Abstract
B cell CLL/lymphoma-2 (BCL-2) and its relatives comprise the BCL-2 family of proteins, which were originally characterized with respect to their roles in controlling outer mitochondrial membrane integrity and apoptosis. Current observations expand BCL-2 family function to include numerous cellular pathways. Here we will discuss the mechanisms and functions of the BCL-2 family in the context of these pathways, highlighting the complex integration and regulation of the BCL-2 family in cell fate decisions.
A Death in the Family
Like all living things, cells die. In animals, the predominant mode of cell death during development and tissue homeostasis is apoptosis. During this process, the caspase proteases effectively package and label (e.g., by inducing cellular blebbing and shrinkage, DNA fragmentation, and plasma membrane changes) dying cells for rapid clearance.
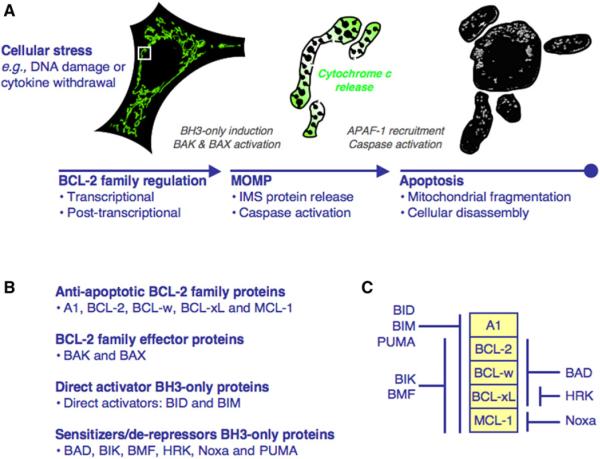
In vertebrates, the BCL-2 family regulates the mitochondrial pathway of apoptosis by complex interactions that dictate the integrity of the outer mitochondrial membrane (OMM) (Green and Evan, 2002). This pathway is initiated by mitochondrial outer membrane permeabilization (MOMP), which allows soluble proteins (e.g., cytochrome c) in the mitochondrial intermembrane space (IMS) to diffuse into the cytosol. Cytochrome c engages apoptotic protease activating factor-1 (APAF-1) to oligomerize into a caspase activation platform termed the apoptosome. This binds and promotes the activation of initiator caspase-9, which then activates executioner caspases-3 and -7. The caspases cleave cellular substrates to elicit the apoptotic phenotype (Figure 1A) (Riedl and Salvesen, 2007). Temporally, MOMP indeterminately occurs following proapoptotic stress, but studies suggest that this timing is dependent on the concentrations of diverse cellular proteins (Spencer et al., 2009). After MOMP, caspase activation and apoptosis ensue often within minutes.
Figure 1. The Mitochondrial Pathway of Apoptosis and the BCL-2 Family.
(A) Cellular stress causes transcriptional and post-transcriptional regulation of the BCL-2 family to promote MOMP.MOMP is induced by interactions between the BH3-only and effector proteins and leads to cytochrome c release, APAF-1 recruitment, and caspase activation. At the time of MOMP (middle), the intact mitochondrial network (green, left) undergoes fragmentation (gray, right), and soon after the cell is disassembled. The mitochondria in the middle are enlarged from the white box.
(B) The BCL-2 family is divided into antiapoptotic, effector, and direct activator/sensitizer/derepressor BH3-only proteins.
(C) The antiapoptotic BCL-2 protein binding profiles for the BH3-only proteins.
Here we discuss the BCL-2 family and its regulation of mitochondrial integrity, apoptosis, and other cellular processes including mitochondrial dynamics, endoplasmic reticulum (ER) calcium stores, and autophagy. Although the BCL-2 family is conserved among animals, its role in controlling the integrity of the OMM is only described for the vertebrates, and limited data exist for functions in invertebrates. In C. elegans, the BCL-2 protein cell death abnormality-9 (CED-9) does not control MOMP, but instead inhibits apoptosis by sequestering the APAF-1 homolog, CED-4 (reviewed in Lettre and Hengartner, 2006). Similarly, the Drosophila BCL-2 homologs do not appear to control cell death, and their functions remain obscure (reviewed in Mollereau, 2009). For these reasons, our discussion focuses on vertebrate BCL-2 family function.
A Family Portrait
BCL-2 and its relatives are functionally classified as either antiapoptotic or proapoptotic (Figure 1B). Most cells express a variety of antiapoptotic and proapoptotic BCL-2 proteins, and the regulation of their interactions dictates survival or commitment to apoptosis (Figure 1C).
Antiapoptotic BCL-2 proteins contain four BCL-2 homology domains (BH1–4) and are generally integrated within the OMM, but may also be in the cytosol or ER membrane (Figure 2A). BCL-2-related gene A1 (A1), BCL-2, BCL-2-related gene, long isoform (BCL-xL), BCL-w, and myeloid cell leukemia 1 (MCL-1) are the major members of the antiapoptotic BCL-2 repertoire and preserve OMM integrity by directly inhibiting the proapoptotic BCL-2 proteins.
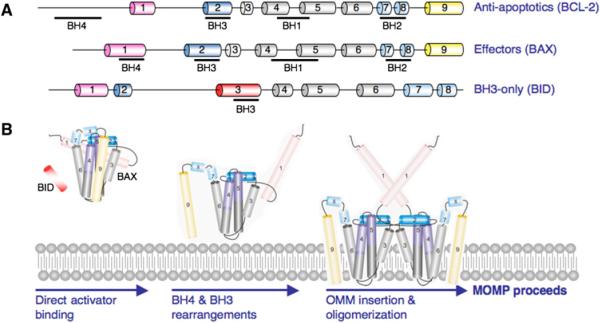
Figure 2. BCL-2 Family Composition and Membrane Permeabilization.
(A) The BCL-2 proteins are comprised of BCL-2 homology (BH) domains. A representation of an antiapoptotic (BCL-2), effector (BAX), and BH3-only (BID) protein is shown with the BH1-4 designated underneath the corresponding α helices.
(B) Proposed model of BAX activation. Soluble BAX interacts with a direct activator and the OMM to promote stable N-terminal exposure, and BAX α5, α6, and α9 insert within the OMM.
The proapoptotic BCL-2 members are divided into the effector proteins and the BH3-only proteins. The effector proteins BCL-2 antagonist killer 1 (BAK) and BCL-2-associated × protein (BAX) were originally described to contain only BH1-3; however, structure-based alignment of globular BCL-2 family proteins revealed a conserved BH4 motif (Figure 2A) (Kvansakul et al., 2008). Upon activation, BAK and BAX homo-oligomerize into proteolipid pores within the OMM to promote MOMP (Figure 2B). There is a potential third effector molecule, BCL-2-related ovarian killer (BOK), but no biochemical evidence supports a function akin to BAK or BAX.
The BH3-only proteins function in distinct cellular stress scenarios and are subdivided based on their ability to interact with the antiapoptotic BCL-2 repertoire or both the antiapoptotic proteins and the effectors (Figures 1C, 3A–3C). BH3-only proteins that only bind to the antiapoptotic repertoire are referred to as “sensitizer” and/or “derepressor” BH3-only proteins; e.g., BAD (BCL-2 antagonist of cell death) and Noxa. BID (BCL-2-interacting domain death agonist) and BIM (BCL-2-interacting mediator of cell death) interact with the antiapoptotic repertoire as well as the effectors, and can directly induce BAK and BAX oligomerization and MOMP. These BH3-only proteins are referred to as “direct activators” (Figure 3B). The interactions between the antiapoptotic repertoire, direct activator/sensitizer/derepressor BH3-only proteins, and effectors determine MOMP and apoptosis (Figures 1C, 3A–3C).
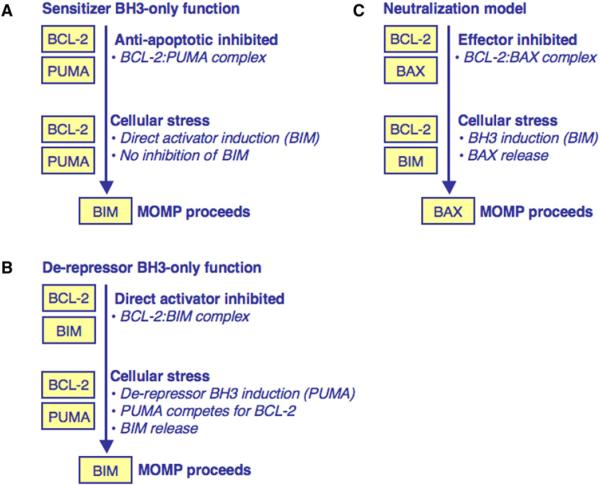
Figure 3. BH3-Only Protein Function.
(A) Sensitizer BH3-only protein function. A sensitizer BH3-only protein inhibits the antiapoptotic BCL-2 repertoire. Following minimal cellular stress, a direct activator is induced but cannot be inhibited and MOMP proceeds.
(B) Derepressor BH3-only protein function. A direct activator is sequestered by an antiapoptotic BCL-2 protein. Following cellular stress, a derepressor BH3-only protein is induced and competes with the direct activator for binding to the antiapoptotic repertoire. When the direct activator is released, MOMP proceeds.
(C) The neutralization model of BCL-2 family function. In this model, BAK/BAX are always competent to promote MOMP but are actively inhibited by the antiapoptotic BCL-2 repertoire to promote survival. Following cellular stress, BH3-only proteins are induced, bind the antiapoptotic proteins, and displace effectors to promote MOMP.
BAK and BAX Activation
Central to the initiation of apoptosis is BAK/BAX activation at the OMM. While there are competing models explaining the control of BAK/BAX activation (Figures 3A–3C), the contribution of BH3-only proteins in this process is undisputed (Chipuk and Green, 2008). At least two of the BH3-only proteins, BID and BIM, are capable of directly inducing effector function. The active form of BID (discussed below) promotes BAK and BAX oligomerization, MOMP, and cytochrome c release (Kuwana et al., 2002; Wei et al., 2000). Similar evidence exists for BIM-mediated BAK/BAX activation; and while PUMA was also suggested to promote BAK/BAX activation, this effect may not be direct (Chipuk et al., 2008; Kim et al., 2006; Kuwana et al., 2005; Letai et al., 2002). The BH3 domains of BH3-only proteins can be synthesized and represent the minimal unit of BH3-only protein function (referred to as BH3 peptides). BID and BIM BH3 peptides induce BAK and BAX oligomerization and pore-forming activity with isolated mitochondria or large unilamellar vesicles (LUVs, lipid vesicles that mimic the OMM) (Kuwana et al., 2002, 2005; Letai et al., 2002).
The series of events leading to BID-mediated activation of BAX has been elucidated in vitro (Lovell et al., 2008). BAX is soluble and undergoes activation in the presence of a direct activator and suitable membrane (e.g., the OMM or LUV); this results in oligomerized BAX and membrane permeabilization. The first step for BID-induced BAX activation is the association of BID with a membrane, followed by BAX recruitment, insertion, and oligomerization (Figure 2B) (Leber et al., 2007). Binding between BID and BAX has been difficult to study, but FRET analysis revealed the interaction in vitro (Lovell et al., 2008).
The interaction between BIM and BAX was demonstrated by NMR (Gavathiotis et al., 2008). Several other proteins are described to directly activate BAX, and it will be interesting to determine if they utilize a similar mechanism. BAK and BAX activation can also be triggered by nonprotein factors: e.g., mild heat, detergents, and high pH (Hsu and Youle, 1997; Khaled et al., 2001; Pagliari et al., 2005). Whether or not these mechanisms physiologically occur remains to be proven. However, observations that BAK/BAX-dependent apoptosis proceeds in the absence of BID and BIM argues that either direct activation by proteins is unnecessary, or alternative mechanisms for activation exist (Willis et al., 2007).
Sensitization and Derepression
Other BH3-only proteins, such as BAD, BCL-2-interacting killer (BIK), Harakiri (HRK), Noxa, and p53-upregulated modulator of apoptosis (PUMA) function predominantly by binding to the anti-apoptotic repertoire and not by directly activating BAK or BAX (Chen et al., 2005; Chipuk et al., 2008; Kuwana et al., 2005; Letai et al., 2002). The terms “sensitizer” and “derepressor” are used to indicate the consequences of binding between a BH3-only protein and an antiapoptotic BCL-2 protein. Each sensitizer/derepressor BH3-only protein has a unique binding profile for the antiapoptotic repertoire, which was primarily determined by using BH3 peptides (Figure 1C). These BH3-only proteins establish two distinct mechanisms that promote BAK/BAX activation: sensitization and derepression (Figures 3A and 3B) (Chipuk et al., 2008; Kuwana et al., 2005; Letai et al., 2002).
Sensitization lowers the threshold for BAK and BAX activation and MOMP but does not cause apoptosis itself (Figure 3A). In this scenario, an antiapoptotic protein is in complex with a sensitizer BH3-only protein, which prevents the inhibition of subsequent direct activators. For example, if BCL-2 is associated with PUMA, any future induction of BIM is not inhibited and MOMP proceeds. In the absence of PUMA, BIM would be sequestered and the cell may survive.
For derepression, a direct activator is bound by an antiapoptotic BCL-2 protein, and a subsequent BH3-only protein releases the direct activator to promote MOMP (Figure 3B). For example, reparable cellular stress can induce BIM function, but this activity is inhibited by the antiapoptotic repertoire and the cell survives. If a derepressor BH3-only protein is induced while the direct activator is sequestered, the latter can be released, allowing for MOMP. Studies using FRET demonstrated that derepression and consequential direct activation occur via protein·protein interactions that are not readily detected in the absence of membranes (Lovell et al., 2008). For example, activated BID was bound by BCL-xL, and this interaction was disrupted by BAD. BID then interacted directly with BAX, followed by BAX oligomerization and LUV permeabilization. These studies highlight the rapidity of the interactions once the conditions for derepression and MOMP are satisfied. Derepression also represents a means of pharmacological regulation in certain tumors, such as chronic lymphocytic leukemia (CLL), which undergo MOMP when treated with a derepressor BH3 mimetic (Certo et al., 2006; Del Gaizo Moore et al., 2007).
An alternative hypothesis to the direct activator requirement is the neutralization model (Figure 3C). In this case, MOMP can proceed following inhibition of the antiapoptotic BCL-2 repertoire independently of direct activator·effector interactions (Uren et al., 2007; Willis et al., 2007). This model assumes that cells harbor activated forms of BAK and BAX that are sequestered by the antiapoptotic BCL-2 repertoire. BH3-only proteins then compete for the antiapoptotic proteins and apoptosis ensues. We recognize that inhibition of the antiapoptotic repertoire contributes to MOMP, but contend it most efficiently occurs following the combined efforts of direct activator and sensitizer/derepressor BH3-only proteins, as recently suggested by elegant experiments in vivo (Merino et al., 2009).
Communicating with the BCL-2 Family
The BH3-only proteins are the major sentinels for cellular stress, and diverse signaling pathways converge upon these proteins. BH3-only proteins share little homology, and this may explain the variety of mechanisms capable of regulating their function. The multidomain BCL-2 proteins can also be regulated during apoptotic signaling by changes in stability or cooperation with other family members. Here we highlight a few of the pathways that regulate the BCL-2 family (see Table S1, available online, for a list of regulators and references).
The proapoptotic function of the BID BH3 domain is revealed by a unique mechanism involving the proteolytic cleavage of the large unstructured loop joining the inhibitory N terminus and the BH3-containing C terminus. Cleavage can be achieved by a variety of proteases; e.g., caspase-8 (via death receptors), granzyme B (cytotoxic lymphocytes), and caspase-2 (via heat shock). The proapoptotic function of BID is also enhanced by N-myristoylation, which promotes OMM targeting and BAK activation.
BIM is regulated both at the transcriptional and posttranslational levels. BIM is expressed as three alternatively spliced isoforms (BIM-S, BIM-L, and BIM-EL), although the latter two are most often observed. Levels of bim mRNA are positively regulated by the forkhead transcription factor FOXO3A upon cytokine deprivation, and by C/EBPα and CHOP following ER stress. Translation of bim mRNA is negatively regulated by the miRNA cluster miRNA-17–92, and overexpression of miRNA-17–92 induces a bim-deficient phenotype (Xiao et al., 2008). BIM function is also controlled by a series of posttranslational modifications via ERK1/2 and βTrCP that differentially regulate the major BIM isoforms.
BAD is involved in apoptotic and nonapoptotic processes, and these dual activities are regulated by posttranscriptional modifications. Growth factors inhibit the proapoptotic function of BAD through phosphorylation by several kinases, such as Akt. Phosphorylation results in cytoplasmic sequestration and inactivation of BAD by 14-3-3 proteins, which prevents interaction with antiapoptotic BCL-2 proteins. The BAD BH3 domain phosphorylation status also regulates a nonapoptotic function of BAD through the direct regulation of glucokinase and glucose-driven mitochondrial respiration. Accordingly, mice deficient in bad or expressing a mutant with mutated phosphorylation residues display abnormal glucose homeostasis and deficient insulin responses, an effect that is reversed by BAD BH3 peptide treatment.
BAK and BAX expression levels are generally sufficient to promote MOMP. Hence, posttranslational modification of BAK and BAX likely regulates interactions within the BCL-2 family. In line with this hypothesis, survival signaling through ERK1/2 causes BAX phosphorylation and inhibition of its proapoptotic activity. Interestingly, the ubiquitously expressed β isoform of human BAX is capable of inducing MOMP and apoptosis, apparently in a BH3-only protein-independent manner. To thwart unwarranted MOMP, BAXβ is constitutively degraded in the absence of proapoptotic stress, suggesting strict posttranslational control (Fu et al., 2009).
Among the antiapoptotic proteins, the stability and function of MCL-1 have been extensively studied. After genotoxic stress, MCL-1 is ubiquitinylated by MULE, a HECT domain-containing E3 ligase, and rapidly degraded. MULE contains a BH3 domain that binds to MCL-1 similarly to the Noxa BH3, which can also promote MCL-1 degradation. MCL-1 stability is regulated by glycogen synthase kinase-3 (GSK-3), which phosphorylates MCL-1 to promote its degradation. The E3 ligase responsible for MCL-1 ubiquitination following GSK-3 phosphorylation is βTrCP, which can regulate BIM-EL stability. Also, removing ubiquitin groups conjugated to MCL-1 thwarts degradation and enhances cellular survival; this can be achieved by the deubiquitinase USP9X (Schwickart et al., 2010).
Family Form: Structural Considerations of the BCL-2 Family
The BCL-2 Core
The BCL-2 family is structurally categorized into folded globular and intrinsically unstructured proteins (IUPs) (see Table S2 for a listing of structures and references). Among the globular proteins, all of the multidomain antiapoptotic and effector BCL-2 proteins share a conserved “BCL-2 core.” This is also preserved in BID, even though it has the least structural homology to the folded members. The remaining BH3-only proteins are IUPs and likely fold upon binding to a globular BCL-2 protein. MCL-1 is structurally distinct, as it contains an N-terminal unstructured domain followed by the BCL-2 core.
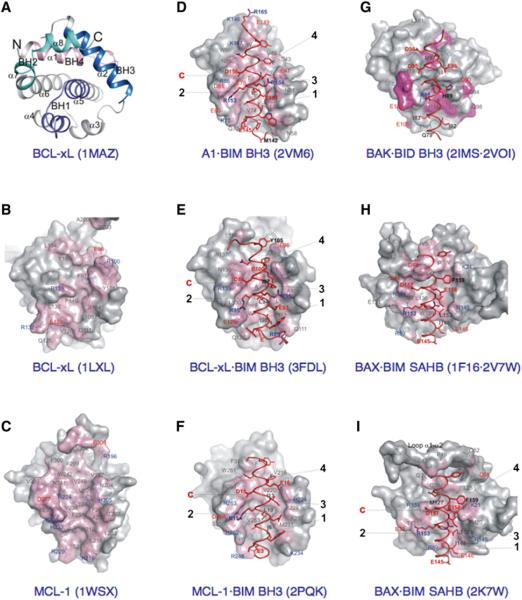
The BCL-2 core was revealed by the X-ray and NMR structures of BCL-xL and represents a ~20 kDa globular domain comprised of seven or eight amphipathic alpha (α) helices, arranged around a central buried helix, α5 (Figure 4A). The BH1 (portions of α4–α5) and BH2 (α7–α8) regions on one side, and the BH3 region (α2) and α3 on the other side, coalesce to delimit a hydrophobic groove (referred to as the BCL-2 family BH3 and C-terminus-binding groove, BC groove) at the “front” of the BCL-2 core. In BCL-w, and probably BCL-xL (as well as BAX, see below) the C-terminal α helix binds to the BC groove. The BH4 region, which is structurally defined by the conserved α1 positioned alongside α6, stabilizes the BH1-BH3 regions. All together, the BC groove and α1/α6 structural components comprise the BCL-2 core. The major differences between antiapoptotic and effector proteins are likely distinguished via the structural features of the individual BCL-2 cores, which reveals how the front pocket geometry, amino acid composition, and degree of obstruction by the C-terminal transmembrane (TM) tail modulate interactions with the BH3-only proteins.
Figure 4. Structural Highlights for the BCL-2 Family.
(A) The “front” view of BCL-xL identifies the BCL-2 core and the respective locations of the four BH regions. The PDB identifier is in parentheses.
(B) Surface representation of free BCL-xL, emphasizing amino acids participating in BH3 peptide binding at the “front” face, identifies the BC groove. Representations are partially transparent, permitting identification, beneath the surface, of contact side chains positioned 4Å from a peptide. With the exception of free BCL-xL and MCL-1 (C), both having surface coloring based on all atoms of peptide amino acids, surface coloring highlights strictly the atoms within 4Å from a peptide. PDB residue numbering has been maintained. Labels of acidic and basic amino acids are colored red and blue, respectively.
(C) Free MCL-1.
(D) A1 bound to BIM BH3. The four conserved hydrophobic residues/sites and the conserved charged interactions are marked. Peptide amino acid labels are bold.
(E) BCL-xL bound to BIM BH3.
(F) MCL-1 bound to BIM BH3.
(G) A model between BID BH3 (from the A1·BID BH3 complex) and BAK-ΔTM, overlapping sites of interaction (colored) identified by NMR spectroscopy.
(H) BIM SAHB modeling to the “back” face of free BAX.
(I) BIM SAHB binding site on BAX.
Conformational Changes during BAK and BAX Activation
In unstimulated cells, BAX is cytosolic and the BC groove accommodates the C-terminal TM region as an amphipathic α helix (Figure 2B). To initiate MOMP, BAX undergoes a cytosolic-to-mitochondrial redistribution, initially implicating the exposure of TM region with OMM targeting. In contrast, BAK is constitutively targeted to the OMM, likely explained by an occluded BC groove, which impedes binding of the TM tail.
Constitutive targeting of BAK or induced redistribution of BAX to the OMM does not imply that effectors are active once the TM regions are embedded in the OMM. Instead, direct activation of BAK and BAX induces numerous conformational changes: (1) α1 exposure, (2) transient BH3 exposure, (3) protection of the membrane-embedded BCL-2 core, and (4) increased proximity of embedded monomers. Furthermore, analysis of BCL-xL/BAX chimeras identified the regions of BAX required for membrane insertion and MOMP (George et al., 2007). Together, these activation-induced conformational changes correlate with MOMP. These studies also revealed that the α5 helix was both necessary and sufficient for BAX oligomerization and MOMP.
Supporting the sequence of events postulated for direct activation of the effectors, subtle conformational changes in BAX instigated by the BIM BH3 peptide were modeled using NMR (Figure 4H) (Gavathiotis et al., 2008). To improve the affinity of the BIM BH3 peptide for BAX, a BIM SAHB (“stabilized α helix of BCL-2 domain” peptide) was used, in which the BH3 α helix is fixed by a chemical staple. The BAX·BIM SAHB complex was nevertheless unstable, precluding structural determination by conventional NMR. Instead, paramagnetic relaxation enhancement NMR oriented the BIM SAHB on the back face opposite the BC groove of BAX. A shallow hydrophobic groove in BAX devoid of pronounced ridges was defined by α1 and α6. This occurred by displacing the α1–α2 loop and rearranging the peripheral side chains (e.g., BAX Lys21 next to BIM Glu158) to accommodate the BIM SAHB; the newly identified groove appears similar to antiapoptotic BC grooves. Binding of the BIM SAHB also induced subtle conformational changes to the BAX BC groove, where α9 became loosely attached compared to the free BAX structure. In addition, the BID and PUMA BH3 can promote remodeling of the BAX structure (Kim et al., 2009).
Whether a similar direct activator-binding strategy mediates BAK activation is unknown. Modeling of the BIM SAHB on the back face identified fewer clashes in BAK compared to BAX (Figure 4I). However, NMR revealed a low-affinity complex between the BID BH3 peptide and BAK-ΔTM (which mimics the globular domain of membrane-targeted BAK) (Figure 4G). The BID BH3 binding site overlapped with the partially occluded BC groove of BAK, and major conformational changes were not detected. The BAK BC groove is also implicated in homodimerization, as illustrated by cross-linking studies at cysteine residues engineered within the BH3 and the BC groove (Dewson et al., 2008). These studies indicated that the formation of BAK dimers involves reciprocal BH3·BC groove interactions; and most recent studies revealed that high-order BAK oligomer formation requires α6·α6 interactions to promote MOMP (Dewson et al., 2009).
Structures of Antiapoptotic and BH3-Only Protein Complexes
The antiapoptotic BCL-2 family members have been extensively studied at the molecular level by both NMR spectroscopy and X-ray crystallography. A comprehensive review of antiapoptotic BCL-2 family structures described free BCL-2, BCL-xL, BCL-w, and the complexes between BCL-xL and the BAK and BAD BH3 peptides (Petros et al., 2004). The current portrait of antiapoptotic proteins now includes A1 and MCL-1 structures in complex with an extensive panel of BH3 peptides (Figures 4C–4F and 5A). These recent structural insights are significant to cancer drug discovery because MCL-1 and A1 are not targeted by the most promising BH3 mimetics (e.g., ABT-737 and its analogs), which only inhibit BCL-2, BCL-w, and BCL-xL (Oltersdorf et al., 2005).
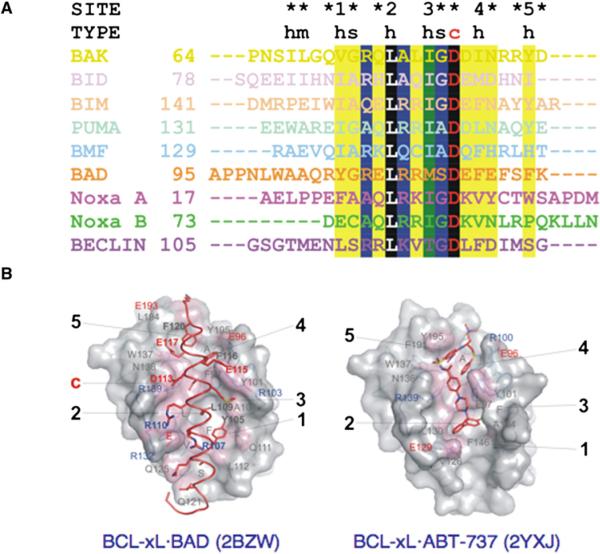
Figure 5. Binding of BH3-Only Peptides, Proteins, and ABT-737 to BC Grooves.
(A) Structure-based alignment of BH3 peptides from complexes with A1 (BAK, 2VOH; BID, 2VOI; BIM, 2VM6; PUMA, 2VOH; BMF, 2VOG), BCL-xL (2BZW, full-length BAD; 2P1L, BECLIN), and MCL-1 (Noxa A, 2ROD; Noxa B, 2NLA; see Table S2). BC grooves accommodate different types of BH3 amino acids: h, hydrophobic; m, mixed; s, small; c, charged. The degree of conservation, illustrated in black (100%), green (>75%), blue (>50%), and yellow (25%), identifies a conserved core defining BH3 peptide specificity. The five conserved hydrophobic residues/sites and the conserved charged interactions are marked.
(B) Structure of BCL-xL illustrates overlapping sites of interaction with full-length BAD and ABT-737.
There are structural similarities and differences between the BCL-2 cores of A1, BCL-xL, and MCL-1 in the free and BH3 peptide-bound states (Figures 4B–4F). Analyses of their BC grooves revealed a deep hydrophobic groove of variable width, stretching across the entire length of the BCL-2 core, lined on either side by ridges composed of distinct combinations of polar and charged amino acids. Most likely, the groove and ridge combinations as captured in these structures define antiapoptotic protein selectivity for BH3 peptides. Compared to the MCL-1 BC groove, which shows a constant width throughout, the BCL-xL BC groove is partially constricted due to obstructions on either side of the groove along its entire length. Large amino acid side chains from α4 (Glu129) and the BH2 region (Tyr195) delimit, respectively, the lower and upper ends of the compacted BCL-xL BC groove, and the conserved BH1 Arg139 side-chain projects centrally within the groove. In addition, α2 and α3 assemble to delimit the tighter groove from the opposite side.
Upon BH3 peptide binding and regardless of the extent of obstruction in the free protein, A1, BCL-xL, and MCL-1 are induced to present a rearranged wide-open BC groove. In contrast to the subtle changes in the preformed groove of MCL-1, binding of BH3 peptide to BCL-xL induces significant rearrangement of the BH3-containing ridge, including winding of additional turns in αqqqqqq2 and concomitant unwinding in α3, along with major side-chain rearrangements on both sides of the BC groove. Five main conserved hydrophobic pockets are induced contiguously along the BC groove to accommodate the hydrophobic residues projected by a snuggly fitting amphipathic helix assumed by incoming BH3 peptides (Figure 5A). Additional interactions from the ridge residues to solvent-exposed, mainly hydrophilic residues of the BH3 peptide may significantly contribute to high-affinity binding. However, with the exception of full-length BAD in complex with BCL-xL, the antiapoptotic BCL-2 protein structures remain to be solved in complex with their putative full-length BH3-only protein partners (Figure 5B).
A Dysfunctional Family
BCL-2 was identified in follicular B cell lymphoma as a translocation to the immunoglobulin heavy-chain locus t(14:18) rendering the gene constitutively hyper-expressed. To determine if BCL-2 was capable of synergizing with oncogenes ex vivo, bcl-2 was retrovirally introduced into the bone marrow of wild-type mice or transgenic mice overexpressing the c-myc oncogene (Eμ-myc). BCL-2 cooperated with c-Myc to promote transformation of B cell precursors (Vaux et al., 1988). However, in the absence of such an additional oncogene, bcl-2 did not promote proliferation, although it did protect against apoptosis induced by cytokine withdrawal. In vivo, this was mirrored by a bcl-2 transgenic model (McDonnell et al., 1989). Further evidence for bcl-2 and c-myc synergy in a double transgenic (Eμ-bcl-2/c-myc) model confirmed that bcl-2 contributes to lymphomagenesis by allowing a cell to survive despite c-myc expression (Strasser et al., 1990).
Although developmentally normal, the adult bcl-2−/− mouse displays thymus and spleen involution, polycystic kidney disease, and hypopigmented hair due to increased apoptosis. Genetic knockouts of the other antiapoptotic proteins revealed specific physiologic roles for the antiapoptotic BCL-2 proteins (see Table S3 for a list of animal models and references). For example, the bcl-x−/− mouse dies at E13.5 due to massive apoptosis of hematopoietic and neuronal cells, while the bcl-w−/− mouse elucidated an important role for BCL-w in spermatogenesis. Loss of MCL-1 blocked embryo implantation and is early embryonic lethal. Although these proteins are classified into one category, each regulates a distinct pathway presumably due to different subsets of proapoptotic molecules. Tissue-specific knockouts for antiapoptotic BCL-2 proteins are currently providing insights where the complete knockouts prevented observation. For example, mcl-1 deletion in the adult mouse revealed a requirement in hematopoietic stem cell survival, although it is not known if this is due to MCL-1's antiapoptotic effects (Opferman et al., 2005).
Bak−/− and bax−/− mice display mild phenotypes compared to the combined bak−/−bax−/− mice. While the bak−/− mouse does not display any striking developmental or homeostatic defects, the bax−/− mouse displays T and B cell hyperplasia and reproductive abnormalities in both sexes. However, it was not until the two null alleles were combined that it was suggested that the two proteins play redundant roles in apoptosis. The vast majority of bak−/− bax−/− mice are embryonic lethal due to an inability to eliminate excess cells during development. The few mice that survive to birth (<10%) display a myriad of apoptosis-related phenotypes, including lymphadenopathy, splenomegaly, and interdigital webbing.
Genetic deletion of the BH3-only proteins revealed tissue-and/or stimulus-specific roles in development and tissue homeostasis. bid−/− mice are resistant to anti-CD95/Fas-induced liver injury but are otherwise developmentally normal. bim−/− mice have lymphoid and myeloid cell hyperplasia (and observable splenomegaly, lymphadenopathy, and systemic lupus erythematosus), and lymphoid cells from these mice are resistant to cytokine withdrawal. The notion that BH3-only proteins function in specific tissues and pathways is also supported by the deletion of hrk, which is required for nerve growth factor withdrawal-induced apoptosis in sensory neurons. However, deletion of other BH3-only proteins, such as PUMA, revealed a broader role in regulating MOMP and apoptosis. Mice deficient in puma are markedly resistant to hematopoietic and gastrointestinal apoptosis following irradiation, and puma−/− MEFs and lymphocytes are resistant to cytokine withdrawal and glucocorticoids. Further analyses indicate a role for PUMA in ER stress, ischemia/reperfusion, and bacterial/viral/ fungal infections.
The removal of one bim allele rescues the renal phenotype and survival of bcl-2−/− mice, whereas loss of both bim alleles further corrects the hypopigmentation phenotype. These observations suggest that basal levels of BIM are sufficient to induce apoptosis in the absence of BCL-2. To dissect the mechanisms of BIM-mediated apoptosis in vivo, the BIM BH3 domain was replaced with the BAD, Noxa, or PUMA BH3 (Merino et al., 2009). When crossed with bcl-2-deficient animals, the BAD, Noxa, and PUMA BH3 replacements, alone or in combination, failed to rescue bcl-2−/− animals. These studies help to delineate differences between effects of neutralization of antiapoptotic proteins and activation of BAK and BAX.
Family Dynamics: Regulation of Mitochondrial Morphology and ER Signaling
Mitochondria are dynamic organelles that undergo fission and fusion to yield an interconnected tubular mitochondrial network (reviewed in Autret and Martin, 2009). Mitochondrial fission is regulated by DRP-1, Fis-1, and Mff. While Fis-1 and Mff are tail anchored in the OMM, DRP-1 is a soluble factor recruited to mitochondria to promote fission. Mitochondrial fusion is controlled by three large GTPases: mitofusins 1 and 2 (MFN1/2) in the OMM, and OPA-1 that is associated with the IMM. MOMP often coincides with fragmentation of the mitochondrial network, suggesting that BCL-2 family function and the mitochondrial dynamics machinery are intimately linked (Frank et al., 2001).
The BCL-2 family modulates the balance between mitochondrial fission and fusion. The combined deletion of BAK and BAX results in reduced fusion whereas fission appears unaffected, yet BAX may also promote fusion by altering MFN2 distribution, mobility, and/or complex assembly (Karbowski et al., 2006). The mechanism of BAX-regulated mitochondrial dynamics remains unclear since this protein is usually cytosolic. One possibility is that BAX shuttles, like DRP-1, between the cytosol and OMM under nonapoptotic conditions. Ectopic BCL-xL expression often induces extensive mitochondrial fusion, which may be due to a direct interaction with MFN2; interestingly, BAK was also shown to interact with MFN1/2 (Brooks et al., 2007; Delivani et al., 2006). Conversely, BAK and BAX can promote mitochondrial fission without inducing MOMP, especially if the latter is antagonized by BCL-xL (Sheridan et al., 2008). A possible explanation for these effects is that BAK and BAX function to promote mitochondrial fusion only when they are not in an activated state. Once activated, perhaps they can be sequestered by antiapoptotic proteins to prevent MOMP, but no longer promote fusion. As a consequence, steady-state mitochondrial fission results in extensively fragmented mitochondria, although a decrease in fission can reestablish normal mitochondrial morphology.
Upon MOMP, the marked increase of mitochondrial fragmentation is caspase independent and coincides with cytochrome c release (Frank et al., 2001; Karbowski et al., 2002). Following proapoptotic stimulation, components of the mitochondrial fusion and fission machinery (e.g., DRP-1 and MFN-2) are recruited to mitochondrial scission sites and colocalize with BAX (Frank et al., 2001; Karbowski et al., 2002). These observations led to the hypothesis that MOMP and mitochondrial fission are linked. Indeed, loss of DRP-1 or Mff function by RNAi, a dominant-negative DRP1, or pharmacological inhibition of DRP-1 delays MOMP, mitochondrial fragmentation, and apoptosis (Cassidy-Stone et al., 2008; Frank et al., 2001; Gandre-Babbe and van der Bliek, 2008; Lee et al., 2004). Downregulation of Fis1 also inhibits apoptosis, while its overexpression triggers mitochondrial fission and MOMP (James et al., 2003; Lee et al., 2004). However, the notion that MOMP and the mitochondrial fission machinery are interdependent remains controversial, as several studies suggest DRP-1 is not required for MOMP (Estaquier and Arnoult, 2007; Ishihara et al., 2009; Parone et al., 2006). Likewise, overexpression of DRP-1 or downregulation of OPA-1 induces mitochondrial fragmentation, but not apoptosis; classical inducers of apoptosis can also trigger cytochrome c release before mitochondrial fission (Arnoult et al., 2005; Karbowski et al., 2002; Lee et al., 2004).
Cytochrome c is not diffuse within the IMS because it is sequestered by mitochondrial cristae, which are formed by invaginations of the IMM. Following MOMP, cytochrome c release from the IMS is postulated to require cristae remodeling to promote free diffusion (Scorrano et al., 2002). However, analysis of mitochondrial morphology during apoptosis revealed that massive cristae rearrangement occurs after MOMP and is caspase dependent (Sun et al., 2007). Nevertheless, in the absence of caspase activation, cristae junctions undergo subtle but significant changes that may be BAK and BAX independent and regulated by cyclosporine A (Scorrano et al., 2002; Yamaguchi et al., 2008). The tight junctions in the IMM that compartmentalize cristae are secured by two splicing variants of OPA1 that are speculated to oligomerize, and this function is independent of OPA-1's regulation of mitochondrial morphology (Cipolat et al., 2006; Frezza et al., 2006). During MOMP, the disruption of OPA1 oligomers is suggested to allow for the complete release of cytochrome c, and BID and BIM are implicated in mediating OPA-1 disassembly, but detailed mechanisms remain unknown (Frezza et al., 2006; Yamaguchi et al., 2008).
Mitochondria continually interact with themselves to sustain metabolic function; however, mitochondria also interact with the ER. The BCL-2 family regulates at least one aspect of the mitochondrial-ER interaction by influencing ER calcium (Ca2+) stores and signaling. The direct impact of Ca2+ signaling on the mitochondrial pathway of apoptosis remains controversial, yet the influence of the BCL-2 family on ER Ca2+ storage and release could certainly influence cellular sensitivity to proapoptotic stimulation by a variety of mechanisms. Examples from each BCL-2 family subtype are described to function at the ER, and while the localization and function of these proteins diverge from generally accepted principles, the cooperation between individual BCL-2 proteins may still hold true (e.g., a BH3-only protein antagonizes antiapoptotic function).
ER Ca2+ is regulated by both entry through the sarco/endoplasmic reticulum Ca2+-ATPase and release via the inositol 1,4,5-trisphosphate receptor (IP3R). ER-localized BCL-2 and BCL-xL are implicated in directly inhibiting IP3R activity and reducing resting ER Ca2+ levels and cytosolic Ca2+ oscillations. Studies suggest that a direct interaction between the BCL-2 BH4 and IP3R is responsible for these effects (Rong et al., 2009). BAK and BAX are also suggested to promote ER Ca2+ storage yet enhance stress-induced ER Ca2+ release, possibly by inactivating the inhibitory functions of BCL-2 and BCL-xL on the IP3R (Oakes et al., 2005; Scorrano et al., 2003). BIM and PUMA are described to promote ER Ca2+ release; however, the mechanism of action (i.e., promoting BAK and BAX activity or inhibiting BCL-2/BCL-xL) remains unknown.
There is another level of complexity between the BCL-2 family and ER function which focuses on disrupted Ca2+ homeostasis leading to the unfolded protein response (UPR). UPR occurs when ER protein modification, folding, and secretory pathways are compromised and stress signaling originating from the ER lumen induces a transcriptional program to promote recovery. Interestingly, BAK and BAX are described to directly bind and regulate IRE1 signaling at the ER, which is responsible for engaging the transcriptionally regulated recovery phase of the UPR (Hetz et al., 2006). The relationship between UPR signaling and BAK and BAX function situates these proteins at both the recovery phase and MOMP-initiating aspects of ER stress.
Several investigations reveal that proteins physically link the OMM to the ER. The proteins responsible for tethering these organelles are components of the mitochondrial dynamic machinery (e.g., DRP-1 and MFN1/2) but may also include proteins not directly implicated in mitochondrial dynamics (e.g., Mmm1) (Csordas et al., 2006; de Brito and Scorrano, 2008; Kornmann et al., 2009). The physically linked regions of mitochondria and ER are best described to regulate Ca2+ signaling, but these unique domains may coordinate lipid synthesis and transport between the organelles.
A Family Dinner: Bcl-2 Proteins and Autophagy
Autophagy is a process in which cytoplasm is engulfed by a double-layered membrane structure which then fuses with lysosomes (referred to as an autolysosome), promoting the degradation of its contents (reviewed in Levine and Kroemer, 2008). Autophagy allows for the recycling of nutrients to preserve bioenergetics and is critical for the clearance of protein aggregates and damaged organelles. The Beclin-1·hVps34 axis represents a regulatory node of the autophagy machinery. Vps34 is a class III phosphatidylinositol 3-kinase that controls the autophagic process from early autophagosome formation to maturation into autolysosomes. Vps34 activity is directly regulated by Beclin-1, a scaffolding protein that is essential for autophagy induction.
Beclin-1 was first identified as a BCL-2-interacting protein, leading to the suggestion that autophagy and apoptosis are mechanistically linked (Liang et al., 1998). Beclin-1 also interacts with BCL-xL, BCL-w, MCL-1, and viral homologs of BCL-2 leading to decreased Vps34 activity and autophagosome formation (Erlich et al., 2007; Maiuri et al., 2007b). It is not clear whether BCL-2 proteins inhibit Beclin-1-induced autophagy by dissociating the Beclin-1-Vps34 complex or by inhibiting its function. The binding of antiapoptotic BCL-2 proteins to Beclin-1 appears to be constitutive, and its dissociation may be sufficient to induce autophagy. Beclin-1 contains a BH3 domain and binds within the BC groove of BCL-xL. Accordingly, mutations in the BCL-2 or BCL-xL BC groove, or in the Beclin-1 BH3 domain, disrupt this complex and promote autophagy (Oberstein et al., 2007).
Two mechanisms are proposed to explain Beclin-1 release from antiapoptotic BCL-2 proteins: (1) posttranslational modification of Beclin-1 and/or BCL-2 proteins, and (2) direct competition for the BCL-2 BC groove by another BH3-only protein (i.e., derepression). Upon autophagy induction, c-Jun N-terminal protein kinase 1 (JNK1) phosphorylates BCL-2 in the α1-α2 unstructured loop, which is sufficient to displace Beclin-1 (Wei et al., 2008; Pattingre et al., 2009). Beclin-1 can also be dissociated from BCL-2/BCL-xL upon phosphorylation of the Beclin-1 BH3 domain (Zalckvar et al., 2009). The second mechanism involves competitive dissociation of Beclin-1 from antiapoptotic BCL-2 proteins by BH3-only proteins. Upon starvation, BAD can displace Beclin-1 from BCL-2/BCL-xL to promote autophagy; likewise, bad-deficient cells have reduced levels of autophagy (Maiuri et al., 2007a).
Several other BH3-only proteins induce autophagy in response to metabolic stress and development. For example, hypoxia causes a reduction in mitochondrial mass by autophagy (referred to as mitophagy) that is thought to prevent increased levels of ROS and cell death (Zhang et al., 2008). This phenomenon is mediated by interactions between BCL-2, the adenovirus E1B 19-kDa-interacting protein 3 (BNIP3), and possibly Beclin-1. Unlike most BH3-only proteins, the binding of BNIP3 to BCL-2 is not via the BH3 but rather the N terminus of the protein; how this affects BCL-2 function is unknown. Similarly, NIX/BNIP3-like (BNIP3L), a BH3-only protein that shares extensive sequence homology with BNIP3, is involved in mitophagy during erythroid cell development. Maturation of erythroid cells involves autophagy-dependent cytocellular remodeling resulting in the loss of the nucleus and other organelles. BNIP3L is upregulated during this process and specifically promotes mitophagy, and bnip3L−/− mice exhibit a lack of mitochondrial clearance in developing erythrocytes (Schweers et al., 2007). The mechanism by which BNIP3L induces mitophagy is not known, but the process is BAK and BAX independent (Sandoval et al., 2008). PUMA is also suggested to be involved in targeting mitochondria for mitophagy (Yee et al., 2009). However, contrary to the BNIP3L-dependent mechanism, PUMA-induced mitophagy occurs following MOMP and is BAX dependent. Surprisingly, this process appears to enhance apoptosis.
The mechanisms and physiological implications of the cross-regulation between autophagy and apoptosis by the BCL-2 family remain unknown. It is not clear how the BH3-only proteins toggle between regulating autophagy and apoptosis. Derepression of Beclin-1·antiapoptotic BCL-2 protein complexes is achieved by a BH3-only protein competitively binding to BCL-2 and/or BCL-xL; how then does derepression regulate autophagy without inducing apoptosis? Perhaps BNIP3 and BNIP3L do not have significant affinity for BCL-2/BCL-xL complexes with other proapoptotic BCL-2 proteins, but can displace Beclin-1. However, this hypothesis does not explain how BAD, which has a high affinity for BCL-2/BCL-xL, can apparently promote autophagy and not apoptosis under certain circumstances.
Family Friends: Regulation of the Bcl-2 Family by “Unrelated” Proteins
In addition to the signaling pathways that influence the BCL-2 family, recent genetic and biochemical data suggest that proteins outside of the family directly regulate MOMP. These proteins include direct activators that engage BAK and BAX function, but also encompass inhibitors to the BCL-2 family, and MOMP inducers with unclear mechanisms of action (see Table S4 for a list and references).
Beyond BID, BIM, and potentially PUMA, a number of proteins are described to directly activate BAK and/or BAX. BCL-2 proteins usually cooperate through BH domain interactions, and some of the non-BCL-2 family direct activators also contain a putative BH3 domain. For example, tissue transglutaminase, MAP-1, the DNA repair protein RAD9, and the 9-2 isoform of 2′-5′ oligoadeny-late synthetase are described to have BH3 domains capable of promoting apoptosis. In addition, proteins without a discernable BH3 domain are suggested to directly engage BAK and/or BAX. ASC, cytosolic p53, ATG5, and nucleophosmin are reported to interact with antiapoptotic BCL-2 proteins and/or BAK and BAX. This suggests that a BH3 domain is not always required to engage the BCL-2 family. Indeed, the BH3 domain sequence is loosely defined, and it is likely that additional features promote BH3-like functions. Furthermore, Nur77, a nuclear receptor with no obvious BH3 domain, is suggested to convert BCL-2 into a proapoptotic molecule. Nur77 translocates to mitochondria and binds between the BCL-2 BH4 and BH3 regions; this pathway has been implicated in the negative selection of thymocytes (Thompson and Winoto, 2008).
Cytosolic p53 is the most biochemically characterized non-BCL-2 family direct activator. Following genotoxic or oncogenic stress, p53 is stabilized and transcriptionally regulates genes involved in cell-cycle arrest, apoptosis, and other cellular functions. However, p53 also possesses a cytosolic activity, which can promote apoptosis in the absence of transcription or translation. The involvement of mitochondria in this pathway was elucidated when a fraction of stabilized p53 localized with mitochondria following genotoxic stress (Marchenko et al., 2000). Cytosolic p53 can be sequestered by antiapoptotic BCL-2 proteins, and directly activates BAK and BAX (Chipuk et al., 2004; Leu et al., 2004; Mihara et al., 2003). Cytosolic p53 is also suggested to function as a derepressor BH3-only protein; and multiple domains within p53 are implicated in these activities (Chipuk et al., 2004; Pietsch et al., 2008). Furthermore, cytosolic p53 is released from BCL-xL by PUMA, indicating cooperation between a derepressor BH3-only protein and a non-BCL-2 family direct activator (Chipuk et al., 2005).
Several non-BCL-2 family proteins inhibit proapoptotic BCL-2 proteins and MOMP; e.g., 14-3-3θ, Humanin, Ku70, and VDAC2. Humanin is a small peptide that binds and inhibits BAX, BID, and BIM-EL. Ku-70 (a DNA end-joining protein) and 14-3-3θ (a conserved regulatory protein) also directly bind BAX and potentially regulate its posttranslational regulation and mitochondrial localization. VDAC2 (voltage-dependent anion channel isoform 2) is an OMM protein suggested to constitutively bind BAK via the BC groove, which maintains BAK in a monomeric, inactive conformation. Following proapoptotic stimulation, direct activator BH3-only proteins disrupt the VDAC2$BAK complex to promote MOMP. Indeed, vdac2−/− cells are more susceptible to apoptosis presumably because lower thresholds of direct activators are required to promote BAK activation.
There are proteins for which a mechanism of MOMP induction has not been determined, either because direct interactions with the BCL-2 family were not investigated or because experiments did not reveal an interaction. For example, histone H1.2 is suggested to translocate to the cytosol to promote BAK-dependent MOMP following DNA damage. Although histone H1.2 promoted the active conformation of BAK in a BCL-xL-regulated manner, no interaction between histone H1.2 and the BCL-2 family was observed. Similarly, breast cancer cell 2 (BRCC2) is capable of inducing BCL-xL-regulated apoptosis in a manner that is dependent upon a putative BRCC2 BH3-like domain. For most of the non-BCL-2 family regulators of MOMP, rigorous biochemical examination of how they cooperate with the BCL-2 family is required to determine if they indeed function as direct activators or sensitizers/derepressors, or offer novel mechanisms to regulate MOMP.
Family Interventions: Pharmacological Regulation of the Bcl-2 Proteins
Although the BCL-2 family is implicated in a wide range of diseases, the best characterized is cancer. Great strides have been made in generating pharmacological regulators of the BCL-2 family for the purpose of single-agent or combination therapies. The most potent and prevalent mechanism of apoptosis dysregulation in cancer is overexpression of antiapoptotic family members. Examples of increased BCL-2 or BCL-xL levels in human malignancy include lymphoma and epithelial cancers, and are generally predictive of aggressive disease, poor prognosis, and chemotherapeutic resistance (reviewed in Letai, 2008).
An emerging theme in cancer biology is that tumor cells are addicted to high levels of antiapoptotic BCL-2 family proteins in order to maintain survival. Accordingly, inhibiting the antiapoptotic proteins in tumors would either induce spontaneous apoptosis of the tumor cells provided the other components of the apoptotic pathway are intact, or marked sensitization to proapoptotic BCL-2 family members. One example is Genasense, a phosphorothioate oligodeoxynucleotide bcl-2 anti-sense, which has been shown to sensitize CLL patients to chemotherapy (Kang and Reynolds, 2009) and phase III trials suggest that Genasense may have efficiency in advanced melanoma with combination therapies (Bedikian et al., 2006).
After the crystal structure of BCL-xL was determined, several drugs were designed to target BCL-xL and the other antiapoptotic BCL-2 proteins (e.g., ABT-737, Figure 5B). A comparison of several inhibitors of the antiapoptotic BCL-2 proteins showed that only ABT-737 causes cell death in a BAK/BAX-dependent manner (van Delft et al., 2006). This small molecule inhibitor neutralizes BCL-2, BCL-xL, and BCL-w with subnanomolar affinity and exhibits marked cytotoxic efficacy in combination with chemotherapeutics and radiation, although healthy primary cells are not killed (Oltersdorf et al., 2005). In preclinical studies, ABT-263 (a bioavailable form of ABT-737) displays single-agent effectiveness against a variety of tumor types, including lymphoma and lung cancer. Overexpression of MCL-1 has been shown to be a potent mechanism of resistance to ABT-737 and ABT-263, and agents that promote degradation of MCL-1 work synergistically to promote apoptosis (van Delft et al., 2006).
Planning the Next Reunion
The functions of the BCL-2 family clearly extend beyond MOMP, but integrating these into cell physiology (and survival) is in the earliest stages. While it is clear that the family impacts on metabolism, for example, the effects and their causes are only beginning to emerge. What other roles will be found for the BCL-2 family, as additional binding partners are discovered and interrogated? As we develop pharmacologic therapies to manipulate these proteins in disease, such integration will take center stage.
The BCL-2 family functions in two worlds, one of water and one of lipid, and while we know a great deal about how these proteins interact in the former, we know almost nothing about their form and function in the latter. This represents several major challenges: How do BAK and BAX specifically disrupt the OMM? How do antiapoptotic BCL-2 proteins inhibit BAK/BAX in the hydrophobic milieu (and can the BC groove exist and function in this environment)? What are the roles for specific lipids in the activation of the effectors? Finally, how do the transitions between hydrophilic and hydrophobic states influence BCL-2 family function in mitochondrial dynamics, ER homeostasis, and autophagy?
As we plan the next reunion, we want to invite some of the “black sheep” of the family that remain obscure. For example, BOK, BCL-G, and BCL-xS are proapoptotic, but how and when they function is largely unknown. The BCL-2 family is large and diverse, and their complex chemistry entices us to explore new approaches to understand the workings of these proteins that hold the keys to life and death in our cells and, ultimately, in us.
Supplementary Material
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes four tables and Supplemental References and can be found with this article online at doi:10.1016/j.molcel.2010.01.025.
REFERENCES
- Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol. Cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J. Clin. Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc. Natl. Acad. Sci. USA. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc. Natl. Acad. Sci. USA. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor R, Duriez PJ, Martin S. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol. Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM. Bak activation for apoptosis involves oligomerization of dimers via their α6 helices. Mol. Cell. 2009;36:696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, Hirsch JA, Stein R, Pinkas-Kramarski R. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–568. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Fu NY, Sukumaran SK, Kerk SY, Yu VC. Baxbeta: a constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol. Cell. 2009;33:15–29. doi: 10.1016/j.molcel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto YI, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0)F(1)-ATPase. J. Biol. Chem. 2001;276:6453–6462. doi: 10.1074/jbc.M006391200. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, Huang DC, Colman PM. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat. Rev. Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Lettre G, Hengartner MO. Developmental apoptosis in C. elegans: a complex CEDnario. Nat. Rev. Mol. Cell Biol. 2006;7:97–108. doi: 10.1038/nrm1836. [DOI] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007a;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain J-C, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007b;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA, Adams JM, Strasser A, Lee EF, Fairlie WD, Bouillet P. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J. Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Mollereau B. Cell death: what can we learn from flies? Editorial for the special review issue on Drosophila apoptosis. Apoptosis. 2009;14:929–934. doi: 10.1007/s10495-009-0383-1. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XLBeclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc. Natl. Acad. Sci. USA. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donzé O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol. Cell. Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J. Biol. Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Pietsch EC, Perchiniak E, Canutescu AA, Wang G, Dunbrack RL, Murphy ME. Oligomerization of BAK by p53 utilizes conserved residues of the p53 DNA binding domain. J. Biol. Chem. 2008;283:21294–21304. doi: 10.1074/jbc.M710539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl. Acad. Sci. USA. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O'Rourke K, Bazan R, Eastham-Anderson J, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Delivani P, Cullen S, Martin S. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome c release. Mol. Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–432. doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Sun MG, Williams J, Munoz-Pinedo C, Perkins G, Brown JM, Ellisman MH, Green DR, Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat. Cell Biol. 2007;9:1057–1065. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J. Exp. Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren RT, Dewson G, Chen L, Coyne SC, Huang DC, Adams JM, Kluck RM. Mitochondrial permeabilization relies on BH3 ligands engaging multiple prosurvival Bcl-2 relatives, not Bak. J. Cell Biol. 2007;177:277–287. doi: 10.1083/jcb.200606065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol. Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135–1145. doi: 10.1038/cdd.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009;5:720–722. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.