Abstract
A method is proposed for the measurement of glycosaminoglycans (GAG) on 5-10 ml of plasma. It is based on the adsorption of GAG on small ECTEOLA columns followed by measurement of the hexuronic acid in the NaCl eluates. Routine use of the method has indicated the presence of a GAG fraction that adsorbs readily on ECTEOLA (“free” GAG) and of another that adsorbs on it only after treatment with papain (“bound” GAG). “Free” and “bound” GAG have been measured in normal adults, normal children, and children affected by mucopolysaccharidosis type I; the results obtained are in good agreement with those previously reported in the literature.
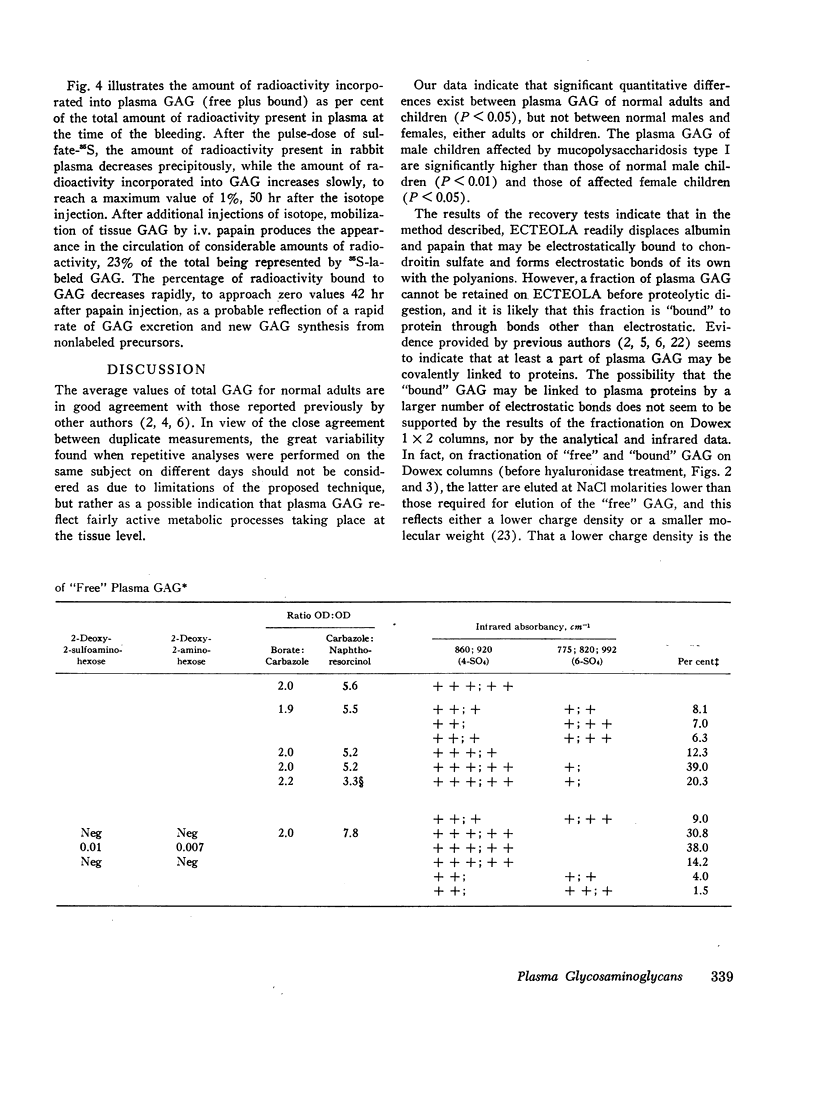
Various analyses performed on purified “free” and “bound” GAG have confirmed that chondroitin-4-sulfate is the main glycosaminoglycan of normal human plasma where it occurs both free and bound to protein and at various levels of sulfation. The presence of small amounts of heparan sulfate and keratan sulfate has also been demonstrated.
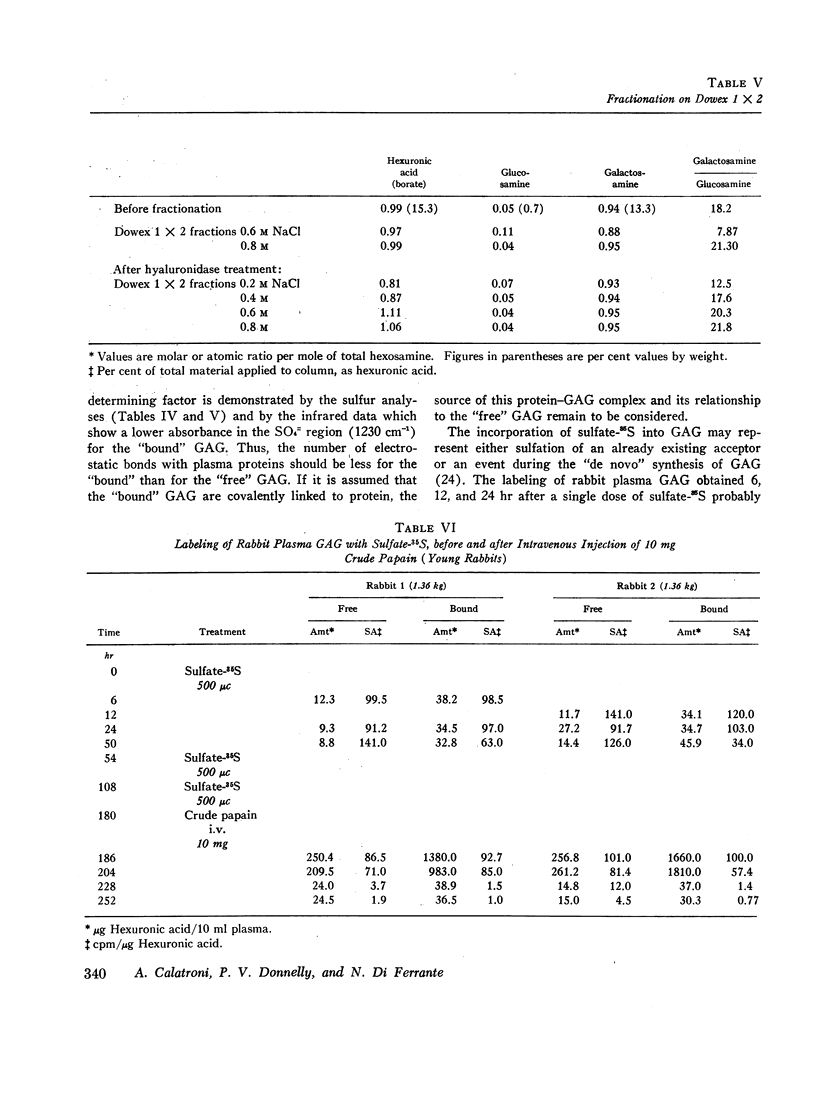
Metabolic experiments performed in rabbits have indicated that plasma GAG derive from peripheral tissues and increase sharply after papain injection. In young animals the “free” GAG have a faster turnover than the “bound,” possibly a reflection of active processes of remodeling and calcification. The synthesis of the “free” and “bound” GAG, as measured with 85S-sulfate incorporation, seems to proceed at the same rate, and the hypothesis has been advanced that as a result of the action of tissue proteases, part of the “bound” GAG may be transformed into “free” GAG, the latter being immediately extruded from the tissues into the circulatory system.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- BADIN J., SCHUBERT M., VOURAS M. Plasma polysaccharide fraction containing uronic acid, in normal subjects and in patients with rheumatoid arthritis. J Clin Invest. 1955 Aug;34(8):1317–1323. doi: 10.1172/JCI103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSIOUNI M. Studies on the acid polysaccharide of the white cells in rheumatic and other diseases showing its similarity to the acid polysaccharide of amyloid. Ann Rheum Dis. 1955 Sep;14(3):288–292. doi: 10.1136/ard.14.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J., SERAYDARIAN M. W., SIMPSON W. F. Acid mucopolysaccharides in normal serum. J Clin Invest. 1957 Sep;36(9):1328–1332. doi: 10.1172/JCI103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman E. R. Separation of connective tissue mucopolysaccharide-protein complexes from unrelated proteins. Nature. 1966 Aug 6;211(5049):640–641. doi: 10.1038/211640a0. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D., DI FERRANTE N. Association of sulfate-S35 with serum proteins in the rat. J Biol Chem. 1957 Jul;227(1):347–356. [PubMed] [Google Scholar]
- Di Ferrante N. M. The measurement of urinary mucopolysaccharides. Anal Biochem. 1967 Oct;21(1):98–106. doi: 10.1016/0003-2697(67)90087-5. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friman C., Brunish R. A zone electrophoretic study of acid mucopolysaccharides (AMPS) in normal human serum. Proc Soc Exp Biol Med. 1966 Jun;122(2):599–601. doi: 10.3181/00379727-122-31201. [DOI] [PubMed] [Google Scholar]
- Hirschman A., Dziewiatkowski D. D. Protein-polysaccharide loss during endochondral ossification: immunochemical evidence. Science. 1966 Oct 21;154(3747):393–395. doi: 10.1126/science.154.3747.393. [DOI] [PubMed] [Google Scholar]
- KERBY G. P. The distribution of acid mucopolysaccharides in Cohn fractions of human plasma proteins. J Clin Invest. 1958 May;37(5):678–681. doi: 10.1172/JCI103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGUNOFF D., WARREN G. Determination of 2-deoxy-2-sulfoaminohexose content of mucopolysaccharides. Arch Biochem Biophys. 1962 Dec;99:396–400. doi: 10.1016/0003-9861(62)90285-0. [DOI] [PubMed] [Google Scholar]
- MUIR H. The nature of the link between protein and carbohydrate of a chondroitin sulphate complex from hyaline cartilage. Biochem J. 1958 Jun;69(2):195–204. doi: 10.1042/bj0690195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKusick V. A., Kaplan D., Wise D., Hanley W. B., Suddarth S. B., Sevick M. E., Maumanee A. E. The genetic mucopolysaccharidoses. Medicine (Baltimore) 1965 Nov;44(6):445–483. doi: 10.1097/00005792-196511000-00001. [DOI] [PubMed] [Google Scholar]
- Meezan E., Davidson E. A. Mucopolysaccharide sulfation in chick embryo cartilage. II. Characterization of the endogenous acceptor. J Biol Chem. 1967 Nov 10;242(21):4956–4962. [PubMed] [Google Scholar]
- Oh S. I., Neff B. J., Block W. D. Soluble collagen in human serum. Clin Chim Acta. 1968 Jul;21(1):1–8. doi: 10.1016/0009-8981(68)90002-8. [DOI] [PubMed] [Google Scholar]
- PELZER H., STAIB W. Steroidkonjugate. I. Hochspannungs-Papierelektrophorese von 17-ketosteroidkonjugaten. Clin Chim Acta. 1957 Oct;2(5):407–415. doi: 10.1016/0009-8981(57)90037-2. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- SCHILLER S. The isolation of chondroitinsulfuric acid from normal human plasma. Biochim Biophys Acta. 1958 May;28(2):413–416. doi: 10.1016/0006-3002(58)90489-x. [DOI] [PubMed] [Google Scholar]
- TELLER W. M., ROSEVEAR J. W., BURKE E. C. Identification of heterozygous carriers of gargoylism. Proc Soc Exp Biol Med. 1961 Nov;108:276–278. doi: 10.3181/00379727-108-26913. [DOI] [PubMed] [Google Scholar]
- THOMAS L., McCLUSKEY R. T., POTTER J. L., WEISSMANN G. Comparison of the effects of papain n vitamin A on cartilage. I. The effects in rabbits. J Exp Med. 1960 May 1;111:705–718. doi: 10.1084/jem.111.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS L. Reversible collapse of rabbit ears after intravenous papain, and prevention of recovery by cortisone. J Exp Med. 1956 Aug 1;104(2):245–252. doi: 10.1084/jem.104.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINSTEIN G. M., SACHS C. R., SCHUBERT M. PROTEIN POLYSACCHARIDE IN CONNECTIVE TISSUE: INHIBITION OF PHASE SEPARATION. Science. 1963 Nov 22;142(3595):1073–1075. doi: 10.1126/science.142.3595.1073. [DOI] [PubMed] [Google Scholar]
- Wessler E. Determination of acidic glycosaminoglycans (mucopolysaccharides) in urine by an ion exchange method. Application to "collagenoses", gargoylism, the nail-patella syndrome and Farber's disease. Clin Chim Acta. 1967 May;16(2):235–243. doi: 10.1016/0009-8981(67)90186-6. [DOI] [PubMed] [Google Scholar]