis. If criteria for escalation of therapy are not met, then continue current therapy along with adjunct nonsteroidal antiinflammatory drugs (NSAIDs) or glucocorticoid joint injections, as needed. Recommendations for reduction of therapy are not addressed. See Table 2 for definitions of disease activity and features of poor prognosis. * = leflunomide may be an appropriate treatment alternative (see text for details); MTX = methotrexate; TNFα = tumor necrosis factor α.
NSAID monotherapy
Initiation of NSAID therapy alone (monotherapy without glucocorticoid joint injection) was uncertain for patients with active arthritis. Continuation of NSAID monotherapy for longer than 2 months was inappropriate for patients with active arthritis, irrespective of poor prognostic features (level C) (80,87).
Methotrexate
Initiation of methotrexate was recommended as initial treatment for patients with high disease activity, irrespective of poor prognostic factors, and for patients with moderate disease activity and features of poor prognosis (level B) (40,77---80). Following approximately 1 month of NSAIDs, initiation of methotrexate was recommended for patients with low disease activity and features of poor prognosis (level B) (40,77---80). Following approximately 1 to 2 months of NSAIDs, initiation of methotrexate was recommended for patients with moderate disease activity without features of poor prognosis (level B) (40,77---80).
Leflunomide
The TFP generally favored the use of methotrexate over leflunomide, owing to greater personal and collective experience with methotrexate. However, initiation of leflunomide was recommended as one treatment approach as initial treatment for patients with high disease activity and features of poor prognosis (level B) (77). Following a brief trial of NSAIDs, initiation of leflunomide was recommended as one treatment approach for patients with high disease activity without features of poor prognosis and for patients with moderate disease activity with features of poor prognosis (level B) (77).
TNFα inhibitors
Initiation of a TNFα inhibitor was recommended for patients who have received methotrexate or leflunomide for 3 months at the maximum tolerated typical dose and have moderate or high disease activity, irrespective of poor prognostic features (level B) (10,15). Initiation of a TNFα inhibitor was also recommended for patients who have received methotrexate or leflunomide for 6 months and have low disease activity, irrespective of poor prognostic features (level B) (10,15).
Switching from one TNFα inhibitor to another was recommended as one treatment approach for patients who have received the current TNFα inhibitor for 4 months and have moderate or high disease activity, irrespective of poor prognostic features (level C) (88,89). Switching to a TNFα inhibitor was recommended as one treatment approach for patients who have received abatacept for 3 months and have high disease activity and features of poor prognosis and for patients who have received abatacept for 6 months and have moderate or high disease activity, irrespective of prognostic features (level D).
Abatacept
Initiation of abatacept was recommended as one treatment approach for patients who have received a TNFα inhibitor for 4 months and have high disease activity, irrespective of features of poor prognosis, or moderate disease activity and features of poor prognosis (level B) (17). Initiation of abatacept was recommended as one treatment approach for patients who have received more than one TNFα inhibitor sequentially and have moderate or high disease activity, irrespective of poor prognostic features, or low disease activity with features of poor prognosis (level B) (17).
Rituximab
Initiation of rituximab was recommended as one treatment approach for patients who have received a TNFα inhibitor and abatacept sequentially and have high disease activity, irrespective of poor prognostic features, or have moderate disease activity and features of poor prognosis (level C) (90---92). Although not formally assessed using the RAND/UCLA Appropriateness Method, there was consensus among the TFP that rituximab may be more appropriate for patients who test positive for RF compared to patients who do not.
Anakinra
Initiation of anakinra was uncertain.
Hydroxychloroquine
Initiation of hydroxychloroquine monotherapy (with or without concurrent NSAIDs) was inappropriate for patients with active arthritis (level A) (86).
Sulfasalazine
Initiation of sulfasalazine was uncertain. Patients with the enthesitis-related arthritis category of JIA and history of arthritis of 5 or more joints were not independently considered by the TFP.
Nonbiologic DMARD combinations
Initiation of nonbiologic DMARD combinations (methotrexate plus sulfasalazine and/or hydroxychloroquine) was uncertain. As stated in Materials and Methods, intolerance of or contraindications to biologic DMARD therapies were not considered by the TFP.
Active sacroiliac arthritis
As stated in Materials and Methods, active sacroiliac arthritis was defined by the presence of clinical and imaging evidence. The definitions of disease activity and features of poor prognosis for this treatment group are listed in Table 3. The only medication class evaluated by the TFP for this treatment group was the TNFα inhibitors. There is no corresponding figure for this treatment group.
Table 3.
Feature of poor prognosis and disease activity for active sacroiliac arthritis
| Feature of poor prognosis |
| Radiographic damage of any joint (erosions or joint space narrowing by radiograph) |
| Disease activity levels |
| Low disease activity (must satisfy all) |
| Normal back flexion |
| Erythrocyte sedimentation rate or C-reactive protein level normal |
| Physician global assessment of overall disease activity <4 of 10 |
| Patient/parent global assessment of overall well-being <2 of 10 |
| Moderate disease activity (does not satisfy criteria for low or high activity) |
| 1 or more features greater than low disease activity level AND fewer than 2 features of high disease activity |
| High disease activity (must satisfy at least 2) |
| Erythrocyte sedimentation rate or C-reactive protein greater than twice upper limit of normal |
| Physician global assessment of overall disease activity ≥7 of 10 |
| Patient/parent global assessment of overall well-being ≥4 of 10 |
TNFα inhibitors
In general, initiation of a TNFα inhibitor was recommended more readily for patients with active sacroiliac arthritis than for patients without this joint affected. Initiation of a TNFα inhibitor was recommended for patients with active sacroiliac arthritis who have received an adequate trial of NSAIDs and have high disease activity and features of poor prognosis (level C) (93,94). Initiation of a TNFα inhibitor was also recommended for patients who have received 3 months of methotrexate and have high disease activity, irrespective of prognostic factors, or moderate disease activity with features of poor prognosis, or 6 months of methotrexate and moderate disease activity without features of poor prognosis (level C) (93,94). Also recommended was initiation of a TNFα inhibitor for patients who have received 3 months of sulfasalazine and have moderate or high disease activity, irrespective of prognostic features, or 6 months of sulfasalazine and low disease activity with features of poor prognosis (level C) (93,94).
Systemic arthritis
As noted in Materials and Methods, the JIA category of systemic arthritis was divided into two treatment groups: active systemic features and active arthritis. The appropriate treatment of patients with concurrent active systemic features and active arthritis may be expected to incorporate elements of both sets of recommendations. Given the variable course of systemic arthritis and the extent to which the prior disease course may influence subsequent treatment decisions, the TFP evaluated the appropriateness of initiating therapies for recently diagnosed patients, as opposed to patients with extensive and potentially influential disease histories.
As noted in Materials and Methods, the initiation of treatment with interleukin-6 inhibitors (such as tocilizumab) or interleukin-1 inhibitors other than anakinra (such as canakinumab or rilonacept) was not considered in the development of these recommendations because these therapeutic agents were not widely commercially available at the time. Options for the treatment of systemic arthritis appear to be increasing. The appropriateness of initiating recently available therapeutic agents for the treatment of systemic arthritis may need to be the focus of a timely update to these recommendations.
Systemic arthritis with active systemic features (and without active arthritis)
The definitions of disease activity and features of poor prognosis for this treatment group are listed in Table 4. A diagram of the overall treatment strategy is shown in Figure 3.
Table 4.
Features of poor prognosis and disease activity for systemic arthritis with active systemic features (and without active arthritis)
| Features of poor prognosis |
| 6-month duration of significant active systemic disease, defined by: fever (35---37), elevated inflammatory markers (35---37), or requirement for treatment with systemic glucocorticoids (36) |
| Disease activity levels (2 levels) |
| Active fever AND physician global assessment of overall disease activity <7 of 10 |
| Active fever AND systemic features of high disease activity (e.g., significant serositis) that result in physician global assessment of overall disease activity ≥7 of 10 |
Figure 3.
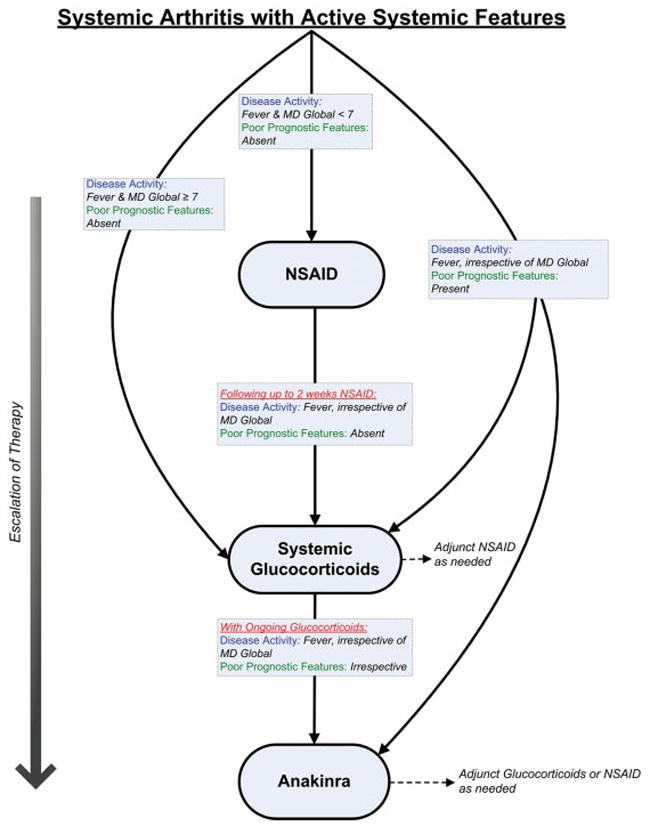
Treatment recommendations for patients with systemic arthritis and active systemic features (and without active arthritis). These recommendations are intended for patients with juvenile idiopathic arthritis who have systemic arthritis with active systemic features and without active arthritis. Recommendations are based upon duration of current therapy, disease activity, and features of poor prognosis. If criteria for escalation of therapy are not met, then continue current therapy along with adjunct nonsteroidal antiinflammatory drugs (NSAIDs), as needed. Recommendations for reduction of therapy are not addressed. See Table 4 for definitions of disease activity and features of poor prognosis. MD Global = physician global assessment of overall disease activity (range 0---10).
These recommendations are not meant to apply to patients with clinical and laboratory evidence of macrophage activation syndrome that warrants specific modification of therapy. Life-threatening clinical scenarios (e.g., cardiac tamponade) were not considered by the TFP and may warrant deviation from these recommendations. TNFα inhibitors were not considered by the TFP in the treatment of active systemic features, owing to their reported relatively poor effectiveness (12,89,95---98).
NSAID monotherapy
Although not formally assessed using the RAND/UCLA Appropriateness Method, there was consensus among the TFP that the use of NSAID monotherapy is appropriate during the clinical evaluation of possible systemic arthritis. The following recommendations apply to patients who have been diagnosed with systemic arthritis.
The initiation (or continuation) of NSAID monotherapy was uncertain for patients with active fever. Initiation of NSAID monotherapy was inappropriate for patients with active fever and physician global assessment of overall disease activity (MD global) of ≥7 of 10 (level D). Continuation of NSAID monotherapy for a duration greater than 1 month was inappropriate for patients with active fever (level C) (99).
Systemic glucocorticoids
Owing to a near complete lack of published evidence, specific systemic glucocorticoid doses or routes of administration were not considered by the TFP.
Initiation of systemic glucocorticoids (with or without additional concurrent therapy) was recommended as initial therapy for patients with active fever and MD global of ≥7 (level D). Initiation of systemic glucocorticoids following up to 2 weeks of NSAIDs was recommended for all patients with active fever (level C) (99,100).
Anakinra
Initiation of anakinra was recommended for all patients with active fever and features of poor prognosis, irrespective of current therapy (level C) (101). Initiation of anakinra was recommended for all patients who sustain or develop active fever while receiving systemic glucocorticoids (level C) (16,101---103).
Calcineurin inhibitors
Initiation of calcineurin inhibitors for patients with active fever and without active arthritis was uncertain for initial management.
Intravenous immunoglobulin
Initiation of intravenous immunoglobulin for patients with active fever and without active arthritis was uncertain for initial management.
Methotrexate
Initiation of methotrexate was inappropriate for initial management of patients with active fever and without active arthritis (level B) (78).
Thalidomide
Initiation of thalidomide for patients with active fever and without active arthritis was uncertain for initial management.
Systemic arthritis with active arthritis (and without active systemic features)
The definitions of disease activity and features of poor prognosis for this treatment group are listed in Table 5. A diagram of the overall treatment strategy is shown in Figure 4. Consistent with the assessment of other treatment groups, the appropriateness of systemic glucocorticoids for the treatment of arthritis in patients with systemic arthritis was not addressed.
Figure 4.
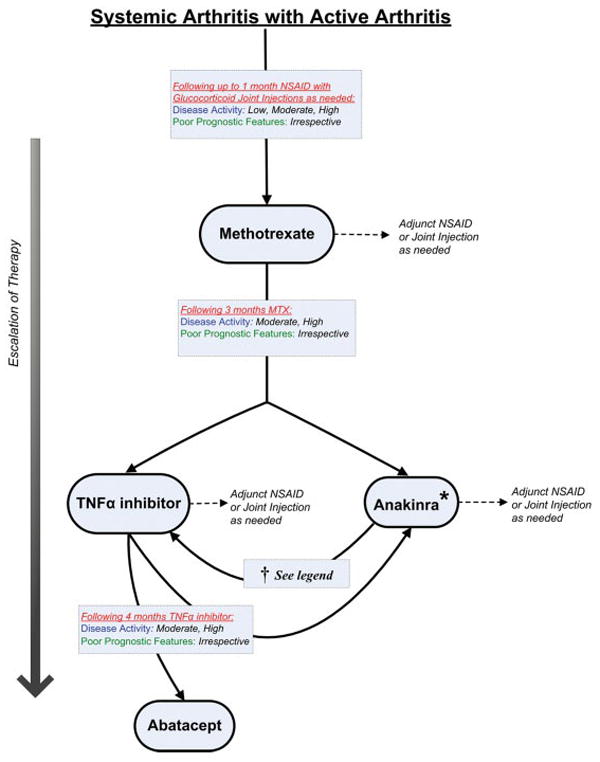
Treatment recommendations for patients with systemic arthritis and active arthritis (and without active systemic features). These recommendations are intended for patients with juvenile idiopathic arthritis who have systemic arthritis with active arthritis and without active systemic features. Recommendations are based upon duration of current therapy, disease activity, and features of poor prognosis. If criteria for escalation of therapy are not met, then continue current therapy along with adjunct nonsteroidal antiinflammatory drugs (NSAIDs), as needed. Recommendations for reduction of therapy are not addressed. See Table 5 for definitions of disease activity and features of poor prognosis. MTX = methotrexate; TFNα = tumor necrosis factor α; * = initiation of anakinra for the treatment of arthritis may be less appropriate later in the disease course compared to nearer the onset of disease; † = switching from anakinra to a TNFα inhibitor may be appropriate for some patients with moderate or high disease activity, irrespective of features of poor prognosis, but there is a possible risk of unmasking latent systemic features when discontinuing anakinra.
NSAID monotherapy
Initiation of NSAID monotherapy (with or without glucocorticoid joint injections) was recommended for patients with low disease activity without features of poor prognosis (level B) (70). It was presumed that most patients with newly diagnosed systemic arthritis would have received NSAIDs during their diagnostic evaluation. Continuation of NSAID monotherapy (without systemic therapy) for a duration greater than 1 month was uncertain for patients with any level of disease activity, irrespective of poor prognostic features (level D).
Methotrexate
Initiation of methotrexate was recommended for all patients with active arthritis following 1 month or less of NSAID monotherapy (with or without glucocorticoid joint injections), irrespective of poor prognostic features (level B) (40,79).
Anakinra
Initiation (addition) of anakinra was recommended for patients who have received methotrexate and who have moderate or high disease activity, irrespective of features of poor prognosis (level C) (16,101---104). Initiation of anakinra was also recommended for patients who have received methotrexate and a TNFα inhibitor or methotrexate and abatacept and have high or moderate disease activity, irrespective of poor prognostic factors (level C) (16,101---103). Although not formally assessed using the RAND/UCLA Appropriateness Method, there was consensus among the TFP that initiation of anakinra for the treatment of arthritis may be less appropriate later in the disease course compared to nearer to the onset of disease.
TNFα inhibitor
Initiation (addition) of a TNFα inhibitor was recommended for patients who have received 3 months of methotrexate and have moderate or high disease activity, irrespective of features of poor prognosis (level B) (10,15). The TFP expressed that it may be appropriate to switch therapy from anakinra to a TNFα inhibitor for patients with moderate or high disease activity, irrespective of features of poor prognosis (level D). However, concern was expressed regarding possible unmasking of latent systemic disease activity when discontinuing anakinra.
Abatacept
Initiation of abatacept was recommended for patients who have received methotrexate and a TNFα inhibitor and have high disease activity, irrespective of features of poor prognosis, or have moderate disease activity and poor prognostic features (level B) (17).
Calcineurin inhibitors
Initiation of calcineurin inhibitors was inappropriate for patients with active arthritis and without active systemic features (level C) (105,106).
Safety monitoring
The TFP evaluated the appropriateness of several safety monitoring interventions by considering the health benefits (minimizing the chance of toxicity or adverse events) and harms (frequent phlebotomies, false-positive test results). As with the evaluation of initiation of therapeutic agents, the TFP did not consider the economic costs of monitoring or adverse events.
NSAID monitoring
Measurement of serum creatinine, urinalysis, complete blood cell count, and liver enzymes was recommended prior to or soon after the initiation of treatment with routine NSAIDs (level D). Periodic repeat measurements of serum creatinine, urinalysis, complete blood cell count, and liver enzymes were recommended approximately twice yearly for patients receiving chronic daily NSAIDs and approximately once yearly for patients receiving NSAIDs routinely (e.g., 3 to 4 days per week) (level D).
Methotrexate monitoring
Measurement of serum creatinine, complete cell blood count, and liver enzymes was recommended prior to initiation of methotrexate (level D). Shortly after initiation of methotrexate, repeat measurements of serum creatinine, complete blood cell count, and liver enzymes were recommended. No single preferred monitoring strategy was recommended. In general, the TFP recommended laboratory measurements approximately 1 month after initiating methotrexate and then approximately 1 to 2 months after any subsequent increase in methotrexate dose (level D). Repeat measurements of serum creatinine, complete blood cell count, and liver enzymes were recommended approximately every 3 to 4 months for patients receiving a stable dose of methotrexate with no recent history of abnormal laboratory monitoring results (level C) (107,108). Although not formally evaluated using the RAND/UCLA Appropriateness Method, there was consensus among the TFP that laboratory measurements be obtained 1 to 2 days prior to the scheduled weekly dose of methotrexate.
The TFP made several recommendations about appropriate responses to elevated liver enzymes for patients receiving methotrexate. In response to liver enzyme elevation of as much as 2 times the upper limit of normal, either no specific action or rechecking liver enzymes at a shorter interval was recommended. In response to liver enzyme elevation more than 2 times the upper limit of normal, decreasing the dose of methotrexate or temporarily withholding methotrexate administration was recommended. If liver enzymes remain at levels more than 3 times the upper limit of normal following a decrease in the methotrexate dose, discontinuation of methotrexate was recommended (all recommendations are level C) (107---110).
TNFα inhibitor monitoring
Measurement of complete blood cell count, liver enzymes, and serum creatinine was recommended prior to initiation of TNFα inhibitors and approximately every 3 to 6 months thereafter for patients who continue to receive TNFα inhibitors (level D).
Tuberculosis screening
Obtaining Mantoux purified protein derivative skin testing for tuberculosis was recommended prior to initiation of TNFα inhibitors for all patients (level C) (111). Repeat testing approximately once yearly thereafter was recommended for all patients who continue to receive TNFα inhibitors (level D). The appropriateness of interferon-γ release assays for detecting tuberculosis was not evaluated. The appropriateness of tuberculosis testing prior to the initiation of biologic agents other than TNFα inhibitors was not evaluated.
Hepatitis B and hepatitis C screening
Antibody testing for infection with hepatitis B or hepatitis C prior to initiating methotrexate or TNFα inhibitors was recommended for patients with risk factors for infection (level D). Risk factors for hepatitis B and hepatitis C infections are shown in Supplementary Appendix C (available in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)2151-4658).
DISCUSSION
Following a systematic review of the literature and using a formal group assessment process, we provide evidence and consensus-based recommendations for the appropriate initiation and safety monitoring of therapeutic agents in the treatment of JIA. These recommendations are meant as a guide to health care providers caring for children with JIA and are not meant to take the place of individualized care or to serve as health care coverage guidelines. These recommendations will require future updates as scientific knowledge expands regarding the benefits and risks of the treatment of JIA.
Addendum
Therapies that were approved after the original literature review are not included in these recommendations.
Supplementary Material
Table 6.
Summary of recommendations for medication safety monitoring
| Nonsteroidal antiinflammatory drugs |
| Complete blood cell count, liver enzymes, serum creatinine |
| Prior to or soon after initiation of routine use |
| Repeat approximately twice yearly for chronic daily use |
| Repeat approximately once yearly for routine use (3---4 days per week) |
| Methotrexate |
| Complete blood cell count, liver enzymes, serum creatinine |
| Prior to initiation |
| Approximately 1 month after initiation |
| Approximately 1---2 months after increase in dose |
| Repeat approximately every 3---4 months if prior results normal and dose stable |
| Tumor necrosis factor α inhibitors |
| Complete blood cell count, liver enzymes, serum creatinine |
| Prior to initiation |
| Repeat approximately every 3---6 months |
| Tuberculosis screening |
| Prior to initiation |
| Repeat approximately once yearly |
Acknowledgments
We thank Ms Amy Miller and Ms Regina Parker of the ACR for administrative support and guidance.
Supported by a grant from the American College of Rheumatology and supported in part by the University of Alabama at Birmingham Deep South Musculoskeletal Center for Education and Research on Therapeutics (Agency for Healthcare Research and Quality grant U18-HS016956). Dr. Beukelman’s work was supported by the NIH (grant 5KL2-RR025776-03) via the University of Alabama at Birmingham Center for Clinical and Translational Science.
Dr. Saag has received consultant fees, speaking fees, and/or honoraria (less than $10,000 each) from Merck, Lilly, Novartis, P&G, Aventis, Genentech, Takeda, AstraZeneca, and Horizon. Dr. Ilowite has received consultant fees, speaking fees, and/or honoraria (less than $10,000) from Genentech. Dr. Kimura has served on the advisory board for Genentech and received a fee (less than $10,000). Dr. Laxer is the Chair of the advisory board for Novartis and receives a fee (less than $10,000). Dr. Martini has received consultant fees, speaking fees, and/or honoraria (less than $10,000) from Bristol-Myers Squibb. Dr. Ruperto has received consultant fees, speaking fees, and/or honoraria (less than $10,000 each) from Bristol-Myers Squibb, Roche, and Novartis.
Members of the Core Expert Panel: Timothy Beukelman, MD, MSCE, Randy Q. Cron, MD, PhD, Esi Morgan DeWitt, MD, MSCE, Norman T. Ilowite, MD, Yukiko Kimura, MD, Ronald M. Laxer, MDCM, FRCPC, Daniel J. Lovell, MD, MPH, Alberto Martini, MD, C. Egla Rabinovich, MD, MPH, Nicolino Ruperto, MD, MPH.
Members of the Task Force Panel: Suzanne L. Bowyer, MD (Indiana University School of Medicine, Indianapolis), Pavla Dolezalova, MD, PhD (Charles University, Prague, Czech Republic), Ciaran M. Duffy, MBBCh, MSc (McGill University, Montreal, Quebec, Canada), James P. Guevara, MD, MPH (Children’s Hospital of Philadelphia, Philadelphia, PA), Anne Murphy, PhD (University of California, San Diego), Murray H. Passo, MD (Medical University of South Carolina, Charleston), Marilynn Punaro, MD (University of Texas Southwestern, Dallas), Rayfel Schneider, MBBCh, FRCPC (University of Toronto and Hospital for Sick Children, Toronto, Ontario, Canada), David D. Sherry, MD (Children’s Hospital of Philadelphia, Philadelphia, PA), Earl D. Silverman, MD, FRCPC (University of Toronto and Hospital for Sick Children, Toronto, Ontario, Canada), Janalee Taylor, RN, MSN, CPNP (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), Carol A. Wallace, MD (Seattle Children’s Hospital Medical Center, Seattle, WA).
Members of the University of Alabama at Birmingham Working Group: Sheree Carter, RN, MSN, Jeffrey R. Curtis, MD, MPH, Archana Jain, MD, Amy Mudano, MPH, Pongthorn Narongroeknawin, MD.
Members of the University of North Carolina at Chapel Hill Working Group: Rachel Fesperman, MD, MPH, Bethany Koestner, MSLS, Lynne Morris, MLS, Karen Rusak, MD, MPH.
American College of Rheumatology Board of Directors Liaison: Raphael Hirsch, MD (University of Pittsburgh, Pittsburgh, PA).
The American College of Rheumatology is an independent professional, medical, and scientific society which does not guarantee, warrant, or endorse any commercial product or service.
Footnotes
AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Beukelman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Beukelman, Patkar, Saag, Tolleson-Rinehart, Cron, DeWitt, Ilowite, Kimura, Laxer, Lovell, Martini, Ruperto.
Acquisition of data. Beukelman, Patkar, Saag, Tolleson-Rinehart, Cron, Ilowite, Kimura, Laxer, Martini, Rabinovich, Ruperto.
Analysis and interpretation of data. Beukelman, Patkar, Saag, Tolleson-Rinehart, Cron, DeWitt, Ilowite, Kimura, Laxer, Lovell, Martini, Rabinovich, Ruperto.
References
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 2.Andersson Gare B. Juvenile arthritis: who gets it, where and when? A review of current data on incidence and prevalence. Clin Exp Rheumatol. 1999;17:367–74. [PubMed] [Google Scholar]
- 3.Hanova P, Pavelka K, Dostal C, Holcatova I, Pikhart H. Epidemiology of rheumatoid arthritis, juvenile idiopathic arthritis and gout in two regions of the Czech Republic in a descriptive population-based survey in 2002-2003. Clin Exp Rheumatol. 2006;24:499–507. [PubMed] [Google Scholar]
- 4.Selvaag AM, Flato B, Dale K, Lien G, Vinje O, Smerdel-Ramoya A, et al. Radiographic and clinical outcome in early juvenile rheumatoid arthritis and juvenile spondyloarthropathy: a 3-year prospective study. J Rheumatol. 2006;33:1382–91. [PubMed] [Google Scholar]
- 5.Van Rossum MA, Zwinderman AH, Boers M, Dijkmans BA, van Soesbergen RM, Fiselier TJ, et al. for the Dutch Juvenile Idiopathic Arthritis Study Group. Radiologic features in juvenile idiopathic arthritis: a first step in the development of a standardized assessment method. Arthritis Rheum. 2003;48:507–15. doi: 10.1002/art.10783. [DOI] [PubMed] [Google Scholar]
- 6.Zak M, Pedersen FK. Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology (Oxford) 2000;39:198–204. doi: 10.1093/rheumatology/39.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schontube M, et al. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2392–401. doi: 10.1002/art.10444. [DOI] [PubMed] [Google Scholar]
- 8.Bowyer SL, Roettcher PA, Higgins GC, Adams B, Myers LK, Wallace C, et al. Health status of patients with juvenile rheumatoid arthritis at 1 and 5 years after diagnosis. J Rheumatol. 2003;30:394–400. [PubMed] [Google Scholar]
- 9.Packham JC, Hall MA. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology (Oxford) 2002;41:1428–35. doi: 10.1093/rheumatology/41.12.1428. [DOI] [PubMed] [Google Scholar]
- 10.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. for the Pediatric Rheumatology Collaborative Study Group. Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342:763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 11.Henrickson M, Reiff A. Prolonged efficacy of etanercept in refractory enthesitis-related arthritis. J Rheumatol. 2004;31:2055–61. [PubMed] [Google Scholar]
- 12.Horneff G, Schmeling H, Biedermann T, Foeldvari I, Ganser G, Girschick HJ, et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:1638–44. doi: 10.1136/ard.2003.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerloni V, Pontikaki I, Gattinara M, Desiati F, Lupi E, Lurati A, et al. Efficacy of repeated intravenous infusions of an anti---tumor necrosis factor α monoclonal antibody, infliximab, in persistently active, refractory juvenile idiopathic arthritis: results of an open-label prospective study. Arthritis Rheum. 2005;52:548–53. doi: 10.1002/art.20793. [DOI] [PubMed] [Google Scholar]
- 14.Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al. for the Pediatric Rheumatology Collaborative Study Group. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 15.Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–20. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 16.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–91. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 18.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, Lacalle JR, Lazaro P. The RAND/UCLA Appropriateness Method user’s manual. Pittsburgh: RAND; 2000. [Google Scholar]
- 19.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 20.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515–26. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 21.The AGREE Collaboration. Appraisal of Guidelines for Research and Education (AGREE) instrument. St George’s Hospital Medical School. 2001 URL: www.agreecollaboration.org.
- 22.Cassidy JT, Petty RE. Systemic arthritis. In: Cassidy JT, Petty RE, Laxer RM, Lindsley CB, editors. Textbook of pediatric rheumatology. 5. Philadelphia: WB Saunders; 2005. pp. 291–303. [Google Scholar]
- 23.Flato B, Lien G, Smerdel A, Vinje O, Dale K, Johnston V, et al. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol. 2003;30:386–93. [PubMed] [Google Scholar]
- 24.Flato B, Smerdel A, Johnston V, Lien G, Dale K, Vinje O, et al. The influence of patient characteristics, disease variables, and HLA alleles on the development of radiographically evident sacroiliitis in juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:986–94. doi: 10.1002/art.10146. [DOI] [PubMed] [Google Scholar]
- 25.Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case---control study. Arthritis Rheum. 2006;54:3573–82. doi: 10.1002/art.22181. [DOI] [PubMed] [Google Scholar]
- 26.Al-Matar MJ, Petty RE, Tucker LB, Malleson PN, Schroeder ML, Cabral DA. The early pattern of joint involvement predicts disease progression in children with oligoarticular (pauciarticular) juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:2708–15. doi: 10.1002/art.10544. [DOI] [PubMed] [Google Scholar]
- 27.Gare BA, Fasth A. The natural history of juvenile chronic arthritis: a population based cohort study. I Onset and disease process. J Rheumatol. 1995;22:295–307. [PubMed] [Google Scholar]
- 28.Ruperto N, Ravelli A, Levinson JE, Shear ES, Murray K, Link Tague B, et al. Long-term health outcomes and quality of life in American and Italian inception cohorts of patients with juvenile rheumatoid arthritis. II Early predictors of outcome. J Rheumatol. 1997;24:952–8. [PubMed] [Google Scholar]
- 29.Guillaume S, Prieur AM, Coste J, Job-Deslandre C. Long-term outcome and prognosis in oligoarticular-onset juvenile idiopathic arthritis. Arthritis Rheum. 2000;43:1858–65. doi: 10.1002/1529-0131(200008)43:8<1858::AID-ANR23>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Flato B, Aasland A, Vinje O, Forre O. Outcome and predictive factors in juvenile rheumatoid arthritis and juvenile spondyloarthropathy. J Rheumatol. 1998;25:366–75. [PubMed] [Google Scholar]
- 31.Magni-Manzoni S, Rossi F, Pistorio A, Temporini F, Viola S, Beluffi G, et al. Prognostic factors for radiographic progression, radiographic damage, and disability in juvenile idiopathic arthritis. Arthritis Rheum. 2003;48:3509–17. doi: 10.1002/art.11337. [DOI] [PubMed] [Google Scholar]
- 32.Gare BA, Fasth A. The natural history of juvenile chronic arthritis: a population based cohort study. II Outcome. J Rheumatol. 1995;22:308–19. [PubMed] [Google Scholar]
- 33.Gilliam BE, Chauhan AK, Low JM, Moore TL. Measurement of biomarkers in juvenile idiopathic arthritis patients and their significant association with disease severity: a comparative study. Clin Exp Rheumatol. 2008;26:492–7. [PubMed] [Google Scholar]
- 34.Habib HM, Mosaad YM, Youssef HM. Anti-cyclic citrullinated peptide antibodies in patients with juvenile idiopathic arthritis. Immunol Invest. 2008;37:849–57. doi: 10.1080/08820130802438057. [DOI] [PubMed] [Google Scholar]
- 35.Schneider R, Lang BA, Reilly BJ, Laxer RM, Silverman ED, Ibanez D, et al. Prognostic indicators of joint destruction in systemic-onset juvenile rheumatoid arthritis. J Pediatr. 1992;120:200–5. doi: 10.1016/s0022-3476(05)80427-5. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel LR, Schneider R, Lang BA, Birdi N, Silverman ED, Laxer RM, et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402–9. doi: 10.1002/1529-0131(200011)43:11<2402::AID-ANR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Singh-Grewal D, Schneider R, Bayer N, Feldman BM. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 2006;54:1595–601. doi: 10.1002/art.21774. [DOI] [PubMed] [Google Scholar]
- 38.Modesto C, Woo P, Garcia-Consuegra J, Merino R, Garcia-Granero M, Arnal C, et al. Systemic onset juvenile chronic arthritis, polyarticular pattern and hip involvement as markers for a bad prognosis. Clin Exp Rheumatol. 2001;19:211–7. [PubMed] [Google Scholar]
- 39.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. for the Paediatric Rheumatology International Trials Organisation. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:658–66. doi: 10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 40.Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, et al. for the Pediatric Rheumatology International Trials Organization. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50:2191–201. doi: 10.1002/art.20288. [DOI] [PubMed] [Google Scholar]
- 41.Ruperto N, Nikishina I, Pachanov ED, Shachbazian Y, Prieur AM, Mouy R, et al. for the Pediatric Rheumatology International Trials Organization. A randomized, double-blind clinical trial of two doses of meloxicam compared with naproxen in children with juvenile idiopathic arthritis: short- and long-term efficacy and safety results. Arthritis Rheum. 2005;52:563–72. doi: 10.1002/art.20860. [DOI] [PubMed] [Google Scholar]
- 42.Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. for the Paediatric Rheumatology International Trials Organisation and the Pediatric Rheumatology Collaborative Study Group. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 43.Magni-Manzoni S, Ruperto N, Pistorio A, Sala E, Solari N, Palmisani E, et al. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2008;59:1120–7. doi: 10.1002/art.23916. [DOI] [PubMed] [Google Scholar]
- 44.Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. for the 20000223 Study Group. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–9. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- 45.Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum. 2006;54:2807–16. doi: 10.1002/art.22070. [DOI] [PubMed] [Google Scholar]
- 46.Oxford Centre for Evidence Based Medicine. Levels of evidence. 2009 URL: http://www.cebm.net/index.aspx?o=1025.
- 47.Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherry DD, Stein LD, Reed AM, Schanberg LE, Kredich DW. Prevention of leg length discrepancy in young children with pauciarticular juvenile rheumatoid arthritis by treatment with intraarticular steroids. Arthritis Rheum. 1999;42:2330–4. doi: 10.1002/1529-0131(199911)42:11<2330::AID-ANR11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 49.Marti P, Molinari L, Bolt IB, Seger R, Saurenmann RK. Factors influencing the efficacy of intra-articular steroid injections in patients with juvenile idiopathic arthritis. Eur J Pediatr. 2008;167:425–30. doi: 10.1007/s00431-007-0525-9. [DOI] [PubMed] [Google Scholar]
- 50.Beukelman T, Arabshahi B, Cahill AM, Kaye RD, Cron RQ. Benefit of intraarticular corticosteroid injection under fluoroscopic guidance for subtalar arthritis in juvenile idiopathic arthritis. J Rheumatol. 2006;33:2330–6. [PubMed] [Google Scholar]
- 51.Eberhard BA, Sison MC, Gottlieb BS, Ilowite NT. Comparison of the intraarticular effectiveness of triamcinolone hexacetonide and triamcinolone acetonide in treatment of juvenile rheumatoid arthritis. J Rheumatol. 2004;31:2507–12. [PubMed] [Google Scholar]
- 52.Zulian F, Martini G, Gobber D, Plebani M, Zacchello F, Manners P. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology (Oxford) 2004;43:1288–91. doi: 10.1093/rheumatology/keh313. [DOI] [PubMed] [Google Scholar]
- 53.Zulian F, Martini G, Gobber D, Agosto C, Gigante C, Zacchello F. Comparison of intra-articular triamcinolone hexacetonide and triamcinolone acetonide in oligoarticular juvenile idiopathic arthritis. Rheumatology (Oxford) 2003;42:1254–9. doi: 10.1093/rheumatology/keg358. [DOI] [PubMed] [Google Scholar]
- 54.Lepore L, del Santo M, Malorgio C, Presani G, Perticarari S, Prodan M, et al. Treatment of juvenile idiopathic arthritis with intra-articular triamcinolone hexacetonide: evaluation of clinical effectiveness correlated with circulating ANA and T γ/δ + and B CD5+ lymphocyte populations of synovial fluid. Clin Exp Rheumatol. 2002;20:719–22. [PubMed] [Google Scholar]
- 55.Neidel J, Boehnke M, Kuster RM. The efficacy and safety of intraarticular corticosteroid therapy for coxitis in juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1620–8. doi: 10.1002/art.10313. [DOI] [PubMed] [Google Scholar]
- 56.Ravelli A, Manzoni SM, Viola S, Pistorio A, Ruperto N, Martini A. Factors affecting the efficacy of intraarticular corticosteroid injection of knees in juvenile idiopathic arthritis. J Rheumatol. 2001;28:2100–2. [PubMed] [Google Scholar]
- 57.Breit W, Frosch M, Meyer U, Heinecke A, Ganser G. A subgroup-specific evaluation of the efficacy of intraarticular triamcinolone hexacetonide in juvenile chronic arthritis. J Rheumatol. 2000;27:2696–702. [PubMed] [Google Scholar]
- 58.Yang MH, Lee WI, Chen LC, Lin SJ, Huang JL. Intraarticular triamcinolone hexacetonide injection in children with chronic arthritis: a survey of clinical practice. Acta Paediatr Taiwan. 1999;40:182–5. [PubMed] [Google Scholar]
- 59.Padeh S, Passwell JH. Intraarticular corticosteroid injection in the management of children with chronic arthritis. Arthritis Rheum. 1998;41:1210–4. doi: 10.1002/1529-0131(199807)41:7<1210::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Remedios D, Martin K, Kaplan G, Mitchell R, Woo P, Rooney M. Juvenile chronic arthritis: diagnosis and management of tibio-talar and sub-talar disease. Br J Rheumatol. 1997;36:1214–7. doi: 10.1093/rheumatology/36.11.1214. [DOI] [PubMed] [Google Scholar]
- 61.Eich GF, Halle F, Hodler J, Seger R, Willi UV. Juvenile chronic arthritis: imaging of the knees and hips before and after intraarticular steroid injection. Pediatr Radiol. 1994;24:558–63. doi: 10.1007/BF02012732. [DOI] [PubMed] [Google Scholar]
- 62.Honkanen VE, Rautonen JK, Pelkonen PM. Intra-articular glucocorticoids in early juvenile chronic arthritis. Acta Paediatr. 1993;82:1072–4. doi: 10.1111/j.1651-2227.1993.tb12815.x. [DOI] [PubMed] [Google Scholar]
- 63.Hertzberger-ten Cate R, de Vries-van der Vlugt BC, van Suijlekom-Smit LW, Cats A. Intra-articular steroids in pauciarticular juvenile chronic arthritis, type 1. Eur J Pediatr. 1991;150:170–2. doi: 10.1007/BF01963559. [DOI] [PubMed] [Google Scholar]
- 64.Earley A, Cuttica RJ, McCullough C, Ansell BM. Triamcinolone into the knee joint in juvenile chronic arthritis. Clin Exp Rheumatol. 1988;6:153–5. [PubMed] [Google Scholar]
- 65.Allen RC, Gross KR, Laxer RM, Malleson PN, Beauchamp RD, Petty RE. Intraarticular triamcinolone hexacetonide in the management of chronic arthritis in children. Arthritis Rheum. 1986;29:997–1001. doi: 10.1002/art.1780290808. [DOI] [PubMed] [Google Scholar]
- 66.Beukelman T, Guevara JP, Albert DA. Optimal treatment of knee monarthritis in juvenile idiopathic arthritis: a decision analysis. Arthritis Rheum. 2008;59:1580–8. doi: 10.1002/art.24190. [DOI] [PubMed] [Google Scholar]
- 67.Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis. 2009;68:519–25. doi: 10.1136/ard.2007.087593. [DOI] [PubMed] [Google Scholar]
- 68.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti---tumor necrosis factor α monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 69.Infliximab. Malvern (PA): Centocor Ortho Biotech; 2010. package insert. [Google Scholar]
- 70.Giannini EH, Brewer EJ, Miller ML, Gibbas D, Passo MH, Hoyeraal HM, et al. for the Pediatric Rheumatology Collaborative Study Group. Ibuprofen suspension in the treatment of juvenile rheumatoid arthritis. J Pediatr. 1990;117:645–52. doi: 10.1016/s0022-3476(05)80708-5. [DOI] [PubMed] [Google Scholar]
- 71.Bhettay E. Double-blind study of sulindac and aspirin in juvenile chronic arthritis. S Afr Med J. 1986;70:724–6. [PubMed] [Google Scholar]
- 72.Kvien TK, Hoyeraal HM, Sandstad B. Naproxen and acetylsalicylic acid in the treatment of pauciarticular and polyarticular juvenile rheumatoid arthritis: assessment of tolerance and efficacy in a single-centre 24-week double-blind parallel study. Scand J Rheumatol. 1984;13:342–50. doi: 10.3109/03009748409111307. [DOI] [PubMed] [Google Scholar]
- 73.Haapasaari J, Wuolijoki E, Ylijoki H. Treatment of juvenile rheumatoid arthritis with diclofenac sodium. Scand J Rheumatol. 1983;12:325–30. doi: 10.3109/03009748309099735. [DOI] [PubMed] [Google Scholar]
- 74.Brewer EJ, Giannini EH, Baum J, Bernstein B, Fink CW, Emery HM, et al. Aspirin and fenoprofen (Nalfon) in the treatment of juvenile rheumatoid arthritis results of the double blind-trial: a segment II study. J Rheumatol. 1982;9:123–8. [PubMed] [Google Scholar]
- 75.Moran H, Hanna DB, Ansell BM, Hall M, Engler C. Naproxen in juvenile chronic polyarthritis. Ann Rheum Dis. 1979;38:152–4. doi: 10.1136/ard.38.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levinson JE, Baum J, Brewer E, Jr, Fink C, Hanson V, Schaller J. Comparison of tolmetin sodium and aspirin in the treatment of juvenile rheumatoid arthritis. J Pediatr. 1977;91:799–804. doi: 10.1016/s0022-3476(77)81045-7. [DOI] [PubMed] [Google Scholar]
- 77.Silverman E, Mouy R, Spiegel L, Jung LK, Saurenmann RK, Lahdenne P, et al. Leflunomide or methotrexate for juvenile rheumatoid arthritis. N Engl J Med. 2005;352:1655–66. doi: 10.1056/NEJMoa041810. [DOI] [PubMed] [Google Scholar]
- 78.Woo P, Southwood TR, Prieur AM, Dore CJ, Grainger J, David J, et al. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum. 2000;43:1849–57. doi: 10.1002/1529-0131(200008)43:8<1849::AID-ANR22>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 79.Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, et al. for the Pediatric Rheumatology Collaborative Study Group and the Cooperative Children’s Study Group. Methotrexate in resistant juvenile rheumatoid arthritis: results of the USA-USSR double-blind, placebo-controlled trial. N Engl J Med. 1992;326:1043–9. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 80.Albers HM, Wessels JA, van der Straaten RJ, Brinkman DM, Suijlekom-Smit LW, Kamphuis SS, et al. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:46–51. doi: 10.1002/art.24087. [DOI] [PubMed] [Google Scholar]
- 81.Brik R, Gepstein V, Berkovitz D. Low-dose methotrexate treatment for oligoarticular juvenile idiopathic arthritis nonresponsive to intra-articular corticosteroids. Clin Rheumatol. 2005;24:612–4. doi: 10.1007/s10067-005-1116-7. [DOI] [PubMed] [Google Scholar]
- 82.Alsufyani K, Ortiz-Alvarez O, Cabral DA, Tucker LB, Petty RE, Malleson PN. The role of subcutaneous administration of methotrexate in children with juvenile idiopathic arthritis who have failed oral methotrexate. J Rheumatol. 2004;31:179–82. [PubMed] [Google Scholar]
- 83.Lin YT, Tsai MJ, Wang LH, Huang MT, Yang YH, Chiang BL. Efficacy and safety of methotrexate therapy for juvenile rheumatoid arthritis. J Formos Med Assoc. 2000;99:623–9. [PubMed] [Google Scholar]
- 84.Van Rossum MA, Fiselier TJ, Franssen MJ, Zwinderman AH, ten Cate R, van Suijlekom-Smit LW, et al. for the Dutch Juvenile Chronic Arthritis Study Group. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Arthritis Rheum. 1998;41:808–16. doi: 10.1002/1529-0131(199805)41:5<808::AID-ART6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 85.Tse SM, Burgos-Vargas R, Laxer RM. Anti---tumor necrosis factor α blockade in the treatment of juvenile spondylarthropathy. Arthritis Rheum. 2005;52:2103–8. doi: 10.1002/art.21121. [DOI] [PubMed] [Google Scholar]
- 86.Brewer EJ, Giannini EH, Kuzmina N, Alekseev L. Penicillamine and hydroxychloroquine in the treatment of severe juvenile rheumatoid arthritis: results of the USA-USSR double-blind placebo-controlled trial. N Engl J Med. 1986;314:1269–76. doi: 10.1056/NEJM198605153142001. [DOI] [PubMed] [Google Scholar]
- 87.Van Rossum MA, van Soesbergen RM, Boers M, Zwinderman AH, Fiselier TJ, Franssen MJ, et al. Long-term outcome of juvenile idiopathic arthritis following a placebo-controlled trial: sustained benefits of early sulfasalazine treatment. Ann Rheum Dis. 2007;66:1518–24. doi: 10.1136/ard.2006.064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prince FH, Twilt M, ten Cate R, van Rossum MA, Armbrust W, Hoppenreijs EP, et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch National Register. Ann Rheum Dis. 2009;68:635–41. doi: 10.1136/ard.2007.087411. [DOI] [PubMed] [Google Scholar]
- 89.Tynjala P, Vahasalo P, Honkanen V, Lahdenne P. Drug survival of the first and second course of anti-tumour necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum Dis. 2009;68:552–7. doi: 10.1136/ard.2007.087130. [DOI] [PubMed] [Google Scholar]
- 90.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. for the REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to anti---tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 91.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. for the DANCER Study Group. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIb randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 92.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 93.Schmeling H, Horneff G. Infliximab in two patients with juvenile ankylosing spondylitis. Rheumatol Int. 2004;24:173–6. doi: 10.1007/s00296-003-0378-0. [DOI] [PubMed] [Google Scholar]
- 94.McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–158. iii–iv. doi: 10.3310/hta11280. [DOI] [PubMed] [Google Scholar]
- 95.Katsicas MM, Russo RA. Use of infliximab in patients with systemic juvenile idiopathic arthritis refractory to etanercept. Clin Exp Rheumatol. 2005;23:545–8. [PubMed] [Google Scholar]
- 96.Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, et al. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48:1093–101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]
- 97.Russo RA, Katsicas MM. Clinical remission in patients with systemic juvenile idiopathic arthritis treated with anti-tumor necrosis factor agents. J Rheumatol. 2009;36:1078–82. doi: 10.3899/jrheum.090952. [DOI] [PubMed] [Google Scholar]
- 98.Kimura Y, Pinho P, Walco G, Higgins G, Hummell D, Szer I, et al. Etanercept treatment in patients with refractory systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2005;32:935–42. [PubMed] [Google Scholar]
- 99.Pay S, Turkcapar N, Kalyoncu M, Simsek I, Beyan E, Ertenli I, et al. A multicenter study of patients with adult-onset Still’s disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol. 2006;25:639–44. doi: 10.1007/s10067-005-0138-5. [DOI] [PubMed] [Google Scholar]
- 100.Picco P, Gattorno M, Buoncompagni A, Pistoia V, Borrone C. 6-methylprednisolone ‘mini-pulses’: a new modality of glucocorticoid treatment in systemic onset juvenile chronic arthritis. Scand J Rheumatol. 1996;25:24–7. doi: 10.3109/03009749609082663. [DOI] [PubMed] [Google Scholar]
- 101.Gattorno M, Piccini A, Lasiglie D, Tassi S, Brisca G, Carta S, et al. The pattern of response to anti---interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–15. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 102.Verbsky JW, White AJ. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2004;31:2071–5. [PubMed] [Google Scholar]
- 103.Zeft A, Hollister R, LaFleur B, Sampath P, Soep J, McNally B, et al. Anakinra for systemic juvenile arthritis: the Rocky Mountain experience. J Clin Rheumatol. 2009;15:161–4. doi: 10.1097/RHU.0b013e3181a4f459. [DOI] [PubMed] [Google Scholar]
- 104.Ilowite N, Porras O, Reiff A, Rudge S, Punaro M, Martin A, et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28:129–37. doi: 10.1007/s10067-008-0995-9. [DOI] [PubMed] [Google Scholar]
- 105.Ruperto N, Ravelli A, Castell E, Gerloni V, Haefner R, Malattia C, et al. Cyclosporine A in juvenile idiopathic arthritis: results of the PRCSG/PRINTO phase IV post marketing surveillance study. Clin Exp Rheumatol. 2006;24:599–605. [PubMed] [Google Scholar]
- 106.Gerloni V, Cimaz R, Gattinara M, Arnoldi C, Pontikaki I, Fantini F. Efficacy and safety profile of cyclosporin A in the treatment of juvenile chronic (idiopathic) arthritis: results of a 10-year prospective study. Rheumatology (Oxford) 2001;40:907–13. doi: 10.1093/rheumatology/40.8.907. [DOI] [PubMed] [Google Scholar]
- 107.Ortiz-Alvarez O, Morishita K, Avery G, Green J, Petty RE, Tucker LB, et al. Guidelines for blood test monitoring of methotrexate toxicity in juvenile idiopathic arthritis. J Rheumatol. 2004;31:2501–6. [PubMed] [Google Scholar]
- 108.Kocharla L, Taylor J, Weiler T, Ting TV, Luggen M, Brunner HI. Monitoring methotrexate toxicity in juvenile idiopathic arthritis. J Rheumatol. 2009;36:2813–8. doi: 10.3899/jrheum.090482. [DOI] [PubMed] [Google Scholar]
- 109.Lahdenne P, Rapola J, Ylijoki H, Haapasaari J. Hepatotoxicity in patients with juvenile idiopathic arthritis receiving long-term methotrexate therapy. J Rheumatol. 2002;29:2442–5. [PubMed] [Google Scholar]
- 110.Wallace CA, Bleyer WA, Sherry DD, Salmonson KL, Wedgwood RJ. Toxicity and serum levels of methotrexate in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1989;32:677–81. doi: 10.1002/anr.1780320604. [DOI] [PubMed] [Google Scholar]
- 111.Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, et al. for the BIOBADASER Group. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–72. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


