Abstract
The herpesvirus entry mediator (HVEM; TNFRSF14) can activate either proinflammatory or inhibitory signaling pathways. HVEM engages two distinct types of ligands, the canonical TNF-related cytokines, LIGHT and Lymphotoxin-α, and the Ig-related membrane proteins, BTLA (B and T lymphocyte attenuator) and CD160. Recent evidence indicates that the signal generated by HVEM depends on the context of its ligands expressed in trans or in cis. HVEM engagement by all of its ligands in trans initiates bidirectional signaling. In contrast, naïve T cells coexpress BTLA and HVEM forming a cis-complex that interferes with the activation of HVEM by extraneous ligands in the surrounding microenvironment. The HVEM network is emerging as a key survival system for effector and memory T cells in mucosal tissues.
Introduction
Herpesviruses are true masters at manipulating the immune system without overtly compromising the host. The molecular mechanisms of immune evasion are revealing new clues on how to selectively modulate immune responses. The persistent lifestyle of these DNA viruses, despite eliciting strong adaptive immunity, demands a sophisticated immune evasion strategy. In contrast to HIV’s dominance over the immune system by global elimination of CD4 T cells, herpesviruses target specific molecules in key signaling pathways including antigen processing and cytokines, providing a subtle, almost tolerant-like impact on host defense. From the point of entry into a cell, Herpes Simplex virus (HSV-1 and 2, α-herpesviradae) manipulates cytokine and cell-to-cell communication pathways [1, 2]. The virus attaches to the cell surface via the herpes virus entry mediator, (HVEM, TNFRSF14), aptly named as the first discovered entry route for HSV[3]. HVEM is a cell surface molecule in the TNF Receptor Superfamily, known to activate cell survival genes through NFκB transcription factors. The cellular ligands for HVEM come from two distinct families: the TNF-related cytokines, LIGHT (TNFSF14) and lymphotoxin-α (LTα)[4] and the Ig superfamily members, BTLA (B and T lymphocyte attenuator) and CD160[5-7]. HSV uses envelope glycoprotein-D (gD) as a viral ligand for HVEM and Nectin-1 to infect epithelial cells – the major site of host entry. The cross utilization of ligands by HVEM, the LTβ receptor and the two receptors for TNF (Figure 1) create a rather complex set of signaling systems that together form a network of pathways regulating inflammation and adaptive immune responses[8]. Viewed within this Network context, the manipulation of HVEM by herpesviruses reveals a deeper appreciation for the evolution of virus-host interactions. Understanding these strategies may provide lessons in how to limit inflammation in autoimmune diseases.
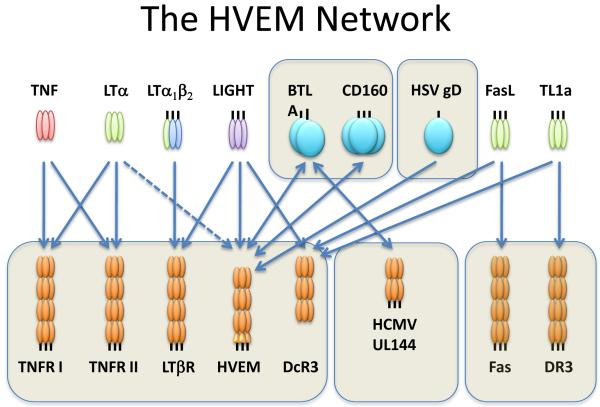
Figure 1. The HVEM Network.
The diagram depicts the specificity of the ligands (upper panel) and cognate receptors (lower panel) in the HVEM network. The arrows define the specific ligand-receptor interaction. The TNF related ligands are shown as trimers (unboxed). LTα is secreted as a homotrimer, and has modest affinity for HVEM (dashed line). The Ig superfamily members, BTLA and CD160, and herpes simplex virus (HSV) glycoprotein D (boxed) are ligands for HVEM. Human cytomegalovirus UL144, an HVEM ortholog, binds BTLA. Decoy receptor-3 (DcR3) binds LIGHT and the paralogous ligands, Fas Ligand and TL1a.
HVEM Connectivity
Much of the action that directs the proinflammatory and inhibitory signaling by HVEM is controlled at the cell surface. These distinct signaling outcomes stem from three key biophysical parameters: the specific pairing of ligand and receptor, the form of the ligand in soluble or membrane-anchored positions, and the cis or trans context of ligand-receptor engagement. The specificity of the ligand and receptor provides the primary mechanism that directs inflammatory and inhibitory signaling. HVEM plays dual roles as both receptor and ligand since BTLA and LIGHT engage distinct sites on HVEM, which is itself an activating ligand for BTLA. The ectodomain of HVEM contains four repeats of a cysteine-rich domain, the signature motif of the TNFR superfamily [9]. BTLA binding site is located in the first cysteine-rich domain at the N-terminus of HVEM, whereas homology modeling indicates LIGHT binds in the second and third domains, but on the opposite face of HVEM[10-13]. LIGHT, a type II transmembrane protein[14], can be proteolyzed releasing a soluble, bioactive form[15]. Soluble LIGHT binds HVEM with high affinity, but does not compete with BTLA, allowing both ligands to simultaneously occupy HVEM. In fact, soluble LIGHT and LTα enhance the binding of BTLA to HVEM, implicating the formation of a trimolecular complex[12, 13]. In striking contrast, the membrane form of LIGHT interferes with HVEM-BTLA binding, by an uncompetitive mechanism, presumably steric hindrance due to proximity of the membrane.
Trans Signaling
Based on the conservation seen in several TNF ligand-receptor crystal structures[16, 17], the stereoconformation of membrane LIGHT must be in trans (in an adjacent cell or membrane) to engage HVEM and activate signaling (Figure 2). The trimeric TNF ligands cluster the TNFR providing the key step in initiating signaling, which is mimicked by bivalent anti-receptor antibody. Surprisingly, BTLA when configured in trans also activates HVEM signaling[18]. BTLA forms dimers in the membrane of viable cells as revealed by flow cytometric-based FRET system. The dimeric conformation of BTLA suggested a mechanism for clustering HVEM and this mechanism was confirmed in experiments demonstrating that BTLA induces activation of HVEM as either a membrane protein or engineered as a soluble dimeric fusion protein with Fc of IgG. CD160 assembles as a trimer [19] providing a multimeric conformation that can function as an activating ligand for HVEM.
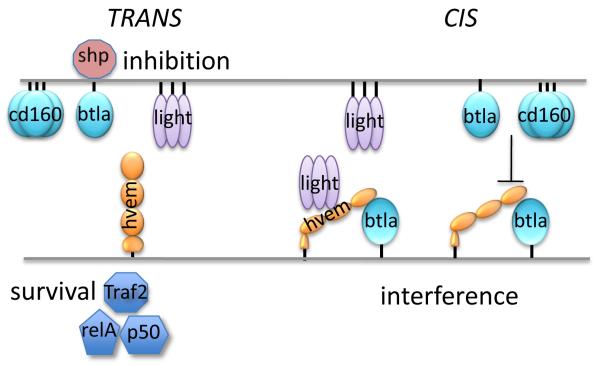
Figure 2. Cis and Trans interactions of HVEM.
The diagram illustrates the signaling outcomes for HVEM with its ligands in the trans (left side) or cis configurations (right side). Engagement of HVEM in trans initiates bidirectional signaling between adjace cells. LIGHT, BTLA or CD160 trigger HVEM to induce formation of the transcription factor NFκB RelA/p50 via the TRAF2 adapter mechanism. Ligation of HVEM with either LIGHT or BTLA promotes survival of effector and memory T cells. HVEM acts as a ligand for BTLA inducing recruitment of the SHP phosphatase1 and 2, to suppress antigen-receptor signaling. The cis configuration, present on naïve T cells, interferes with signaling via LIGHT, BTLA or CD160 when those ligands are in trans. Soluble LIGHT enhances the binding between HVEM and BTLA, but cannot activate HVEM in the cis complex. Membrane LIGHT can drive the disassociation of the HVEM-BTLA cis complex alleviating inhibitory signaling by BTLA.
Despite the location of the binding regions on the opposite sides of HVEM, both LIGHT and BTLA induce the canonical NFκB RelA pathway through a TRAF2-dependent mechanism, indicating signaling outcome is qualitatively the same for both ligands [18]. Whether this conclusion will apply to other HVEM signaling pathways such as the AKT/AP1 pathway, is not yet clear. Nonetheless, this result suggests both ligands provide HVEM with sufficient conformational reorganization to initiate the TRAF2 E3 ligase pathway that activates NFκB. HVEM does not recruit TRAF3 into its signaling complex as do other TNFR such as the LTβR, and thus the signaling activity of HVEM is limited to the activation NFκB RelA. TRAF3 is an essential component that limits NIK availability through ubiquitin-dependent degradation[20-22]. This limited signaling capacity for only the RelA/p50 NFκB may reflect HVEM’s restriction to the cell surface, in contrast to LTβR, which is internalized following ligation[23, 24].
The Cis and Trans of HVEM
Coexpression of HVEM and BTLA in T cells creates an intrinsic mechanism that interferes with the ability of LIGHT and the other ligands to access and activate HVEM [25](Figure 2). Previous studies had largely assumed that the behavior of the soluble form of LIGHT was representative of its membrane bound form. This assumption is indeed the case in the ability of soluble LIGHT to activate the LTβR or HVEM in epithelia cells. However, a distinction between soluble and membrane LIGHT emerged in studies of T cells, which in contrast to epithelia cells, coexpress HVEM with its inhibitory receptor BTLA. T cells derived from human blood and naïve mouse spleens constitutively coexpress HVEM and BTLA. HVEM and BTLA are expressed independently of each other as shown in mice genetically deficient in either gene. Together the HVEM-BTLA cis complex is stable and expressed at the cell surface, although no signaling activity is associated with HVEM. The formation of the cis complex required only HVEM to bind the ectodomain of BTLA and mutations that affect BTLA binding to HVEM in trans, also impacted binding in cis, indicating the HVEM-BTLA cis complex occupies the same binding sites as in trans. Indeed, cells coexpressing HVEM and BTLA are unable to bind HVEM or BTLA-Fc, which provided a method to quantify the HVEM and BTLA on the cell surface of T cells at a ratio of 1:1 with >85% of the molecules in the cis complex. Some antibodies that recognize an epitope in the HVEM-BTLA binding site likely underestimate the expression of these molecules in lymphocyte populations[26]. Importantly, the addition of soluble LIGHT or LTα failed to activate HVEM as detected by induction of NFκB, although binding of LIGHT was detectable as expected, and in cells expressing HVEM alone, these ligands were fully functional. BTLA or CD160 in either soluble or membrane positions also failed to activate HVEM in cells expressing the cis complex. Thus, BTLA functions as an inhibitor when coexpressed with HVEM.
The membrane form of LIGHT is the only cellular ligand capable of activating HVEM within the cis complex with BTLA. However, this activation is reduced compared to membrane LIGHT activation of HVEM in the absence of BTLA. The presence of antibodies to BTLA that disrupt the cis-complex synergizes with LIGHT to activate HVEM, which suggests the disassociation of HVEM from BTLA is a key biophysical feature of membrane LIGHT’s ability to activate HVEM. Together, these results indicate that membrane LIGHT has four distinct signaling functions: activation of HVEM, disruption of HVEM-BTLA cis complex, activation of the LTβR, and in soluble form enhancing HVEM-BTLA complex formation [4] .
The viral ligand, gD also forms complex in cis with HVEM, but in contrast to BTLA, does activate NFκB signaling, reflecting evolutionary mastery of this pathway [25]. It is reasonable to think that the regulation of prosurvival genes by NFκB may provide HSV with a selective advantage in mucosal epithelium during the early phase of the primary infection or following reactivation from latency. The ability of HSV gD to activate HVEM in cis suggests gD may induce appropriate conformational reorganization of HVEM, which BTLA cannot in the cis complex. gD also inhibited the binding of soluble and membrane LIGHT to HVEM even though gD engages the same site on HVEM as BTLA. We suggested that the HVEM-BTLA cis complex is a cellular representation of viral interference[25]. Herpesvirus gD mediates viral interference in which the initial infecting pathogen blocks additional pathogen infection by occupying the entry route [27, 28], and thus, functionally resisting influence from the surrounding microenvironment. The remarkable diversity in viral mimicry of the HVEM-BTLA complex [29] suggests this viral strategy serves as a key selective pressure driving evolution of host defenses.
The HVEM Network in Disease Models
The survival of memory and effector T cells has emerged as an important immune function of the HVEM Network. Btla−/− CD4 or CD8 T cells proliferate more when activated in vitro consistent with the inhibitory signaling of BTLA[25]. Inclusion of BTLA-Fc as a surrogate ligand for HVEM to cultures of CD4 and CD8 T cells substantially enhanced proliferation of Btla−/− T cells, but not the rate of division, suggesting HVEM impacted cell survival. Rel A nuclear translocation correlated with the survival of Btla−/− T cells treated with BTLA-Fc providing a mechanism linking BTLA-activated HVEM signaling to cell survival gene expression.
T cell driven, intestinal [30] and lung [31, 32] inflammatory disease models in mice have shown distinct roles for HVEM-BTLA and LIGHT-HVEM pathways. Transfer of CD4+T cells into lymphopenic mice (Rag−/−) induces colitis over 6-10 weeks. HVEM expressed in the host, likely in mucosal epithelium, is required to prevent the disease from dramatic acceleration (two weeks). BTLA expression in T cells and host innate cells was also essential to limit the accelerated colitis. This result is consistent with the idea that T lymphocyte-epithelia cell interactions establish a bidirectional signaling pathway via HVEM-BTLA. The mechanisms that HVEM activation in mucosal epithelium might play to protect intestinal functions remain to be elucidated. Interestingly, the transferred Btla−/− effector CD4+ T cells failed to survive in this colitis model [30]. More importantly, the cotransfer of wild-type T cells with T cells deficient in both Btla and Hvem into Rag−/− recipient mice showed a similar loss of gene-deficient T cells indicating that pathogenic effector T cells required intrinsic expression of both BTLA and HVEM. This observation implicates the HVEM-BTLA cis complex in sustaining survival effector CD4+ T cells.
Recent studies in a T cell dependent, lung inflammation model implicate the LIGHT-HVEM pathway in the survival of memory CD4+ Th2 cells[31]. Antigen-specific memory CD4+ T cells deficient in Hvem failed to persist following exposure to recall antigen, although the initial response to antigen was normal. Furthermore, both Th2 and Th1 effector cells depended on LIGHT and HVEM signaling to differentiate into memory cells. Importantly, therapeutic intervention with LTβR-Fc decoy, a competitive antagonist of LIGHT and LTαβ ligands, inhibited effector Th2 accumulation and disease symptoms. The LIGHT-HVEM pathway seems to be the dominant pathway in the lung, although BTLA remains to be fully explored in this model. The different roles played by HVEM-BTLA and LIGHT-HVEM as T cell survival pathways in these two different organ systems is striking. Together, perhaps these models are showing that different organ systems utilize different ligands. Or possibly LIGHT or BTLA may be more relevant to the generation of memory T cells because of distinct cellular interactions, involving T cells with dendritic cells vs macrophages, or perhaps, a yet unknown feature of the HVEM network.
It is cogent to recall that HVEM-BTLA functions as an inhibitory pathway in the homeostasis of dendritic cells. The HVEM-BTLA pathway counter-regulates the proliferation inducing activity of the LTαβ-LTβR pathway that stimulates CD8α-dendritic cell subsets in the lymph organs[33, 34]. Adoptive transfer experiments revealed that expression of both HVEM and BTLA were required in the hematopoietic cells and in host, implicating both cis and trans signaling configurations are operative in the regulation of dendritic cell proliferation.
Conclusions
The HVEM network controls the behavior of lymphocytes as they engage cells in their niches during the recognition, effector and memory phases of immune responses. Both the TNF and Ig superfamily ligands of HVEM can initiate the activation of HVEM survival signaling in trans via the NFκB pathway. HVEM-BTLA cis complex provides intrinsic regulation in T cells serving as an interference mechanism silencing signals coming from the microenvironment. Each ligand-receptor pairing in the HVEM network provides both proinflammatory and inhibitory signaling. The complexity of the HVEM network in regulating lymphocyte behavior has been thoroughly exploited by herpesviruses, which offer insights into selectively modulating pathogenic immune responses.
Highlights.
HVEM has five distinct ligands: LIGHT and LTα in the TNF family and BTLA, CD160 and herpesvirus gD in the Ig family.
HVEM-BTLA forms a bidirectional signaling pathway in trans.
Naïve T cells coexpress HVEM and BTLA forming an inhibitory complex in cis interfering with extraneous ligands in the microenvironment.
The HVEM Network is critical for the survival of memory CD4 T cells in persistent mucosal inflammation.
Acknowledgements
The authors wish to thank Tim Cheung, Paula Norris and Marcos Steinberg for their contributions to this study. The US Public Health Service, National Institutes of Health, National Institute of Allergy and Infectious Disease grants AI033068, AI067890, AI048073 and AI088445 supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344:17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Sedy JR, Spear PG, Ware CF. Cross-regulation between herpesviruses and the TNF superfamily members. Nat Rev Immunol. 2008;8:861–873. doi: 10.1038/nri2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery RI, Warner MS, Lum B, Spear PG. Herpes simplex virus 1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 4.Ware CF. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev. 2008;223:186–201. doi: 10.1111/j.1600-065X.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 6.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaye J. CD160 and BTLA: LIGHTs out for CD4+ T cells. Nat Immunol. 2008;9:122–124. doi: 10.1038/ni0208-122. [DOI] [PubMed] [Google Scholar]
- 8.Ware CF. NETWORK COMMUNICATIONS: Lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 9.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27:19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 10.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 11.Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D, Hymowitz SG. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J Biol Chem. 2005;280:39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci U S A. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 15.Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF. Genomic Characterization of LIGHT Reveals Linkage to an Immune Response Locus on Chromosome 19p13.3 and Distinct Isoforms Generated by Alternate Splicing or Proteolysis. J Immunol. 2001;167:5122–5128. doi: 10.4049/jimmunol.167.9.5122. [DOI] [PubMed] [Google Scholar]
- 16.Banner DW, D’Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 17.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor HveA. Molecular Cell. 2001;8:169–179. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- *18.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D’Souza C, Norris PS, Pfeffer K, Murphy KM, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. Defined BTLA and CD160 as activating Iigands for HVEM and role in T cell survival.
- 19.Le Bouteiller P, Tabiasco J, Polgar B, Kozma N, Giustiniani J, Siewiera J, Berrebi A, Aguerre-Girr M, Bensussan A, Jabrane-Ferrat N. CD160: A unique activating NK cell receptor. Immunol Lett. 2011;138:93–96. doi: 10.1016/j.imlet.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 21.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanjo H, Zajonc DM, Braden R, Norris PS, Ware CF. Allosteric regulation of the ubiquitin:NIK and ubiquitin:TRAF3 E3 ligases by the lymphotoxin-{beta} receptor. J Biol Chem. 2010;285:17148–17155. doi: 10.1074/jbc.M110.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Force WR, Glass AA, Benedict CA, Cheung TC, Lama J, Ware CF. Discrete signaling regions in the lymphotoxin-beta receptor for tumor necrosis factor receptor-associated factor binding, subcellular localization, and activation of cell death and NF-kappaB pathways. J Biol Chem. 2000;275:11121–11129. doi: 10.1074/jbc.275.15.11121. [DOI] [PubMed] [Google Scholar]
- 24.Rooney IA, Butrovich KD, Glass AA, Borboroglu S, Benedict CA, Whitbeck JC, Cohen GH, Eisenberg RJ, Ware CF. The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem. 2000;275:14307–14315. doi: 10.1074/jbc.275.19.14307. [DOI] [PubMed] [Google Scholar]
- *25.Cheung TC, Oborne LM, Steinberg MW, Macauley MG, Fukuyama S, Sanjo H, D’Souza C, Norris PS, Pfeffer K, Murphy KM, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183:7286–7296. doi: 10.4049/jimmunol.0902490. Identifed the HVEM-BTLA cis complex expressed in naïve T cells as an inhibitor.
- *26.del Rio ML, Kaye J, Rodriguez-Barbosa JI. Detection of protein on BTLAlow cells and in vivo antibody-mediated down-modulation of BTLA on lymphoid and myeloid cells of C57BL/6 and BALB/c BTLA allelic variants. Immunobiology. 2010;215:570–578. doi: 10.1016/j.imbio.2009.09.008. Highlights the importance of different assays in defining antagonist and agonist functions of antibodies to BTLA.
- 27.Nicola AV, Peng C, Lou H, Cohen GH, Eisenberg RJ. Antigenic structure of soluble herpes simplex virus (HSV) glycoprotein D correlates with inhibition of HSV infection. J Virol. 1997;71:2940–2946. doi: 10.1128/jvi.71.4.2940-2946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dean HJ, Warner MS, Terhune SS, Johnson RM, Spear PG. Viral determinants of the variable sensitivity of herpes simplex virus strains to gD-mediated interference. J Virol. 1995;69:5171–5176. doi: 10.1128/jvi.69.8.5171-5176.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinkade A, Ware CF. The DARC conspiracy - virus invasion tactics. Trends Immunol. 2006;27:362–367. doi: 10.1016/j.it.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg MW, Turovskaya O, Shaikh RB, Kim G, McCole DF, Pfeffer K, Murphy KM, Ware CF, Kronenberg M. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205:1463–1476. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.Soroosh P, Doherty TA, So T, Mehta AK, Khorram N, Norris PS, Scheu S, Pfeffer K, Ware C, Croft M. Herpesvirus entry mediator (TNFRSF14) regulates the persistence of T helper memory cell populations. J Exp Med. 2011;208:797–809. doi: 10.1084/jem.20101562. Provides evidence that LIGHT-HVEM pathway is crucial for memory T cell survival.
- **32.Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011 doi: 10.1038/nm.2356. Identifies the importance of LIGHT as a driving cytokine in chronic lung inflammation.
- 33.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic Lymphotoxin-beta Receptor Requirement for Homeostasis of Lymphoid Tissue Dendritic Cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 34.De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, et al. The Inhibitory HVEM-BTLA Pathway Counter Regulates Lymphotoxin Receptor Signaling to Achieve Homeostasis of Dendritic Cells. J Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]