Abstract
Activation of the vagal afferents by noxious gastrointestinal stimuli suggests that vagal afferents may play a complex role in visceral pain processes. The contribution of the vagus nerve to visceral pain remains unresolved. Previous studies reported that patients following chronic vagotomy have lower pain thresholds. The patient with irritable bowel syndrome has been shown alteration of vagal function. We hypothesize that vagal afferent nerves modulate visceral pain. Visceromotor responses (VMR) to graded colorectal distension (CRD) were recorded from the abdominal muscles in conscious rats. Chronic subdiaphragmatic vagus nerve sections induced 470, 106, 51, and 54% increases in VMR to CRD at 20, 40, 60 and 80 mmHg, respectively. Similarly, at light level of anesthesia, topical application of lidocaine to the subdiaphragmatic vagus nerve in rats increased VMR to CRD. Vagal afferent neuronal responses to low or high-intensity electrical vagal stimulation (EVS) of vagal afferent Aδ or C fibers were distinguished by calculating their conduction velocity. Low-intensity EVS of Aδ fibers (40 μA, 20 Hz, 0.5 ms for 30 s) reduced VMR to CRD at 40, 60, and 80 mmHg by 41, 52, and 58%, respectively. In contrast, high-intensity EVS of C fibers (400 μA, 1 Hz, 0.5 ms for 30 s) had no effect on VMR to CRD. In conclusion, we demonstrated that vagal afferent nerves modulate visceral pain. Low-intensity EVS that activates vagal afferent Aδ fibers reduced visceral pain. Thus EVS may potentially have a role in the treatment of chronic visceral pain.
Keywords: colorectal distension, vagal afferent Aδ or C fibers, visceromotor responses
Although it is generally held that pain arising from the viscera is mediated exclusively by spinal afferents, vagal afferents primarily convey interoceptive information that is important in regulating autonomic function but do not contribute to the perception of pain. However, there is growing evidence that the vagus nerve may play a complex role in these processes (5, 32, 35, 46). Electrical physiological studies have demonstrated that electrical or chemical stimulation of thoracic vagal and sympathetic afferent fibers activated C1–C3 spinothalamic tract (STT) neurons, which received input from noxious mechanical stimulation of somatic fields including the neck and jaw regions (6, 7). Studies have shown that vagal afferents respond to nociceptive mechanical and chemical stimulation and this leads to brain stem representation of nociceptive signals (35, 46, 51). Noxious gastric distension resulted in c-Fos expression in the nucleus of the solitary tract (NTS), the location of second order neurons receiving vagal afferent input from the stomach. This increase in c-Fos response is blunted by vagotomy but persists after spinal cord transection (51). Although vagal afferents are activated by gastrointestinal noxious stimuli, the contribution of the vagus nerve to visceral pain remains unresolved. It is well known that a hot drink or a nourishing meal (stimulation of various abdominal receptors) is relaxing and helps to calm anxiety, suggesting that enhanced sensory vagal inputs originating from the gut modulate attitude and behavior (56). It has been proposed that nociceptive input through the vagus nerve may contribute to the affective-emotional rather than to the sensory-discriminative aspect of pain (21, 51). Thus the vagus nerve may indirectly modulate abdominal hyperalgesia. However, these ideas need verification by further experimentation.
Holtmann et al. (20) described lower thresholds for the perception of pain in patients who had previously undergone vagotomy in the course of a Billroth I gastrectomy compared with pain thresholds in healthy controls. In healthy human volunteers, the thresholds for pain induced by heat and noxious laser stimulation were increased after a rapid filling of the stomach with water (45). Altered vagal function in patients with irritable bowel syndrome has been reported (48). These previous reports suggested that abdominal vagus nerve may contribute for modulation of visceral pain.
Electrical vagal nerve stimulation (EVS), already used clinically as a treatment for refractory epilepsy (16) and gastric dysrhythmia (32), has been assessed for its analgesic effect. In early clinical literature, EVS performed intraoperatively gives rise to nausea but not pain (53). Recent human studies indicate that high-frequency, low-energy gastric pacing enhances tolerance to gastric distension in patients with diabetic gastroparesis. Similar electrostimulation also reduces dyspeptic symptoms independent of gastric emptying in another group of diabetic patients (12, 34). These observations implicate the vagal afferents in the modulation of visceral pain in humans. In animal studies, electrical stimulation of abdominal vagal afferents exerts inhibition or facilitation of somatic nociceptive impulse transmission in the spinal dorsal horn and depresses nociceptive behavior depending on whether unmyelinated or myelinated vagal afferents are excited (38). Using anesthetized monkeys, Hobbs et al. (19) have shown that higher intensity electrical stimulation (33 V) of cervical vagal nerves reduced resting neuronal activities and urinary bladder pressure-induced lumbosacral STT neuronal activities. But stimulation of abdominal vagal afferents overall did not significantly affect STT neuronal activities (19). However, studies in the anesthetized rat have demonstrated that dorsal subdiaphragmatic EVS (20–200 μA) intensity dependently reduced lumbosacral spinal dorsal horn neuronal responses induced by noxious heating of the hindpaw (38). To date, the influences of electrical stimulation of subdiaphragmatic vagal afferent nerves on visceral pain evoked by viscera nociceptive stimuli have not been investigated. It is unclear whether a specific group of vagal afferents are involved in the protective visceral pain reactions.
Rodents do not have the forebrain structures to generate the cognitive emotional feelings of humans. Nonetheless, the use of behavioral paradigms to assess spinal nociceptive reflexes that do not include the assessment of cognitive perception in the conscious rat may help to identify the modulatory role of the vagal afferents in visceral pain sensation. The present studies explore the visceral analgesic properties of subdiaphragmatic vagus nerve stimulation in rats and show that low-intensity EVS that activates vagal afferent Aδ fibers reduces visceral pain, suggesting that a group of vagal afferents innervating viscera may have remarkable functions that are related to visceral pain inhibition. Because rats lack the cognitive emotional ability of humans to subjectively experience a reduction of pain as a consequence of emotional comfort, our results suggest that stimulation of the vagal nerve inhibits visceral pain.
MATERIALS AND METHODS
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). All protocols were approved by the University Committee on Use and Care of Animals at the University of Michigan. Experiments were performed on adult male Sprague-Dawley rats (275–300 g). For surgical procedures, rats were anesthetized with a mixture of xylazine and ketamine according to the protocol described in our laboratory’s previous publication (29).
VMRs to CRD
Rats were maintained on a 12:12-h light-dark cycle. Measurement of visceral sensitivity in animals is mainly based on brain stem reflexes, which have been described as “pseudoaffective” responses (33). The visceromotor responses (VMR) were recorded by quantifying a reflex contraction of the abdominal musculature induced by colorectal distension (CRD). The animals were anesthetized with a mixture of xylazine and ketamine (13 and 87 mg/kg body wt, respectively). EMG electrodes made from Teflon-coated, 32-gauge stainless steel wires were implanted into the external oblique pelvic muscles 4–6 days prior to the beginning of the experimental procedures. The skin was sutured over the strain gauge, and the lead wires were looped around the animal’s flank and secured with a single suture in the skin. To reduce stress and motion artifacts, rats were habituated to Plexiglas tubes (length 21 cm, diameter 8 cm; Braintree Scientific, Braintree, MA) 30 min per day for 3 consecutive days prior to experiments. During the experiment, the strain gauge was connected by way of a shielded cable to a chart recorder to monitor the number of abdominal muscle contractions. A latex balloon (7 cm long) was be inserted into the colon. A catheter was fixed at the tail with adhesive tape. CRD was obtained by injecting saline into the balloon. Graded-pressure CRD was produced by rapidly injecting saline into the colonic balloon over 1 s and maintaining the distension for 20 s. Pressure was regulated with a distension control device and monitored by use of a pressure transducer (14). Graded-intensity stimulation trials (20–40-60–80 mmHg CRD) were conducted to establish stimulus-response curves. Each distension trial consisted of three segments: a 20-s predistension baseline period, a 20-s distension period, and a 20-s post-CRD termination period with a 4-min inter-stimulus interval. The responses were considered stable if there was less than 20% variability between two consecutive trials of CRD at 60 mmHg. The results of electromyography were amplified and filtered (5,000×, 300–5,000 Hz; A-M System), digitized, and integrated by using the SPIKE2/CED 1401 data-acquisition interface. Both raw and integrated EMGs were continuously displayed on an oscilloscope and recorded. Spike bursts higher than 0.3 mV were regarded as significant and therefore used to estimate the pain response. Data were presented as the number of contractions that surpassed the threshold. The results of electromyography were also quantified by calculating the area under the curve (AUC), which is the sum of all recorded data points multiplied by the sample interval (in seconds) after baseline subtraction.
Bilateral subdiaphragmatic vagotomy
To determine whether subdiaphragmatic vagus nerves are involved in the modulation of visceral pain chronic bilateral subdiaphragmatic vagotomy was performed. Through a midline incision of the abdominal wall, the stomach was carefully manipulated to expose the esophagus. The subdiaphragmatic vagal trunks were exposed halfway between the diaphragm and the gastric cardia. Both anterior and posterior trunks of the vagal nerves were transected. For control experiments, the abdominal vagal nerves were exposed but not cut. VMR studies were conducted 5 to 7 days after vagotomy as described.
Application of lidocaine to abdominal vagal nerves
To rule out the possibility that the neuroplasticity changes after chronic vagotomy mediate the observed effect on nociception, we examined the effect of acute vagal functional denervation using topical application of 1% lidocaine. Rats were maintained at a light level of anesthesia (corneal and flexion reflexes present) by intravenous infusion of pentobarbital (5–8 mg · kg−1 · h−1), and abdominal vagal trunks were exposed. VMR induced by a trial of CRD was performed. Then a small piece of gauze soaked in saline or 1% lidocaine was left on the vagal trunk for 10 min before the VMR study. Each rat served as it own control. In separate group of rats, successful temporary vagal denervation was confirmed in electrophysiological studies showing the absence of nodose ganglia neuronal responses to EVS.
EVS
The dorsal and ventral subdiaphragmatic vagus nerves were isolated by using a retroperitoneal approach just rostral to the accessory branch of the vagus. The nerves were placed on bipolar silver stimulating electrodes. A nontoxic silicone gel was placed around the nerve and the electrode. VMR studies were conducted 5 days after surgery.
By using electrophysiological recording of vagal afferent neurons in nodose ganglia, we have shown previously that electrical stimulation of subdiaphragmatic vagal afferent fibers enhanced the conduction of afferent signals (30). The ability to depolarize nerve fibers by means of electrical stimulation depends on the intensity and duration (e.g., magnitude of the current and the width of the pulse) of the stimulus. If the pulse width is kept constant, a stepwise increase in stimulus intensity first depolarizes large, low-threshold nerve fibers, and, as the intensity is increased, higher threshold, smaller fibers will be gradually be depolarized as well (27). Nonetheless, as the stimulus intensities increase, side effects such as hoarseness, cough, throat tightness, and shortness of breath worsen. Higher currents are required to depolarize a nerve when pulse duration is reduced. However, the relationship between intensity and duration is not linear. A pulse of 250 ms requires only a slightly higher stimulus current than a 500-ms pulse for similar activation of the vagus nerve, but further reduction in pulse width requires much higher currents. Studies of adult functional MRI brain activation by stimulation of the vagus nerve have shown similar activation using pulse widths of 500 and 250 ms, but pulses of 130 ms produced significantly less overall activation. Pulses of 250 ms are often tolerated better, with minimal change in effectiveness. In our pilot studies, we performed a series of experiments to examine the effects of vagal nerve stimulation (VNS) on CRD having set various parameters. The intensities of EVS were set to 40 or 80 μA, 20 Hz (low-intensity EVS) and then to 300 or 600 μA, 1 Hz (high-intensity EVS). On the basis of our preliminary electricophysiological recording and VMR studies, the following stimulation parameters were used: to recruit Aδ fibers, 40 μA, 20 Hz, delivered in 0.5-ms biphasic pulses, for 30 s (conduction velocity ~8.5 m/s); to recruit C fibers, 400 μA, 1 Hz, 0.5-ms biphasic pulses, for 30 s (conduction velocity ~0.8 m/s). Once the VMRs to CRD were stabilized, EVS was elicited in repeated VMR trials. Two different parameters of EVS were applied on different days to the same rat. Each rat was tested twice for one parameter. In our unpublished observations we have observed that electrical stimulation of vagal efferent had no effects of VMRs induced by CRD.
Postoperative care
Postoperatively, animals were given 15 ml/kg of sterile 5% glucose and 0.9% sodium chloride intraperitoneally, and, to prevent infection, 0.5 ml bicillin (300,000 U/ml) was administered intramuscularly (29). Each rat was also given diluted cherry-flavored Tylenol (50 mg/kg) to drink for 24 h after surgery. Rats exhibited normal exploratory behavior after surgery. Body weight was monitored daily. During the initial 3-day postoperative period, rat weight decreased a mean of 3% ± 0.1.5 of the preoperative weight. In the subsequent 5 days, rats gained 3–4 g, reestablishing their preoperative weight.
Recording of single nodose neuronal activity to identify the A fiber or C fiber vagal afferent neurons
The animals were anesthetized with a mixture of xylazine and ketamine (13 and 87 mg/kg body wt, respectively). Supplemental doses of the anesthetic agents were administered as needed to maintain a deep level of anesthesia and muscle relaxation. The animals were ventilated with a respirator, and a tracheal tube permitted artificial ventilation with room air (75–85 strokes/min, 3.5–4.0 cm3 tidal volume). A midline abdominal incision exposed the abdominal vagus, the stomach, and the duodenum. Stimulation of the subdiaphragmatic vagus afferent nerves was accomplished by placing a pair of Teflon-coated, pure gold wire electrodes (outside diameter, 76 μm) around the anterior and posterior trunks, ~2–3 cm above the gastroesophageal junction and above the accessory and celiac branches of the vagus nerve. These stimulating electrodes were loosely sutured to the esophagus to limit displacement. At the end of each experiment, an overdose of anesthetic was administered to kill the animals.
Rats were placed in a small Kopf animal stereotaxic frame. Body temperature was maintained with a special heating pad. The right nodose ganglion was exposed by a short dorsal approach as previously described (30, 57). The beveled glass recording micropipette filled with 1.0 M KCl was lowered into the nodose ganglion. A reference electrode was placed on a skin incision near the recording electrode. In general, low stimulus frequencies are believed to facilitate repetitive stimulation of the slow conducting C fibers that have a longer refractory period. Very fine fibers have lower maximal firing rates and cannot follow higher frequency stimulation as faithfully as larger diameter fibers. As stimulation frequency is increased, fibers of a given caliber cease after each stimulus and often exhibit blocking. C fiber blocking may begin to occur between 1 and 10 Hz, whereas large myelinated fibers may follow rates up to at least 50 Hz before blocking. In this study, we distinguished vagal A or C fibers by their conduction velocity (30). Conduction velocity was estimated by using the distance and conduction delay between the stimulating and recording electrodes. In the rat, the estimation of conduction velocity will be adapted from a classification reported (26). A cutoff of 2.0 m/s was used to distinguish between myelinated and unmyelinated fibers. Units with a conduction velocity greater than 10.0 m/s were considered to be large myelinated (Aβ) fibers, whereas units with conduction velocity 2.0–10.0 m/s were considered to be thin myelinated (Aδ) fibers. In our preliminary studies, we could not find Aβ innervated units as vagal nociceptors. Neuronal discharges recorded were amplified by an A-M System high-input-impedance preamplifier, monitored with an oscilloscope and audio monitor, displayed, and stored on a computer using Axon tape software.
Statistical analyses
Statistical comparisons of the VMR in various groups were made by one-way repeated-measures ANOVA, followed by multiple comparisons adjusted by the Bonferroni test using baseline values as a covariate and two main factors (i.e., distension level as the repeated factor and group as the independent factor). Results were expressed as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
VMRs to CRD in the normal control and the vagotomized rats
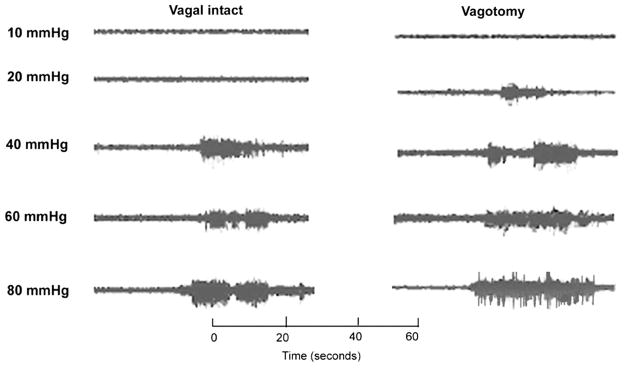
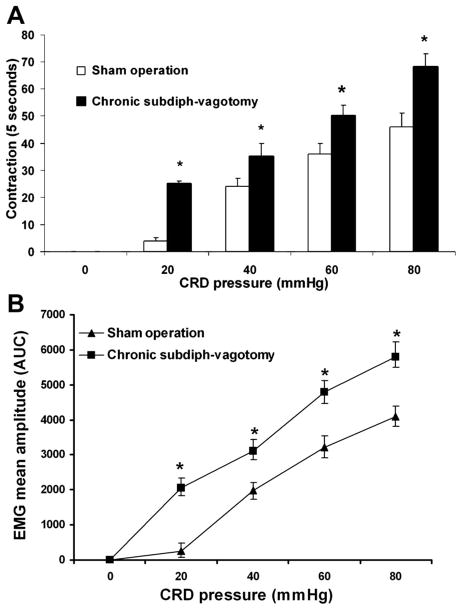
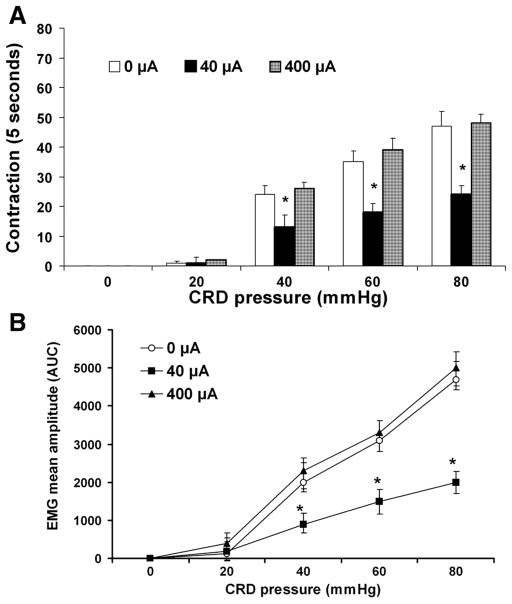
The VMR can be measured as a sudden, sustained increase in the electrical activity of the abdominal muscles. When a ramped pressure stimulus was used, a distending pressure threshold for the VMR could be determined that was relatively constant within a given animal over time and normally distributed in two groups of animals. Under basal conditions when there was no CRD (0 mmHg), there was no significant difference between normal control rats (sham operation, vagal intact) and the rats after vagotomy. Both control and the vagotomized rats showed pressure-dependent increases in the VMR to CRD. Original EMGs are shown in Fig. 1. These responses were enhanced in vagotomized rats. A significant VMR to the lowest distension pressure tested (20 mmHg) in vagotomized rats and an absence of response to the lowest distension pressure in normal rats suggests a reduced pressure threshold (i.e., allodynia) in rats after vagotomy. Graded CRD pressures of 20, 40, 60, and 80 mmHg caused an increase in the number of muscle contractions to 1 ± 0.5, 24.5 ± 3.5, 35 ± 3, and 47 ± 5 contractions per 5 s, respectively, in normal rats, and to 24 ± 3, 36.5 ± 5, 50 ± 4, and 58 ± 6 contractions per 5 s in vagotomized rats, which represent 470, 106, 51, and 54% increases in VMR to CRD at 20, 40, 60, and 80 mmHg (Fig. 2A). The mean amplitude of the EMG (AUC, μV/s) is shown in Fig. 2B. These results provide evidence that vagotomy enhanced visceral pain responses (i.e., hyperalgesia) in rats.
Fig. 1.
Visceromotor responses (VMR) to graded distension pressures in vagal intact (sham control) rats and rats after chronic subdiaphragmatic vagotomy. Representative tracings of VMR activities recorded from the external oblique-pelvic muscles in normal rats and the rats after chronic vagotomy. Enhanced responses were observed following vagotomy.
Fig. 2.
Effects of subdiaphragmatic (subdiph) vagotomy on the VMR to colorectal distension (CRD) in conscious rats. Data were collected from 8 sham-operation control rats and 10 rats after vagotomy. The VMR was quantified as the number of abdominal muscle contractions (A) and the mean amplitude, expressed as the area under the curve (AUC) after baseline subtraction (B). CRD evoked a dose-dependent increase in VMR in control sham operation rats. Chronic subdiaphragmatic vagotomy produced a marked increase in the number of abdominal muscle contractions. *P < 0.05 compared with sham operation. ANOVA showed a significant effect for distension level, as well as a significant interaction between distension level and group (*P < 0.05). Stimulus-response functions were shifted to the left in vagotomized rats, indicating group differences in the VMR response. Values are means ± SE.
Application of lidocaine to the abdominal vagal nerves
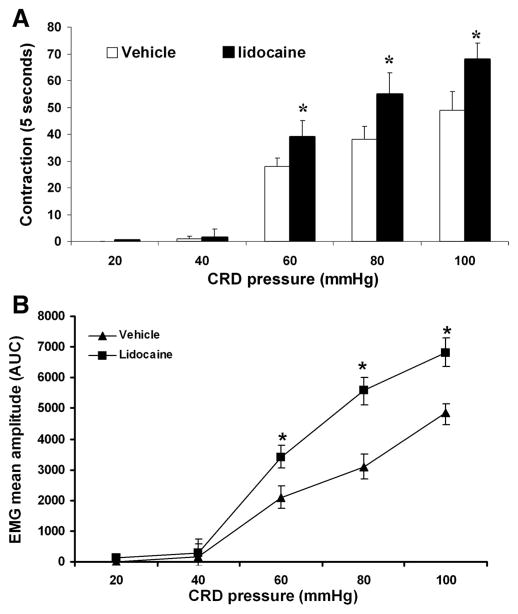
We and other investigators have observed that CRD-evoked VMR are barely apparent in rats lightly anesthetized by continuous intravenous infusion of pentobarbital (5–8 mg · kg−1 · h−1); the pressure threshold, however, was increased to 50–60 mmHg. We demonstrated that similar to responses of vagotomized rats, those subjected to acute topical application of lidocaine showed decreased pressure threshold and enhanced VMRs to CRD (Fig. 3). These results suggest that acute interruption of vagal afferent input from the abdominal viscera to the central nervous system can affect spinal nociceptive responses (VMR) to CRD.
Fig. 3.
Effects of abdominal vagal nerve application of lidocaine on the VMR to CRD in anesthetized rats. Data were collected from 6 vehicle control rats and 6 rats after topic lidocaine application. The VMR was quantified as the number of abdominal muscle contractions produced by graded-pressure CRD (A) and the mean amplitude, expressed as the AUC after baseline subtraction (B). CRD evoked a dose-dependent increase in VMR in lightly anesthetized rats; pressure threshold, however, was increased to 60 mmHg. Similar with the VMR to CRD in rats after chronic vagotomy, acute lidocaine treatment markedly enhanced VMR to CRD. *P < 0.05 compared with vehicle.
Responses of nodose ganglia neurons to electrical stimulation of subdiaphragmatic vagal afferent nerves
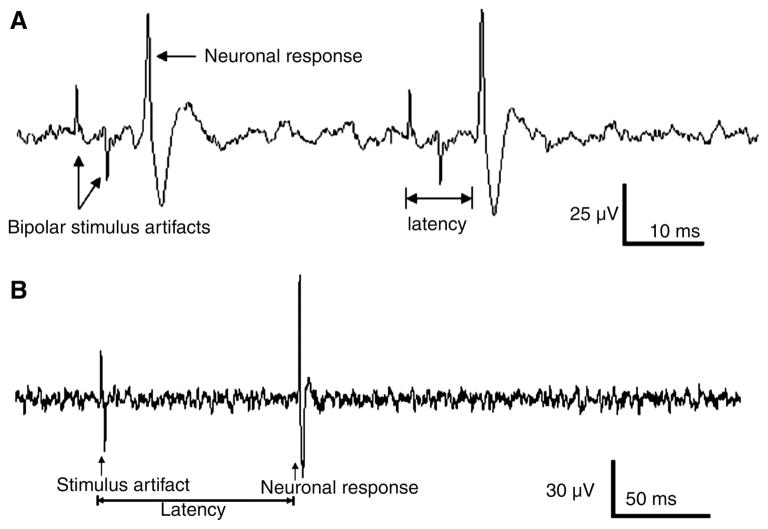
We identified the parameters of electrical stimulation capable of evoking responses in different types of vagal afferent neurons (30). A total of 18 neurons in four rats were examined. Electrical stimulation of the subdiaphragmatic vagal afferent nerves at a low intensity (40 μA, 20 Hz) elicited the action potential with latency 0.007 ± 0.001 s. Since the conduction distance = 0.06 m (between stimulating electrode and recording electrode), thus conductive velocity = 8.5 ± m/s, suggesting that we were recording from a gastrointestinal Aδ fiber. Action potential elicited from another nodose ganglia neuron in response to high-intensity EVS (400 μA, 1 Hz) had latency = 0.072 ± 0.005 s and conduction velocity = 0.8 ± m/s, suggesting that a gastrointestinal C fiber was recorded (Fig. 4).
Fig. 4.
Vagal nodose ganglia neuronal responses to electrical stimulation of subdiaphragmatic vagal afferent nerves (SDVAS). A: action potential elicited from a nodose ganglia neuron in response to 2 consecutive electrical subdiaphragmatic vagal stimulations (100 μA, 20 Hz). Conduction distance = 0.06 m, latency = 0.007 s, conductive velocity = 8.5 m/s, suggesting that we were recording a gastrointestinal Aδ fiber. B: action potential elicited from a nodose ganglia neuron in response to high-intensity electrical vagal stimulation (EVS) (400 μA, 1 Hz). The conductive distance = 0.06 m, latency = 0.07 s, conduction velocity = 0.8 m/s, suggesting that a gastrointestinal C fiber was recorded.
EVS
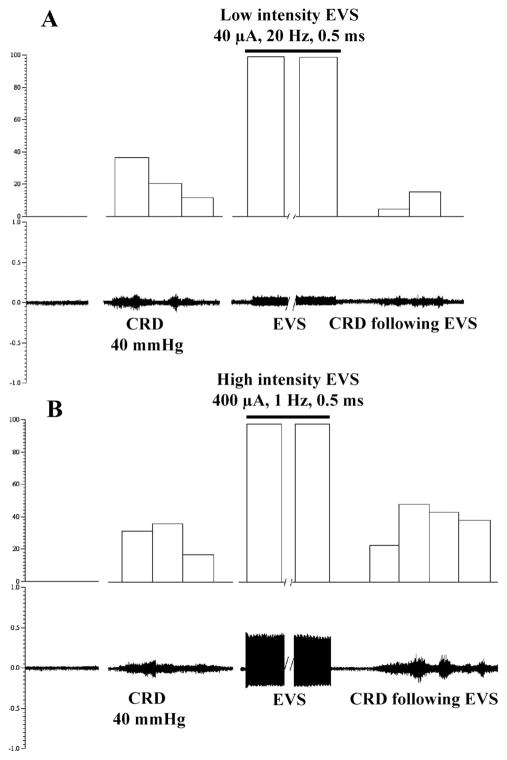
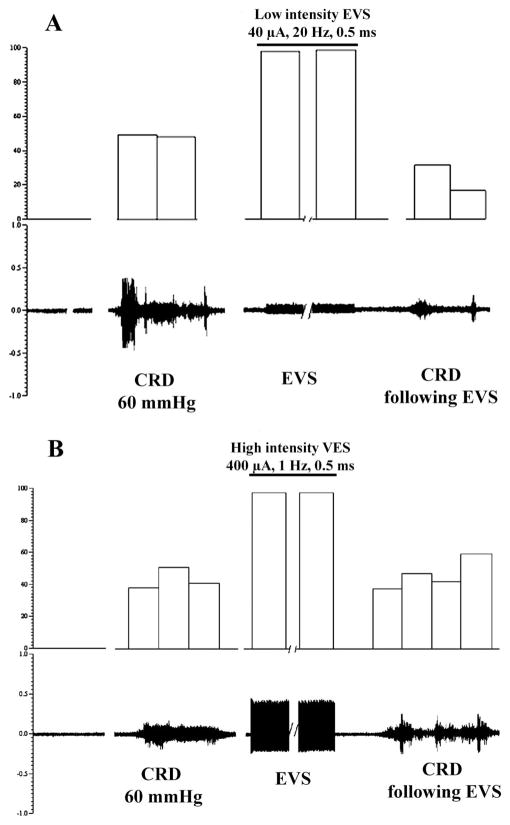
In our pilot studies, we performed a series of experiments to systematically examine the effects of EVS on CRD. The intensities of EVS were set to 40 or 100 μA, 20 Hz (low-intensity EVS) and then to 300 or 600 μA, 1 Hz (high-intensity EVS). The analysis of the compound action potential of the nodose neurons in our electrophysiological studies showed that a stimulus of 40 –100 μA activates predominantly myelinated fibers with calculated conduction velocities above 2.0 m/s (Aδ range), whereas 300- and 600-μA stimuli activate nonmyelinated vagal afferents. Trains of 100-μA EVS (20 s) caused no noticeable change in arterial blood pressure. Higher intensity VNS led to a transient increase in mean arterial pressure in some cases. We identified the parameters of abdominal vagal nerve electrical stimulation capable of modulating the VMR evoked by CRD. Electrical stimulation of the vagal afferent nerves at a low-intensity (40 μA, Figs. 5A and 6A) suppressed VMR to all intensities of CRD (Fig. 7) (from 22.5 ± 2.0, 35 ± 3 and 47 ± 5 contractions per 5 s, respectively, after sham stimulation, to 13 ± 1.0, 18 ± 3 and 24 ± 2.5 contractions per 5 s after low-intensity stimulation in response to 40, 60, and 80 mmHg CRD, respectively, which represent 41, 49, and 53% inhibition). The stimulus-produced inhibition began to recover 8 min after termination of the stimulation. In contrast, high-intensity electrical stimulation (400 μA) of the vagal afferent nerves did not suppress VMR in response to 40, 60, and 80 mmHg CRD (Figs. 5B, 6B, and 7).
Fig. 5.
Effect of electrical stimulation of the SDVAS on the VMR to CRD 40 mmHg in rats. EMG records of VMR to CRD 40 mmHg from external oblique-pelvic muscles. A: histogram at top displays the number of muscle contractions per 5 s. Low-intensity EVS of the vagal afferent nerve (40 μA) suppressed VMR induced by CRD (40 mmHg). B: compared with preelectrical vagal stimulation, higher intensity EVS (400 μA) has no effect on VMR induced by CRD.
Fig. 7.
Effects of electrical stimulation of vagal afferent nerves on VMR induced by CRD in rats. Twelve rats were used in this study. Each rat had preelectrical stimulation trial and immediately after electrical stimulation trial. Different parameters of EVS were applied on different days for the same rat. Graded CRD evoked reproducible VMR (0 μA). Low-intensity EVS (40 μA) reduced VMR in response to all intensities of CRD. In contrast, higher intensity EVS (400 μA) had no effect on VMR induced by CRD. Data are presented as means ± SE. *P < 0.05. ANOVA showed a significant effect for distension level and group interaction. Post hoc comparisons of means revealed a significant difference between the group receiving electrical stimulation at 40 μA, 20 Hz (*P < 0.05) and 400 μA, 20 Hz at 40, 60, and 80 mmHg CRD. Data are shown as means ± SE. *P < 0.05 compared with 0 Hz and 10 Hz electrical stimulation (1-way repeated-measures ANOVA followed by the Bonferroni test).
DISCUSSION
The modulation of nociception by vagal afferents was first investigated about 25 years ago (49). Cervical vagal afferent stimulation altered the response of spinal dorsal horn neurons to noxious somatic stimuli (38) and suppressed induction of c-Fos in dorsal horn neurons by noxious heating of the hind-paw (11). Under certain conditions, vagal afferent stimulation had an somatic analgesic potential (37, 38, 45). To date, the role of the vagus nerve in the modulation of visceral pain responses remains controversial. Previous studies have showed that vagal manipulation failed to affect the VMR evoked by esophageal distension (23). Another study showed that a slight but insignificant increase in the VMR to noxious gastric distension after chronic vagotomy (51). However, other studies have shown that vagotomy blunts the VMR to upper cervical esophageal distensions (22) and significantly enhanced the VMR to tonic CRD in conscious rats (17). In this study, we demonstrated that chronic subdiaphragmatic vagotomy decreases the threshold and enhances the VMR to all grades of CRD (470, 106, 51, and 54% increases in VMR to CRD at 20, 40, 60, and 80 mmHg, respectively). The observed effects of vagotomy on nociception are not due to the neuroplasticity changes after vagotomy. In a separate study, we performed acute vagal functional denervation using topical application of lidocaine in anesthetized rats. Similar to the results after vagotomy, we observed that topical application of lidocaine facilitated VMR induced by CRD. These observations suggest that subdiaphragmatic vagal nerves are involved in the inhibitory modulation of visceral pain responses.
The analgesic effect of the vagal nerve may be partly mediated by the opioidergic pathway. μ-Opiate receptors are present in vagal sensory neurons (1), and μ-opioid agonists inhibit voltage-gated calcium currents in vagal afferent neurons (43). Previous animal studies have demonstrated that vagal afferent integrity is essential to the efficacy of morphine (40), whereas a antinociceptive effect of morphine was significantly attenuated following subdiaphragmatic vagotomy (39).
The vagus nerve, like all cranial nerves, contains three types of fibers (A–C), distinguished by their physical and electrical conductance properties (7, 24). Recruited at the lowest thresholds (0.02–0.2 mA) are the large, myelinated, A fibers. At thresholds of 0.04–0.6 mA, smaller, myelinated B fibers are recruited. C fibers are small (0.4–1.2) unmyelinated fibers with the highest stimulation thresholds of above 2.0 mA.
Twenty years ago, researchers discovered that intermittent electrical stimulation of the vagus nerve produces inhibition of neural processes, which can alter brain electrical activity and terminate seizures (55). Vagal nerve stimulation therapy has also been used for treatment of depression and certain eating disorders (16, 34). Recently, gastric electrical stimulation has been used for normalizing gastric dysrhythmia, accelerating gastric emptying and improving nausea and vomiting (32).
In the literature, a wide range of EVS parameters were reported with respect to the cervical EVS vs. abdominal EVS, the spinal neuronal activity vs. behavioral responses, the somatic nociception vs. visceral nociception, and the anesthetized vs. conscious rats in different species (2, 8, 13, 19, 24, 41, 42, 50). Previous studies have demonstrated that cervical EVS modulated sacral spinal neuronal responses to noxious visceral stimulation. Stimulation intensities <25 μA produced a mild facilitation; however, intensities at 50–100 μA nearly abolished the CRD-induced responses (38). Electrical stimulation of dorsal subdiaphragmatic afferent vagal fibers produced intensity-dependent (20–200 μA) inhibition of lumbosacral spinal dorsal horn neuronal responses to noxious heat. No facilitatory effect was observed (38). Behavioral measure of somatic nociception, previous studies have demonstrated that low-intensity stimulation of cervical vagal afferents facilitates, but high-intensity stimulation inhibits nociceptive reflexes, such as the jaw-opening reflex (8) or the tail-flick (TF) reflex in capsaicin-treated rats (42). However, subdiaphragmatic vagotomy decreases the threshold for mechanically induced hindpaw withdrawal in rats (24), increases sensitivity to various noxious lesions (8), and enhances bradykinin-induced hyperalgesic behavior (24). In this study, we found that low- and high-level EVS might have different effects on visceral nociception in conscious rats. Low-intensity EVS of Aδ fibers (40 μA, 20 Hz, 0.5 ms for 30 s) reduced VMRs to CRD at 40, 60, and 80 mmHg by 41, 52, and 58%, respectively.
In our pilot studies, the intensities of EVS were set to 40 or 100 μA, 20 Hz (low-intensity EVS) and then to 300 or 600 μA, 1 Hz (high-intensity EVS). Data collected from the rats by using 40- and 400-μA EVS were reported. Although we did not observe a facilitatory effect on VMRs to CRD, the intensity of subdiaphragmatic EVS that inhibited VMRs in conscious rats was compatible with the intensity of cervical EVS (50–100 μA), which has been shown to inhibit spinal neuronal responses to noxious CRD (38), and the intensity of dorsal subdiaphragmatic EVS (20–200 μA), which inhibits spinal neuronal responses to heating of the hindpaw, respectively (38). It is also compatible with previous reports in rats with a light level of anesthesia (40), which showed that subdiaphragmatic EVS (25–64 μA, 2.0 ms, 20 Hz) inhibited TF reflex but did not facilitate the TF reflex at the intensity <32 μA as did cervical EVS (40). Our findings demonstrated that activation of vagal Aδ fibers can suppress visceral pain. In contrast, high-intensity EVS of C fiber (400 μA, 20 Hz, 0.5 ms for 30 s) had no effects on VMR induced by all intensities of CRD.
Recently, the functional properties of vagal A-type neuron has been investigated by neurophysiological methods. It has been well established that CCK activates vagal afferent neurons. Although most vagally mediated actions of CCK are blocked by capsaicin treatment (30, 47), a recent patch-clamp electrophysiological study found that subpopulations of both A- and C-type neurons responded to CCK. Thus some vagally mediated actions of CCK may be mediated by capsaicin-insensitive A-type neurons (47). Further study suggested that CCK directly activates capsaicin-resistant A-type afferents to facilitate vagal afferent responses to gastric distension (52). In behavioral studies of conscious rats, we find that topical application of CCK-8 on abdominal vagal tracts, or intravenous infusion of CCK-8, had no effects of VMRs induced by CRD (data not shown).
Previous studies have shown that stimulation parameters that are effective for seizure suppression are below those that would recruit C fibers in humans (27) and rats (28). Furthermore, weak stimulation of the vagus, which recruits the A fibers, causes synchronization of the EEG (3), whereas high stimulation, which additionally recruits C fibers, results in desynchronization of the EEG (9). In fact, activation of C fibers not only may be unnecessary but is probably also undesirable, since animal studies show that it leads to autonomic effects such as bradycardia (28). Together, the data suggest that C fiber activation is probably not required for clinical benefit of VNS.
The findings of this study should raise the question as to what physiological stimuli activate the subdiaphragmatic vagal afferents that produce this modulation of visceral pain under physiological and pathophysiological conditions. Investigators have reported that after a 48-h fast female rats exhibited increased nociceptive behavior in the formalin test. The fasting-induced effect on nociception appears to be mediated by the vagus nerve since it is prevented by subdiaphragmatic vagotomy (25). Furthermore, increasing the bulk content of the stomach (without providing nutrients) by infusion of petrolatum significantly attenuated the effect of fasting during the interphase period of the formalin response, suggesting that decreased gut distension, and possibly decreased motility, are important in fasting-induced enhancement of somatic nociception. Further studies are needed to determine whether natural periodic changes in gut distension and motility may control an ongoing vagus-mediated adjustment in the visceral nociceptive sensitivity. In particular, could dysfunction of this system cause visceral hypersensitivity?
The mechanisms responsible for the analgesic effects of EVS are unclear. Vagal stimulation affects widespread brain structures involved in regulation of mood and cognition (18, 44). The central terminals of vagal afferents are located in the NTS. In addition, ~5% of projections terminate in the upper cervical spinal cord (C1–2), where they are believed to contribute to referred pain originating in the heart (6, 7). Neurons in the NTS project to the parabrachial nucleus and the information is further transmitted to the amygdala, hypothalamus, and limbic cortex, likely influencing autonomic responses and emotional reactions to noxious visceral stimuli. Electrical stimulation of cervical vagus nerve has been shown to modulate neuronal activity in the parietal cortex (36). Using functional magnetic resonance imaging in humans has revealed that VNS at different frequencies likely has frequency-dependent modulatory effects on brain activities (31). Recently, we have demonstrated that anterior cingulate cortex (ACC) plays a critical role in the modulation of pain reflex in viscerally hypersensitive rats (4). In our electrophysiological studies, we have showed that CRD-induced firing of neurons of the ACC depends on glutamatergic neurotransmission via N-methyl-D-aspartate receptors in rats with experimental visceral hypersensitivity (54); low-intensity electrical vagus nerve stimulation reduces ACC neuronal firings in response to CRD in rats (15). A fundamental limitation of VNS at present is the lack of understanding of the definite functional anatomy of VNS as modified and controlled by its use parameters (intensity, pulse-width, frequency, duty cycle). Therefore, the key question is whether VNS, applied with different use parameters, might be selectively “targeted” to modify different brain regions, with attendant “focusing” of behavioral effects.
In conclusion, we demonstrated that vagal afferent nerves modulate visceral pain. Low-intensity electrical vagal stimulation that activates vagal afferent Aδ fibers reduced visceral pain. Thus EVS may potentially have a role in the treatment of chronic visceral pain.
Fig. 6.
Effect of electrical stimulation of the SDVAS on the VMR to CRD 60 mmHg in rats. A: low-intensity EVS of the vagal afferent nerve (40 μA) markedly suppressed VMR induced by CRD (60 mmHg). However, B shows that higher intensity EVS (400 μA) has no effect on VMR induced by CRD.
Acknowledgments
Present address of S. L. Chen and Z. J. Cao: Dept. of Gastroenterology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, China.
GRANTS
This research was supported by the National Institute of Neurological Disorders and Stroke Grant RO1 NS051466-01 (Y. Li) and the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-51717 (Y. Li) and P30-DK-34933 (C. Owyang).
References
- 1.Aicher SA, Goldberg A, Sharma S, Pickel VM. Mu-opioid receptors are presented in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Com Neurol. 2000;422:181–190. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Ammons WS, Blair RW, Foreman RD. Vagal afferent inhibition of primate thoracic spinothalamic neurons. J Neurophysiol. 1983;50:926–940. doi: 10.1152/jn.1983.50.4.926. [DOI] [PubMed] [Google Scholar]
- 3.Barnes A, Duncan R, Chisholm JA, Lindsay K, Patterson J, Wyper D. Investigation into the mechanisms of vagus nerve stimulation for the treatment of intractable epilepsy, using 99mTc-H.MPAO SPET brain images. Eur J Nucl Med Mol Imaging. 2003;30:301–305. doi: 10.1007/s00259-002-1026-8. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Wu X, Chen S, Fan J, Zhang R, Owyang C, Li Y. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008;134:535–543. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 6.Chandler MJ, Zhang J, Foreman RD. Vagal, sympathetic and somatic sensory inputs to upper cervical (C1–C3) spinothalamic tract neurons in monkeys. J Neurophysiol. 1996;76:2555–2567. doi: 10.1152/jn.1996.76.4.2555. [DOI] [PubMed] [Google Scholar]
- 7.Chandler MJ, Zhang J, Qin C, Yuan Y, Foreman RD. Intrapericardiac injections of algogenic chemicals excite primate C1–C2 spinothalamic tract neurons. Am J Physiol Regul Integr Comp Physiol. 2000;279:R560–R568. doi: 10.1152/ajpregu.2000.279.2.R560. [DOI] [PubMed] [Google Scholar]
- 8.Chase MH, Nakamura Y, Torii S. Afferent vagal modulation of brain stem somatic reflex activity. Exp Neurol. 1970;27:534–544. doi: 10.1016/0014-4886(70)90114-7. [DOI] [PubMed] [Google Scholar]
- 9.Chase MH, Sterman MB, Clemente CD. Cortical and subcortical patterns of response to afferent vagal stimulation. Exp Neurol. 1966;16:36–49. doi: 10.1016/0014-4886(66)90084-7. [DOI] [PubMed] [Google Scholar]
- 10.Erlanger J, Gasser ILS. The action potential in fibers of slow conduction in spinal roots and somatic nerves. Am J Physiol. 1930;92:43–81. [Google Scholar]
- 11.Evans AR, Jones SL, Blair RW. Effects of vagal afferent nerve stimulation on noxious heat-evoked Fos-like immunoreactivity in the rat lumbar spinal cord. J Comp Neurol. 1994;346:490–498. doi: 10.1002/cne.903460403. [DOI] [PubMed] [Google Scholar]
- 12.Forster J, Sarosiek I, Delcore R, Lin Z, Raju GS, McCallum RW. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–681. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- 13.Fu QG, Chandler MJ, McNeill DL, Foreman RD. Vagal afferent fibers excite upper cervical neurons and inhibit activity of lumbar spinal cord neurons in the rat. Pain. 1992;51:91–100. doi: 10.1016/0304-3959(92)90013-2. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Wu X, Owyang C, Li Y. Enhanced responses of the anterior cingulate cortex neurons to colonic distension in viscerally hypersensitive rats. J Physiol. 2006;570:169–184. doi: 10.1113/jphysiol.2005.096073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Gao J, Wu X, Owyang C, Li Y. Vagal afferent modulation of the anterior cingulate cortex activated by colorectal distension is mediated by vagal A fibers containing gaba (Abstract) Gastroenterology. 2004;126:W1053. [Google Scholar]
- 16.George MS. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- 17.Gschossmann JM, Mayer EA, Miller JC, Raybould HE. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterol Motil. 2002;14:403–408. doi: 10.1046/j.1365-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 18.Henry TR. Vagus nerve stimulation for epilepsy: anatomical, experimental, and mechanistic investigations. In: Schachter SC, Schmidt D, editors. Vagus Nerve Stimulation. 2. London: Dunitz; 2003. pp. 1–32. [Google Scholar]
- 19.Hobbs SF, Oh UT, Chandler MJ, Foreman RD. Cardiac and abdominal vagal afferent inhibition of primate T9–S1 spinothalamic cells. Am J Physiol Regul Integr Comp Physiol. 1989;257:R889–R895. doi: 10.1152/ajpregu.1989.257.4.R889. [DOI] [PubMed] [Google Scholar]
- 20.Holtmann G, Goebell H, Jockenhoevel F, Talley NJ. Altered vagal and intestinal mechanosensory function in chronic unexplained dyspepsia. Gut. 1998;42:501–506. doi: 10.1136/gut.42.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzer P. Afferent signalling of gastric acid challenge. J Physiol Pharmacol. 2003;54:43–53. [PubMed] [Google Scholar]
- 22.Hummel T, Barz S, Hölscher TL, Neuhuber W. Differences in responses to nociceptive stimulation of the oral and aboral oesophagus. J Clin Neurosci. 2003;10:223–225. doi: 10.1016/s0967-5868(02)00332-6. [DOI] [PubMed] [Google Scholar]
- 23.Jou CJ, Farber JP, Qin C, Foreman RD. Convergent pathways for cardiac- and esophageal-somatic motor reflexes in rats. Autonom Neurosci Basic Clin. 2002;99:70–77. doi: 10.1016/s1566-0702(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 24.Khasar SG, Miao JP, Janig W, Levine JD. Modulation of bradykinin-induced mechanical hyperalgesia in the rat by activity in abdominal vagal afferents. Eur J Neurosci. 1998;10:435–444. doi: 10.1046/j.1460-9568.1998.00030.x. [DOI] [PubMed] [Google Scholar]
- 25.Khasar SG, Reichling DB, Green PG, Isenberg WM, Levine JD. Fasting is a physiological stimulus of vagus-mediated enhancement of nociception in the female rat. Neuroscience. 2003;119:215–221. doi: 10.1016/s0306-4522(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 26.Koltzenburg M, Kress M, Reeh PW. The nociceptor sensitization by bradykinin does not depend on sympathetic neurons. Neuroscience. 1992;46:465–473. doi: 10.1016/0306-4522(92)90066-b. [DOI] [PubMed] [Google Scholar]
- 27.Koo B, Ham SD, Sood S, Tarver B. Human vagus nerve electrophysiology—a guide to vagus nerve stimulation parameters. J Clin Neurophysiol. 2001;18:429–433. doi: 10.1097/00004691-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Krahl SE, Senanayake SS, Handforth A. Destruction of peripheral C fibers does not alter subsequent vagus nerve stimulation-induced seizure suppression in rats. Epilepsia. 2001;42:586–589. doi: 10.1046/j.1528-1157.2001.09700.x. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Wu XY, Zhu JX, Yan J, Owyang C. Hypothalamic regulation of pancreatic secretion is mediated by central cholinergic pathway in rat. J Physiol. 2003;552:571–587. doi: 10.1113/jphysiol.2003.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y. Sensory signal transduction in the vagal primary afferent neurons. Curr Med Chem. 2007;14:2552–2563. doi: 10.2174/092986707782023334. [DOI] [PubMed] [Google Scholar]
- 31.Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219–27. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 32.Lin Z, Chen JDZ. Advances in gastrointestinal electrical stimulation. Crit Rev Biomed Eng. 2002;30:419–457. doi: 10.1615/critrevbiomedeng.v30.i456.70. [DOI] [PubMed] [Google Scholar]
- 33.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang H, Yin J, Chen JDZ. Therapeutic potential of gastric electrical stimulation for obesity and its possible mechanisms: a preliminary canine study. Dig Dis Sci. 2003;48:698–705. doi: 10.1023/a:1022824406648. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- 36.Radna RJ, MacLean PD. Vagal elicitation of respiratory-type and other unit responses in basal limbic structures of squirrel monkeys. Brain Res. 1981;213:45–61. doi: 10.1016/0006-8993(81)91247-6. [DOI] [PubMed] [Google Scholar]
- 37.Randich A, Aicher A. Medullary substrates mediating antinociception produced by electrical stimulation of the vagus. Brain Res. 1998;445:68–76. doi: 10.1016/0006-8993(88)91075-x. [DOI] [PubMed] [Google Scholar]
- 38.Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 39.Randich A, Thurston CL, Ludwig PS, Timmerman MR, Gebhart GF. Antinociception and cardiovascular responses produced by intravenous morphine: the role of vagal afferents. Brain Res. 1991;543:256–270. doi: 10.1016/0006-8993(91)90036-u. [DOI] [PubMed] [Google Scholar]
- 40.Ren K, Randich A, Gebhart GF. Vagal afferent modulation of a nociceptive reflex in rats: involvement of spinal opioid and monoamine receptors. Brain Res. 1988;446:285–294. doi: 10.1016/0006-8993(88)90887-6. [DOI] [PubMed] [Google Scholar]
- 41.Ren K, Randich A, Gebhart GF. Effects of electrical stimulation of vagal afferents on spinothalamic tract cells in the rat. Pain. 1991;44:311–319. doi: 10.1016/0304-3959(91)90102-4. [DOI] [PubMed] [Google Scholar]
- 42.Ren K, Zhuo M, Randich A, Gebhart GF. Vagal afferent stimulation-produced effects on nociception in capsaicin-treated rats. J Neurophysiol. 1993;69:1530–1540. doi: 10.1152/jn.1993.69.5.1530. [DOI] [PubMed] [Google Scholar]
- 43.Rusin KI, Moises HC. Mu-opioid and GABA(B) receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons. Neuroscience. 1998;85:939–956. doi: 10.1016/s0306-4522(97)00674-x. [DOI] [PubMed] [Google Scholar]
- 44.Schachter SC, Saper CB. Progress in epilepsy research: vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 45.Sedan O, Sprecher E, Yarnitsky D. Vagal stomach afferents inhibit somatic pain perception. Pain. 2005;113:354–359. doi: 10.1016/j.pain.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Sengupta JN, Petersen J, Peles S, Shaker R. Response properties of antral mechanosensitive afferent fibers and effects of ionotropic glutamate receptor antagonists. Neuroscience. 2004;125:711–723. doi: 10.1016/j.neuroscience.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Simasko SM, Ritter RC. Cholecystokinin activates both A- and C-type vagal afferent neurons. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1204–G1213. doi: 10.1152/ajpgi.00132.2003. [DOI] [PubMed] [Google Scholar]
- 48.Smart HL, Atkinson M. Abnormal vagal function in irritable bowel syndrome. Lancet. 1987;2:475–478. doi: 10.1016/s0140-6736(87)91792-2. [DOI] [PubMed] [Google Scholar]
- 49.Thies R, Foreman RD. Descending inhibition of spinal neurons in the cardiopulmonary region by electrical stimulation of vagal afferent nerves. Brain Res. 1981;207:178–183. doi: 10.1016/0006-8993(81)90690-9. [DOI] [PubMed] [Google Scholar]
- 50.Thies R, Foreman RD. Inhibition and excitation of thoracic spinoreticular neurons by electrical stimulation of vagal afferent nerves. Exp Neurol. 1983;82:1–16. doi: 10.1016/0014-4886(83)90238-8. [DOI] [PubMed] [Google Scholar]
- 51.Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- 52.Van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin-insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;289:R695–R703. doi: 10.1152/ajpregu.00809.2004. [DOI] [PubMed] [Google Scholar]
- 53.White JC, Sweet WH. Pain and the Neurosurgeon: A Forty-Year Experience. Springfield, IL: Thomas; 1969. Abdominal visceral disease. [Google Scholar]
- 54.Wu X, Gao J, Yan J, Fan J, Owyang C, Li Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G918–G927. doi: 10.1152/ajpgi.00452.2007. [DOI] [PubMed] [Google Scholar]
- 55.Zabara J. Peripheral control of hypersynchronous discharge in epilepsy (Abstract) Electroencephalogr Clin Neurophysiol. 1985;61:S162. [Google Scholar]
- 56.Zagon A. Does the vagus nerve mediate the sixth sense? Trends Neurosci. 2001;24:671–673. doi: 10.1016/s0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- 57.Zhu JX, Wu XY, Owyang C, Li Y. Electrophysiological evidence that intestinal 5-HT acts as a paracrine to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431– 442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]