Abstract
PURPOSE
Mammalian programmed cell death-1 (PD-1) is a membrane-associated receptor regulating the balance between T cell activation, tolerance and immunopathology, however its role in neurons has not yet been defined. We investigate the hypothesis that PD-1 signaling actively promotes retinal ganglion cell (RGC) death within the developing mouse retina.
METHODS
Mature retinal cell types expressing PD-1 were identified by immunofluorescence staining of vertical retina sections; developmental expression was localized by immunostaining and quantified by Western analysis. PD-1 involvement in developmental RGC survival was assessed in vitro using retina explants and in vivo using PD-1 knockout mice. PD-1 ligand gene expression was detected by RT-PCR.
RESULTS
PD-1 is expressed in most adult RGCs, and undergoes dynamic upregulation during the early postnatal window of retinal cell maturation and physiological programmed cell death (PCD). In vitro blockade of PD-1 signaling during this time selectively increases survival of RGCs. Furthermore, PD-1 deficient mice show a selective increase in RGC number in the neonatal retina at the peak of developmental RGC death. Lastly, throughout postnatal retina maturation, we find gene expression of both immune PD-1 ligand genes, PD-L1 and PD-L2.
CONCLUSIONS
These findings collectively support a novel role for a PD-1-mediated signaling pathway in developmental PCD during postnatal RGC maturation.
Introduction
Modulation of signaling elicited by cell-cell interactions is critical for the formation and remodeling of neuronal synaptic networks in development and learning, as well as for the generation of immunity towards environmental and endogenous antigens.1, 2 In some cases, molecules involved in such selection may be shared by both the immune and central nervous systems. For example, the MHC class I ligand and its receptor component CD3ξ, a canonical receptor-ligand immune recognition pair, are required to establish functional connections between the retina and brain during development3, and the initiating complement protein C1q marks neural retina synapses for elimination in both development and degenerative disease4.
In the immune system, cell-cell interaction molecules are critical for regulating lymphocyte function. PD-1 (CD279) is a key immunoregulatory receptor, inducibly expressed on T cells, B cells, NK T cells, activated monocytes, and dendritic cells.5 PD-1 transduces an inhibitory signal when engaged in combination with the T cell receptor (TCR). These immunoinhibitory signals regulate the extent of T cell activation, attenuate anti-microbial immunity, facilitate chronic viral infections, and provide inhibitory signals that regulate both central and peripheral T cell tolerance.2 During the establishment of central tolerance, PD-1 is expressed on developing thymocytes as they progress through several maturational stages, where PD-1 signaling modifies signaling thresholds in thymocytes during both positive and negative selection stages of maturation.6, 7 In addition, PD-1 regulates both the induction and maintenance of peripheral T cell tolerance by limiting mature self-reactive T cell function.5
We recently observed constitutive neuronal expression of PD-1 in retinal ganglion cells (RGCs)8, suggesting that PD-1 may also provide inhibitory signals important for physiologic loss of neurons during retinal maturation. In this study, we tested whether PD-1 has a parallel role in negative selection during neuronal network formation in the developing and adult retina, an organ with well-defined cytological architecture that has been an important model for investigating molecular mechanisms of neurogenesis.
Materials and Methods
Animals
Mice were purchased from Charles River Laboratory unless otherwise noted. Embryonic and adult CD1 mice were used for PD-1 blocking experiments. C57BL/6 mice were used for PD-1 immunoblotting and PD-1 ligand gene expression studies. For the PD-1-/- characterization, PD-1-/- mice were constructed in the C57BL/6 background, as previously described9, and wildtype C57BL/6 age-matched mice were purchased from the Charles River Laboratory. All animal experiments were reviewed and approved by the UCLA Chancellor’s Animal Research Committee in adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tissue Preparation
Eyes were rapidly enucleated from adult and embryonic mice. Posterior eye cups were fixed in 4% paraformaldehyde at room temperature for one hour, followed by cryoprotection in 30% sucrose/PBS and OCT embedding. Cryostat sectioning was performed at a thickness of 6-8 μm. The sections were selected to represent the equivalent regions of the globes and taken just adjacent to the optic nerve plane. In order to assure validity of analysis, sections were used for quantification only if the retina architecture represented a vertical section through the retina.
RGC Isolation
Positive selection of RGCs was performed using magnetic beads coated with a Thy-1.1 monoclonal antibody (Millipore/Chemicon), as previously described10.
Retinal Explants
Neural retinas were dissected from newborn P0 mice and cultured as previously described11. Briefly, retinas were placed on polycarbonate filter discs (Millipore) and cultured for 24 hours at 37°C/5% CO2 in DMEM/F12 (Mediatech) containing 10% fetal bovine serum (Omega Scientific), and 1 × N2 supplement (Gibco). For the antibody blocking experiment, the following functional grade antibodies were used at a final concentration of 7μg/ml: anti-mouse PD-1 (clone J43, hamster IgG, eBioscience) for which specificity12 and ability to block interaction with PD-L1 and PD-L213 have previously been described, or isotype control hamster IgG (eBioscience). After 24 hours in culture, explants were fixed, cryoprotected, embedded in OCT, and cryosectioned, as described above.
Immunostaining
Sections were incubated at 4°C overnight with the following primary antibodies: anti-mouse PD-1 (clone 29F.1A12, rat IgG2a) for which specificity has previously been described14; RGC markers anti-Brn3a (clone 5A3.2, mouse IgG1, Millipore/Chemicon)15 and anti-NeuN (mouse IgG1, clone A60, Millipore/Chemicon)16; amacrine cell marker anti-mouse AP2α (3B5, mouse IgG2b, Developmental Studies Hybridoma Bank)17; or apoptotic cell marker rabbit anti-mouse activated caspase-3 (R&D Systems)18. For immunohistochemistry, the Rat IgG Vectastain ABC Kit, AEC substrate kit, and hematoxylin QS counterstain (Vector) were used according to product instructions. For immunofluorescence staining, AlexaFluor 488 or AlexaFluor 594 conjugated secondary fluorescent antibodies and 1.4μM DAPI counterstain (Invitrogen) were used.
Quantification of Immunofluorescence Staining
Retina sections were imaged at 200X magnification, using a Nikon E800 microscope and a SPOTII digital camera, yielding a retina image width of 0.63mm. Within the GCL, immunostained nuclei or cells and DAPI positive nuclei were counted, for each retina section, by two investigators masked to the identity of samples and experiment. Values are reported as the mean and standard error of the mean (S.E.M.). The Mann-Whitney, non-parametric t-test was used to analyze differences between experimental groups, and p<0.05 was considered significant. Prism 5 (GraphPad) was used for graphing and statistical analysis. For the PD-1 blocking study, each of three experimental repeats examined 4 retinal explants; for each explant, 5 sections (n=5) were co-stained with caspase-3, Brn3a, and DAPI. For the PD-1-/- study, 3 animals were examined at each time point, for both PD-1-/- and wildtype; at least 3 retina sections (n≥3) animal were stained with Brn3a or AP2, and DAPI.
Western Blot Analysis
Retinas were isolated from C57BL/6 and PD-1-/- mice. Retinas were sonicated on ice in lysis buffer (30 mM Tris-HCl, 10 mM EGTA, 5 mM EDTA, 1% Triton X-100, 250 mM sucrose)19 with complete protease inhibitor cocktail (Roche), for 2 min (4 × 30 second pulses). 30μg of each lysate was analyzed by SDS-PAGE as previously described20. These primary antibodies were used: rat anti-mouse PD-1 (clone 29F.9A2, rat IgG2a,k) or mouse anti-β-actin (clone 2A2.1, mouse IgG1, US Biologicals). PD-1 and β-actin were detected with anti-rat IgG-HRP and anti-mouse IgG-HRP (Southern Biotech), respectively.
RT-PCR
Neural retinas were dissected or primary thymocytes were isolated. Retinas from 3 animals were pooled for each time point; 3 experimental repeats were performed. Tissues or cells were stabilized in RNA later (Qiagen). Total RNA was extracted using an RNeasy Mini Kit (Qiagen) and purified using the RNeasy MinElute Cleanup Kit (Qiagen). 0.5μg total RNA was used as a template for reverse transcription using the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) and amplified using Platinum Quantitative PCR SuperMix-UDG (Invitrogen) on an ABI 7500 platform. The following 20X Taqman assays (Applied Biosystems) were used: Mouse GAPDH Endogenous Control, FAM-MGB (GenBank Accession No. NM_008084.2); PD-L1-FAM (Mm00452055_m1, GenBank NM_021893.2); PD-L2-FAM (Mm01208507_m1, GenBank NM_021396.1).
Results
PD-1 is Expressed in RGCs and Displaced Amacrine Cells of the Adult Mouse Retina
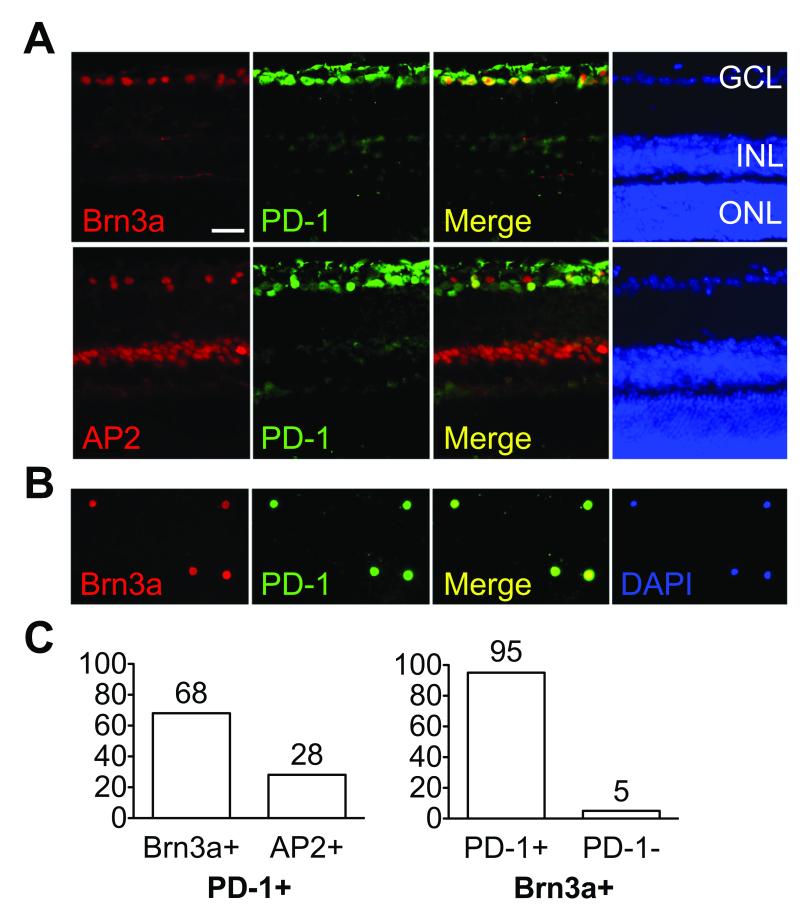
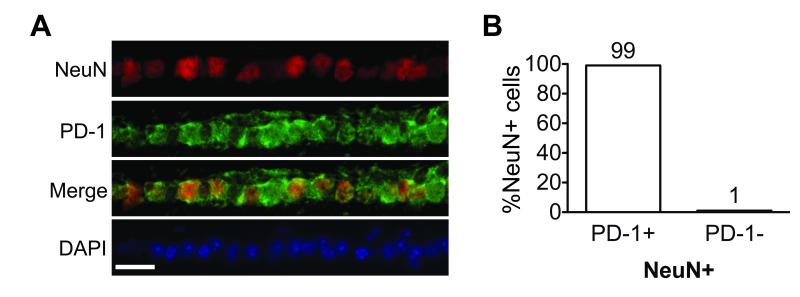
In the adult retina, PD-1 expression was detected mainly in the ganglion cell layer (GCL) and a sub-population of cells in the inner nuclear layer (INL)8. Since the mouse retina consists of six functionally distinct neuronal cell types distributed in three cell layers, we were interested to determine the identity of the cell populations that express PD-1. Immunofluorescent double labeling with antibodies directed against specific retinal cell makers revealed colocalization of PD-1 with a specific RGC marker, Brn3a, and an amacrine cell marker AP2α, hereafter referred to as AP2 (Fig. 1A). To further validate RGC-specific PD-1 expression, we used another well-established RGC marker Thy-121. Thy-1.1 conjugated magnetic beads were used to isolate RGCs, followed by PD-1 immunostaining (Fig. 1B), and our results confirm that Thy-1 positive cells are PD-1 positive. Quantification of co-staining demonstrated that over 95% of the Brn3a positive cells were PD-1 positive, and among the PD-1 positive cells in GCL, 68% were Brn3a positive RGCs and 28% were AP2 positive amacrine cells (Fig. 1C). To assess the possibility that PD-1 expression is coupled to Brn3a expression, we assessed colocalization of PD-1 with an independent RGC marker NeuN16 (Fig. 2A). Amongst NeuN positive RGCs, we found 99±0.2% PD-1 positive and 1.2±0.2% PD-1 negative cells (Fig. 2B). Thus, we conclude that authentic PD-1 is expressed by mature retinal neurons, predominantly in the RGC population.
Figure 1.
PD-1 expression in the adult mouse retina. (A) Double staining of PD-1 with Brn3a or AP2 on vertical retina sections. Left to right panels represent Brn3a or AP2 (red), PD-1 (green), merge (yellow) and nuclear DAPI (blue) staining of retinal sections. (B) Isolated RGCs using Thy-1 coated magnetic beads. Left to right panels represent Brn3a (red), PD-1 (green), and nuclear DAPI (blue). (C) Quantification of PD-1 positive population in vivo. Percentage of Brn3a (68% ±11%) or AP2 (28% ±7%) cells among PD-1 positive cells (left). Percentage of PD-1 positive (95% ±3.4%) and PD-1 negative (5% ±1.6%) cells among Brn3a positive RGCs (right). Percentages represent mean ±S.E.M. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 50μm. These findings are representative of four experiments.
Figure 2.
Confirmation of PD-1 expression in adult RGCs. (A) Double staining of PD-1 with NeuN on vertical retina sections. GCL is shown, with top to bottom panels representing NeuN (red), PD-1 (green), merge (yellow), and nuclear DAPI (blue) staining. Scale bar, 50μm. (B) Quantification of NeuN positive population in vivo. Percentage of PD-1 positive (99±0.2%) and PD-1 negative (1.2±0.2%) cells among NeuN positive RGCs is shown. These findings are representative of three experiments.
Dynamic Developmental Expression of PD-1 in the Retina
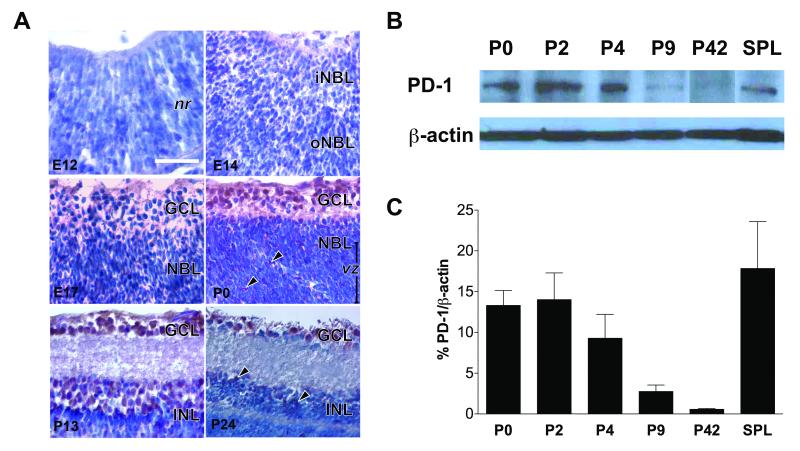
We next characterized the regulation of PD-1 expression in developing retina. RGCs are the first retinal neurons, emerging after embryonic day 11 (E11) in mice and completing maturation after a postnatal retinal maturation process22. We therefore examined the spatio-temporal expression pattern of PD-1 at defined times during the period of retinal development. Low levels of PD-1 expression were initially detected in the inner and outer neuroblast layers by immunohistochemistry at E14 (Fig. 3A), around the peak period of RGC genesis. Weak expression of PD-1 appeared throughout both the GCL, containing RGCs, and the neuroblast layer, containing migrating RGC precursors and retinal progenitors (Fig. 3A, E17). By postnatal day 0 (P0), relatively high levels of PD-1 were present at the inner retina, where postmigratory RGC precursors undergo differentiation; substantial PD-1 expression was also detected in a subset of proliferating neuroblasts (arrows) occupying the ventricular zone (vz). In contrast to the embryonic and adult time points, PD-1 expression appeared greatest at P0 and P13 (Fig. 3A). In the adult retina, the PD-1 expression appeared decreased, with expression retained in the GCL and in a subset of INL neurons (Fig. 3A, P24, arrows). These observations by immunohistochemistry were confirmed through quantification of postnatal PD-1 protein expression by Western blot analysis (Fig. 3B). Retinal PD-1 expression was greatest during P0-P4, coinciding with a very active time of neuronal culling23; total PD-1 expression decreased dramatically, 85% by P9, and 96% P42, as compared to P0 (Fig. 3C). Together, these findings indicate that PD-1 is present in the developing retina, especially in the differentiating RGCs, and the dramatic increase in PD-1 expression coincides with the period of retinal cell maturation and synaptogenesis.
Figure 3.
Dynamic regulation of PD-1 expression during retinal development. (A) Immunohistochemical staining on retinal sections from embryonic days E12, E14, E17, and postnatal days P0, P13, P24. PD-1 (red) and hematoxylin (blue). nr, neural retina; iNBL & oNBL, inner & outer neuroblast layer; GCL, ganglion cell layer; vz, ventricular zone; INL, inner nuclear layer. Scale bar, 50μm. These findings are representative of three experiments. (B) Western blot for PD-1 at P0, P2, P4, P9, P42, and spleen as a positive control. β-actin was used as a loading control. (C) Quantification of PD-1 immunoblots. PD-1 expression decreases by 80±3.8% by P9, and 96±0.9% by P42, as compared to P0 expression; values reported as mean±S.E.M. p=0.03 by ANOVA analysis across all retina samples. SPL, spleen. Negative controls for reagent (secondary antibody only) and tissue (skeletal muscle) showed no detectable staining (data not shown). These findings are representative of three animals per time point.
PD-1 Signaling Modulates Apoptosis of Neonatal RGCs
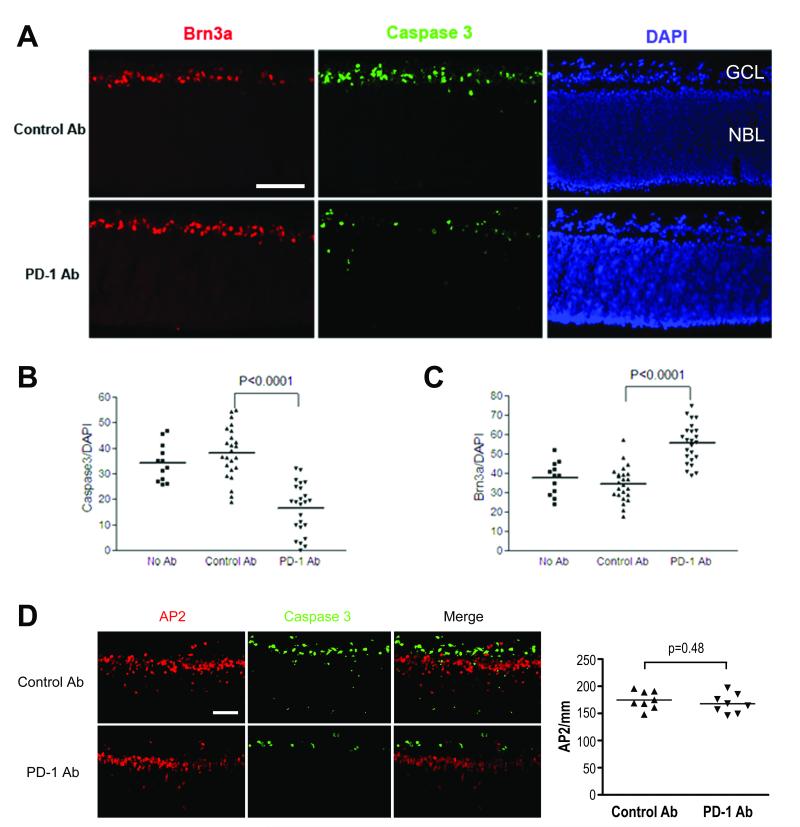
During murine postnatal retina maturation, 50% of RGCs undergo programmed cell death (PCD)23, representing one of the key mechanisms regulating cell-number homeostasis. To test whether functional PD-1 signaling is involved in apoptosis of neonatal RGCs, we performed a study on postnatal retinal explants, a culture system which preserves neuronal connections and retinal cytological architecture and serves as an in vitro model to study the developing retina. Neonatal retinal explants were treated with an antibody that blocks PD-1 function and compared to both isotype control antibody treated and untreated explants. After a 24-hour incubation, explants were evaluated for expression of cleaved caspase-3, Brn3a, and AP2, as markers for apoptosis, RGCs, and amacrine cells, respectively.
When retinal explants were cultured in the presence of a PD-1 blocking antibody, there was a significant decrease in caspase-3-mediated apoptosis in the GCL of retinal explants (p<0.0001; Fig. 4A, B). In contrast, isotype control antibody and untreated controls had indistinguishable levels of apoptosis (p=0.25, Fig. 4B). Brn3a, a specific marker for RGCs, is observed to be downregulated or absent during RGC apoptosis24. A significant increase in the number of Brn3a positive cells was observed in the explants that were exposed to the PD-1 functional blocking antibody (p<0.0001; Fig. 4A, C), concordant with the decreased apoptosis in the GCL. We also assessed the effect of blocking PD-1 in amacrine cells, a subset of which express PD-1 (Fig. 4D). We considered amacrine cells as a specificity control, since unlike RGCs, amacrine cells are resistant to apoptosis during the first two days after birth25. Amacrine cells did not undergo apoptosis in our in vitro system (Fig. 4D), and there were no significant changes in numbers of amacrine cells after administration of antibodies (p=0.48; Fig. 4D). Taken together, the data suggest that PD-1 signaling is important in murine postnatal retinal maturation, where active signaling through the PD-1 axis may be critical for caspase-3-mediated RGC apoptosis during this developmental period.
Figure 4.
PD-1 blockade inhibits apoptosis of retinal ganglion cells in the developing retina. Neonatal retinal explants were incubated for 24 hours in basal medium containing isotype control antibody (top panel) or PD-1 blocking antibody (bottom panel) (A and D). (A, D) Immunofluorescent staining was performed with antibodies against Brn3a (red, A) or AP2 (red, D), activated form of caspase-3 (green), and DAPI (blue). (B, C) Quantification of percentage of Brn3a positive cells among DAPI positive cells (B) or caspase-3 positive cells among DAPI positive cells (C) in the GCL in three experimental groups, as indicated in the figure. (D) Amacrine cell survival is not affected by PD-1 blockade. Immunofluorescent staining was performed and the merge image supports lack of colocalization of cleaved caspase-3 and AP2 positive amacrine cells. Quantification of AP2 positive cells also revealed no difference between the anti-PD-1 and control antibody treatments. Scale bars, 100μm. All findings are representative of three experiments.
Absence of PD-1 Selectively Increases RGC Cellularity During the Peak of Developmental RGC Apoptosis
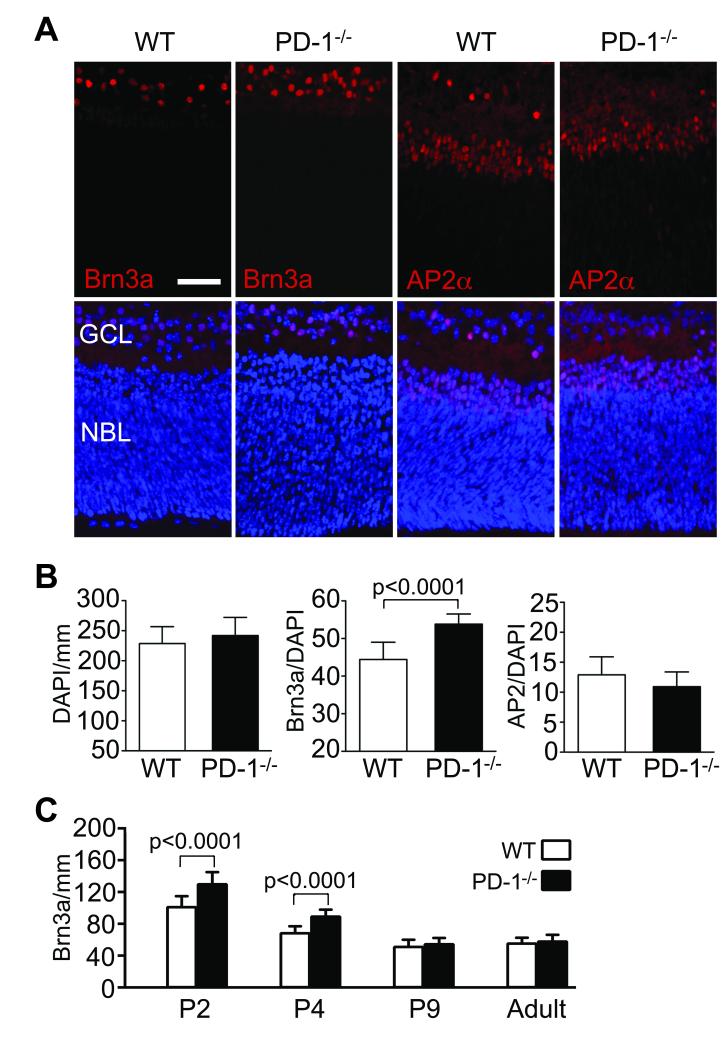
To test the physiological importance of PD-1 in eye development, we compared retinal structure and cellular composition in the developing PD-1-/- mouse retina to aged-matched wildtype C57BL/6 controls. To assess GCL composition, immunofluorescent staining was performed using the RGC and amacrine cell specific nuclear markers Brn3a and AP2, respectively (Fig. 5A). Total cell number in the GCL, as measured by the number of DAPI positive cells/mm, was not significantly changed in the absence of PD-1 at P2 (Fig. 5B). However, during this critical developmental window the PD-1-/- retina had a significant increase in the fraction of Brn3a positive RGCs (p<0.0001) within the GCL, where “fraction” refers to Brn3a/DAPI and will hereafter be referred to as “RGC fraction”. In contrast, there was no significant change in the fraction of AP2 positive amacrine cells (p=0.15) within this layer (Fig. 5B). In the PD-1-/- animals, at P2 and P4, the peak of naturally occurring PCD, there was an increase in number of Brn3a positive cells in the GCL (p<0.0001 for each, Fig. 5C); RGC fraction was also significantly increased at P2 and P4 (p≤0.0003 for each, data not shown). The early developmental increase in RGC number did not persist: by P9 and subsequent time points, the PD-1-/- retina showed no significant difference in either RGC number (P9 p=0.62, adult p=0.052, Fig. 5C) or RGC fraction (P9 p=0.40, adult p=0.29, data not shown), as compared to wildtype. These data show that the absence of PD-1 is associated with a transiently increased RGC number during the peak of post-natal neuronal culling in the mouse retina, providing further evidence for the importance of PD-1 function during the critical window of retinal maturation.
Figure 5.
Increased RGCs during postnatal retinal development in the PD-1-/- mouse. (A) Immunofluorescence staining was performed on both wildtype and PD-1-/- P2 retina cryosections using antibodies against either Brn3a (red) or AP2 (red). Nuclei were visualized with DAPI (blue). NBL, neuroblast layer. (B) Within the GCL, quantification of total cells (DAPI/mm), Brn3a+ cells (Brn3a/DAPI), and AP2+ cells (AP2/mm), at postnatal day P2, in wildtype and PD-1-/-. p-values denote significant differences between WT and PD-1-/-. (C) Quantification of Brn3a in wildtype and PD-1-/- retinal sections from P2, P4, P9, and adult (8 week old) animals was performed as noted. p-values denote significant differences, at P2 and P4, between WT and PD-1-/-. Scale bar, 50μm.
PD-1 ligand Genes Are Expressed Throughout Postnatal Retina Maturation
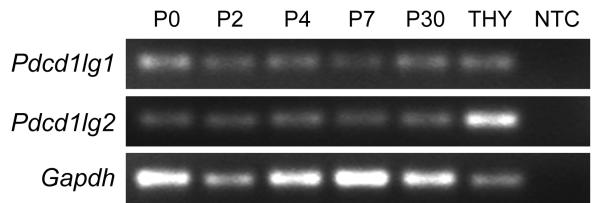
To investigate the cell surface interactions responsible for PD-1 mediated PCD in the developing mouse retina, we wished to characterize expression of the PD-1 ligands present during retina development. There are two known PD-1 ligands in the immune system, PD-1 ligand 1 and ligand 2 (PD-L1 and PD-L2), which upon ligation with PD-1, function to regulate the balance between lymphocyte activation and suppression5. In mice, PD-L2 expression is thought to be restricted to hematopoietic cell types, while PD-L1 protein is widely expressed in many organs, including immunoprivileged regions such as placenta5, cornea endothelium and ciliary body of the eye26, and brain27. PD-L1 is transcribed in the mature mouse retina26, making it a strong candidate for the PD-ligand functioning during developmental culling. We examined PD-L1 and PD-L2 expression during postnatal development at P0, 2, 4, 7, and 30 by reverse transcription PCR (RT-PCR) and found both ligands to be expressed at all ages studied (Fig. 6), supporting the idea that PD-L1 and/or PD-L2 are the activating signal for PD-1 mediated PCD.
Figure 6.
Expression of PD-L1 and PD-L2 throughout retina maturation. RT-PCR products showing Pdcd1lg1 (PD-L1 gene) and Pdcd1lg2 (PD-L2 gene) expression at various ages, with Gapdh as an endogenous control and thymus as a positive control. NTC, no template control.
Discussion
Programmed cell death (PCD) regulates central nervous system (CNS) cell-number homeostasis and formation of functional neuronal networks28, 29, through a balance of survival factors and death signals22. In particular, it has been well established that neurotrophic factors and electrical activity contribute to postnatal RGC survival25, 30-34. This study introduces a distinct hypothesis that PCD may also result from an active negative selection process through the PD-1 pathway. PD-1 protein is transiently up-regulated in the GCL and INL during the critical postnatal period of RGC target-finding and synaptogenesis. This spatio-temporal pattern is consistent with an important role for PD-1 receptor function in RGC culling. Our functional perturbations, using a PD-1 receptor-neutralizing antibody in vitro and PD-1 deficient mice in vivo, have demonstrated selective increases in RGC survival, thus strongly supporting a role for PD-1 signaling during the peak period of RGC physiological PCD. PD-1 is also developmentally expressed in a subset of cells located within the ventricular zone of the neonatal retina, where retinal progenitors are localized, so it is possible that PD-1 ligation has an additional role in regulating the survival of progenitors. Whether the observed RGC protective effect functions directly through a blockade on RGCs, or indirectly through an effect on progenitors, is an important consideration. Since no overt altered phenotypes have been detected in other postnatally generated cell types, such as amacrine cells, in PD-1 null retinas, it is unlikely that PD-1 plays a significant developmental role in controlling amacrine cell number. The role of PD-1 expression and definition of the amacrine cell subset expressing this molecule requires additional investigation. Our results thus support a direct function for PD-1 signaling in RGC survival. We present the first evidence that PD-L1 and PD-L2 are transcribed throughout postnatal retina maturation. PD-Ligands may be expressed by either neuronal, endothelial, or resident immune (e.g., macrophage or dendritic) cell types; spatio-temporal localization of these ligands will be key to understanding which cell compartments experience the consequences of PD-1 mediated signaling during development. However, currently we cannot rule out the possibility that, in addition to the known PD-ligands, novel PD-ligand-like molecules could be involved in CNS-specific functions of PD-1 signaling.
Since PD-1 ligation does not directly engage apoptotic pathways in T cells, but rather acts as a co-signaling event, reducing signal strength from the TCR during thymocyte selection6, we propose a similar mechanism for PD-1 signaling during RGC culling: PD-1 may act to modulate the strength of neuronal survival signals. We have determined that the TCR subunits TCRβ and CD3e are not expressed in the developing or adult retina, by RT-PCR and immunostaining (data not shown). Thus, TCR is not the neuronal co-receptor and identification of such molecules will be an important next step. It should be noted that the PD-1-/- retina shows only a transient increase in RGCs, with normal cellularity after retinal maturation is complete. This implies the role for additional mechanisms that determine the overall outcome of neuronal culling in the retina, presumably including those previously delineated25, 30-34. Since immune PD-ligands are known to have functional interactions with molecules other than PD-135, we cannot rule out the possibility that one or both PD-ligands could be necessary for RGC culling. While normal RGC cellularity is eventually achieved in PD-1-/- mice, it will be important to determine if the temporal disorder of RGC formation has consequences for definitive retina organization, retinogeniculate synapse formation, and visual function.
Programmed cell death of RGCs is prominent during the first 11 days after birth, peaking during the first 5 days28, 36, with a key role for caspase-318, 37. Our findings are consistent with a PD-1 mediated mechanism for physiological apoptosis through a caspase-dependent pathway, as a PD-1 blockade decreases caspase-3 induction. The retinal explant system used in this study involves experimental axotomy, but axotomy-induced RGC death is an unlikely explanation for our observations since it is independent of caspase activation in the neonatal retina.38 While the precise PD-1-mediated apoptotic mechanism has yet to be defined, two possible mediators are pro-apoptotic Bax39 and anti-apoptotic Bcl-240, both known to be critical intracellular effector molecules regulating developmental RGC apoptosis in mouse models41. Furthermore, Bcl-2 is negatively regulated by PD-1 mediated signaling during immunological thymocyte maturation6.
According to the trophic factor theory for neuronal survival, RGCs die after unsuccessful competition for target-derived trophic factors, with stable connectivity also influenced by neuronal activity.33, 34 The present study provides evidence for an additional regulatory mechanism: RGC death can also involve an active process of negative selection. It is notable that developmental RGC apoptosis may be similar to the molecular pathogenesis of RGC degeneration.42, 43 Intriguingly, the PD-1 receptor is constitutively expressed and PD-1 ligands are transcribed in the adult retina, a state where there is no active cell death, and we speculate that PD-1 ligation may trigger RGC death upon optic nerve damage. In addition, since PD-1 is constitutively expressed across neuronal populations in many cerebral compartments8, PD-1 signaling might also act to augment neuronal injury in cerebral disease. Accordingly, the present study gives impetus to investigation of the role of PD-1 in neurodegenerative diseases and in specific therapies for neurodegenerative disorders.
Acknowledgments
Funding Information: Supported by the Research to Prevent Blindness Foundation (LKG); a generous gift from Richard and Barbara Braun (LC); National Institutes of Health (NIH) Grant T32 GM08042, UCLA Medical Scientist Training Program (CWS); NIH/National Institute of Allergy and Infectious Diseases Grant RO1 AI021256, UCLA Immunopathology Training Grant (CWS); and NIH Grant PO1 AI056299 (AHS and GJF).
References
- 1.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 2.Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–327. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Pai V, Levinson R, et al. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Ocul Immunol Inflamm. 2009;17:47–55. doi: 10.1080/09273940802491884. [DOI] [PubMed] [Google Scholar]
- 9.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 10.Kwong JM, Lalezary M, Nguyen JK, et al. Co-expression of heat shock transcription factors 1 and 2 in rat retinal ganglion cells. Neurosci Lett. 2006;405:191–195. doi: 10.1016/j.neulet.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 11.Goureau O, Rhee KD, Yang XJ. Ciliary neurotrophic factor promotes muller glia differentiation from the postnatal retinal progenitor pool. Dev Neurosci. 2004;26:359–370. doi: 10.1159/000082278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem. 2002;277:24466–24474. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 13.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Khare SL, Liang X, et al. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127:3237–3247. doi: 10.1242/dev.127.15.3237. [DOI] [PubMed] [Google Scholar]
- 16.Buckingham BP, Inman DM, Lambert W, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West-Mays JA, Zhang J, Nottoli T, et al. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- 18.Gashegu J, Ladha R, Vanmuylder N, et al. HSP110, caspase-3 and -9 expression in physiological apoptosis and apoptosis induced by in vivo embryonic exposition to all-trans retinoic acid or irradiation during early mouse eye development. J Anat. 2007;210:532–541. doi: 10.1111/j.1469-7580.2007.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podesta F, Romeo G, Liu WH, et al. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000;156:1025–1032. doi: 10.1016/S0002-9440(10)64970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277:41094–41100. doi: 10.1074/jbc.M206868200. [DOI] [PubMed] [Google Scholar]
- 21.Barnstable CJ, Drager UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- 22.Cellerino A, Bahr M, Isenmann S. Apoptosis in the developing visual system. Cell Tissue Res. 2000;301:53–69. doi: 10.1007/s004410000178. [DOI] [PubMed] [Google Scholar]
- 23.Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229:362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- 24.Weishaupt JH, Klocker N, Bahr M. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. J Mol Neurosci. 2005;26:17–25. doi: 10.1385/JMN:26:1:017. [DOI] [PubMed] [Google Scholar]
- 25.ElShamy WM, Ernfors P. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 complement and cooperate with each other sequentially during visceral neuron development. J Neurosci. 1997;17:8667–8675. doi: 10.1523/JNEUROSCI.17-22-08667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 27.Lipp M, Brandt C, Dehghani F, Kwidzinski E, Bechmann I. PD-L1 (B7-H1) regulation in zones of axonal degeneration. Neurosci Lett. 2007;425:156–161. doi: 10.1016/j.neulet.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 28.Vecino E, Hernandez M, Garcia M. Cell death in the developing vertebrate retina. Int J Dev Biol. 2004;48:965–974. doi: 10.1387/ijdb.041891ev. [DOI] [PubMed] [Google Scholar]
- 29.Davies AM. Regulation of neuronal survival and death by extracellular signals during development. EMBO J. 2003;22:2537–2545. doi: 10.1093/emboj/cdg254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res. 2003;22:483–543. doi: 10.1016/s1350-9462(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 31.Du JL, Poo MM. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature. 2004;429:878–883. doi: 10.1038/nature02618. [DOI] [PubMed] [Google Scholar]
- 32.Dunker N, Krieglstein K. Reduced programmed cell death in the retina and defects in lens and cornea of Tgfbeta2(-/-) Tgfbeta3(-/-) double-deficient mice. Cell Tissue Res. 2003;313:1–10. doi: 10.1007/s00441-003-0761-x. [DOI] [PubMed] [Google Scholar]
- 33.Lipton SA. Blockade of electrical activity promotes the death of mammalian retinal ganglion cells in culture. Proc Natl Acad Sci U S A. 1986;83:9774–9778. doi: 10.1073/pnas.83.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 35.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Prog Retin Eye Res. 2001;20:139–174. doi: 10.1016/s1350-9462(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 37.Mayordomo R, Valenciano AI, de la Rosa EJ, Hallbook F. Generation of retinal ganglion cells is modulated by caspase-dependent programmed cell death. Eur J Neurosci. 2003;18:1744–1750. doi: 10.1046/j.1460-9568.2003.02891.x. [DOI] [PubMed] [Google Scholar]
- 38.Spalding KL, Dharmarajan AM, Harvey AR. Caspase-independent retinal ganglion cell death after target ablation in the neonatal rat. Eur J Neurosci. 2005;21:33–45. doi: 10.1111/j.1460-9568.2004.03826.x. [DOI] [PubMed] [Google Scholar]
- 39.Pequignot MO, Provost AC, Salle S, et al. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003;228:231–238. doi: 10.1002/dvdy.10376. [DOI] [PubMed] [Google Scholar]
- 40.Donovan M, Doonan F, Cotter TG. Decreased expression of pro-apoptotic Bcl-2 family members during retinal development and differential sensitivity to cell death. Dev Biol. 2006;291:154–169. doi: 10.1016/j.ydbio.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Nickells RW. The molecular biology of retinal ganglion cell death: caveats and controversies. Brain Res Bull. 2004;62:439–446. doi: 10.1016/j.brainresbull.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Doonan F, Cotter TG. Apoptosis: a potential therapeutic target for retinal degenerations. Curr Neurovasc Res. 2004;1:41–53. doi: 10.2174/1567202043480215. [DOI] [PubMed] [Google Scholar]
- 43.Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]