Abstract
Objectives. We evaluated a theory-based lifestyle intervention targeting physical activity and dietary fat intake among African American women at high risk for cardiovascular disease.
Methods. The Heart Healthy and Ethnically Relevant Lifestyle trial (2005–2008) randomly assigned 266 low-income African American women aged 35 years and older who were patients of South Carolina community health care centers into comprehensive or standard care interventions. Comprehensive participants received standard care (stage-matched provider counseling and assisted goal setting) plus 12 months of telephone counseling and tailored newsletters. Primary outcomes were 6- and 12-month self-reported physical activity and dietary fat intake.
Results. Comprehensive participants were more likely than were standard care participants to decrease total physical activity (odds ratio [OR] = 3.13; 95% confidence interval [CI] = 1.18, 8.25) and increase leisure-time physical activity (OR = 3.82; 95% CI = 1.41, 10.3) at 6 months (no 12-month differences). Mean reductions in Dietary Risk Assessment score occurred in both groups but were greater among comprehensive participants than among standard care participants (6 months, −8.50 vs −5.34; 12 months, −7.16 vs −3.37; P < .001).
Conclusions. The comprehensive intervention improved women's leisure-time physical activity and dietary fat intake, highlighting a replicable model to help primary care providers implement lifestyle counseling.
African American women are at increased risk for morbidity and mortality from cardiovascular disease (CVD) compared with White women1 because of their higher prevalence of CVD risk factors and lower socioeconomic status.1–3 Interventions embedded in primary care settings, such as locally based, patient-driven community health care centers, have the unique potential to address these health disparities because they provide a large proportion of comprehensive health care services to medically underserved, vulnerable populations, regardless of ability to pay. About 66% of these centers’ patients are members of minority groups, 90% have incomes below 200% of the federal poverty line, and 39% lack health insurance.4,5 The delivery of health behavior change interventions through these centers holds additional promise because providers are trusted sources of health information6 and can reach underserved populations that are more likely than the general population to suffer from CVD risk factors. Despite this great potential, interventions have not been widely tested in this setting.
Some evidence exists that lifestyle counseling based on the transtheoretical model7 and social cognitive theory8 delivered through primary care settings can yield small but significant improvements in CVD risk factors.9 Such counseling is recommended by various health organizations, especially for overweight or obese individuals and those with chronic diseases.10–15 Because of the many barriers (e.g., inadequate time, reimbursement, training, skills, and organizational support)16 faced by primary care providers, however, lifestyle counseling is often suboptimal or abandoned.17–20 In addition, few studies conducted in primary care settings have targeted underserved populations,16,21,22 been integrated into routine office visits,16,23 or used multidisciplinary models in which primary care providers delivered brief lifestyle counseling and made time-saving referrals to other professionals or community resources.21 Telephone counseling has proven effective in changing physical activity and dietary behaviors in many populations and has been recommended for dissemination testing,24 especially in clinical settings.25 This approach is flexible for providers and underserved populations because it does not require transportation and can occur at convenient times for each party.
In response to these literature gaps and to provide a novel, replicable method to help primary care providers implement lifestyle counseling for minority women at high risk of CVD, our Heart Healthy and Ethnically Relevant (HHER) Lifestyle trial compared the effectiveness of a standard care intervention (brief primary care provider counseling, nurse-assisted goal setting, community resource guide, and educational materials) with that of a comprehensive intervention (standard care intervention plus 12 months of tailored telephone counseling and tailored print materials) at increasing moderate-to-vigorous physical activity and reducing dietary fat intake (primary outcomes) among financially disadvantaged African American women patients at 2 community health centers in South Carolina. Because behavior change is a difficult process that requires new behavioral skills that must be practiced over time, we hypothesized that the comprehensive intervention would lead to significantly greater improvements in these modifiable CVD risk factors than the standard care intervention.
METHODS
The HHER Lifestyle randomized controlled trial's design and methods, which have been described in detail elsewhere,26 are briefly summarized here.
Setting
HHER Lifestyle trial participants were recruited from 9 community clinics within 2 federally funded community health care centers in South Carolina between 2005 and 2008. We selected these centers because their patient profiles matched the priority population targeted by the HHER Lifestyle trial.27–29 Center-provided reports showed that patients were predominantly members of ethnic minorities (70% African American), on Medicaid or Medicare (70%), and self-paid or uninsured (25%). Patients’ primary diagnoses were hypertension and diabetes.
Participant eligibility and recruitment.
Patients were eligible for the trial if they
were self-identified African American women aged 35 years or older;
had no physical disability or orthopedic problem that would prevent them from meeting physical activity goals;
had baseline blood pressure below 160/95;
did not have insulin-controlled diabetes;
were not pregnant or planning to become pregnant during the study;
had access to a telephone; and
were able and willing to complete survey instruments and assessment procedures.
Each week, the community health care centers’ clinics used a computerized patient scheduling system to identify African American women aged 35 years and older who had nonurgent medical appointments scheduled with a primary care provider trained in the HHER standard care protocol. The HHER team mailed women with appointments in the coming 4 to 6 weeks a personalized recruitment letter, study brochure, and postage-paid refusal postcard. If a refusal postcard was not received within 2 weeks, the HHER team telephoned participants for an eligibility screening after which they scheduled eligible and interested women for a home baseline assessment visit at least 1 week before their medical appointment. To enroll, a participant had to complete the baseline visit and attend her medical appointment. All participants provided informed consent before enrolling.
After the baseline visit and medical appointment, the HHER team randomized participants to the trial's standard care or comprehensive interventions. We used a stratified randomization procedure with blocking by primary care provider to balance randomization across providers for every 4 patients. Primary care providers, nurses, and research assistants responsible for data collection were blind to treatment assignment. We notified study participants of their treatment assignment by a mailed letter followed by a telephone call.
The intervention.
The basic tenets of the HHER Lifestyle trial have been explained elsewhere.26,30 Briefly, this randomized controlled trial assessed the effectiveness of a culturally appropriate, theory-based intervention delivered in primary health care settings to reduce dietary fat and increase moderate-to-vigorous physical activity among financially disadvantaged African American women. We modeled both the trial's standard care and comprehensive interventions in part after the Physician-Based Assessment and Counseling for Exercise project31 and the Activity Counseling Trial,32 2 primary care–based interventions that successfully increased women's physical activity through lifestyle counseling. Like these trials, our intervention strategies were based on integrating the transtheoretical model7 and social cognitive theory.8 We added dietary change content, and, as described elsewhere in more detail,26 we adapted the intervention for financially disadvantaged African American women in South Carolina by
using telephone calls and print materials to address topics of concern to the population (e.g., finding safe walking areas, identifying affordable healthy food options, and addressing cultural beliefs regarding food, activity, and body size),
creating or modifying print materials for less than an eighth-grade reading level,
culturally tailoring materials at the surface and deep levels33 (e.g., using photos, common foods, and testimonials of African Americans to emphasize cultural values and norms),
recruiting and delivering the standard care intervention via a community health center, and
conducting home visits for measurement, pairing the intervention with an existing clinic visit, and delivering the intervention via telephone to reduce participant burden and travel.
Intervention groups.
Research staff notified the clinic of the patient's participation and stage of readiness for change regarding both physical activity and diet (on the basis of the baseline assessment). All participants received the standard care intervention during their appointment: motivational, stage-based behavioral counseling from their primary care provider; nurse-assisted goal setting; a community resource guide featuring free or low-cost programs and facilities; and ethnically tailored educational materials. Comprehensive intervention participants received standard care plus the following: 12 motivational, stage-matched, ethnically tailored newsletters over 1 year; an in-depth, introductory telephone call; and up to 14 brief, motivationally tailored telephone counseling calls from research staff over 1 year. We modeled the telephone counseling after Stanford University's Active Choices program, a behavior-change program that successfully increased moderate-to-vigorous physical activity in randomized trials34–37 in diverse settings and populations.38 Telephone calls were brief and low-cost to enhance generalizability to routine primary care clinical practices. We chose telephone counseling over in-person meetings because it is more flexible, avoids transportation problems common in this population, and has proven effective in many populations.24
Provider and nurse training.
At study onset, the HHER team invited all clinic primary care providers and nurses to a kickoff event to recruit them to participate. The team contacted new employees who later joined the clinics and invited them to join. Of 30 providers invited, 17 (57%) completed the required training. Of 28 nurses invited, 16 (57%) completed the training. A detailed description of provider and nurse recruitment, training, and study participation is available elsewhere.30
To ease training completion, providers and nurses received a CD-ROM with training materials, a supplemental training manual, and a pocket-sized counseling reference tool. The CD-ROM featured videos demonstrating motivational, stage-matched, patient-centered provider counseling and nurse-assisted goal setting for patients in different stages of change. Providers were trained to give 2- to 4-minute, motivational, stage-matched counseling for physical activity and dietary fat intake during a patient's scheduled medical appointment. Nurses were trained to engage participants in stage-matched goal setting sessions lasting 5 to 10 minutes and to provide a community resource guide and ethnically tailored educational materials on moderate-to-vigorous physical activity and healthy diet. Those who completed training, posttests, and training evaluations received continuing medical education credits (providers) or continuing education units (nurses). Only providers and nurses who completed training participated in the study.
Measures
To minimize participant burden and remove transportation barriers, we conducted baseline, 6-month, and 12-month assessments in participants’ homes. Participants received a $40 incentive after each assessment. Detailed study measures26 are briefly described here.
Primary outcomes.
The trial's primary outcomes were self-reported minutes per week of moderate-to-vigorous physical activity and self-reported dietary fat intake. We measured physical activity with the 41-item Community Health Activities Model Program for Seniors (CHAMPS) physical activity questionnaire.39 The interviewer-administered CHAMPS covers activities undertaken for exercise, physical-in-nature activities undertaken in the course of one's day, and physically active recreational activities during “a typical week in the past 4 weeks.” Activity frequency is assessed in times per week. Duration is classified by use of 6 categories, ranging from “less than 1 hour per week” to “9 or more hours per week.”
For the analyses, we calculated the number of hours per week spent in all types of physical activity covered in the CHAMPS questionnaire. Because the trial emphasized purposeful activity or exercise, we also computed hours per week spent in moderate-to-vigorous physical activity during leisure time (excluding activities related to gardening and housework). CHAMPS has strong psychometric properties, including demonstrated validity,40 test-retest reliability,40 and sensitivity to change.36,39,41–43 Resnicow et al.44 validated a modified CHAMPS version in a population of adult African Americans.
We assessed diet with the 52-item New Leaf Dietary Risk Assessment (DRA).45 The DRA incorporates a food frequency approach and provides an assessment of dietary fat and cholesterol intake that is correlated (r = 0.60) with the Keys score, which measures the potential of the diet to raise serum cholesterol levels.46 The questionnaire was designed specifically for a low-income, rural, southeast US population. Each item is scored from 0 to 2, with a lower score indicating a more healthful dietary pattern (lower saturated fat and cholesterol). Scores from all questions are summed for a total DRA score, ranging from 0 to 104. Higher scores indicate a diet higher in saturated fat and cholesterol. Secondary analyses focused on 4 subscales produced by the DRA: (1) meats, (2) side dishes and snacks, (3) dairy products and eggs, and (4) spreads, dressings, and oils. The original DRA score for spreads, dressings, and oils is the sum of 12 items (range = 0–24). Through instrumentation error, 2 items from the score for spreads, dressings, and oils were dropped and replaced with the participant mean of the remaining items to keep within the original scale range.
Other measures.
The HHER team collected self-reported demographic variables (e.g., age, income, education, marital status, and employment) during telephone eligibility screening. In addition to self-reported attitudinal and behavioral measures, in-home assessments collected physiological data (e.g., height, weight, waist circumference, blood pressure, and capillary blood draw). Weight was measured (to the nearest 0.1 kg) with a Seca scale and height (to the nearest 0.1 cm) with a Seca stadiometer (Seca, Hanover, MD). We computed body mass index (BMI; defined as weight in kg divided by height in m2).47
Statistical Analysis
We used the χ2 test to examine differential attrition by treatment group and demographic characteristics for each time period. We used significant variables as covariates in regression analysis to minimize bias between treatment groups. Originally, we treated CHAMPS outcomes as continuous variables that were transformed to square-root values because of skewness in the distributions. After further evaluation of the data, it was determined that traditional longitudinal analysis (such as repeated measures) would not be possible because of the large proportion of participants with zero change or a decline in physical activity over time. To handle this data limitation and present findings in a meaningful way, we created a 3-level variable in which participants who improved were assigned a 1 (> 1 hour increase), participants who stayed the same a 2 (a difference of −1 to +1 hours), and those who declined in activity a 3 (> 1 hour decrease). Multinomial logistic regression analysis examined whether the odds of improvement or decline differed by treatment group from baseline to 6 and 12 months, relative to staying the same. We adjusted all models for covariates (age, income, employment, education, and baseline BMI).
For both intervention groups, we calculated and compared averages of each DRA outcome at each time period. We also calculated average DRA decreases—decreases signify improvements in dietary intake—from baseline to 6 months and baseline to 12 months. We calculated maximum likelihood estimates to determine the average change in DRA scores over the trial. We treated DRA scores as nested within each subject. We adjusted all estimates for covariates (age, income, employment, education, and baseline BMI). In addition, a group × time interaction evaluated differences in change between the standard care and comprehensive interventions. Finally, we included a quadratic term to account for nonlinearity that may exist over time in DRA scores. We conducted all analyses with Stata 10 SE (StataCorp, College Station, TX).
RESULTS
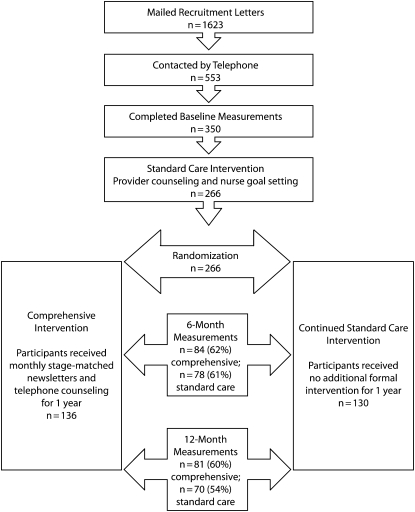
The HHER team identified 1623 patients through the clinics’ computerized patient scheduling system. Participant recruitment and retention is summarized in Figure 1. A detailed study recruitment flowchart has been reported.26 In summary, 553 of the targeted patients (34%) could be contacted by telephone before their scheduled medical appointment. Of those contacted, 465 (84%) completed the telephone screening. We conducted baseline assessments with 350 patients, of whom 266 (76%) were randomized. Most of the remaining 84 patients were not enrolled because they did not attend their scheduled clinic visit. Of the 266 randomized women (130 standard care, 136 comprehensive intervention), assessments were completed by 162 (61%) at 6 months and 151 (57%) at 12 months. There was no difference between intervention groups in retention rates at 6 and 12 months; however, we observed significant differences in baseline characteristics between those who completed the study and those who dropped out or were lost to follow-up. Overall, younger, employed participants were more likely to be lost to follow-up. Standard care intervention participants who were normal weight (18.5–24.9 kg/m2) or overweight (25–29.9 kg/m2) also were more likely to drop out than their comprehensive intervention peers.
FIGURE 1.
Study design and recruitment and retention of participants: Heart Healthy and Ethnically Relevant Lifestyle trial, South Carolina, 2005–2008.
Participant characteristics, by intervention group, are shown in Table 1. The majority of participants were obese. Most participants were aged 35 to 64 years, with only a small percentage aged 65 years or older. About one third were married, and more than one third were divorced or separated. The sample was roughly split between those with a high school education or less and those who had attended at least some college. Most participants had annual incomes of less than $30 000, and about half were employed.
TABLE 1.
Participant Characteristics, by Randomization Status: Heart Healthy and Ethnically Relevant Lifestyle Trial, South Carolina, 2005–2008
| Characteristic | Comprehensive, No.a (%) or Mean ±SD | Standard Care, No.a (%) or Mean ±SD | Pb |
| Age, y | |||
| 35–49 | 63 (47.0) | 64 (49.6) | .39 |
| 50–64 | 59 (44.0) | 48 (37.2) | |
| ≥ 65 | 12 (9.0) | 17 (13.2) | |
| Marital status | |||
| Married or living together | 43 (31.9) | 43 (33.1) | .58 |
| Divorced or separated | 50 (37.0) | 56 (43.1) | |
| Not married | 15 (11.1) | 12 (9.2) | |
| Widowed | 27 (20.0) | 19 (14.6) | |
| Education | |||
| < high school | 26 (19.4) | 25 (19.4) | .21 |
| Completed high school or equivalent | 49 (36.6) | 37 (28.7) | |
| Some college or degree | 54 (40.3) | 55 (42.6) | |
| Some graduate or degree | 5 (3.7) | 12 (9.3) | |
| Annual income, $ | |||
| Missing | 20 (14.7) | 13 (10.0) | .32 |
| 0–9999 | 29 (25.0) | 20 (17.1) | |
| 10 000–19 999 | 29 (25.0) | 35 (29.9) | |
| 20 000–29 999 | 22 (19.0) | 29 (24.8) | |
| ≥ 30 000 | 36 (26.5) | 33 (25.4) | |
| Employment status | |||
| Unemployed | 25 (18.5) | 25 (18.2) | .91 |
| Employed (full- or part-time) | 78 (57.8) | 71 (54.6) | |
| Disabled (permanent or temporary) | 15 (11.1) | 18 (13.9) | |
| Retired | 17 (12.6) | 16 (12.3) | |
| Body mass index, kg/m2 | |||
| Normal (18.5–24.9) | 16 (12.4) | 13 (10.6) | .08 |
| Overweight (25–29.9) | 31 (24.0) | 17 (13.8) | |
| Obese (≥ 30) | 82 (63.6) | 93 (75.6) | |
| CHAMPS Physical Activity Score, h/wk | |||
| Total MVPA | 3.5 ±4.8 | 3.9 ±4.3 | .46 |
| Leisure-time MVPA | 2.7 ±4.1 | 2.9 ±2.5 | .6 |
| DRA scoresc | |||
| Total DRA | 32.0 ±9.1 | 32.1 ±8.5 | .93 |
| Meat | 11.35 ±3.9 | 10.8 ±3.7 | .24 |
| Side dishes and snacks | 9.6 ±2.9 | 10.3 ±3.3 | .08 |
| Dairy and eggs | 5.0 ±2.8 | 5.0 ±2.8 | .91 |
| Spreads, dressings, and oils | 6.1 ±3.3 | 6.0 ±2.9 | .89 |
Note. CHAMPS = Community Health Activities Model Program for Seniors; DRA = Dietary Risk Assessment; MVPA = moderate-to-vigorous physical activity. The sample size for comprehensive care was n = 136 and for standard care, n = 130.
Numbers may vary because of missing data.
Differences in proportions evaluated by χ2 (2-tailed) and means by t test.
The DRA includes 52 items, each of which is scored from 0 to 2. A lower score indicates a more healthful dietary pattern.
Of the 136 comprehensive intervention participants, 3.6% were never reached for any intervention contact, and 7.4% received only the initial overview call. The percentage of participants receiving the overview call plus at least 1 subsequent call was 6.6% for 1 to 3 subsequent calls, 14.0% for 4 to 6 calls, 11.8% for 7 to 9 calls, 22.1% for 10 to 12 calls, and 34.6% for 13 to 14 calls. The mean number of calls delivered was 10.0 ±3.9 out of 14 possible calls. The mean duration of the initial overview call was 73.7 ±12.3 minutes, and subsequent calls were 21.7 ±8.0 minutes.
There were significant group differences for total and leisure-time moderate-to-vigorous physical activity at 6 months but not at 12 months (Table 2). Comprehensive intervention participants were significantly more likely than were those in standard care to decline in total physical activity at 6 months (adjusted odds ratio [OR] = 3.13; 95% confidence interval [CI] = 1.18, 8.25), but they were also significantly more likely to improve in leisure-time physical activity (adjusted OR = 3.82; 95% CI = 1.41, 10.3).
TABLE 2.
Odds of Increasing Moderate-to-Vigorous Physical Activity: Heart Healthy and Ethnically Relevant Lifestyle Trial, South Carolina, 2005–2008
| Follow-Up Interval and Intervention | Improvement |
No Change (Ref), No. (%) | Decline |
||
| No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | ||
| Total moderate-to-vigorous physical activity | |||||
| 6 mo (n = 150) | |||||
| Comprehensive | 21 (26.6) | 1.02 (0.41, 2.55) | 22 (27.9) | 36 (45.6) | 3.13 (1.18, 8.25) |
| Standard care | 22 (39.4) | 24 (33.8) | 19 (26.8) | ||
| 12 mo (n = 142) | |||||
| Comprehensive | 23 (30.7) | 0.63 (0.24, 1.68) | 23 (30.7) | 29 (38.7) | 1.90 (0.64, 5.58) |
| Standard care | 30 (44.8) | 24 (35.8) | 13 (19.4) | ||
| Leisure-time moderate-to-vigorous physical activity | |||||
| 6 mo (n = 150) | |||||
| Comprehensive | 37 (44.0) | 3.82 (1.41, 10.30) | 30 (35.7) | 17 (20.2) | 0.56 (0.22, 1.43) |
| Standard care | 17 (22.0) | 32 (41.6) | 28 (36.4) | ||
| 12 mo (n = 142) | |||||
| Comprehensive | 29 (35.8) | 1.76 (0.62, 5.00) | 29 (35.8) | 23 (28.4) | 0.52 (0.20, 1.33) |
| Standard care | 13 (18.6) | 26 (37.1) | 31 (44.3) | ||
Note. CI = confidence interval; OR = odds ratio. ORs are adjusted for demographic characteristics and baseline body mass index; 95% CIs are for odds of improving or declining relative to staying the same, compared with baseline.
Table 3 presents DRA scores at baseline, 6 months, and 12 months across intervention groups and results from the repeated-measures analyses. Group × time interactions were significant for the DRA total score and the meat and the dairy products and eggs subscales. As expected, the comprehensive intervention group showed significantly greater improvements (reduction in risk score) over time than did the standard care group for the DRA total score and for the meat and the dairy products and eggs subscales. Group × time interactions were not significant for the DRA side dishes and snacks subscale or the spreads, dressings, and oils subscale.
TABLE 3.
Dietary Risk Assessment Scores: Heart Healthy and Ethnically Relevant Lifestyle Trial, South Carolina, 2005–2008
| Dietary Risk Assessment Score |
|||||
| Follow-Up Interval and Intervention | Total Scorea | Meats | Side Dishes and Snacks | Dairy Products | Spreads, Dressings, and Oils |
| Baseline, mean (SD) | |||||
| Comprehensive (n = 136) | 32.0 (9.1) | 11.3 (4.0) | 9.6 (3.0) | 5.0 (2.8) | 6.1 (3.3) |
| Standard care (n = 127) | 32.1 (8.5) | 10.8 (3.7) | 10.2 (3.3) | 5.0 (2.8) | 6.0 (2.9) |
| 6 mo, mean (SD) | |||||
| Comprehensive (n = 84) | 24.1 (7.4) | 8.8 (3.6) | 8.3 (2.4) | 3.2 (2.2) | 3.8 (2.5) |
| Standard care (n = 78) | 27.5 (7.2) | 9.8 (3.6) | 9.3 (2.5) | 4.3 (2.5) | 4.1 (2.3) |
| 12 mo, mean (SD) | |||||
| Comprehensive (n = 80) | 21.3 (6.9) | 7.2 (3.2) | 7.8 (2.7) | 2.8 (1.9) | 3.6 (2.3) |
| Standard care (n = 71) | 26.8 (7.3) | 9.5 (2.6) | 9.5 (2.6) | 3.9 (2.4) | 3.9 (2.3) |
| Change, 0–6 mob | |||||
| Comprehensive | −8.50 | −2.45 | −0.94 | −2.09 | −3.02 |
| Standard care | −5.34 | −0.34 | −0.60 | −1.18 | −3.21 |
| Change, 0–12 mob | |||||
| Comprehensive | −7.16 | −3.32 | 0.35 | −2.06 | −3.43 |
| Standard care | −3.37 | −0.90 | 1.06 | −1.72 | −3.21 |
| Group × time interaction Pc | < .001 | < .001 | .15 | .04 | .5 |
Note. The Dietary Risk Assessment includes 52 items, each of which is scored from 0 to 2. A lower score indicates a more healthful dietary pattern.
Sum of parts may not equal total due to rounding.
Mean change adjusted for age, income, employment status, education, and body mass index at baseline.
Repeated-measures analysis adjusted for age, income, employment status, education, body mass index, and time squared.
DISCUSSION
Health behavioral counseling interventions in primary care settings that help patients improve their physical activity and dietary behaviors have the potential to improve population health. The HHER Lifestyle trial extends this body of evidence in an innovative direction by targeting an understudied, financially disadvantaged population of African American women who suffer disproportionately from CVD. Despite the challenging nature of the study population (low income and lack of transportation), high levels of intervention delivery were achieved, along with modest improvements in several dietary outcomes and leisure-time physical activity. Although DRA scores—the total score as well as the 4 subscale scores—improved for women in both study groups, the magnitude of change was greater in the comprehensive intervention group than in the standard care intervention group (although statistically significant effects were achieved only for the total DRA and the subscales for meat and for dairy products and eggs). Other studies conducted with low-income women in other settings have reported similar differences in DRA effects between intervention and control participants.48–50 Keyserling et al., the primary developers of the DRA, note that a change of this magnitude suggests substantially improved dietary quality.51 Comparisons between our findings and other studies in the literature are more difficult to make for the CHAMPS scores because there are fewer studies of African American samples that use this measure, and these studies more commonly report mean changes over time rather than a categorical outcome.
Women in the comprehensive intervention were more likely than were those in standard care to improve their leisure-time physical activity at 6 months (44% vs 22%). A similar pattern, although not statistically significant, occurred at 12 months (35.8% vs 18.6%). The contradictory finding that women in the comprehensive intervention were more likely to decline in total moderate-to-vigorous physical activity than were those in standard care was unexpected. This pattern is consistent with a behavioral compensatory mechanism52 whereby participants in an intervention to increase exercise53–55 or reduce calorie intake56 actually decrease overall energy expenditure by increasing sedentary behaviors. This issue merits further study among African American women.
For both diet and physical activity, the most dramatic improvements occurred between baseline and 6 months, with change attenuating somewhat by 12 months. In this study, given that there was not a true no-treatment control, the improvement in DRA scores for both interventions was not unexpected. The standard care group received a level of intervention that was more intensive than would transpire in usual clinical practice—brief physician counseling with nurse-assisted goal setting and educational materials. Thus, observed differences between the groups stemmed from the added value of additional, more intensive telephone counseling and tailored materials. The inclusion of a no-treatment control group would have aided the interpretation of findings.
Limitations
Despite its success, this trial had some limitations. First, overall study attrition was high (43% at 12 months), and data were not missing at random, making analyses based on the initial treatment intent unfeasible. The analyses presented include the subset of patients who received the intervention and did not leave the study. Younger and employed participants were more likely to leave the study. Attrition also differed by study group; standard care participants who were normal weight or overweight were more likely to drop out than were their comprehensive intervention peers. Our analyses did control for these variables to reduce potential biases, but perhaps the intervention format—telephone counseling—provided a needed outlet for communication and social support to older women who did not work outside the home. Conversely, younger employed women may have felt more social role strain. Regarding higher attrition among normal and overweight women in the standard care intervention, we believe women who were less overweight may have been less motivated to stay involved in the study, especially after learning they were not going to receive the comprehensive intervention. Although we designed the telephone intervention and home measurement visits to maximize retention, and although we provided reminders and monetary incentives, additional incentives might have aided retention.
A second limitation is the use of self-reported diet and physical activity measures that can be subject to overreporting (physical activity) or underreporting (dietary intake). In addition, the CHAMPS physical activity measure does not specifically assess occupational activity, which could be a serious omission in this population. In the comprehensive intervention group, a health educator's telephone contact may have introduced a social desirability bias that resulted in participants reporting higher physical activity and lower dietary fat intake. In addition, through telephone counseling, the importance of eating a low-fat diet and increasing physical activity was repeatedly emphasized; therefore, these participants were more mindful of these factors’ importance and may have overestimated healthy behaviors. Although attempts were made to collect objective physical activity data through accelerometers, poor compliance forced us to drop this measure. Despite the question of the accuracy of self-reported measures, however, all our trial's measures have been subjected to extensive validation26 and have been routinely used in population-based epidemiological and intervention research.
A third limitation is that we did not study postintervention maintenance of behavior change. Indeed, few community-based physical activity interventions have studied behavior change maintenance,57 a limitation of the larger field. This type of analysis is important because it has implications for the feasibility and generalizability of this type of intervention in clinical settings.
Conclusions
The HHER Lifestyle trial was unique in using a primary care setting to target low-income African American women, a patient sample with multiple comorbid chronic conditions (e.g., hypertension, hyperlipidemia, diabetes) whose baseline health behavior profile indicated a strong need for intervention. The trial's comprehensive intervention, which achieved consistent contact with individually tailored telephone counseling and mailed newsletters, demonstrated significant change in diet and leisure-time physical activity (but not total physical activity) compared with standard care. The results of the current trial, combined with the growing number of studies supporting the efficacy of telephone counseling interventions,24 suggest that it is time for dissemination trials. It remains to be seen whether the intensity of this trial's intervention is feasible in current clinical practice and whether postintervention behavior change is maintained. Nonetheless, it is less intensive than other behavioral interventions (e.g., Diabetes Prevention Program58,59 and Look AHEAD59) and very similar to another intervention delivered in clinical practice.25 Telephone delivery also makes it flexible for staff and patients and lends itself well to “booster” sessions.25 Future trials should examine strategies to sustain initial treatment gains and enhance retention rates. Alternative approaches that consider the time restrictions on younger, employed, and financially disadvantaged African American women may also be required.
In summary, this trial provided novel evidence that lifestyle interventions can be delivered effectively in community-based primary care settings to reach underserved, disadvantaged women and stimulate them to improve their physical activity levels and dietary intake. This intervention approach, if replicated broadly in primary care settings, might be able to reach large numbers of patients at high risk for chronic diseases.
Acknowledgments
This project was supported by the National Heart, Lung, and Blood Institute (award R01HL073001).
We are especially grateful to all of the women who participated in this study. We also thank the providers and nurses at the Eau Claire Cooperative Health Center and Family Health Center Inc for their participation as well as their time and feedback regarding the study protocols. In addition, we thank Alice Ammerman, DrPH, of the University of North Carolina at Chapel Hill for providing consultation and feedback regarding the development of the nutrition training content and Barbara Ainsworth, PhD, MPH, FACSM, of Arizona State University for providing consultation on measurement of physical activity. Finally, we acknowledge the substantial contributions of staff and investigators who participated in the HHER Lifestyle program: Tiffany N. Barker, Alisa Brewer, Shamika Brown, Tina Devlin, Elizabeth Fallon, Gwen Felton, Monetha Gaskin, Desireé Hammond, Amanda McClain, Genova McFadden, Edena Meetze, Keri Norris, and Lisa Wigfall.
Note. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Human Participant Protection
This study was approved by the University of South Carolina institutional review board.
References
- 1.American Heart Association Heart Disease and Stroke Statistics—2009 Update. Dallas, TX: American Heart Association; 2009 [Google Scholar]
- 2.Chu KC, Miller BA, Springfield SA. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc. 2007;99(10):1092–1100, 1102–1104 [PMC free article] [PubMed] [Google Scholar]
- 3.Sudano JJ, Baker DW. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: the roles of socioeconomic status, health behaviors, and health insurance. Soc Sci Med. 2006;62(4):909–922 [DOI] [PubMed] [Google Scholar]
- 4.Health Resources and Services Administration, Bureau of Primary Health Care The Health Center Program: national aggregate UDS data, Table 3B: patients by race/ethnicity/language. 2007. Available at: http://bphc.hrsa.gov/uds/2007data/National/NationalTable3BUniversal.htm. Accessed February 21, 2009
- 5.Health Resources and Services Administration, Bureau of Primary Health Care The Health Center Program: national aggregate UDS data, Table 4: patients by socioeconomic characteristics. 2007. Available at: http://bphc.hrsa.gov/uds/2007data/National/NationalTable4Universal.htm. Accessed February 21, 2009
- 6.Parra-Medina D, Wilcox S, Thompson-Robinson M, Sargent R, Will JC. A replicable process for redesigning ethnically relevant educational materials. J Womens Health (Larchmt). 2004;13(5):579–588 [DOI] [PubMed] [Google Scholar]
- 7.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. Am Psychol. 1992;47(9):1102–1114 [DOI] [PubMed] [Google Scholar]
- 8.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986 [Google Scholar]
- 9.Goldstein MG, Evelyn PW, Judith D. Multiple behavioral risk factor interventions in primary care: summary of research evidence. Am J Prev Med. 2004;27(2 suppl):61–79 [DOI] [PubMed] [Google Scholar]
- 10.Healthy People 2010: Understanding and Improving Health. 2nd ed Washington, DC: US Dept of Health and Human Services; 2000 [Google Scholar]
- 11.US Preventive Services Task Force Behavioral counseling in primary care to promote a healthy diet: recommendations and rationale. Am J Prev Med. 2003;24(1):93–100 [DOI] [PubMed] [Google Scholar]
- 12.US Preventive Services Task Force Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139(11):930–932 [DOI] [PubMed] [Google Scholar]
- 13.Fletcher GF. How to implement physical activity in primary and secondary prevention: a statement for healthcare professionals from the task force on risk reduction, American Heart Association. Circulation. 1997;96(1):355–357 [DOI] [PubMed] [Google Scholar]
- 14.Jacobson DM, Strohecker L, Compton MT, Katz DL. Physical activity counseling in the adult primary care setting: position statement of the American College of Preventive Medicine. Am J Prev Med. 2005;29(2):158–162 [DOI] [PubMed] [Google Scholar]
- 15.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093 [DOI] [PubMed] [Google Scholar]
- 16.Eakin EG, Smith BJ, Bauman AE. Evaluating the population health impact of physical activity interventions in primary care: are we asking the right questions? J Phys Act Health. 2005;2(2):197–215 [Google Scholar]
- 17.Glasgow RE, Eakin EG, Fisher EB, Bacak SJ, Brownson RC. Physician advice and support for physical activity: results from a national survey. Am J Prev Med. 2001;21(3):189–196 [DOI] [PubMed] [Google Scholar]
- 18.Fallon EA, Wilcox S, Laken M. Health care provider advice for African American adults not meeting health behavior recommendations. Prev Chronic Dis. 2006;3(2):A45. [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton C, Goodwin M, Stange K. Direct observation of nutrition counseling in community family practice. Am J Prev Med. 2002;23(3):174–179 [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Urizar G, Jr, Alehegn T, Stafford R. Diet and physical activity counseling during ambulatory care visits in the United States. Prev Med. 2004;39(4):815–822 [DOI] [PubMed] [Google Scholar]
- 21.Tulloch H, Fortier M, Hogg W. Physical activity counseling in primary care: who has and who should be counseling? Patient Educ Couns. 2006;64(1–3):6–20 [DOI] [PubMed] [Google Scholar]
- 22.Wilcox S, Parra-Medina D, Thompson-Robinson M, Will J. Nutrition and physical activity interventions to reduce cardiovascular disease risk in health care settings: a quantitative review with a focus on women. Nutr Rev. 2001;59(7):197–214 [DOI] [PubMed] [Google Scholar]
- 23.Smith BJ. Promotion of physical activity in primary health care: update of the evidence on interventions. J Sci Med Sport. 2004;7(1 suppl):67–73 [DOI] [PubMed] [Google Scholar]
- 24.Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med. 2007;32(5):419–434 [DOI] [PubMed] [Google Scholar]
- 25.Eakin E, Reeves M, Lawler S, et al. Telephone counseling for physical activity and diet in primary care patients. Am J Prev Med. 2009;36(2):142–149 [DOI] [PubMed] [Google Scholar]
- 26.Parra-Medina D, Wilcox S, Wilson DK, Addy CL, Felton G, Poston MB. Heart Healthy and Ethnically Relevant (HHER) Lifestyle trial for improving diet and physical activity in underserved African American women. Contemp Clin Trials. 2010;31(1):92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra-Medina D, Wilcox S, Evans A, Watkins K, Rafirou C, Thatch S. Feasibility study of provider counseling for financially disadvantaged African American women: HHER Lifestyle Pilot Program [abstract]. Ann Behav Med. 2002;22:S152 [Google Scholar]
- 28.Parra-Medina D, Smith S, D'Antonio A, et al. Weight management in type 2 diabetes: Pounds Off With Empowerment (POWER) [abstract]. Ann Behav Med. 2002;24:S159. [DOI] [PubMed] [Google Scholar]
- 29.Parra-Medina D, D'Antonio A, Smith SM, Levin S, Kirkner G, Mayer-Davis E. Successful recruitment and retention strategies for a randomized weight management trial for people with diabetes living in rural, medically underserved counties of South Carolina: the POWER study. J Am Diet Assoc. 2004;104(1):70–75 [DOI] [PubMed] [Google Scholar]
- 30.Wilcox S, Parra-Medina D, Felton G, Poston MB, McClain A. Adoption and implementation of physical activity and dietary counseling by community health center providers and nurses. J Phys Act Health. 2010;7(5):602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med. 1996;25(3):225–233 [DOI] [PubMed] [Google Scholar]
- 32.Activity Counseling Trial Writing Group Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA. 2001;286(6):677–687 [DOI] [PubMed] [Google Scholar]
- 33.Resnicow K, Braithwaite RL. Cultural sensitivity in public health. : Braithwaite RL, Taylor SE, Health Issues in the Black Community. San Francisco, CA: Jossey-Bass; 2001:516–542 [Google Scholar]
- 34.King AC, Taylor CB, Haskell WL. Effects of differing intensities and formats of 12 months of exercise training on psychological outcomes in older adults. Health Psychol. 1993;12(4):292–300 [DOI] [PubMed] [Google Scholar]
- 35.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults: a randomized controlled trial. JAMA. 1997;277(1):32–37 [PubMed] [Google Scholar]
- 36.King AC, Pruitt LA, Phillips W, Oka R, Rodenburg A, Haskell WL. Comparative effects of two physical activity programs on measured and perceived physical functioning and other health-related quality of life outcomes in older adults. J Gerontol A Biol Sci Med Sci. 2000;55(2):M74–M83 [DOI] [PubMed] [Google Scholar]
- 37.King AC, Baumann K, O'Sullivan P, Wilcox S, Castro C. Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2002;57(1):M26–M36 [DOI] [PubMed] [Google Scholar]
- 38.Wilcox S, Dowda M, Leviton LC, et al. Active for life: final results from the translation of two physical activity programs. Am J Prev Med. 2008;35(4):340–351 [DOI] [PubMed] [Google Scholar]
- 39.Stewart AL, Mills KM, King AC, Haskell WL, Gillis, Ritter PL. CHAMPS Physical Activity Questionnaire for Older Adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141 [DOI] [PubMed] [Google Scholar]
- 40.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33(6):962–970 [DOI] [PubMed] [Google Scholar]
- 41.Stewart AL, Mills KM, Sepsis PG, et al. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med. 1997;19(4):353–361 [DOI] [PubMed] [Google Scholar]
- 42.Stewart AL. Community-based physical activity programs for adults age 50 and older. J Aging Phys Act. 2001;9(suppl):S71–S91 [Google Scholar]
- 43.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465–M470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Resnicow K, McCarty F, Blissett D, Wang T, Heitzler C, Lee RE. Validity of a modified CHAMPS physical activity questionnaire among African-Americans. Med Sci Sports Exerc. 2003;35(9):1537–1545 [DOI] [PubMed] [Google Scholar]
- 45.Keyserling TC, Samuel-Hodge CD, Ammerman AS, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care. 2002;25(9):1576–1583 [DOI] [PubMed] [Google Scholar]
- 46.Ammerman AS, Haines PS, DeVellis RF, et al. A brief dietary assessment to guide cholesterol reduction in low-income individuals: design and validation. J Am Diet Assoc. 1991;91(11):1385–1390 [PubMed] [Google Scholar]
- 47.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968–976 [DOI] [PubMed] [Google Scholar]
- 48.Ammerman AS, Keyserling TC, Atwood JR, Hosking JD, Zayed H, Krasny C. A randomized controlled trial of a public health nurse directed treatment program for rural patients with high blood cholesterol. Prev Med. 2003;36(3):340–351 [DOI] [PubMed] [Google Scholar]
- 49.Keyserling TC, Ammerman AS, Davis CE, Mok MC, Garrett J, Simpson R., Jr A randomized controlled trial of a physician-directed treatment program for low-income patients with high blood cholesterol: the Southeast Cholesterol Project. Arch Fam Med. 1997;6(2):135–145 [DOI] [PubMed] [Google Scholar]
- 50.Rosamond WD, Ammerman AS, Holliday JL, et al. Cardiovascular disease risk factor intervention in low-income women: the North Carolina WISEWOMAN project. Prev Med. 2000;31(4):370–379 [DOI] [PubMed] [Google Scholar]
- 51.Keyserling TC, Samuel Hodge CD, Jilcott SB, et al. Randomized trial of a clinic-based, community-supported, lifestyle intervention to improve physical activity and diet: the North Carolina enhanced WISEWOMAN project. Prev Med. 2008;46(6):499–510 [DOI] [PubMed] [Google Scholar]
- 52.Tremblay MS. Assessing the level of sedentarism. : Bouchard C, Katzmarzyk PT, Physical Activity and Obesity. 2nd ed Champaign, IL: Human Kinetics; 2010:13–17 [Google Scholar]
- 53.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol. 1992;263(5 pt 1):E950–E957 [DOI] [PubMed] [Google Scholar]
- 54.Manthou E, Gill JM, Wright A, Malkova D. Behavioral compensatory adjustments to exercise training in overweight women. Med Sci Sports Exerc. 2010;42(6):1121–1128 [DOI] [PubMed] [Google Scholar]
- 55.King NA, Caudwell P, Hopkins M, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring). 2007;15(6):1373–1383 [DOI] [PubMed] [Google Scholar]
- 56.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE. 2009;4(2):e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcus BH, Williams DM, Dubbert PM, et al. Physical activity intervention studies: what we know and what we need to know: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114(24):2739–2752 [DOI] [PubMed] [Google Scholar]
- 58.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Look AHEAD Research Group, Wing RR Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]