Abstract
The dynamics of hydration water in several phospholipid membranes of different compositions is studied by 2D 1H-31P heteronuclear correlation NMR under magic-angle spinning. By using a 1H T2 filter and 1H mixing time before and after the evolution period, inter-bilayer water is selectively detected without resonance overlap from bulk water outside the multilamellar vesicles. Moreover the 1H T2 relaxation time of the inter-bilayer water is measured. Lipid membranes with labile protons either in the lipid headgroup or in sterols exhibit water-31P correlation peaks while membranes free of exchangeable protons do not, indicating that the mechanism for water-lipid correlation is chemical exchange followed by relayed magnetization transfer to 31P. In the absence of membrane proteins, the inter-bilayer water 1H T2’s are several tens of milliseconds. Incorporation of charged membrane peptides shortened this inter-bilayer water T2 significantly. This T2 reduction is attributed to the peptides’ exchangeable protons, molecular motion and intermolecular hydrogen bonding, which affect the water dynamics and the chemically relayed magnetization transfer process.
Keywords: water, lipid membranes, solid-state NMR, 1H-31P correlation, membrane proteins, dynamics
1. Introduction
Water is essential to the structure and dynamics of biological molecules. The folding, dynamics and function of proteins and nucleic acids are strongly influenced by water. The self-assembly of amphipathic lipid molecules to form the bilayer that protects all cells also requires water. The hydration force between lipid bilayers has long been recognized as an important factor that influences the physical properties of lipid membranes [1; 2]. A wide range of biophysical techniques, including 2H NMR [3; 4; 5; 6], 1H NMR [6; 7; 8; 9], neutron scattering [10; 11], x-ray scattering [12; 13], Raman scattering [14], osmotic stress and surface force measurements [2], and molecular dynamics simulations, have been used to characterize the interaction of water with lipid membranes. The most extensively characterized lipid membranes are the phosphatidylcholines (PC), for which both the water dynamics [3; 4] and lipid dynamics [15] have been investigated as a function of hydration level and the membrane phase. However, so far few spectroscopic studies have directly compared the dynamics of water in lipids of different headgroups, and the effect of membrane proteins on water-membrane interactions has been scarcely investigated.
High-resolution magic-angle spinning (MAS) NMR spectroscopy is an excellent approach for probing the structure and dynamics of lipid membranes [16; 17]. Due to the fast uniaxial rotational diffusion of lipid molecules, hydrated lipid membranes exhibit well resolved 1H spectra under moderate magic-angle spinning, making 1H 1D and 2D MAS NMR the method of choice for investigating membrane dynamics and disorder [18]. The heteronuclear 1H-31P 2D correlation technique is particularly sensitive to membrane-associated water. Since water residence time on the membrane surface is only on the order of 100 ps based on 1H NOESY experiment [7], direct dipolar coupling of water with the lipid phosphate group is not sufficiently strong to be detectable by NMR. Instead, water-31P correlation peaks in 2D spectra reflect water magnetization transferred to some lipid headgroup protons, then relayed to lipid protons closest to 31P before cross polarization (CP) to 31P.
In this work, we probe the water-lipid interaction using the 1H-31P 2D correlation experiment and examine the dynamics of the lipid-correlated water by measuring its T2 relaxation times. A number of membranes with different headgroup structures are studied. They include phosphatidylcholine, phosphatidylethanolamine (PE), and phosphatidylglycerol (PG). Cholesterol is added to one of the PC samples to study the effect of this important sterol on hydration water dynamics. Mixed PE/PG membranes containing two cationic antimicrobial peptides are then studied to examine the influence of membrane proteins containing polar charged residues on hydration water dynamics. These membrane composition variations allow us to understand the effects of labile lipid protons, hydrogen bonding, membrane surface charge, sterol, and proteins on the hydration water dynamics.
2. Materials and Methods
Membrane sample preparation
All lipids were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Most samples were prepared by dissolving and mixing the lipids in chloroform, evaporating chloroform under a stream of dry nitrogen gas, then resuspending the lipid mixture in cyclohexane and lyophilizing overnight. Chloroform is necessary for complete mixing of the lipids, while cyclohexane is necessary for complete removal of the organic solvent after mixing. The dried lipid powder was packed into 4-mm MAS rotors and directly hydrated. The amount of water added was approximate 35 wt% of the total mass. The exact hydration level was determined by integration of the 1H NMR spectra. Antimicrobial peptides TP-I and PG-1 were synthesized using standard FMOC chemistry as described before [19; 20], and were reconstituted into POPE/POPG membranes by mixing lipid vesicle solutions with the appropriate amount of the peptide solution. The mixed solution was centrifuged at 150,000 g to obtain wet pellets, which were then lyophilized, packed into the rotor, and rehydrated to ~35 wt% water.
Solid-state NMR experiments
Magic-angle spinning (MAS) NMR experiments were carried out on a Bruker DSX-400 spectrometer (Karlsruhe, Germany) operating at Larmor frequencies of 400.49 MHz for 1H and 162.12 MHz for 31P. An MAS probe equipped with a 4 mm spinner was used for all experiments. The samples were spun at 4.0 kHz in most experiments. Typical 1H-31P cross polarization (CP) contact times were 4 ms and the Hartmann-Hahn match was established at 50 kHz. The 1H 90° pulse length was 5 μs, and the 1H decoupling field was 42–50 kHz during 31P detection. Recycle delays for the 1H-31P 2D correlation experiments were 2.5–3.0 s. The 1H chemical shifts were internally referenced to the lipid chain CH3 signal at 0.9 ppm [21].
1H-detected water T2’s were measured using a 1D Hahn-echo experiment. 31P-detected water T2’s were measured using the 2D 1H-31P correlation experiment with a 1H mixing period and a pre-evolution T2 filter (Figure 1) [22]. In this experiment, 31P magnetization is first destroyed by several 90° pulses. A 1H 90° excitation pulse is then applied, followed by a Hahn-echo period with a variable delay 2τ. 1H chemical shift evolution (t1) ensues, then the 1H magnetization is stored along the z-axis for a period tm, during which magnetization transfer occurs by either spin diffusion or nuclear Overhauser effect (NOE). Finally, the 1H magnetization is cross-polarized to 31P for detection in t2. A series of 2D experiments with varying echo delays 2τ was conducted to measure the T2 of the water protons that correlate with the lipid 31P. Mixing times of 1 ms to 225 ms were used in the 2D experiments.
Figure 1.

Pulse sequence for the 2D 1H-31P correlation experiment with a 1H T2 filter period of 2τ and a mixing period of tm. Filled and open rectangles denote 90° and 180° pulses.
3. Results
1D 1H MAS spectra – bulk water and inter-bilayer water
The goal of this study is to investigate the interaction between water and lipid membranes with different headgroups and measure the dynamics of the hydration water of the membrane. Phospholipids containing palmitoyl and oleoyl chains were used in all samples because these acyl chains are the most abundant in biological membranes. The PC, PE and PG headgroup chemical structures and their nomenclatures are shown in Figure 2. The three lipids have different gel to liquid-crystalline phase transition temperatures (Tm): −2°C for POPC and POPG and 25°C for POPE. The dynamics of hydration water should depend both on the bulk water property and the membrane dynamics. We chose to conduct the NMR experiments at similar temperatures with respect to their phase transition temperatures (Tm). This reduced temperature, ΔT = T−Tm, was set to be 5–7°C for the various membranes studied (Table 1). For mixed POPE/POPG membranes the weighted molar average of lipids is used to find the Tm of the mixture.
Figure 2.

Headgroup structures of the phospholipids used in this study. (a) POPC. (b) POPE. (c) POPG. R and R′ denote oleoyl and palmitoyl chains, respectively.
Table 1.
Water 1H T2 (ms) values observed from 1D 1H and 2D 1H-31P spectra.
| Membrane | 1D, Narrow | 1D, Broad | 2D | T (°C) | ΔT (°C) |
|---|---|---|---|---|---|
| POPC | - | 59±1 | - | 5 | 7 |
| POPC/cholesterol (3:2) | 375±40 | 23±1 | 15±1 | 5 | 7 |
| POPE | 410±70 | 18±1 | 16±2 | 30 | 5 |
| POPG | - | 32±1 | 30±3 | 5 | 7 |
| POPE/POPG (3:2), POPE | 81±4 | - | 30±2 | 20 | 6 |
| POPG | 40±1 | 20 | 6 | ||
| POPE/POPG/TP-I (9:6:1) | 82±1 | 4.5±0.5 | 3.3±0.3 | 20 | 6 |
| POPE/POPG/PG-1 (8:4:1) | - | 16±1 | 0.4±0.2, 12±1 | 25 | 7 |
Figure 3 shows the 1D 1H MAS spectra of the POPC, POPE, and POPE/POPG mixed membranes. The 1H peaks are assigned based on literature chemical shift values [23; 24; 25] and additional 1H-13C 2D correlation spectra measured directly on these samples (not shown). Close inspection of the water region of the 1H spectra show two partially resolved peaks – a narrow downfield component and a broad upfield component - for POPC/cholesterol, POPE, and POPG membranes but not for the POPC membrane (Figure 4). The chemical shift difference between the two components is about 0.05 ppm (Table 2). We assign the sharp peak to mobile bulk water outside the multilamellar vesicles and the broad peak to inter-bilayer water that interacts intimately with the lipids. The partial spectral resolution means that the two types of water are in slow exchange with rates less than 0.05 × 400 × 2π = 125 s−1, consistent with prior experiments on membranes packed in spherical inserts [8]. Hahn echo detection with a long echo period preferentially suppressed the broad upfield peak, supporting the presence of two types of water. The 1H T2 relaxation times of the two water peaks are listed in Table 1: the broad water T2 (~ 20 ms) is an order of magnitude shorter than the narrow water T2 (~400 ms) for the POPC/cholesterol and POPE membranes. For the peptide-containing POPE/POPG membranes, the 1H T2’s of the broad water peak is generally shorter than the pure membranes. The TP-I sample retains the order-of-magnitude difference between the bulk water and inter-bilayer water T2. The PG-1 sample shows only a single water peak, with a short 1H T2 of 16 ms.
Figure 3.

1D 1H MAS spectra of three hydrated lipid membranes. (a) POPC. (b) POPE. (c) POPE/POPG membrane. (d) Inset for the POPE/POPG membrane showing the assignment for the 3.0–4.6 ppm region.
Figure 4.
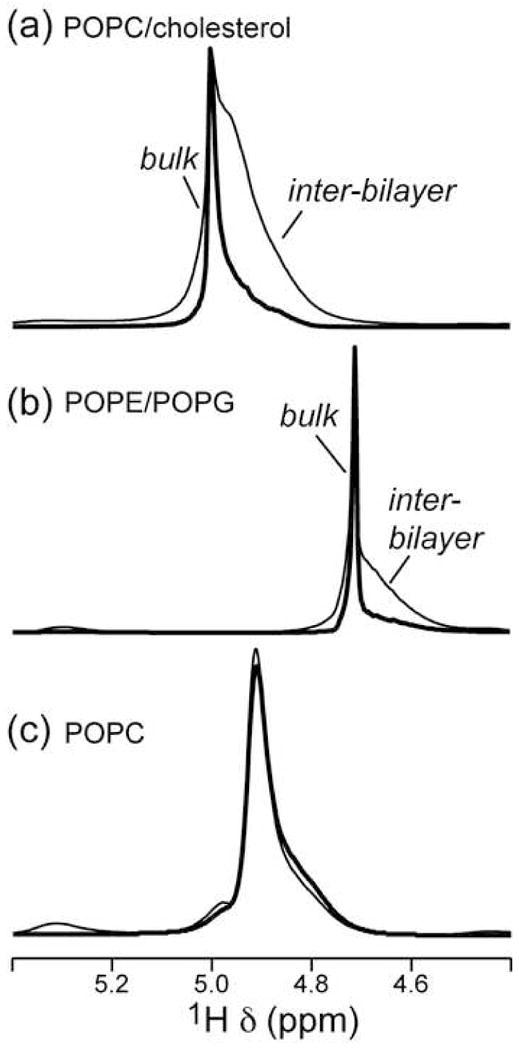
Water region of the 1D direct-excitation 1H MAS spectra of lipid membranes without (thin line) and with a T2 filter (thick line). (a) POPC/cholesterol membrane. The echo delay (2τ) is 80 ms. (b) POPE/POPG (3:2) membrane. 2τ = 30 ms. (c) POPC membrane. 2τ = 80 ms. Note the one-component nature of the POPC spectrum.
Table 2.
Water 1H chemical shifts (ppm) in various lipid membranes. The bulk water frequencies are obtained from 1D 1H spectra, and the inter-bilayer water frequencies are obtained from 2D 1H-31P spectra.
| Membrane | Bulk | Inter-bilayer |
|---|---|---|
| POPC | - | 4.92 |
| POPC/cholesterol (3:2) | 5.00 | 4.94 |
| POPE | 4.71 | 4.66 |
| POPE/POPG (3:2) | 4.79 | 4.71 |
| POPE/POPG/TP-I (9:6:1) | 4.91 | 4.86 |
| POPE/POPG/PG-1 (8:4:1) | 4.80 | 4.76 |
1H- 31P 2D correlation spectra – 1H T2 of the inter-bilayer water
Since 1D 1H spectra do not completely resolve the inter-bilayer water signal from the bulk water signal, it is of interest to selectively detect the inter-bilayer water in the absence of the dominant bulk water peak. The 2D 1H-31P correlation experiment with 1H mixing [22; 26] achieves this purpose and at the same time verifies the assignment of the broad water peak to inter-bilayer water. In this experiment, the 1H spins closest to the lipid phosphate group, Hα and G3, cross-polarize to 31P. Protons further from the phosphate group, including water, first transfer their magnetization to Hα and G3 by a number of possible mechanisms, including chemical exchange, spin diffusion, and dipolar cross relaxation (i.e. NOE), then cross-polarize to 31P. A mixing time of 64 ms and a 1H-31P CP contact time of 4 ms were typically used in the 2D experiments. When water cross peaks are not detected under these conditions, the mixing time was extended to 225 ms.
Figure 5 shows two representative 1H-31P 2D spectra, for the POPC membrane and the POPE/POPG membrane, and Figure 6 compares the 1H cross sections for six lipid membranes with their respective 1D 1H direct-excitation spectra. Several common features are observed in the 2D spectra. First, the strongest 1H-31P cross peaks come from the headgroup Hα and glycerol G3, as expected due to the proximity of these methylene groups to 31P. The G3 cross peak is broader and lower than Hα which is expected because no 1H homonuclear decoupling is applied during the evolution time and 1H-1H dipolar coupling for G3 are a factor of 3.5 times stronger than Hα due to the nearly parallel orientation of the G3 geminal H-H vector to the lipid motional axis [27]. On the other hand, G3 protons do not cross polarize much more efficiently than Hα to 31 P since the 1H-31P dipolar coupling for G3 is only a factor of 1.5 stronger than for Hα [27]. The second common feature among the spectra is that the acyl chain CH2 also exhibits a cross peak with 31P, indicating chain-headgroup contacts. A significant contribution to this cross peak is intermolecular 1H-1H NOE due to chain upturns in the fluid bilayer, as shown before for non-cholesterol-containing membranes [28; 29]. Finally, water-31P cross peaks are observed between 4.7 and 5.1 ppm for all membranes except for POPC, and in most cases have lower intensities than the Hα peaks. This is consistent with the intermolecular nature of the water-31P magnetization transfer and the high mobility of water.
Figure 5.
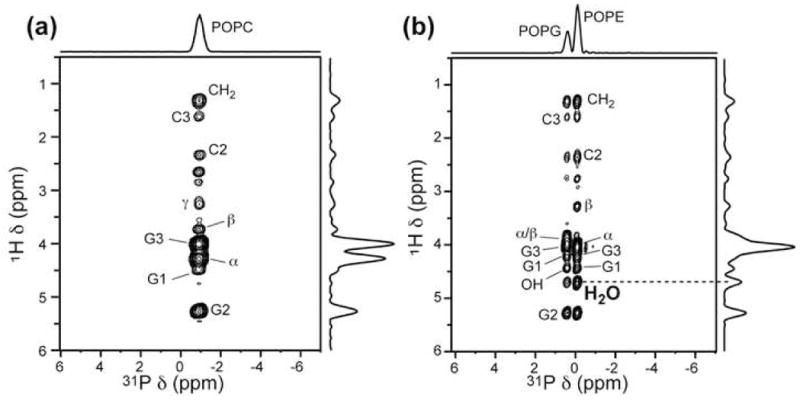
Representative 2D 1H-31P correlation spectra of hydrated lipid membranes with a mixing time of 64 ms. (a) POPC. (b) POPE/POPG (3:2) membrane. 1H peak assignment is indicated. POPC lacks a water-31P cross peak. Extending the mixing time to 225 ms still yields no water cross peak. Spectra were measured under 4.0 kHz MAS.
Figure 6.

1H direct-excitation spectra (top) and cross sections from 1H-31P 2D spectra (bottom) of various lipid membranes. Dashed lines guide the eye for the water peak. The most significant lipid peaks are assigned. (a) POPC membrane. (b) POPC/cholesterol membrane. (c) POPE membrane prepared from organic solution. (d) POPE membrane prepared from aqueous solution. (e) POPE/POPG membrane with TP-I. (f) POPE/POPG membrane with PG-1.
In the following we describe the water-31P cross peak for each lipid membrane. The POPC membrane does not exhibit any water-31P cross peak up to 225 ms mixing (Figure 6a). We attribute this absence to the lack of exchangeable protons in the POPC headgroup, since all other membranes studied here contain labile protons and exhibit water cross peaks in 2D spectra. Negative water-headgroup 1H-1H cross peaks in 2D NOESY spectra, corresponding to positive water-headgroup cross relaxation rates, have been reported for POPC membranes [7; 9]. Thus, the lack of a water-31P cross peak suggests that the water-headgroup dipolar coupling, while present, is not strong enough to be detected by the current 2D 1H-31P correlation experiment. In addition, the 1D 1H spectrum of POPC shows only a single water peak, thus the water signal must come from the inter-bilayer hydration water rather than the bulk water outside the multilamellar vesicle.
In contrast to the pure POPC membrane, the addition of cholesterol to the POPC membrane gave rise to a strong water cross peak (Figure 6b) that matches the position of the broad water peak in the 1D spectrum (Figure 4a). Varying the T2 filter time of the 2D experiment yielded a 31P-detected water T2 of 15 ms, in qualitative agreement with the 1D-detected T2 (Table 1). We attribute the water cross peak in the POPC/cholesterol membrane to the combined effect of exchange between water and the cholesterol hydroxyl proton and the condensing effect of cholesterol on lipid membranes, which facilitates 1H spin diffusion.
The POPE membrane exhibits a weak water cross peak at 4.66 ppm (Figure 6c) in the 2D spectrum with a 16 ms T2, consistent with the 18 ms T2 found in the 1D spectra. Figure 7 shows several water 1H T2 decay curves detected using the 1D 1H and 2D 31P-detected experiments. The POPE data highlights the spectral simplification by 2D correlation: the 1D T2 decay is bi-exponential due to the partial overlap of the inter-bilayer and bulk water signals, while the 2D-detected T2 decay is single exponential, reflecting only the inter-bilayer water dynamics.
Figure 7.

Representative 1H T2 curves from 1H 1D and 1H-31P 2D correlation spectra. Left column: POPE membrane. (a) 1D 1H-detected T2 decay of the narrow water peak, (b) 2D 31P-detected water 1H T2 decay. Right column: POPE/POPG membrane. (c) 1D 1H-detected T2 decay of the water peak, (d) 2D 31P-detected water 1H T2 decay. Filled squares: POPE. Open squares: POPG. Note that the time axis is not the same for all panels.
For the POPE membrane, the water cross peak most likely results from chemical exchange between water and the headgroup amine protons (Hγ) followed by relayed magnetization transfer to 31P. The native Hγ-31P cross peak, if protected from exchange, would be negligible, since the Hβ-31P cross peak is already very weak. The monotonic intensity decrease of 1H-31P cross peaks from Hα to Hβ and Hγ is clearly seen in the 2D POPC spectrum (Figure 6a), which does not have resonance overlap between water and Hγ.
To examine the influence of sample preparation methods on hydration-water dynamics, we prepared another POPE sample by making a vesicle solution, subjecting it to several freeze-thaw cycles, then centrifuging the solution to give a pellet. This aqueous sample gave a broad water peak at 5.09 ppm (Figure 6d), which is 0.43 ppm downfield from the broad water peak in the “organic” sample. This downfield water peak shows a T2 of 4.9 ms from the 2D experiments. The chemical shift of the lipid-associated water peak is the weighted average of the NH3 chemical shift and the water proton chemical shift. Lys NH3 protons in proteins protected from exchange have a chemical shift of 7 – 8 ppm [30], thus the downfield displacement of the inter-bilayer water peak in the aqueous POPE sample indicates that the amount of the inter-bilayer water is smaller in the aqueous sample than in the organic sample. Similarly, the shorter water T2 (4.9 ms) of the aqueous POPE sample compared to the organic sample (16 ms) can be attributed to the stronger influence of the NH3 proton dynamics in the exchange-average T2. The 1H T2’s of the POPE headgroup decrease from Hα to Hβ, in contrast to the POPC headgroup, which has increasing T2’s from Hα (27 ms) to Hβ (38 ms) and Hγ (71 ms). The shorter 1H T2 towards the end of the POPE headgroup most likely reflects intermolecular hydrogen bonding between NH3+ and PO4− of neighboring lipid molecules, which restricts the headgroup mobility [31; 32].
To obtain further insight into the nature of the POPE hydration water, we examined the temperature dependence of the water cross peak in the 2D 1H-31P spectra. Figure 8a shows the 1D cross sections of the organic POPE sample from 20°C to 40°C. The spectra were collected with identical scans and plotted on the same intensity scale after taking into account small CP efficiency differences. The water cross peak decreases with increasing temperature, with the most significant change occurring across the phase transition temperature of 25°C. We also examined the mixing-time dependence of the water cross peak at 30°C. The water cross peak was detected as early as 4 ms, as shown in Figure 8b.
Figure 8.

1H cross sections of the 2D 1H-31P spectra of hydrated POPE membrane. (a) Temperature dependence of the water cross peak intensity. Mixing time was 64 ms for all spectra. (b) Mixing time dependence of the water cross peak intensity at 30°C.
The POPG membrane shows a single water peak in the 1D spectra with a T2 of 32 ms and a water-31P cross peak in the 2D spectrum with a similar T2 of 30 ms. The high salt content of this lipid made the samples susceptible to rf heating and degradation so that variability in the cross peak intensity was observed. Buffering the membrane pH to 7 stabilized the sample to some extent and gave rise to a clear water-31P cross peak in the 64 ms 2D spectra. Mixing POPG with POPE lipids also created stable membranes, with reproducible water-31P cross peak intensities for both the POPE and POPG components. Figure 5b shows the 2D spectrum of the POPE/POPG (3:2) membrane, exhibiting two well resolved 31P peaks along with their respective water cross peaks. The water 1H T2 values are 30 ms for POPE and 40 ms for POPG (Table 1). Since the organic POPE membrane alone has a water T2 of 16 ms, the mixture result indicates that POPG lengthened the T2 of the POPE component.
Effect of cationic membrane peptides on inter-bilayer water T2
We next examined the inter-bilayer water dynamics in the presence of two cationic membrane peptides. Tachyplesin-I (TP-I) and protegin-1 (PG-1) are Arg-rich cationic β-hairpin antimicrobial peptides that have recently been extensively characterized by solid-state NMR [19; 20; 33; 34]. We measured the 2D 1H-31P spectra of POPE/POPG membrane containing these peptides. The 31P spectra no longer resolve the two lipids due to line broadening by the peptides. The 1H cross sections are shown in Figure 6e, f. For the TP-I sample, the water cross peak is relatively broad and is lower than the main Hα/G3 peak, similar to the other membranes. In contrast, the PG-1 sample exhibits a narrow and much stronger water peak with similar intensity as the Hα/G3 peak. The T2 decay of these 2D-detected water peaks are shown in Figure 9. Both peptide-containing samples exhibit much shorter water T2’s than the pure POPE/POPG membrane: 3.3 ms for the TP-I sample and 0.4 ms (20%) and 12 ms (80%) for the PG-1 sample (Table 1).
Figure 9.

31P-detected water 1H T2 decays of the POPE/POPG membrane containing cationic peptides. Open squares: TP-I. Filled squares: PG-1. Open circle: PG-1 13C-detected water cross peak intensity.
The double-exponential nature of the water T2 decay for the PG-1-containing membrane is noteworthy. The large value of 12 ms is similar to the 1D-detected water T2 of 16 ms. Since the water cross peak is much higher in this sample than in the other samples, we assign the longer T2 component to highly mobile water between bilayers, whose magnetization is transferred to 31P as a result of the immobilized β-barrel assembly of PG-1 molecules [34]. In other words, the rigid peptide oligomers provide an efficient spin diffusion pathway from water to the lipid 31P. This assignment is confirmed by 13C-1H 2D correlation spectra that correlate the 13C labeled residues in PG-1 with water 1H. The spectra showed similar water T2 dephasing as the 31P-detected experiment (Figure 9), indicating that the same water molecules correlate with the lipid phosphate and with the peptide. The implication of this assignment is that the main water peak in the 1D 1H spectra of the PG-1 sample results from near-isotropic inter-bilayer water rather than bulk water outside the liposomes, similar to the POPC membrane. The absence of this long-T2 in the TP-I sample can be attributed to the extensive dynamics of TP-I that prevents the detection of the highly mobile inter-bilayer water [19].
4. Discussion
The lipid membranes used in this study are multilamellar vesicles that can have two very different types of water: bulk water outside the vesicles and inter-bilayer water within the vesicles. The observation of two partially resolved water 1H peaks in the slow-exchange limit with very different T2’s supports the assignment of these two types of water.
The nature of water-membrane interaction has been extensively discussed in the literature. Early 2H NMR studies [4] led to the proposal of as many as three types of membrane-bound water, including tightly bound, weakly bound, and trapped water with fast exchange between the layers. More recent studies monitoring hydration-dependent 2H quadrupolar couplings indicate that the inter-bilayer water dynamics is a continuous function of hydration level [3]. A single quadrupolar splitting was observed for up to ~15 water molecules per POPC molecule (n = 15), above which a zero-frequency peak grows in that corresponds to bulk water in slow exchange with the inter-bilayer water. The single-component nature of the 2H spectra below n = 15 indicates that all inter-bilayer water undergoes rapid exchange on the 2H NMR timescale. Thus, phenomenologically, we do not further distinguish among inter-bilayer water molecules [3; 35], even though the middle of the hydration layer has more isotropic water than the region near the membrane surface.
While water 2H NMR spectra of hydrated phospholipids give information on the residual quadrupolar coupling due to the inter-bilayer water anisotropy induced by the membrane, the 2D 1H- 31P correlation technique is more sensitive to chemical exchange between water and labile lipid protons and to water-lipid dipolar interactions. The fact that the only lipid membrane that does not exhibit a water-31P correlation peak, POPC, is also the only lipid without any labile protons proves the essential role that chemical exchange plays in intermolecular magnetization transfer. POPE and POPG headgroups possess labile NH3 and OH protons, whose exchange rates have been measured in amino acids to be in the range of 1000–4000 s−1 at 36°C and pH 7.0 [36]. The exchanged water 1H magnetization can then be relayed to Hα before cross-polarizing to 31P. This mechanism was termed chemically relayed nuclear Overhauser (or spin diffusion) effect, and its dependence on exchange rate and molecular motional correlation time have been analyzed in detail by 2D 1H-1H correlation NMR [37].
The rate of chemical exchange increases with temperature while the rate of dipolar magnetization transfer decreases with temperature. Thus, the change of the water cross peak intensity with temperature depends on the relative sensitivity of the two processes on temperature [36]. For the POPE membrane, we found that the water-31P cross peak increases as the temperature decreases and upon entering the gel phase the cross peak intensity increases dramatically. This indicates that the more efficient 1H spin diffusion in the gel-phase membrane outweighs the reduction of the proton exchange rate at low temperature. However, this does not mean that exchange is unnecessary for the detection of the water-31P cross peak. At 30°C, the water-NH3 exchange rate of several thousand times per second [36] is much faster than the rate of 1H spin diffusion and cross-polarization from NH3 to 31P. The 1H-31P dipolar couplings to the nearest methylene groups of Hα and G3 are 200–300 Hz in liquid-crystalline PC and PE membranes [27], thus the magnetization transfer rate from the more remote Hγ is at most several tens of hertz. Thus, the limiting factor in the chemically relayed nuclear Overhauser or spin diffusion process is the dipolar transfer rather than chemical exchange, and the temperature dependence of the dipolar transfer determines the overall intensity of the water cross peak. Recently it was shown that the 2D 1H-31P experiment is able to detect a water cross peak in sphingomyelin (SM) membranes but not in PC membranes [38]. While this difference is partly due to the rigidity of the SM membrane over the PC membrane, the presence of two labile protons in the SM backbone, which are absent in glycerophospholipid backbones, is almost certainly necessary for the observation of the water cross peak.
POPC differs from other membranes not only in having no labile protons in the headgroup, but also in having a single water signal with a T2 (~60 ms) that falls between the bulk water T2 (~400 ms) and inter-bilayer T2 (15–40 ms) of the other membranes. We assign this signal to inter-bilayer water for the following reasons. First, PC is much more hygroscopic than PE and PG lipids, as reflected by a thicker hydration layer and stronger repulsive hydration forces [2; 31]. The higher hydration of PC compared to similarly zwitterionic PE lipids is attributed to the methylation of the primary amine in the PC headgroup, which weakens the attractive inter-bilayer forces resulting from hydrogen-bonded water bridges between apposing bilayers. Further, molecular dynamics simulations showed that the PC trimethylamine group has a much larger hydration shell than the PE amine [32] [39] due to the absence of hydrogen bonding. Thus, more inter-bilayer water is required to hydrate PC than PE. Finally, our POPC sample has 15–18 water molecules per lipid based on the 1H spectral integration. This is in the regime of little bulk water based on 2H NMR [3], thus supporting the assignment of the single water 1H peak to inter-bilayer water.
The lipid-correlated water 1H T2’s increase in the direction of POPC/cholesterol ≤ POPE < POPG. Although it is tempting to interpret this trend as reflecting the interaction strengths between water and the various lipid membranes, the water T2 is the weighted average of the inter-bilayer water and labile lipid proton T2’s, thus the lipid proton T2 affects the measured water cross peak T2. For example, POPE Hβ has a shorter T2 (~ 20 ms in the organic sample and ~ 7 ms in the aqueous sample) than the Hα protons (~25 ms in all samples), thus POPE Hγ protons should have an even shorter intrinsic T2, which should shorten the measured water cross peak T2. Among all the lipid membranes studied here, the POPC/cholesterol bilayer is the most rigid and thus its water cross peak should have the largest contribution from direct dipolar effects between water and the lipid. Even so, the magnetization transfer pathway most likely involves an initial step of exchange from water to the cholesterol hydroxyl proton, followed by back transfer to the lipid chains and then to the lipid headgroup. Mixing time dependence of the POPC/cholesterol 2D spectra (not shown) indicates that the water cross peak buildup is slower than the POPE membrane, consistent with a magnetization transfer pathway that involves lipid chain protons next to the rigid sterol rings.
Inclusion of cationic membrane peptides reduced the lipid-correlated water T2 to a few milliseconds. The number of labile protons in TP-I and PG-1 is similar. TP-I has 17 residues while PG-1 has 18, with the corresponding labile backbone amide protons. TP-I has five Arg residues and one Lys, each with exchangeable sidechain NHn protons, while PG-1 contains six Arg residues. TP-I and PG-1 contain two and one hydroxyl-containing Tyr residues, respectively. However, the exposure of these labile protons to water and the efficiency of 1H-1H dipolar transfer differ significantly between the two peptides due to their different topological structures in the membrane. TP-I binds to the membrane-water interface near the glycerol backbone [33], is oriented roughly parallel to the membrane plane [40], and is highly dynamic [19]. In contrast, PG-1 forms immobilized transmembrane β-barrels in the anionic membrane [34; 41] (Figure 10), whose extensive intermolecular hydrogen bonding should shield some backbone NH protons from exchange. Thus, TP-I should experience more efficient water exchange than PG-1. But since the limiting factor in the water-31P cross peak detection is the dipolar transfer rate rather than the exchange rate, the immobilized PG-1 backbone transfers whatever level of exchanged water magnetization to the lipid 31P much more efficiently than the dynamic TP-I. PG-1 thus shows a higher water cross peak than TP-I, and allows the observation of more isotropic inter-bilayer water, which is not observed in the TP-I sample.
Figure 10.

Topological structures of TP-I and PG-1 in POPE/POPG membranes. (a) TP-I is monomeric and mobile and lies at the membrane-water interface. (b) PG-1 is transmembrane and forms immobilized β-barrels.
5. Conclusion
The current 2D 1H-31P correlation study indicates that chemical exchange plays an essential role in the dynamics of hydration water in lipid membranes. The presence of a 31P-correlated water peak in the 2D spectra requires exchangeable lipid protons, while the intensity of the cross peak is mainly determined by the 1H-1H dipolar transfer efficiency. The 31P-detected 1H T2 of the inter-bilayer water is the exchange-averaged T2 of all inter-bilayer water and the labile lipid proton, and depends on the hydration level of the membrane and the property of the labile proton. Cholesterol facilitates the detection of the inter-bilayer water through its condensing effect on the membrane. Cationic membrane proteins affect the hydration water dynamics through intermolecular hydrogen bonding and protein dynamics.
Acknowledgments
The authors thank Prof. Alan J. Waring for providing the TP-I and PG-1 samples used in this study. T. Doherty is a grateful recipient of a Roy J. Carver Trust predoctoral training fellowship. The authors thank M. Tang for discussions and help with lipid 1H chemical shift assignment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parsegian VA, Fuller N, Rand RP. Measured work of deformation and repulsion of lecithin bilayers. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:2750–4. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rand RP, Arsegian VA. Hydration forces between phospholipid bilayers. Biochim Biophys Acta. 1989;988:351–376. [Google Scholar]
- 3.Volke F, Eisenblatter S, Galle J, Klose G. Dynamic properties of water at phosphatidylcholine lipid-bilayer surfaces as seen by deuterium and pulsed field gradient proton NMR. Chem Phys Lipids. 1994;70:121–131. doi: 10.1016/0009-3084(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 4.Finer EG, Darke A. Phospholipid hydration studied by deuterium magnetic resonance spectroscopy. Chemistry and Physics of Lipids. 1974;12:1–16. doi: 10.1016/0009-3084(74)90064-4. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi A, Takizawa T, Nakata Y. A Deuteron NMR Study of the Dynamics of Water Molecules Bound Tightly to the Phosphate Group in Dipalmitoyl-Phosphatidylcholine-D2O System. Journal of the Physical Society of Japan. 1996;65:635–642. [Google Scholar]
- 6.Ceckler TL, Wolff SD, Yip V, Simon SA, Balaban RS. Dynamic and Chemical Factors Affecting Water Proton Relaxation by Macromolecules. Journal of Magnetic Resonance. 1992;98:637–645. [Google Scholar]
- 7.Gawrisch K, Gaede HC, Mihailescu M, White SH. Hydration of POPC bilayers studied by 1H-PFG-MAS-NOESY and neutron diffraction. Eur Biophys J. 2007;36:281–291. doi: 10.1007/s00249-007-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Sayer BG, Hughes DW, Stark RE, Epand RM. Studies of phospholipid hydration by high-resolution magic-angle spinning nuclear magnetic resonance. Biophys J. 1999;76:387–399. doi: 10.1016/S0006-3495(99)77205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volke F, Pampel A. Membrane hydration and structure on a subnanometer scale as seen by high resolution solid state nuclear magnetic resonance: POPC and POPC/C12EO4 model membranes. Biophys J. 1995;68:1960–1965. doi: 10.1016/S0006-3495(95)80373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitter J, Lechner RE, Dencher NA. Interactions of Hydration Water and Biological Membranes Studied by Neutron Scattering. J Phys Chem B. 1999;103:8036–8050. [Google Scholar]
- 11.Konig S, Sackmann E, Richter D, Zorn R, Carlie C, Bayerl TM. Molecular dynamics of water in oriented DPPC multilayers studied by quasielastic neutron scattering and deuterium-nuclear magnetic resonance relaxation. Journal of Chemical Physics. 1994;100:3307–3316. [Google Scholar]
- 12.Pearson RH, Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979;281:499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- 13.Wiener MC, White SH. Structure of a fluid DOPC bilayer determined by joint refinement of x-ray and neutron diffraction data III Complete structure. Biophys J. 1992;61:434–447. doi: 10.1016/S0006-3495(92)81849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng JX, Pautot S, Weitz DA, Xie XS. Ordering of water molecules between phospholipid bilayers visualized by coherent anti-Stokes Raman scattering microscopy. Proc Natl Acad Sci U S A. 2003;100:9826–9830. doi: 10.1073/pnas.1732202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulrich AS, Watts A. Molecular response of the lipid headgroup to bilayer hydration monitored by 2H-NMR. Biophys J. 1994;66:1441–1449. doi: 10.1016/S0006-3495(94)80934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong M. Oligomeric structure, dynamics, and orientation of membrane proteins from solid-state NMR. Structure. 2006;14:1731–1740. doi: 10.1016/j.str.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Hong M. Structure, topology, and dynamics of membrane peptides and proteins from solid-state NMR spectrosco py. J Phys Chem B. 2007;111:10340–10351. doi: 10.1021/jp073652j. [DOI] [PubMed] [Google Scholar]
- 18.Gawrisch K, Eldho NV, Polozov IV. Novel NMR tools to study structure and dynamics of biomembranes. Chemistry and Physics of Lipids. 2002;116:135–151. doi: 10.1016/s0009-3084(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 19.Doherty T, Waring AJ, Hong M. Dynamic structure of disulfide-removed linear analogs of tachyplesin-I in the lipid bilayer from solid-state NMR. Biochemistry. 2008;47:1105–1116. doi: 10.1021/bi701390t. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi S, Waring A, Hong T, Lehrer R, Hong M. Solid-State NMR Investigations of Peptide-Lipid Interaction and Orientation of a beta-Sheet Antimicrobial Peptide, Protegrin. Biochemistry. 2002;41:9852–9862. doi: 10.1021/bi0257991. [DOI] [PubMed] [Google Scholar]
- 21.Li KL, Tihal CA, Guo M, Stark RE. Multinuclear and magic-angle spinning NMR investigations of molecular organization in phospholipid-triglyceride aqueous dispersions. Biochemistry. 1993;32:9926–9935. doi: 10.1021/bi00089a008. [DOI] [PubMed] [Google Scholar]
- 22.Huster D, Yao XL, Hong M. Membrane Protein Topology Probed by 1H Spin Diffusion from Lipids Using Solid-State NMR Spectroscopy. J Am Chem Soc. 2002;124:874–883. doi: 10.1021/ja017001r. [DOI] [PubMed] [Google Scholar]
- 23.Forbes J, Bowers J, Shan X, Moran L, Oldfield E, Moscarello MA. Some new developments in solid-state nuclear magnetic resonance spectroscopic studies of lipids and biological membranes, including the effects of cholesterol in model and natural systems. J Chem Soc Faraday Trans I. 1988;84:3821–3849. [Google Scholar]
- 24.Forbes J, Husted C, Oldfield E. High-field, high-resolution proton “magic-angle” sample-spinning NMR spectroscopic studies of gel and liquid crystalline lipid bilayers and the effects of cholesterol. J Am Chem Soc. 1988;110:1059–1065. [Google Scholar]
- 25.Husted C, Montez B, Le C, Moscarello MA, Oldfield E. Carbon-13 “magic-angle” sample-spinning nuclear magnetic resonance studies of human myelin, and model membrane systems. Magn Res Med. 1993;29:168–178. doi: 10.1002/mrm.1910290204. [DOI] [PubMed] [Google Scholar]
- 26.Tang M, Waring AJ, Lehrer RI, Hong M. Effects of Guanidinium-Phosphate Hydrogen Bonding on the Membrane-Bound Structure and Activity of an Arginine-Rich Membrane Peptide from Solid-State NMR. Angew Chem Int Ed Engl. 2008;47:3202–3205. doi: 10.1002/anie.200705993. [DOI] [PubMed] [Google Scholar]
- 27.Hong M, Schmidt-Rohr K, Nanz D. Study of phospholipid structure by 1H, 13C, and 31P dipolar couplings from 2D NMR. Biophys J. 1995;69:1939–1950. doi: 10.1016/S0006-3495(95)80064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huster D, Arnold K, Gawrisch K. Investigation of lipid organization in biological membranes by two-dimensional nuclear Overhauser enhancement spectroscopy. J Phys Chem. 1999;103:243–251. [Google Scholar]
- 29.Huster D, Gawrisch K. NOESY NMR crosspeaks between lipid headgroups and hydrocarbon chains: Spin diffusion or molecular disorder? Journal of the American Chemical Society. 1999;121:1992–1993. [Google Scholar]
- 30.Iwahara J, Jung YS, Clore GM. Heteronuclear NMR spectroscopy for lysine NH(3) groups in proteins: unique effect of water exchange on (15)N transverse relaxation. Journal of the American Chemical Society. 2007;129:2971–2980. doi: 10.1021/ja0683436. [DOI] [PubMed] [Google Scholar]
- 31.Rand RP, Fuller N, Parsegian VA, Rau DC. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988;27:7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- 32.Suits F, Pitman MC, Feller SE. Molecular dynamics investigation of the structural properties of phosphatidylethanolamine lipid bilayers. Journal of Chemical Physics. 2005;122:244714. doi: 10.1063/1.1899152. [DOI] [PubMed] [Google Scholar]
- 33.Doherty T, Waring AJ, Hong M. Membrane-bound conformation and topology of the antimicrobial peptide tachyplesin I by solid-state NMR. Biochemistry. 2006;45:13323–13330. doi: 10.1021/bi061424u. [DOI] [PubMed] [Google Scholar]
- 34.Mani R, Cady SD, Tang M, Waring AJ, Lehrer RI, Hong M. Membrane-dependent oligomeric structure and pore formation of a b-hairpin antimicrobial peptide in lipid bilayers from solid-state NMR. Proc Natl Acad Sci USA. 2006;103:16242–16247. doi: 10.1073/pnas.0605079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen ZJ, Van Gorkom LC, Epand RM, Stark RE. Nuclear magnetic resonance studies of lipid hydration in monomethyldioleoylphosphatidylethanolamine dispersions. Biophys J. 1996;70:1412–1418. doi: 10.1016/S0006-3495(96)79700-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liepinsh E, Otting G. Proton exchange rates from amino acid side chains--implications for image contrast. Magn Reson Med. 1996;35:30–42. doi: 10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- 37.van der Ven FJM, Janssen HGJM, Graslund A, Hilbers CW. Chemically relayed nuclear overhauser effects. Connectivities between resonances of nonexchangeable protons and water. J Magn Reson. 1988;79:221–235. [Google Scholar]
- 38.Holland GP, Alam TM. Unique backbone-water interaction detected in sphingomyelin bilayers with 1H/31P and 1H/13C HETCOR MAS NMR spectroscopy. Biophys J BioFAST. 2008 April 4; doi: 10.1529/biophysj.108.130724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez CF, Nielsen SO, Klein ML, Moore PB. Hydrogen bonding structure and dynamics of water at the dimyristoylphosphatidylcholine lipid bilayer surface from a molecular dynamics simulation. J Phys Chem. 2004;108:6603–6610. [Google Scholar]
- 40.Hong M, Doherty T. Orientation determination of membrane-disruptive proteins using powder samples and rotational diffusion: a simple solid-state NMR approach. Chem Phys Lett. 2006;432:296–300. doi: 10.1016/j.cplett.2006.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buffy JJ, Waring AJ, Lehrer RI, Hong M. Immobilization and Aggregation of Antimicrobial Peptide Protegrin in Lipid Bilayers Investigated by Solid-State NMR. Biochemistry. 2003;42:13725–34. doi: 10.1021/bi035187w. [DOI] [PubMed] [Google Scholar]


