Abstract
A challenge in the application of solid-state NMR spectroscopy to membrane peptides and proteins is the relatively broad linewidths compared to solution NMR spectra. To understand the linewidth contributions to membrane protein NMR spectra, we have measured the inhomogeneous and homogeneous linewidths of several well-studied membrane peptides under immobilized conditions. 13C T2 relaxation times of uniformly 13C-labeled residues show that the homogeneous linewidths of the peptides are comparable to those of crystalline model compounds under identical 1H decoupling and magic-angle-spinning conditions, indicating that the homogeneous linewidths are determined by conformation-independent factors, including residual dipolar coupling, J coupling and intrinsic T2 relaxation. However, the membrane peptides exhibit larger apparent linewidths than the crystalline compounds, indicating conformational disorder. A cationic cell-penetrating peptide, the human immunodeficiency virus TAT, exhibits the largest apparent linewidths, which are about 5-fold larger than the homogeneous linewidths, while the transmembrane helix of the influenza M2 peptide and the β-hairpin antimicrobial peptide PG-1 show moderately larger apparent linewidths than the crystalline compounds. These results are consistent with the random coil nature of the TAT peptide, which contrasts with the intramolecularly hydrogen-bonded M2 and PG-1. Cross peak lineshapes of 2D double-quantum correlation spectra show that the conformational disorder can occur at the residue level and can result from three origins: lipid-peptide interaction, intrinsic conformational disorder encoded in the amino acid sequence, and sidechain rotameric averaging. A particularly important lipid-peptide interaction for cationic membrane peptides is guanidinium-phosphate ion pair interaction. Thus, NMR linewidths and lineshapes are useful for understanding the conformational disorder of membrane peptides and proteins.
Introduction
Solid-state NMR (SSNMR) spectroscopy has become a powerful probe of the molecular structure and dynamics of insoluble biological macromolecules such as membrane proteins 1,2, amyloid fibrils 3–5, and cell walls 6,7. A persistent challenge in structure determination of these biological solids is spectral resolution. While biomolecules in solution have narrow linewidths due to their fast isotropic tumbling, solid systems, without such motion, exhibit broad NMR linewidths due to the presence of orientation-dependent nuclear spin interactions such as chemical shift anisotropy (CSA) and dipolar coupling. Magic-angle-spinning (MAS) 8 and hetero- and homo-nuclear dipolar decoupling techniques 9–14 eliminate most of this orientational broadening. Nevertheless, residual dipolar couplings between protons and heteronuclei due to imperfect decoupling still contribute sizeable linewidths, and dipolar couplings between isotopically enriched 13C spins broaden lines when MAS rates are insufficient. In addition, 13C-13C J couplings are difficult to remove in directly detected 13C spectra, thus contribute a fixed amount of linewidth. These coherent line broadening mechanisms are ameliorated by the use of higher magnetic fields 15, faster MAS 14,16 and reduction of the proton density by perdeuteration of proteins followed by H/D exchange 17–19.
In contrast to the coherent linewidth contributions, an incoherent line broadening mechanism is transverse T2 relaxation, which results from random fluctuations of local magnetic fields induced by molecular motion 20,21. When motional rates are comparable to the strength of the dipolar decoupling field strengths or MAS rate, extreme line broadening occurs that abolishes the spectral intensity altogether. This intermediate-timescale motional broadening has been studied in both small molecules 22,23 and membrane peptides 24–26.
The third line broadening mechanism, which cannot be removed by radio-frequency pulses or higher magnetic fields, is conformational disorder. Conformational distribution gives rise to multiple isotropic chemical shifts for each chemically unique nuclear spin, in the same manner that chemically inequivalent spins cause different isotropic shifts. This inhomogeneous line broadening causes characteristic 2D correlation lineshapes 27,28 and can give very broad peaks, even if the homogeneous linewidths due to residual couplings and T2 relaxation are small. The large difference between inhomogeneous and homogeneous linewidths has been exploited for designing J-coupling based polarization transfer methods for solids 29,30.
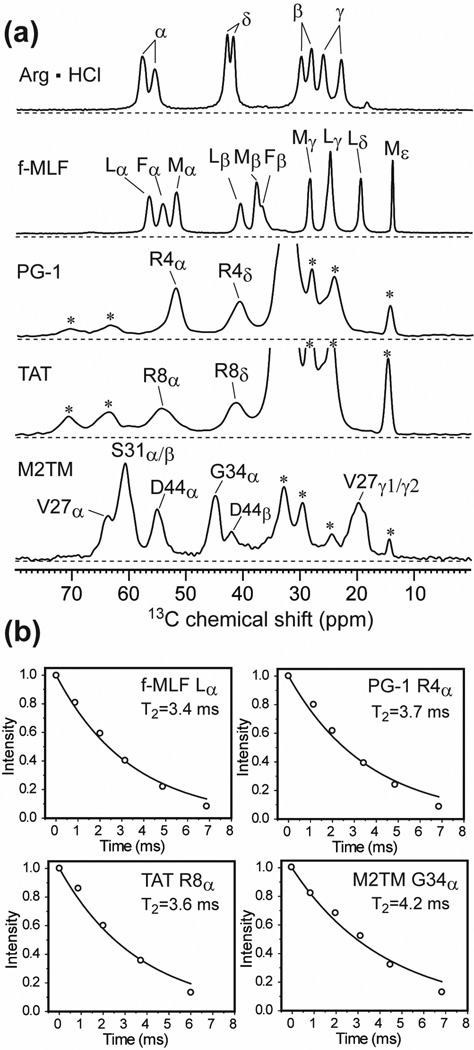
To date, the line broadening mechanisms of only a few biological solids have been investigated. These include cellulose in wood 27,28,30, elastin 31, and a crystalline tripeptide, formyl-Met-Leu-Phe-OH (f-MLF-OH) 32,33. No systematic study of the linewidth contributions of membrane-bound peptides and proteins has been reported. The amphipathic lipid bilayer surrounding membrane proteins 34 presents a complex anisotropic environment in which a distribution of lateral pressures and dielectric constants exist across the bilayer normal 35, and abundant thermal disorder of the lipid chains is present. Thus, membrane-bound peptides and proteins have additional mechanisms of conformational disorder compared to crystalline molecules, globular proteins, and fibrous biopolymers. Fig. 1 gives an example of the 13C linewidth differences between crystalline compounds and membrane peptides. The 1D 13C MAS spectra of two crystalline molecules, arginine hydrochloride (Arg ˙ HCl) and f-MLF-OH, are compared with the spectra of three hydrated membrane peptides: the transmembrane (TM) domain of the influenza M2 protein (M2TM), the disulfide-bonded β-hairpin antimicrobial peptide PG-1, and the Arg-rich cell-penetrating peptide (CPP) HIV TAT. All amino acid residues examined are uniformly labeled in 13C. The membrane peptide spectra were measured at a temperature (238 K) well below the gel-to-liquid-crystalline phase transition temperature to suppress any large-scale motion of the peptide backbone but not low enough to freeze methyl rotations or phenylene ring flips. At these temperatures, the linewidth contribution from intrinsic T2 relaxation should be comparable between the crystalline compounds and membrane peptides. We applied the same 1H decoupling field strength (71 kHz) and MAS rate (7 kHz) to maintain the same coherent line broadening contributions to the linewidths. We found that arginine hydrochloride and f-MLF-OH exhibit Cα full widths at half maximum (FWHM) of 120–140 Hz, M2TM and PG-1 show modestly larger Cα linewidths of 160 – 210 Hz, while TAT exhibits a very large linewidth of ~480 Hz. Thus, these membrane peptides exhibit varying degrees of static conformational disorder that is additional to the crystalline model compounds. Among the three membrane peptides, the linewidths of M2TM and PG-1 are largely independent of temperature between 238 K and 293 K (Fig. 1c, d), whereas TAT has strikingly narrower linewidths at ambient temperature due to near isotropic motion as described recently 36.
Fig. 1.
Representative Cα peaks from the 13C CP-MAS spectra of (a) Arg · HCl at 296 K; (b) f-MLF-OH at 296 K; (c) LAGI-M2TM in viral membranes at 238 K (black) and at 293 K (red); (d) Arg4 labeled PG-1 in POPE/POPG lipids at 238 K (black) and at 283 K (red). (e) Lys4 labeled TAT in POPE/POPG lipids at 238 K.
In this work, we investigate the line broadening mechanisms of these representative membrane peptides by measuring the apparent and homogeneous linewidths and by analyzing lineshapes in 2D correlation spectra 37. We measured these linewidths at the moderate low temperature of 238 K, where the peptide backbones are immobilized in the gel phase of the membranes. Low-temperature linewidth is interesting for both technological and structural reasons, since an increasing number of solid-state NMR experiments now utilize low temperature to enhance the spectral sensitivity 38 and to elucidate protein-folding pathways 39. Understanding the origins of membrane protein line broadening is thus important for developing new NMR methods to enhance spectral resolution and sensitivity and for harvesting the inherent structural information in the NMR linewidths and lineshapes. Our experiments reveal several mechanisms of conformational disorder that contribute to the inhomogeneous linewidths, and shed light on the relationship between dynamic exchange at physiological temperature and static conformational distribution at low temperature.
Materials and Methods
Sample Preparation
All phospholipids, including POPE, POPG, DMPC, DPPC, DPPE, and egg sphingomyelin (SPM) and cholesterol were purchased from Avanti Polar Lipids and used without further purification. Uniformly 13C, 15N-labeled Arg · HCl was purchased from Sigma-Aldrich. Fmoc-protected uniformly 13C, 15N-labeled amino acids were either prepared in-house 40 or purchased from Sigma-Aldrich and Cambridge Isotope Laboratories. All peptides, including PG-1 (RGGRLCYCRRRFCVCVGR), the CPP domain of the HIV Tat protein (residues 48–60, GRKKRRQRRRPPQ), and M2TM (residues 22–46, SSDPLVVAASIIGILHLILWILDRL), were synthesized using Fmoc chemistry and purified by HPLC.
Hydrated proteoliposomes were prepared in aqueous solution as described before 41,42. Lipids were mixed in chloroform and dried under nitrogen gas. The lipid film was redissolved in cyclohexane and lyophilized to remove trace organic solvent. The lipid vesicles were prepared by suspending the dry powder in phosphate buffer and freeze-thawed for six cycles. For the water-soluble PG-1 and TAT, the peptide solution was directly added to the lipid vesicle solution and incubated overnight before ultracentrifugation. For the insoluble M2TM, the peptide was reconstituted into lipid bilayers by dialysis using octyl- β-D-glucopyranoside 41. All proteoliposomes have peptide/lipid molar ratios of 1: 15 or 1:12.5 and were hydrated to ~40% by mass using a pH 7.0 or 7.5 phosphate buffer.
All peptides contain uniformly 13C and 15N labeled residues. One PG-1 sample contained labeled Gly3 and Leu5 while another PG-1 sample contained labeled Arg4. Both samples were incorporated into POPE/POPG (3:1) membranes. One M2TM sample contained labeled Val27, Ser31, Gly34 and Asp44 (VSGD-M2TM) and was reconstituted into DMPC bilayers. Another M2TM sample contained Leu26, Ala29, Gly34 and Ile35 labels (LAGI-M2TM) and was reconstituted into a virus-mimetic membrane consisting of DPPC, DPPE, SPM and cholesterol (6 : 6 : 4.5 : 6.4). Two TAT samples containing a Lys4 label and an Arg8 label were bound to POPE/POPG (8:7) membranes.
Solid-State NMR experiments
All solid-state NMR experiments were carried out on a Bruker DSX-400 spectrometer (Karlsruhe, Germany) operating at Larmor frequencies of 400.49 MHz for 1H, 100.70 MHz for 13C, and 40.58 MHz for 15N. A double-resonance MAS probe tuned to 13C/1H or 15N/1H modes was used. A Kinetics Thermal System XR air-jet sample cooler (Stone Ridge, NY) was used for cooling samples using dry air or nitrogen gas as input. Sample temperatures were direct readings from the thermocouple and were estimated to be within 1°C of the true temperature due to the moderate MAS rates (< 8 kHz) of all experiments. 13C and 15N chemical shifts were externally referenced to the α-glycine 13CO resonance at 176.49 ppm on the TMS scale and 15N-acetylvaline (NAV) at 122.0 ppm on the NH3 scale, respectively.
One-dimensional 13C and 15N cross polarization (CP) MAS spectra were measured using CP contact times of 0.5–1.5 ms. T2 relaxation times were measured using the Hahn-echo experiment 43 under 7 kHz MAS. The temperature was 238 K for membrane peptides and 296 K for crystalline model compounds. 1H TPPM decoupling 9 at 71.4 kHz was applied during detection. An acquisition time of ~17 ms, which was sufficiently long to avoid truncation of the time signal, was used for most membrane peptides.
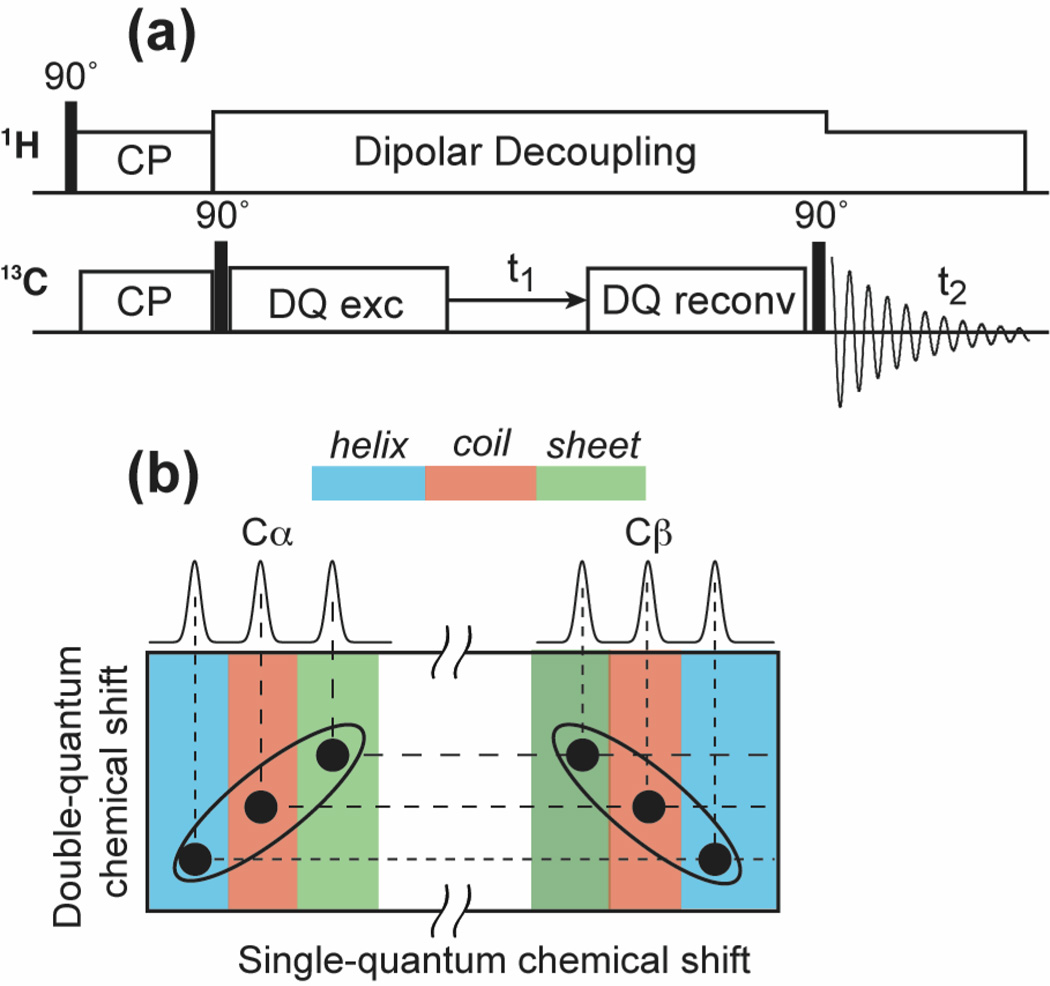
1D CP-MAS or 1D double-quantum (DQ) filtered MAS spectra were measured to extract the apparent 13C linewidth as a function of temperature. The DQ experiment suppresses the natural-abundance lipid 13C signals and gives well-resolved peptide 13C signals. No apodization was applied to the time signals when reading off the apparent linewidths. In a few cases where two peaks partially overlap, we obtained the FWHM as twice the half width at half maximum. For 2D correlation spectra, modest line broadening was used as indicated in the figure caption. The SPC-5 sequence 44 was used to recouple the 13C-13C dipolar coupling in the DQ experiments. All DQ spectra were measured under 5333 Hz MAS. The 2D DQ dipolar INADEQUATE experiment 37 correlates mostly directly bonded carbon signals due to the short DQ mixing time used and the fact that the one-bond 13C-13C dipolar coupling is 5-fold stronger than two-bond couplings. In our experience the dipolar INADEQUATE experiment has higher sensitivity than the J-coupling analog 30.
Results
We chose three membrane peptides with different secondary structures and dynamics to examine the conformational dependence of SSNMR linewidths and lineshapes. The TM domain of the influenza M2 protein forms a four-helix bundle in the virus envelope and conducts protons for the virus lifecycle 45–47. The antimicrobial peptide PG-1 is a disulfide-linked β-hairpin 48,49 that associates into β-barrels in anionic lipid membranes 42,50,51 to kill bacterial cells. The cell-penetrating peptide, TAT, from HIV crosses the membrane of eukaryotic cells to transport molecular cargos 52,53. Both PG-1 and TAT are cationic due to a large fraction of Arg residues in their amino acid sequences 34 while M2TM is mostly hydrophobic 47,54. The linewidths of Arg4 and Arg11 in PG-1 have been reported before 55.
Immobilized membrane peptides have similar homogeneous linewidths as crystalline compounds
We measured the 13C T2 relaxation times using the Hahn-echo experiment. The echodetected T2 gives the homogeneous linewidth (Δ) according to Δ = 1/πT2. In this work, we use the term homogeneous linewidth to denote the linewidth caused by both residual couplings and true T2 relaxation, since the two effects cannot be separated without infinitely strong 1H decoupling, very fast MAS, and J-decoupling. The J-coupling results from the fact that the crystalline compounds and the examined residues in the membrane peptides are all uniformly labeled in 13C, and neither MAS nor the hard-pulse spin-echo experiment removes 13C-13C J coupling. Thus, two- and three-spin 13C-13C J couplings contribute a fixed amount of 50–80 Hz to the 13C homogeneous linewidths. The apparent linewidth (Δ*) read off from the FWHM of the spectral peaks includes both the homogeneous contribution and the inhomogeneous contribution, and translates to an apparent T2 relaxation time according to . All spectra were measured at a magnetic field of 9.4 Tesla and all 13C T2 relaxation times were measured under a 1H decoupling field strength of 71 kHz. Stronger 1H decoupling will give narrower Δ, while higher magnetic fields will result in larger apparent linewidths Δ* (in frequency units) due to the field dependence of chemical shift.
Fig. 2a shows the aliphatic region of the 13C CP-MAS spectra of the crystalline compounds and the immobilized membrane peptides. Only resolved 13C signals were used to report the T2 values. Arginine hydrochloride shows two peaks for each 13C site due to two inequivalent molecules in the asymmetric unit cell 56,57. Representative T2 decays are shown in Fig. 2b. All data fit to single-exponential decays with time constants of 3–4 ms, indicating that the J-coupled 13C homogeneous linewidths are ~100 Hz (~1.0 ppm) for both the crystalline compounds and the hydrated membrane peptides under the conditions of the experiments.
Fig. 2.
13C CP-MAS spectra and T2 relaxation decays. (a) Representative 13C CP spectra of Arg · HCl, f-MLF-OH, Arg4-labeled PG-1 in POPE/POPG membranes, Arg8-labeled TAT in POPE/POPG bilayers and VSGD-M2TM in DMPC bilayers. Asterisks denote lipid 13C peaks. Experiments were conducted at 293 K for the model compounds and at 238 K for the membrane peptides. (b) Representative fittings of exponential T2 decays, measured under 7 kHz MAS and 71 kHz 1H TPPM decoupling.
Table 1 lists Δ and Δ* of all measured sites, distinguished by their numbers of directly bonded protons. For Cα, the homogeneous linewidths of all samples range from 75 to 100 Hz (0.75 – 1.0 ppm), irrespective of the crystalline or membrane-bound nature of the samples. At stronger 1H decoupling fields the Δ values decrease modestly 16,30,58 (Table S1). In comparison, the membrane peptides exhibit larger Δ* than crystalline compounds. For Cα, the membrane peptide Δ* ranges from 160 to 500 Hz while the model compound Δ* spans 110–140 Hz. The Δ*/Δ ratio is an indicator of the conformational heterogeneity of the samples 30: larger conformational disorder gives multiple uncorrelated chemical shift frequencies, whose homogeneous linewidth can be much smaller. Table 1 shows that the two crystalline compounds have CαΔ*/Δ ratios of 1.3–1.5, M2TM and PG-1 Cα have moderately larger Δ*/Δ ratios of 1.8–2.5. In contrast, TAT has large Δ*/Δ ratios of 5–6, indicating severe conformational disorder. The TAT peptide disorder approaches the reported Δ*/Δ ratios of 5 – 17 for cellulose in wood, measured under 10 kHz MAS and 100 kHz 1H decoupling 30.
Table 1.
13C apparent (Δ*) and homogeneous (Δ) linewidths of 13C, 15N-labeled compounds obtained from 1D 13C spectra and T2 measurements, respectively.
| Peptide | Site | CHn | Δ* (Hz) | Δ (Hz) | Δ*/Δ |
|---|---|---|---|---|---|
| f-MLF-OH | L Cα | CH | 120 | 93 | 1.3 |
| F Cα | CH | 140 | 96 | 1.5 | |
| M Cα | CH | 116 | 78 | 1.5 | |
| L Cγ | CH | 90 | 64 | 1.4 | |
| L Cβ | CH2 | 112 | 98 | 1.1 | |
| M Cβ | CH2 | 106 | 68 | 1.6 | |
| M Cγ | CH2 | 73 | 43 | 1.7 | |
| L Cδ1/2 | (CH3)2 | 64 | 47 | 1.4 | |
| Arg · HCl | R Cζ | C | 75 | 45 | 1.7 |
| R Cα | CH | 137 | 102 | 1.3 | |
| R Cδ | CH2 | 112 | 70 | 1.6 | |
| R Cβ | CH2 | 125 | 95 | 1.3 | |
| R Cγ | CH2 | 125 | 99 | 1.3 | |
| M2TM | G34 Cα | CH2 | 158 | 75 | 2.1 |
| V27 Cβ | CH | 178 | 116 | 1.5 | |
| D44 Cα | CH | 167 | 89 | 1.9 | |
| D44 Cβ | CH2 | 180 | 105 | 1.7 | |
| PG-1 | R4 Cζ | C | 187 | 49 | 3.8 |
| R4 Cα | CH | 215 | 86 | 2.5 | |
| L5 Cα | CH | 160 | 90 | 1.8 | |
| R4 Cδ | CH2 | 290 | 97 | 3.0 | |
| TAT | R8 Cζ | C | 212 | 51 | 4.3 |
| K4 Cα | CH | 479 | 100 | 4.8 | |
| R8 Cα | CH | 506 | 89 | 5.7 | |
| R8 Cδ | CH2 | 358 | 100 | 3.6 | |
| K4 Cε | CH2 | 354 | 145 | 2.4 |
Sidechain CH2 carbons show similar trends of Δ* among the different samples, with TAT exhibiting the largest Δ*. Fig. 3 plots the homogeneous (open bars) and apparent (filled bars) linewidths of the different carbons, organized according to CH, CH2, CH3 and quaternary carbons. The average homogeneous linewidth is the smallest for quaternary carbons, 45–51 Hz, as expected because of their weak 1H dipolar coupling. Methine and methylene carbons show similar homogeneous linewidths of ~90 Hz. CH2 carbons are expected to have larger Δ due to stronger residual 1H dipolar couplings; however many CH2 groups examined here are from the amino acid sidechains, whose mobility may attenuate the 1H-13C dipolar coupling.
Fig. 3.
Summary of the 13C apparent linewidths (Δ*, filled bars) and homogeneous linewidths (Δ, open bars) of CHn (n = 0–3) in crystalline compounds and membrane peptides.
The crystalline Arg · HCl have smaller Δ*/Δ ratios (1.3–1.7) than arginines in membrane-bound PG-1 and TAT (2.5–5.7). Arg4 in PG-1 also has 1.4 times larger Δ*/Δ than its neighboring residue Leu5. Another Arg in PG-1, Arg11, exhibits an even larger Cα linewidth Δ* of 460 Hz (not shown). Thus, Arg in membrane peptides experiences additional line broadening mechanisms that are absent in the crystalline environment and have larger local conformational disorder than neutral residues in the peptides. 13C-31P distance measurements showed that many Arg-rich membrane peptides form guanidinium-phosphate complexes with lipid headgroups 34,36,50,51,59 to carry out their functions. Thus we attribute the larger linewidths of Arg’s in membrane peptides to this specific peptide-lipid interaction.
Conformational disorder of membrane peptides from 2D correlation spectra
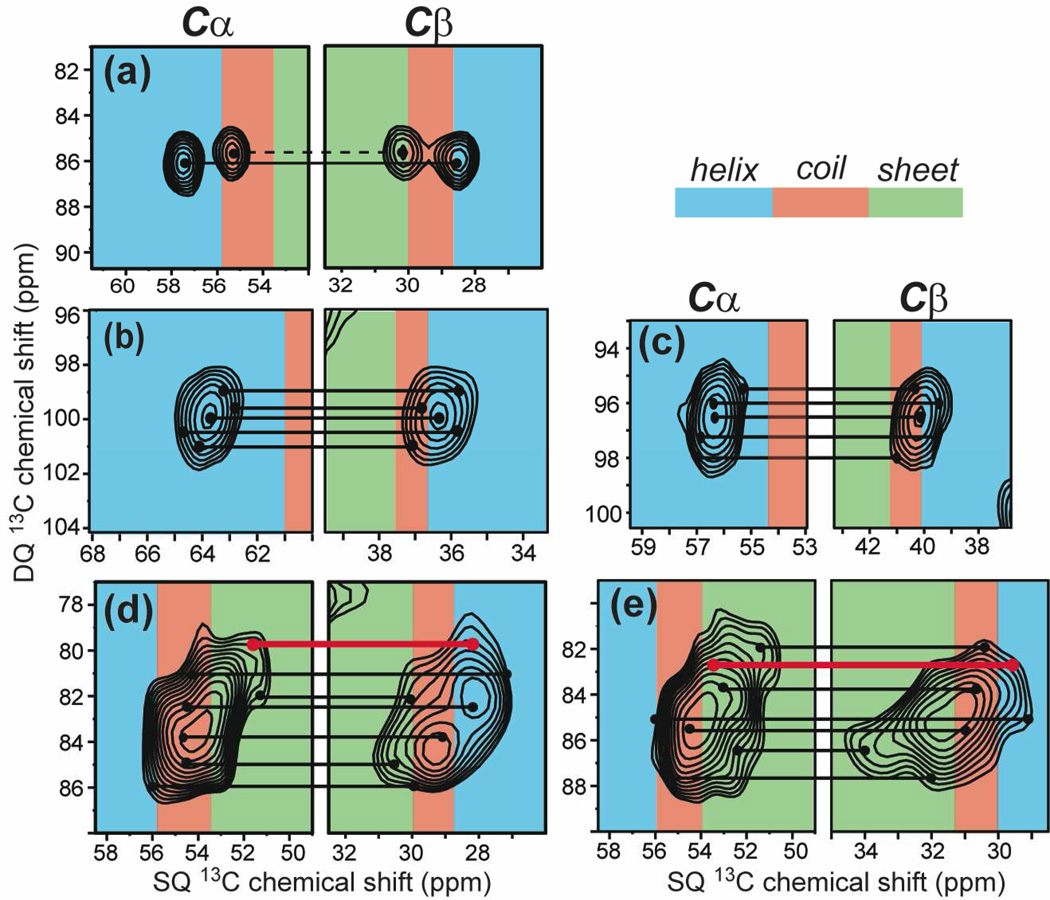
The 1D 13C spectra above show strong variations in the apparent linewidths (Δ*/Δ) of the three membrane peptides. M2TM has the smallest Δ* while the cationic residues in PG-1 and TAT have the largest Δ*. To gain insight into the nature of the conformational disorder represented by these linewidths, we measured the 2D double-quantum (DQ) filtered single-quantum (SQ) correlation spectra of these peptides. Fig. 4 shows the aliphatic regions of representative 2D spectra. Database chemical shift ranges for α-helical, β-sheet and random coil structures are distinguished by color. Peak assignments are based on the connectivity patterns as well as literature reports 60,61. Along the spectral diagonal, the crystalline compounds exhibit round lineshapes while the membrane peptide lineshapes are elongated. The latter is a sign of the inhomogeneous nature of the line broadening, where the homogeneous linewidth is reflected by the narrower linewidth in the direction perpendicular to the spectral diagonal. The specific conformations of the residues can be gleaned from the chemical shifts spanned by the Cα peaks. One of the two Cα peaks of Arg · HCl resonates at a coil/sheet chemical shift while the other Cα signal resonates at the α-helical frequency. In f-MLF-OH, the three Cα chemical shifts correspond to conformations that are consistent with the results of direct (ϕ, ψ) torsion angle measurements 62. For M2TM, the D44 Cα peak spans the α-helical and coil chemical shifts when the peptide for a DMPC-bound sample at 238 K, but becomes only α-helical when the peptide is bound to a virus-mimetic membrane at 273 K (Fig. 4e, f). This conformational difference is mostly due to the membrane composition and not the temperature difference, since viral-membrane bound M2TM shows the same linewidths between 238 K and 293 K (Fig. 1c) 63. Arg8 Cα of TAT exhibits the largest chemical shift distribution among all sites examined, with Δ* more than two times larger than those of M2TM.
Fig. 4.
Conformational dispersion of membrane-bound peptides revealed by 2D DQ filtered correlation spectra. (a) VSGD-M2TM in DMPC membrane at 238 K. (b) Arg8-labeled TAT in POPE/POPG membranes at 238 K. (c) Cα region of Arg · HCl at 296 K. (d) f-MLF-OH at 296 K. (e) VSGD-M2TM in DMPC membranes at 238 K. (f) VSGD-M2TM in viral membranes at 273 K. (g) Arg8-labeled TAT in POPE/POPG lipids at 238 K. Colors indicate the chemical shift ranges of α-helix (blue), random-coil (red) and β-sheet (green) conformations.
To examine how the lineshape of one carbon is correlated with the lineshape of its directly bonded carbon, we measured the 2D INADEQUATE spectra, which correlate DQ chemical shifts with SQ frequencies 37,64 (Fig. 5a). The indirect dimension of the spectrum represents the sum of the chemical shifts of the two coupled 13C spins. Compared to the SQ correlation spectra shown in Fig. 4, the INADEQUATE experiment has the advantage that it not only suppresses natural-abundance 13C signals but also removes the spectral diagonal so that carbons with similar chemical shifts are well resolved. Fig. 5b depicts the hypothetical lineshapes expected for a Cα-C β spin pair. For conformationally disordered proteins, multiple cross peaks are expected for each 13C pair. It is well known that Cα and C β have opposite signs for their secondary-structure dependent chemical shifts 65,66. For example, in α-helices, Cα has larger chemical shifts than random coil values while C β has smaller chemical shifts than random coil values. As a result, if each molecule exhibits a well-defined conformation but the structure varies from one molecule to another, then the pair of Cα-C β cross peaks should be elongated and tilted in opposite directions in the INADEQUATE spectrum (Fig. 5b). On the other hand, if each molecule already dynamically samples a range of conformations at high temperature, and the ensemble is frozen at low temperature, then a Cα chemical shift can correlate with multiple C β chemical shifts and vice versa, leading to round lineshapes for the pair of broadened cross peaks. Finally, a conformationally ordered ensemble of molecules should exhibit narrow linewidths due to only T2 relaxation and residual dipolar and J couplings. If the DQ 13C T2 can be approximated as the sum of the two SQ T2’s 67, then the pair of cross peaks should exhibit elliptical lineshapes with the long axis parallel to the ω1 axis.
Fig. 5.
(a) Pulse sequence of the dipolar INADEQUATE experiment 37. (b) Predicted INADEQUATE Cα-C β cross peak lineshapes if a residue has well defined secondary structures within each molecule, but the secondary structure varies from one molecule to another.
Fig. 6 shows three representative 2D INADEQUATE spectra. The Arg · HCl spectrum resolves two sets of 13C connectivities for the two inequivalent molecules (Fig. 6a) and shows oval lineshapes that are parallel to the ω1 axis for each pair of cross peaks. This lineshape is consistent with the ordered nature of this crystalline compound. The viral-membrane bound M2TM has modestly larger linewidths than arginine hydrochloride but most pairs of cross peaks exhibit oval lineshapes with the long axis parallel to the ω1 axis, with the exception of the sidechain carbons of Ala29 and Leu26, which show tilted lineshapes indicative of sidechain disorder (Fig. 6b). Membrane-bound TAT exhibits by far the broadest peaks with ill-defined shapes (Fig. 6c). Fig. 7 zooms in the Cα-C β region of the 2D spectra to examine the backbone conformational distribution. Characteristic chemical shift ranges for α-helix, β-sheet and random coil 68 are shaded in blue, green, and red, respectively. The two dimensions of the spectra are drawn with the same ppm range per unit length to reflect the relative linewidths of the DQ and SQ dimensions. In Arg · HCl (Fig. 7a), one pair of Cα-C β cross peaks corresponds to the α-helical conformation while the other pair falls in the coil/sheet secondary shift region. This is consistent with the crystal structure, which shows one molecule with (ϕ, ψ) angles of (−50°, −51°), corresponding to a helical backbone, while the other molecule has (ϕ, ψ) angles of (0°, −41°), outside the helix or sheet regions of the Ramachandran diagram. The apparent linewidths in the DQ dimension are 40–50 Hz smaller than the sum of the SQ linewidths for the two crystalline model compounds, due to the fact that DQ coherence commutes with the 13C-13C coupling so that the ω1 linewidth is not broadened by 13C-13C J-coupling.
Fig. 6.
2D 13C INADEQUATE spectra of (a) Arg · HCl at 296 K, (b) viral-membrane-bound LAGI-M2TM at 238 K, and (c) Arg8-labeled TAT in POPE/POPG membranes at 238 K. All spectra were measured under 5333 Hz MAS and 71 kHz 1H TPPM decoupling. Gaussian multiplication was applied to both dimensions with LB/GB values of −3/0.1, −7/0.07 and −15/0.04 for (a), (b) and (c), respectively. The single-quantum Cα linewidths of Arg hydrochloride, M2TM Ile35 and TAT Arg8 are 126 Hz, 138 Hz and 360 Hz, respectively.
Fig. 7.
Amplified Cα-Cβ regions of the INADEQUATE spectra. (a) Arg · HCl at 296 K. (b) Ile35 of viral-membrane bound M2TM at 238 K. (c) Leu26 of viral-membrane bound M2TM at 238 K. (d) Arg8 of TAT in POPE/POPG bilayers at 238 K. (e) Lys4 of TAT in POPE/POPG bilayers at 238 K. Helix, coil and sheet chemical shift regions are shaded in blue, red and green, respectively. Selected correlated Cα-Cβ positions are connected by lines to guide the eye. Sheet-helix chemical shift correlations are colored by red lines.
In viral-membrane bound M2TM, the Ile35 and Leu26 lineshapes show moderate conformational disorder (Fig. 7b, c): both Cα peaks fall well within the α-helical range while the C β chemical shifts are found in both the helix and coil regions. In contrast, the TAT Lys4 and Arg8 peaks span all three secondary structures (Fig. 7d, e). In addition to the expected coil – coil and sheet – sheet Cα-C β correlations (black lines), we observed β-sheet Cα shifts correlated with α-helical C β shifts (red lines). This random correlation creates tilted lineshapes that differ from the case depicted in Fig. 5b. Thus, these residues adopt (ϕ, ψ) torsion angles far from the canonical secondary structures in some of the molecules. This indicates large conformational distributions at the residue level, which suggests dynamically interconverting conformers at physiological temperature.
Sidechain conformational disorder
An inhomogeneously broadened peak can result from a continuous distribution of many frequency components or the overlap of a few discrete peaks. Bajaj et al. studied the temperature-dependent linewidths of the solvent-free microcrystalline f-MLF-OH and found discrete peaks per carbon between 90 K and 200 K 69. They attributed the temperature-induced spectral changes to slowing down of the phenylene ring flips at low temperature, which affected the Met and Leu sidechain conformations.
We also observed temperature-dependent discrete sidechain disorder in PG-1. At 238 K, Arg4 of PG-1 in POPE/POPG membranes shows two Cβ peaks (29.5 ppm and 33.1 ppm) correlated with the same Cα chemical shift (Fig. 8a). When the temperature is increased to 283 K, a single C β peak was observed at the averaged position (31.7 ppm) of the two low-temperature chemical shifts (Fig. 8b, c). Thus, Arg4 undergoes equal-population two-site exchange that is slow at 238 K but fast at 283 K. Since PG-1 backbone is disulfide bonded to be a robust β-hairpin, and Arg4 Cα does not display this peak doubling, the nature of the conformational exchange is most likely sidechain rotameric averaging, as reflected by the low order parameters measured for this sidechain 55. The existence of the sidechain conformational distribution is also consistent with the fact that the 13Cζ-31P distance between Arg4 guanidinium and lipid phosphates shows a distribution in the previously reported REDOR data 51. Assuming a Gaussian distribution, we had obtained a best-fit distance of 5.7 Å with a distribution of 1.5 Å. The present observation of two discrete C β peaks suggests that the conformational distribution may be more accurately described as bimodal rather than a single Gaussian distribution. Indeed, the REDOR dephasing data can be equally well fit by a 1:1 combination of a short distance of 4.8 Å and a long distance of 8.5 Å (Fig. S1). The revised fitting, with one of the two distances being much shorter than the average distance of 5.7 Å from the single-Gaussian fit, strengthens the model that guanidinium-phosphate interaction is strong for membrane-bound PG-1 50,51,70. Arg4 lies in the β-strand part of the peptide, far from the tip of the hairpin near the membrane surface. Thus, the fact that Arg4 guanidinium can approach lipid 31P to 4.8 Å means that the guanidinium ions are very effective in dragging lipid headgroups into the traditionally hydrophobic region of the bilayer, causing toroidal pore defects.
Fig. 8.
2D 13C INADEQUATE spectra of POPE/POPG-bound Arg4-labeled PG-1 at (a) 238 K and (b) 283 K. (c) Enlarged region of the Cβ peak at the two temperatures.
Discussion
We measured the linewidths and conformational distribution of the three membrane peptides at 238 K, a temperature at which all peptides are immobilized in the gel phase of their respective membranes but do not undergo any glass transition, which is a phenomenon largely observed in globular proteins at lower temperatures of 180–220 K 32,71,72. The similar linewidths of M2TM and PG-1 between 238 K and 293 K (Fig. 1c, d) also confirm that the conformational distribution of the peptides at 238 K is similar to the distribution at more moderate temperatures of the gel phase.
In the gel phase, all examined 13C sites of the membrane peptides exhibit larger apparent linewidths and Δ*/Δ ratios than the crystalline compounds, indicating larger static disorder. On the other hand, the homogeneous linewidths Δ are similar between the membrane peptides and the crystalline compounds, verifying the hypothesis that Δ is mainly dictated by intrinsic T2 relaxation and residual dipolar and J broadening.
Both 2D INADEQUATE lineshapes and Δ*/Δ ratios indicate that the three membrane peptides have different degrees of conformational disorder. M2TM and neutral residues in PG-1 have relatively small disorder, while cationic residues in PG-1 and TAT have much larger inhomogeneous broadening. These differences can be understood from the oligomeric structure and lipid interactions of these peptides. M2TM forms a water-filled tetrameric helical bundle 1,73–75 that is immobilized in the virus-mimetic membrane 63. The interhelical interactions stabilize the peptide backbone, and the high viscosity of the cholesterol-rich viral membrane reduces the conformational plasticity of the helices 63. The conformational landscape of M2TM has been investigated extensively. It is known that the peptide adopts several discrete “basis” conformations whose equilibria depend on the membrane composition, pH and drug binding 76,77. In the virus-mimetic membrane, a single dominant conformation was previously observed with small (ϕ, ψ) angle distributions 76, consistent with the relatively narrow linewidths seen in the 2D spectra here.
PG-1 is constrained by two disulfide bonds to adopt a well-defined β-hairpin structure 78. In addition, it oligomerizes into a transmembrane β-barrel in the POPE/POPG membrane 42, thus the peptide-peptide interaction should also reduce conformational distribution. Countering this influence is the abundant peptide-lipid interactions, peptide-water interactions and sidechain conformational averaging. Among these mechanisms, guanidinium-phosphate salt bridge interaction appears to be the main contributor to line broadening, as reflected by the narrower linewidths of neutral residues in PG-1 (Table 1).
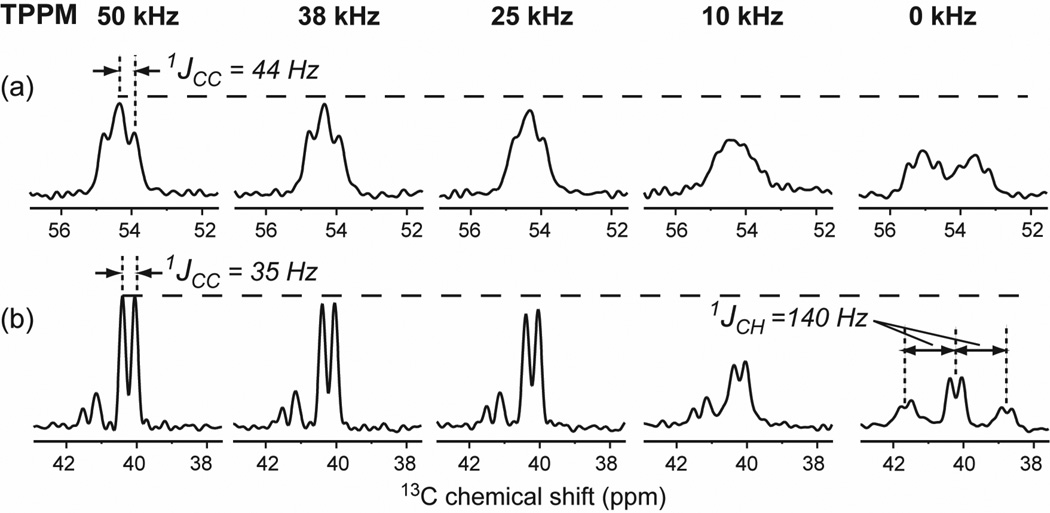
The Arg residues of TAT also exhibit large inhomogeneous line broadening, but charge-charge interaction is not the dominant cause. Instead, the TAT amino acid sequence encodes for an intrinsic lack of a single conformation. The Tat protein from which the CPP domain is derived is an RNA-binding protein central for HIV replication, and interacts with a variety of intracellular and extracellular molecules. Solution NMR studies showed that the Tat protein does not have a fixed conformation in solution 79, similar to a number of other intrinsically disordered proteins, which adopt specific structures only upon binding to substrates 80. The current data show that the highly basic cell-penetrating domain of the Tat protein remains unstructured upon binding to the lipid membrane. The broad low-temperature Cα/C β peaks correlate with extremely narrow high-temperature peaks, both of which are centered at random coil chemical shifts 36 in several membrane compositions (Table S2). Thus, TAT undergoes nearly isotropic motion and samples a large conformational space at high temperature, and the ensemble of conformations is captured by slow freezing. The nearly isotropic motion of TAT structure gives rise to resolved 13C-13C J-splittings in the high-temperature spectra (Fig. 9), even when the 1H decoupling strength is weak (50 and 25 kHz). In DMPC/DMPG bilayers the C-H order parameters for Cα and C β sites are only 0.15–0.18 36,81, further confirming the dynamic nature of this peptide in the lipid bilayer.
Fig. 9.
Cα (a) and Cε (b) peaks of Lys4-labeled TAT in POPE/POPG bilayers at 303 K. The spectra were measured by direct 13C polarization under 5 kHz MAS with varying 1H decoupling field strengths. (a) Cα peaks. (b) Cε peaks.
We posit that the unstructured nature of the TAT peptide in the membrane is functionally important. By having no fixed conformation due to the lack of intramolecular H-bonds, TAT can better form transient intermolecular H-bonds with lipid phosphates and water to facilitate its translocation across the lipid bilayer 36. The lack of a stable amphipathic conformation also prevents TAT from forming long-lasting hydrophobic interactions with the lipid bilayer, which would prohibit its membrane translocation activity.
The homogeneous linewidths reported here are specific for the 1H decoupling field strength of ~70 kHz. Stronger 1H decoupling lengthens the T2 and decreases the homogeneous linewidths 82,83. As noted above, 13C-13C J-coupling and residual dipolar coupling also contribute a fixed amount to the homogeneous linewidths of these uniformly 13C-labeled residues. For comparison, the 15N spins, without any J-coupling, show limiting homogeneous linewidths of ~10 Hz (0.25 ppm) for crystalline compounds (Table S3). For singly 13C-labeled crystalline compounds, the 13C homogeneous linewidths are 20–30 Hz for backbone Cα (Table S4), much smaller than the linewidths of uniformly 13C-labeled samples.
4. Conclusions
We have investigated the conformational disorder of several membrane peptides by comparing their homogeneous and apparent linewidths and by examining their 2D spectral lineshapes. At low temperature the membrane peptides exhibit similar homogeneous linewidths as crystalline compounds, consistent with the origins of homogeneous linewidths in residual coherent couplings and T2 relaxation. But membrane peptides exhibit larger apparent linewidths than crystalline compounds, and the extent of the line broadening is a function of the peptide conformation. The largest apparent linewidths are observed for non-oligomeric and cationic TAT, due to its intrinsic near random conformation and extensive peptide-lipid interaction. The main source of disordering peptide-lipid interactions in cationic membrane peptides is guanidinium-phosphate interaction, which causes multiple sidechain conformations and distance distribution in addition to chemical shift distribution.
The current study indicates that strong peptide-peptide interactions through oligomerization (as for M2TM and PG-1) promote relatively homogeneous conformations, while extensive peptide-lipid interactions for monomeric peptides such as TAT cause larger linewidths and conformational disorder. This insight may be useful for freeze trapping experiments for studying protein folding intermediates 84 and protein photoreactions 69,85. Low-temperature experiments have become increasingly important in biological SSNMR 39,84,86–88 due to the maturation of the dynamic nuclear polarization technique 69,89. Our data indicate that the linewidths of membrane proteins without strong charge interactions with lipids are not excessively broadened at temperatures down to about 230 K. Investigation of membrane protein linewidths at even lower temperatures (to about 100 K) will further elucidate the applicability of DNP to membrane proteins.
Supplementary Material
Acknowledgement
This work is funded by NIH grant GM66976 to M.H.
References
- 1.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, Degrado WF, Hong M. Nature. 2010;463:689. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M. Nature. 2006;440:959. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]
- 3.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. Proc. Natl. Acad. Sci. USA. 2002;99:16742. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Science. 2008;319:1523. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 5.Helmus JJ, Surewicz K, Nadaud PS, Surewicz WK, Jaroniec CP. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6284. doi: 10.1073/pnas.0711716105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cegelski L, O'Connor RD, Stueber D, Singh M, Poliks B, Schaefer J. J. Am. Chem. Soc. 2010;132:16052. doi: 10.1021/ja104827k. [DOI] [PubMed] [Google Scholar]
- 7.Dick-Pérez M, Zhang Y, Hayes J, Salazar A, Zabotina OA, Hong M. Biochemistry. 2011;50:989. doi: 10.1021/bi101795q. [DOI] [PubMed] [Google Scholar]
- 8.Maricq MM, Waugh JS. J. Chem. Phys. 1979;70:3300. [Google Scholar]
- 9.Bennett AE, Rienstra CMAM, Lakshmi KV, Griffin RG. J. Chem. Phys. 1995;103:6951. [Google Scholar]
- 10.Fung BM, Khitrin AK, Ermolaev K. J. Magn. Reson. 2000;142:97. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 11.Detken A, Hardy EH, Ernst M, Meier BH. Chem. Phys. Lett. 2002;356:298. [Google Scholar]
- 12.Sakellariou D, Lesage A, Hodgkinson P, Emsley L. Chem. Phys. Lett. 2000;319:253. [Google Scholar]
- 13.De Paëpe G, Giraud N, Lesage A, Hodgkinson P, Böckmann A, Emsley L. J. Am. Chem. Soc. 2003;125:13938. doi: 10.1021/ja037213j. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkinson P. Prog. Nucl. Magn. Reson. Spectrosc. 2005;46:197. doi: 10.1016/j.pnmrs.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Sperling LJ, Nieuwkoop AJ, Lipton AS, Berthold DA, Rienstra CM. J. Biomol. NMR. 2010;46:149. doi: 10.1007/s10858-009-9389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zorin VE, Brown SP, Hodgkinson P. J. Chem. Phys. 2006;125:144508. doi: 10.1063/1.2357602. [DOI] [PubMed] [Google Scholar]
- 17.Akbey U, Lange S, Trent Franks W, Linser R, Rehbein K, Diehl A, van Rossum BJ, Reif B, Oschkinat H. J. Biomol. NMR. 2010;46:67. doi: 10.1007/s10858-009-9369-0. [DOI] [PubMed] [Google Scholar]
- 18.Hologne M, Chevelkov V, Reif B. Prog. Nucl. Magn. Reson. Spectrosc. 2006;48 [Google Scholar]
- 19.Morcombe CR, Gaponenko V, Byrd RA, Zilm KW. J. Am. Chem. Soc. 2005;127:397. doi: 10.1021/ja045581x. [DOI] [PubMed] [Google Scholar]
- 20.Mehring M. Principles of High Resolution NMR in Solids. New York: Springer-Verlag; 1983. [Google Scholar]
- 21.Rothwell WP, Waugh JS. J. Chem. Phys. 1981;74:2721. [Google Scholar]
- 22.Long JR, Sun BQ, Bowen A, Griffin RG. J. Am. Chem. Soc. 1994;116:11950. [Google Scholar]
- 23.deAzevedo ER, Saalwachter K, Pascui O, de Souza AA, Bonagamba TJ, Reichert D. J. Chem. Phys. 2008;128:104505. doi: 10.1063/1.2831798. [DOI] [PubMed] [Google Scholar]
- 24.Cady SD, Goodman C, Tatko C, DeGrado WF, Hong M. J. Am. Chem. Soc. 2007;129:5719. doi: 10.1021/ja070305e. [DOI] [PubMed] [Google Scholar]
- 25.Cady SD, Hong M. J. Biomol. NMR. 2009;45:185. doi: 10.1007/s10858-009-9352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Y, Waring AJ, Ruchala P, Hong M. Biochemistry. 2011;50:2072. doi: 10.1021/bi101975v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakellariou D, Brown SP, Lesage A, Hediger S, Bardet M, Meriles CA, Pines A, Emsley L. J. Am. Chem. Soc. 2003;125:4376. doi: 10.1021/ja0292389. [DOI] [PubMed] [Google Scholar]
- 28.Cadars S, Lesage A, Emsley L. J. Am. Chem. Soc. 2005;127:4466. doi: 10.1021/ja043698f. [DOI] [PubMed] [Google Scholar]
- 29.Duma L, Hediger S, Brutscher B, Böckmann A, Emsley L. J. Am. Chem. Soc. 2003;125:11816. doi: 10.1021/ja036893n. [DOI] [PubMed] [Google Scholar]
- 30.Lesage A, Bardet M, Emsley L. J. Am. Chem. Soc. 1999;121:10987. [Google Scholar]
- 31.Yao XL, Hong M. J. Am. Chem. Soc. 2004;126:4199. doi: 10.1021/ja036686n. [DOI] [PubMed] [Google Scholar]
- 32.Bajaj VS, van der Wel PC, Griffin RG. J. Am. Chem. Soc. 2009;131:118. doi: 10.1021/ja8045926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong M, Griffin RG. J. Am. Chem. Soc. 1998;120:7113. [Google Scholar]
- 34.Hong M, Su Y. Protein Sci. 2011;20:641. doi: 10.1002/pro.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross TA, Sharma M, Yi M, Zhou HX. Trends Biochem. Sci. 2010;36:117. doi: 10.1016/j.tibs.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Y, Waring AJ, Ruchala P, Hong M. Biochemistry. 2010;49:6009. doi: 10.1021/bi100642n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong M. J. Magn. Reson. 1999;136:86. doi: 10.1006/jmre.1998.1631. [DOI] [PubMed] [Google Scholar]
- 38.Maly T, GT D, Bajaj VS, Hu KN, Joo CG, Mak-Jurkauskas ML, Sirigiri JR, van der Wel PCA, Herzfeld J, Temkin RJ, Griffin RG. J. Chem. Phys. 2008;128 doi: 10.1063/1.2833582. 052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu KN, Tycko R. Biophys. Chem. 2010;151:10. doi: 10.1016/j.bpc.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpino LA, Han GY. Org. Chem. 1972;37:3404. [Google Scholar]
- 41.Cady SD, Hong M. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1483. doi: 10.1073/pnas.0711500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mani R, Cady SD, Tang M, Waring AJ, Lehrer RI, Hong M. Proc. Natl. Acad. Sci. U S A. 2006;103:16242. doi: 10.1073/pnas.0605079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn EL. Phys. Rev. 1950;80:580. [Google Scholar]
- 44.Hohwy M, Rienstra CM, Jaroniec CP, Griffin RG. J. Chem. Phys. 1999;110:7983. [Google Scholar]
- 45.Pinto LH, Holsinger LJ, Lamb RA. Cell. 1992;69:517. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 46.Pinto LH, Lamb RA. J. Biol. Chem. 2006;281:8997. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 47.Cady SD, Luo W, Hu F, Hong M. Biochemistry. 2009;48:7356. doi: 10.1021/bi9008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kokryakov VN, Harwig SS, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV, Korneva HA, Lehrer RI. FEBS Lett. 1993;327:231. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 49.Bellm L, Lehrer RI, Ganz T. Exp. Opin. Invest. Drugs. 2000;9:1731. doi: 10.1517/13543784.9.8.1731. [DOI] [PubMed] [Google Scholar]
- 50.Tang M, Hong M. Mol. Biosyst. 2009;5:317. doi: 10.1039/b820398a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang M, Waring AJ, Hong M. J. Am. Chem. Soc. 2007;129:11438. doi: 10.1021/ja072511s. [DOI] [PubMed] [Google Scholar]
- 52.Frankel AD, Pabo CO. Cell. 1988;55:1189. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 53.Vives E, Brodin P, Lebleu B. J. Biol. Chem. 1997;272:16010. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Qiu JX, Soto CS, DeGrado WF. Curr. Opin. Struct. Biol. 2011;21:68. doi: 10.1016/j.sbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang M, Waring AJ, Hong M. Chembiochem. 2008;9:1487. doi: 10.1002/cbic.200800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khawas B. Acta. Cryst. 1971;B27:1517. [Google Scholar]
- 57.Mazumdak SK, Venkatesan K. Zeitschrift für Kristallographie. 1969;130:328. [Google Scholar]
- 58.Cowans BA, Grutzner JB. J. Magn. Reson. 1993;105:10. [Google Scholar]
- 59.Su Y, Doherty T, Waring AJ, Ruchala P, Hong M. Biochemistry. 2009;48:4587. doi: 10.1021/bi900080d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petkova AT, Hu JG, Bizounok M, Simpson M, Griffin RGHJ. Biochemistry. 1999;38:1562. doi: 10.1021/bi981968z. [DOI] [PubMed] [Google Scholar]
- 61.Ladizhansky V, Jaroniec CP, Diehl A, Oschkinat H, Griffin RG. J. Am. Chem. Soc. 2003;125:6827. doi: 10.1021/ja029082c. [DOI] [PubMed] [Google Scholar]
- 62.Rienstra CM, Tucker-Kellogg L, Jaroniec CP, Hohwy M, Reif B, McMahon MT, Tidor B, Lozano-Pérez T, Griffin RG. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10260. doi: 10.1073/pnas.152346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo W, Cady SD, Hong M. Biochemistry. 2009;48:6361. doi: 10.1021/bi900716s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bax A, Freeman R, Kempsell SP. J. Am. Chem. Soc. 1980;102:4849. [Google Scholar]
- 65.Spera S, Bax A. J. Am. Chem. Soc. 1991;113:5490. [Google Scholar]
- 66.Wishart DS, Sykes BD, Richards FM. J. Mol. Biol. 1991;222:311. doi: 10.1016/0022-2836(91)90214-q. [DOI] [PubMed] [Google Scholar]
- 67.Levitt MH, Raleigh DP, Creuzet F, Griffin RG. J. Chem. Phys. 1990;92:6347. [Google Scholar]
- 68.Wang Y, Jardetzky O. Protein Sci. 2002;11:852. doi: 10.1110/ps.3180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9244. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang M, Waring AJ, Lehrer RI, Hong M. Angew. Chem. Int. Ed. Engl. 2008;47:3202. doi: 10.1002/anie.200705993. [DOI] [PubMed] [Google Scholar]
- 71.Rasmussen BF, Stock AM, Ringe D, Petsko GA. Nature. 1992;357:423. doi: 10.1038/357423a0. [DOI] [PubMed] [Google Scholar]
- 72.Doster W, Cusack S, Petry W. Nature. 1989;337:754. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- 73.Acharya A, Carnevale V, Fiorin G, Levine BG, Polishchuk A, Balannick V, Samish I, Lamb RA, Pinto LH, DeGrado WF, Klein ML. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15075. doi: 10.1073/pnas.1007071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Nature. 2008;451:596. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo W, Hong M. J. Am. Chem. Soc. 2010;132:2378. doi: 10.1021/ja9096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu F, Luo W, Cady SD, Hong M. Biochim. Biophys. Acta. 2011;1808:415. doi: 10.1016/j.bbamem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C, Qin H, Gao FP, Cross TA. Biochim. Biophys. Acta. 2007;1768:3162. doi: 10.1016/j.bbamem.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J. Chem. & Biol. 1996;3:543. doi: 10.1016/s1074-5521(96)90145-3. [DOI] [PubMed] [Google Scholar]
- 79.Shojania S, O'Neil JD. J. Biol. Chem. 2006;281:8347. doi: 10.1074/jbc.M510748200. [DOI] [PubMed] [Google Scholar]
- 80.Wright PE, Dyson HJ. J. Mol. Biol. 1999;293:321. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 81.Hong M, Gross JD, Rienstra CM, Griffin RG, Kumashiro KK, Schmidt-Rohr K. J. Magn. Reson. 1997;129:85. doi: 10.1006/jmre.1997.1242. [DOI] [PubMed] [Google Scholar]
- 82.De Paëpe G, Lesage A, Emsley L. J. Chem. Phys. 2003;119:4833. [Google Scholar]
- 83.Tang M, Comellas G, Mueller LJ, Rienstra CM. J. Biomol. NMR. 2010;48:103. doi: 10.1007/s10858-010-9442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu KN, Yau WM, Tycko R. J. Am. Chem. Soc. 2010;132:24. doi: 10.1021/ja908471n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mak-Jurkauskas ML, Bajaj VS, Hornstein MK, Belenky M, Griffin RG, Herzfeld J. Proc. Natl. Acad. Sci. U. S. A. 2008;105:883. doi: 10.1073/pnas.0706156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Havlin RH, Tycko R. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3284. doi: 10.1073/pnas.0406130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu KN, Havlin RH, Yau WM, Tycko R. J. Mol. Biol. 2009;292:1055. doi: 10.1016/j.jmb.2009.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siemer AB, Huang KY, McDermott AE. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17580. doi: 10.1073/pnas.1009369107. (2010) 107(41), - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Griffin RG. Nature. 2010;468:381. doi: 10.1038/468381a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.