Abstract
Objectives. The United States has the highest prevalence of obesity and one of the lowest life expectancies among high-income countries. We investigated the relationship between these 2 phenomena.
Methods. We estimated the fraction of deaths attributable to obesity by country, age, and sex and reestimated life tables after removing these deaths. To allow for a possible secular decline in obesity risks, we employed alternative risks from a more recent period.
Results. In our baseline analysis, obesity reduced US life expectancy at age 50 years in 2006 by 1.54 years (95% confidence interval [CI] = 1.37, 1.93) for women and by 1.85 years (95% CI = 1.62, 2.10) for men. Removing the effects of obesity reduced the US shortfall by 42% (95% CI = 36, 48) for women and 67% (95% CI = 57, 76) for men, relative to countries with higher life expectancies. Using more recently recorded risk data, we estimated that differences in obesity still accounted for a fifth to a third of the shortfall.
Conclusions. The high prevalence of obesity in the United States contributes substantially to its poor international ranking in longevity.
Life expectancy in the United States has fallen below that of most other industrialized countries and ranked 32nd in the world in 2008.1 As President Obama has noted, the relatively low level of life expectancy in the United States coexists with the highest per capita expenditure on health care in the world.2 Explanations of the low US ranking range from a history of high levels of cigarette smoking to low levels of physical activity, a poorly performing health care sector, high levels of income inequality, and high levels of obesity.3 Identifying the responsible factors would help to clarify the critical public health domains where the United States has fallen furthest behind its peers.
We estimated the extent to which the high level of obesity in the United States is contributing to its low longevity. According to World Health Organization estimates, men and women in the United States had a higher prevalence of obesity in 2005—defined as having a body mass index (BMI; defined as weight in kilograms divided by the square of height in meters) of 30.00 or higher—than did any other country in Europe, North America, or East Asia.4 Because many studies demonstrate that obese individuals suffer an elevated risk of death,5 it is reasonable to suppose that the high level of obesity in the United States is contributing to its comparatively low life expectancy.
The years of life lost by an individual as a result of his or her obesity have been estimated in several studies.5,6 In this study, we ask a question about population health rather than individual health: how many years of life are forfeited, on average, by members of a population as a result of the level of obesity in that population? Answering this question involves combining the prevalence of obesity in a population with the risks of mortality for people in a particular BMI category to estimate the effects of obesity on age-specific mortality rates. Estimates of the impact of obesity on a population's level of life expectancy are uncommon; an exception is Olshansky et al., whose effort was limited to the United States.7 However, these estimates are important because they provide a basis for conducting cross-national comparisons that can be used to determine why some countries achieve better health outcomes than others.
We estimated the fraction of deaths attributable to obesity by age and sex for 16 countries, including the United States, and the extent of international variation in life expectancy attributable to differences in BMI distributions. We focused on persons aged 50 years and older because 94% of newborns survive to age 50 years in the current US life table, and variation in life expectancy at birth is dominated by variation in mortality above this age.8,9
METHODS
We used BMI as our basic indicator of obesity. Flegal and Graubard have shown that the proportion of deaths attributable to obesity does not vary significantly with the indicator chosen.10 In our baseline analysis, we assumed that the relative mortality risks in various BMI categories by age and sex that were recorded in a study by the Prospective Studies Collaboration (PSC) are applicable to all countries considered.5 The PSC study is the largest and most detailed of several large compilations of data on obesity and mortality.11 The synthesis includes data on 895 000 participants from 57 prospective studies, of whom 63% were from Europe and Israel, 29% were from the United States and Australia, and 8% were from Japan. Results of the PSC investigation have been presented by sex, age group (35–59, 60–69, 70–79, and 80–89 years), and detailed BMI categories (2.5-unit intervals within the range 15.00–34.99 and a single interval for 35.00–49.99).5
We estimated population distributions of BMI from nationally representative survey data. Height and weight data for estimating an individual's BMI are based on self-reports obtained through in-person interviews, except in Canada and England, where we used data on measured height and weight. For the United States, we used both self-reported and measured values.
We obtained data for European countries, excluding England, from the Survey of Health, Ageing and Retirement in Europe, including individuals interviewed in wave 1 (2004) as well as a refresher sample from wave 2 (2006–2007). We obtained data for England from wave 2 (2004–2005) of the English Longitudinal Study of Ageing. US data came from the National Health and Nutrition Examination Survey (NHANES) cycles 2003–2004, 2005–2006, and 2007–2008. Previous research has found no significant national trend in adult obesity for either sex during this period in the United States.12 Data for Canada were taken from cycle 3.1 (2005) of the Canadian Community Health Survey. We constructed period life tables by country, age (in single-year age intervals), and sex using data from the Human Mortality Database (HMD)13 on deaths and population in 2006.
To identify the proportion of deaths in a particular country, age, and sex category that were attributable to obesity, we hypothetically redistributed the population above the optimal BMI category (i.e., the lowest-mortality category) in that group to the optimal category and calculated the proportional reduction in mortality that would occur under this redistribution. This is known as the population attributable fraction (PAF). We constructed estimates of BMI prevalence in the same age-sex-BMI groupings as used by the PSC except that we applied the PSC mortality values for ages 35 through 59 years to ages 50 through 59 years. In the PSC, the lowest-risk BMI category is 22.50 to 24.99, except for males aged 80 through 89, for whom it is 20.00 to 22.49, and women aged 70 through 79, for whom it is 25.00 to 27.49. We used the term obesity to refer to all weight categories above the optimal, including overweight (BMI = 25.00–29.99). We did not change the proportion of persons below the optimal BMI category because our interest was in the effect of obesity on mortality. Throughout our analysis, we assumed the mortality risk from obesity to be zero after age 90. We estimated the PAF for population i (where i is an indicator for each country, age, and sex combination) as
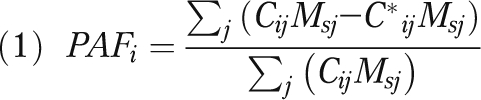 |
where Cij = proportion of population i in BMI category j, Msj = death rate in BMI category j in the standard drawn from PSC data, and C*ij = proportion of population i in BMI category j if all individuals above the optimal BMI were redistributed to the optimal category.
Equation 1 would give the same value of the PAF if the death rates were in the form of relative risks (e.g., if numerator and denominator were divided by the death rate in the optimal category).
We applied the country-, age-, and sex-specific PAFs to death rates in the HMD in single-year age intervals to estimate what these rates would be if no one were obese. We then calculated life expectancy at age 50 years using the modified death rates. Conventional methods of calculating life tables were used.14 We then compared hypothetical life expectancies obtained in this manner with the actual values, also computed from the HMD, by country and sex. To identify the extent to which the US shortfall in life expectancy is attributable to obesity, we compared differences in actual life expectancy between the United States and each country with the differences that would be expected in the absence of obesity. When Canada and England were compared with the United States, we used measured rather than self-reported heights and weights.
We conducted analysis of uncertainty for PAFs and life expectancy estimates using a bootstrapping procedure.15 We combined uncertainty estimates from 2 sources: uncertainty in the BMI data resulting from sampling variability and uncertainty in estimation of the relative risks. For each country, age, and sex combination, we sampled BMI values randomly with replacement as many times as there were nonmissing observations on BMI in that country, age, and sex category. To incorporate uncertainty from the relative risks, vectors of the underlying effect parameters of relative risks of length corresponding to the number of BMI intervals were drawn from independent normal distributions with age- and sex-specific standard errors provided to us by the PSC. We applied the resulting vectors of risks to the simulated BMI distribution data to obtain country-, age-, and sex-specific PAFs. We repeated these steps to obtain 500 estimates of each country-, age-, and sex-specific attributable fraction from which we extracted the 2.5 and 97.5 percentile values as 95% confidence intervals.
We explored the sensitivity of results to the assumed set of risks associated with obesity and to misreporting of height and weight. Flegal et al. have suggested that the relative risks of death associated with obesity have declined in the United States.16 To investigate the effect of a possible reduction in obesity risks on international comparisons, we introduced an alternative set of risk factors adapted from Adams et al. that applies to a more recent period.17 These were derived from a large study of 527 000 enrollees in the National Institutes of Health–American Association for Retired Persons Diet and Health Study,17 which was conducted in 6 US states and 2 cities. Enrollees were followed from enrollment in 1995 and 1996 through the end of 2005. As in the PSC results, relative risks were adjusted for smoking. In contrast to PSC procedure, relative risks in Adams et al. were also adjusted for social status and physical activity.
We used the published results of Adams et al. to estimate relative risks in the age categories that were used in the baseline analysis using data from the PSC. To do so, we fit a linear age trend using weighted least squares to risks that were originally reported in 4 age intervals (50–65, 56–70, 61–75, and 66–81 years). From primary data, we recalculated the proportions in various BMI intervals in each country to align with the categories used by Adams et al. We approximated standard errors for uncertainty estimation because of the smoothing procedure we employed to obtain risks for the relevant ages.
Analysis of NHANES data shows that American women tend to underestimate their weight, whereas both men and women tend to overestimate height at older ages.18 To explore whether our results were sensitive to error in self-reports of height and weight, we replicated all analyses after correcting self-reported height and weight for misreporting, using an approach similar to one applied elsewhere.19 Using data on adults aged 50 years and older from NHANES 2003–2008, for each sex, we estimated linear regression models of measured height (weight) vs self-reported height (weight), age, and the square of age.
We conducted analyses using Stata 10.1 (Stata Corp, College Station, TX) and R 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Table 1 presents sample sizes in each country and the proportion of persons who were in or above the standard BMI categories of overweight (BMI = 25.00–29.99), obese class I (BMI = 30.00–34.99), obese class II (BMI = 35.00–39.99), and obese class III (BMI ≥ 40.00). The proportion of individuals exceeding the thresholds for obesity classes I, II, and III was higher in the United States than in any comparison country for both men and women. The proportionate difference between the United States and other countries grew larger as BMI increased. In Canada and England, prevalence rates for obese class I and above exceeded rates found in other countries, but remained consistently lower than those in the United States. The difference was most pronounced for severe obesity, with the prevalence of class III obesity in Canada and England being about half of the level found in the United States.
TABLE 1.
Cumulative Prevalence of Overweight and Obesity by Country Among Adults Aged 50–89 Years
| Women Aged 50–89 Years |
Men Aged 50–89 Years |
||||||||
| Country | Sample Size, No. | BMI ≥ 25, % (95% CI) | BMI ≥ 30, % (95% CI) | BMI ≥ 35, % (95% CI) | BMI ≥ 40, % (95% CI) | BMI ≥ 25, %(95% CI) | BMI ≥ 30, % (95% CI) | BMI ≥ 35, % (95% CI) | BMI ≥ 40, % (95% CI) |
| Estimates based on self-reported height and weight | |||||||||
| Comparison countries | |||||||||
| Austria | 1840 | 57.0 (53.8, 60.2) | 21.0 (18.5, 23.8) | 4.7 (3.5, 6.3) | 1.5 (0.9, 2.6) | 69.2 (65.5, 72.6) | 19.3 (16.4, 22.6) | 3.9 (2.6, 5.8) | 1.1 (0.5, 2.5) |
| Belgium | 2933 | 53.2 (50.6, 55.8) | 17.7 (15.8, 19.7) | 4.1 (3.2, 5.2) | 0.8 (0.4, 1.3) | 63.8 (61.2, 66.4) | 17.4 (15.5, 19.6) | 2.9 (2.1, 3.9) | 0.5 (0.2, 1.1) |
| Czech Republic | 1768 | 67.7 (63.4, 71.7) | 22.8 (19.2, 26.7) | 4.6 (3.1, 6.9) | 1.0 (0.5, 2.1) | 75.9 (71.6, 79.7) | 21.8 (18.3, 25.8) | 2.7 (1.7, 4.2) | 0.8 (0.3, 1.9) |
| Denmark | 1756 | 43.7 (40.4, 47.1) | 13.0 (10.9, 15.5) | 3.0 (2.1, 4.3) | 0.6 (0.3, 1.3) | 59.0 (55.4, 62.5) | 13.2 (10.9, 15.8) | 1.9 (1.1, 3.0) | 0.4 (0.1, 1.3) |
| France | 2774 | 46.0 (42.7, 49.3) | 15.5 (13.0, 18.4) | 3.2 (2.3, 4.6) | 1.2 (0.6, 2.3) | 61.6 (57.8, 65.4) | 16.5 (13.8, 19.7) | 3.0 (1.7, 5.1) | 0.3 (0.1, 1.8) |
| Germany | 2885 | 54.8 (51.9, 57.6) | 15.8 (13.8, 17.9) | 4.4 (3.4, 5.7) | 1.4 (0.8, 2.2) | 67.9 (64.9, 70.7) | 16.4 (14.3, 18.9) | 3.8 (2.8, 5.3) | 0.8 (0.4, 1.7) |
| Israel | 2146 | 57.9 (48.7, 66.6) | 19.0 (13.9, 25.4) | 3.5 (2.0, 6.2) | 1.0 (0.3, 2.7) | 64.3 (57.5, 70.5) | 14.0 (10.1, 19.1) | 3.3 (1.3, 7.9) | 0.6 (0.1, 2.7) |
| Italy | 2751 | 53.2 (50.0, 56.4) | 15.9 (13.8, 18.3) | 3.1 (2.2, 4.2) | 0.6 (0.3, 1.1) | 67.3 (63.9, 70.5) | 14.9 (12.6, 17.5) | 3.1 (2.1, 4.6) | 0.2 (0.1, 0.5) |
| Netherlands | 2812 | 52.7 (49.8, 55.6) | 15.9 (14.0, 18.1) | 4.3 (3.3, 5.7) | 1.5 (0.9, 2.4) | 62.5 (59.1, 65.8) | 12.7 (10.9, 14.9) | 2.4 (1.7, 3.5) | 0.4 (0.2, 0.9) |
| Poland | 1681 | 68.3 (64.9, 71.6) | 27.3 (24.4, 30.5) | 6.2 (4.8, 8.1) | 1.1 (0.6, 1.9) | 64.5 (60.7, 68.1) | 20.1 (17.3, 23.3) | 4.0 (2.7, 5.8) | 0.8 (0.3, 1.9) |
| Spain | 1994 | 66.5 (63.2, 69.6) | 23.9 (21.2, 26.8) | 7.5 (6.0, 9.4) | 1.9 (1.1, 3.1) | 71.9 (68.4, 75.1) | 20.6 (17.8, 23.7) | 3.7 (2.4, 5.4) | 0.3 (0.1, 1.0) |
| Sweden | 2966 | 52.1 (49.1, 55.0) | 16.4 (13.8, 19.2) | 3.3 (2.4, 4.4) | 0.9 (0.5, 1.7) | 56.7 (52.6, 60.6) | 13.9 (10.4, 18.3) | 4.2 (1.6, 10.3) | 0.5 (0.2, 1.2) |
| Switzerland | 1615 | 41.4 (38.0, 44.9) | 12.5 (10.4, 15.0) | 3.1 (2.1, 4.6) | 0.7 (0.3, 1.6) | 61.8 (58.2, 65.4) | 14.2 (11.7, 17.1) | 2.5 (1.6, 4.1) | 0.0 (0.0, 0.0) |
| Average | 2302 | 55.0 (54.0, 56.2) | 18.2 (17.5, 19.1) | 4.2 (3.9, 4.7) | 1.1 (0.9, 1.3) | 65.1 (64.0, 66.1) | 16.5 (15.7, 17.3) | 3.2 (2.7, 3.7) | 0.5 (0.4, 0.7) |
| United States | 7526 | 61.9 (59.7, 64.0) | 31.0 (29.1, 32.9) | 12.3 (11.6, 13.2) | 5.1 (4.3, 6.0) | 73.3 (71.3, 75.2) | 30.9 (28.6, 33.2) | 8.8 (7.5, 10.3) | 2.5 (1.9, 3.3) |
| Estimates based on measured height and weight | |||||||||
| Comparison countries | |||||||||
| Canada | 1979 | 65.5 (60.9, 69.9) | 28.6 (24.9, 32.7) | 11.8 (9.2, 15.1) | 3.2 (2.0, 5.3) | 79.3 (75.0, 83.0) | 32.9 (27.9, 38.4) | 5.9 (4.1, 8.4) | 1.7 (0.8, 3.7) |
| England | 7153 | 69.6 (68.1, 71.1) | 31.0 (29.5, 32.6) | 9.8 (8.9, 10.9) | 3.0 (2.5, 3.6) | 75.9 (74.3, 77.4) | 27.2 (25.5, 28.9) | 6.0 (5.1, 6.9) | 1.0 (0.7, 1.5) |
| United States | 6511 | 67.4 (65.2, 69.4) | 36.3 (34.0, 38.6) | 16.5 (15.0, 18.1) | 7.0 (6.0, 8.1) | 76.9 (75.2, 78.5) | 34.6 (32.5, 36.9) | 11.7 (10.2, 13.3) | 3.3 (2.6, 4.2) |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); CI = confidence interval. Table presents data on the cumulative distribution of overweight and obesity based on various thresholds of BMI. Prevalence rates are age-standardized to the US 2000 Census population using age groups 50–59, 60–69, 70–79 and 80–89 years. Sampling weights were used to adjust BMI estimates for unequal selection probabilities and standard errors were adjusted for cluster design and stratification where this information was available.
Source. Sources and years of data are as follows: United States, National Health and Nutrition Examination Survey, cycles 2003–2004, 2005–2006, and 2007–2008; Canada, Canadian Community Health Survey, cycle 3.1 (2005); European countries excluding England, Survey of Health, Ageing and Retirement in Europe, wave 1 (2004) and refresher sample from wave 2 (2006–2007); England, English Longitudinal Study of Ageing, wave 2 (2004–2005).
Figure A (available as a supplement to the online version of this article at http://www.ajph.org) shows smoothed frequency distributions of BMI by sex based on self-reports for the United States and a set of countries selected to show the range of variation present in the sample. The US distribution had larger variance and was markedly right skewed with respect to the comparison countries.
Effects of Obesity on Longevity
Fractions of all-cause mortality attributable to obesity (PAF) by country, age, and sex are presented in Table 2. Confidence intervals for PAFs reflect sampling uncertainty in BMI data and estimation uncertainty in the risks of obesity. The use of measured rather than self-reported values of height and weight in the United States led to PAFs that were higher by approximately 3%. The discrepancy between the PAFs in the United States and other countries was typically greatest at ages 50 to 59 years for both men and women, reflecting the unusually large proportion of individuals in the United States who were obese at those ages. As determined through self-reported data, the fraction of deaths attributable to obesity for US women aged 50 to 59 years was 0.20 (95% confidence interval [CI] = 0.17, 0.27), compared with an average of 0.10 (95% CI = 0.08, 0.15) in comparison countries. Mortality attributable to obesity declined significantly with age for both men and women. For women, the greatest effects were found in the age group 60 to 69 years, whereas for men, the impact of obesity was highest at ages 50 to 59 years. After reaching these levels, the effects of obesity on mortality declined by about two thirds across the age range in both sexes.
TABLE 2.
Estimated Proportion of All-Cause Mortality Attributable to Obesity, by Country, Age, and Sex
| Women |
Men |
|||||||
| Country | 50–59 Years, PAF (95% CI) | 60–69 Years, PAF (95% CI) | 70–79 Years, PAF (95% CI) | 80–89 Years, PAF (95% CI) | 50–59 Years, PAF (95% CI) | 60–69 Years, PAF (95% CI) | 70–79 Years, PAF (95% CI) | 80–89 Years, PAF (95% CI) |
| Estimates based on self-reported height and weight | ||||||||
| Comparison countries | ||||||||
| Austria | 0.13 (0.10, 0.19) | 0.18 (0.13, 0.23) | 0.08 (0.06, 0.14) | 0.06 (0.02, 0.16) | 0.19 (0.15, 0.23) | 0.13 (0.10, 0.16) | 0.10 (0.07, 0.13) | 0.05 (0.00, 0.12) |
| Belgium | 0.09 (0.07, 0.15) | 0.16 (0.11, 0.20) | 0.10 (0.08, 0.15) | 0.06 (0.02, 0.17) | 0.16 (0.13, 0.20) | 0.12 (0.09, 0.15) | 0.10 (0.07, 0.13) | 0.07 (0.00, 0.14) |
| Czech Republic | 0.10 (0.07, 0.17) | 0.19 (0.13, 0.25) | 0.14 (0.10, 0.21) | 0.07 (0.02, 0.18) | 0.18 (0.14, 0.22) | 0.16 (0.12, 0.20) | 0.12 (0.08, 0.15) | 0.09 (0.00, 0.17) |
| Denmark | 0.08 (0.06, 0.13) | 0.11 (0.06, 0.14) | 0.09 (0.07, 0.15) | 0.03 (0.01, 0.14) | 0.13 (0.10, 0.17) | 0.11 (0.08, 0.14) | 0.07 (0.05, 0.10) | 0.06 (0.00, 0.13) |
| France | 0.08 (0.06, 0.14) | 0.12 (0.08, 0.17) | 0.08 (0.06, 0.14) | 0.04 (0.01, 0.15) | 0.15 (0.11, 0.19) | 0.13 (0.09, 0.17) | 0.10 (0.07, 0.13) | 0.06 (0.00, 0.13) |
| Germany | 0.09 (0.08, 0.15) | 0.16 (0.11, 0.21) | 0.11 (0.09, 0.17) | 0.05 (0.01, 0.15) | 0.15 (0.12, 0.19) | 0.13 (0.11, 0.16) | 0.11 (0.08, 0.14) | 0.08 (0.00, 0.15) |
| Israel | 0.10 (0.06, 0.18) | 0.14 (0.09, 0.19) | 0.15 (0.10, 0.22) | 0.05 (0.00, 0.15) | 0.15 (0.10, 0.21) | 0.13 (0.09, 0.18) | 0.11 (0.06, 0.17) | 0.05 (0.00, 0.12) |
| Italy | 0.09 (0.07, 0.15) | 0.14 (0.10, 0.19) | 0.09 (0.07, 0.15) | 0.05 (0.01, 0.16) | 0.14 (0.11, 0.18) | 0.13 (0.10, 0.16) | 0.10 (0.07, 0.13) | 0.07 (0.00, 0.14) |
| Netherlands | 0.10 (0.08, 0.16) | 0.13 (0.08, 0.18) | 0.11 (0.08, 0.17) | 0.05 (0.01, 0.14) | 0.13 (0.10, 0.16) | 0.13 (0.10, 0.15) | 0.07 (0.05, 0.10) | 0.04 (0.00, 0.11) |
| Poland | 0.14 (0.11, 0.21) | 0.22 (0.16, 0.27) | 0.16 (0.13, 0.22) | 0.06 (0.02, 0.17) | 0.16 (0.13, 0.21) | 0.16 (0.13, 0.20) | 0.10 (0.08, 0.13) | 0.06 (0.00, 0.12) |
| Spain | 0.12 (0.10, 0.19) | 0.21 (0.15, 0.27) | 0.14 (0.11, 0.20) | 0.09 (0.03, 0.18) | 0.18 (0.14, 0.23) | 0.15 (0.12, 0.18) | 0.11 (0.08, 0.14) | 0.09 (0.00, 0.16) |
| Sweden | 0.09 (0.07, 0.16) | 0.13 (0.08, 0.17) | 0.10 (0.08, 0.16) | 0.05 (0.02, 0.16) | 0.16 (0.10, 0.24) | 0.11 (0.09, 0.14) | 0.08 (0.06, 0.10) | 0.04 (0.00, 0.10) |
| Switzerland | 0.06 (0.04, 0.10) | 0.11 (0.07, 0.16) | 0.09 (0.06, 0.14) | 0.05 (0.01, 0.14) | 0.14 (0.11, 0.18) | 0.11 (0.08, 0.15) | 0.09 (0.06, 0.13) | 0.06 (0.00, 0.13) |
| Average | 0.10 (0.08, 0.15) | 0.15 (0.11, 0.20) | 0.11 (0.09, 0.17) | 0.05 (0.02, 0.16) | 0.16 (0.13, 0.19) | 0.13 (0.11, 0.16) | 0.10 (0.07, 0.12) | 0.06 (0.00, 0.12) |
| United States | 0.20 (0.17, 0.27) | 0.23 (0.18, 0.28) | 0.14 (0.12, 0.19) | 0.06 (0.03, 0.16) | 0.24 (0.21, 0.29) | 0.21 (0.18, 0.24) | 0.14 (0.11, 0.17) | 0.07 (0.00, 0.13) |
| Estimates based on measured height and weight | ||||||||
| Comparison countries | ||||||||
| Canada | 0.15 (0.12, 0.22) | 0.26 (0.20, 0.33) | 0.14 (0.11, 0.20) | 0.09 (0.04, 0.19) | 0.23 (0.19, 0.28) | 0.22 (0.18, 0.26) | 0.14 (0.10, 0.17) | 0.07 (0.00, 0.14) |
| England | 0.17 (0.14, 0.23) | 0.22 (0.17, 0.27) | 0.17 (0.15, 0.22) | 0.09 (0.04, 0.19) | 0.22 (0.19, 0.26) | 0.18 (0.15, 0.21) | 0.13 (0.10, 0.15) | 0.11 (0.00, 0.17) |
| United States | 0.22 (0.20, 0.30) | 0.26 (0.20, 0.31) | 0.18 (0.15, 0.23) | 0.09 (0.04, 0.19) | 0.26 (0.22, 0.31) | 0.23 (0.20, 0.26) | 0.17 (0.13, 0.20) | 0.10 (0.00, 0.16) |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); CI = confidence interval; PAF = population attributable fraction. Source of relative risks is Prospective Studies Collaboration.5 The 95% CIs for PAFs incorporate sampling uncertainty in estimates of the distribution of BMI and estimation uncertainty in relative risks of obesity.
Source. Sources and years of data are as follows: United States, National Health and Nutrition Examination Survey, cycles 2003–2004, 2005–2006, and 2007–2008; Canada, Canadian Community Health Survey, cycle 3.1 (2005); European countries excluding England, Survey of Health, Ageing and Retirement in Europe, wave 1 (2004) and refresher sample from wave 2 (2006–2007); England, English Longitudinal Study of Ageing, wave 2 (2004–2005).
Table 3 presents the impacts on life expectancy that are implied by the estimates of deaths attributable to obesity presented in Table 2. Reallocating individuals with higher-than-optimal BMI to the lowest-risk BMI for their age and sex would increase US life expectancy at age 50 years by an estimated 1.28 years (95% CI = 1.14, 1.70) for women and by 1.61 years (95% CI = 1.44, 1.82) for men when self-reported BMI data are used. In other countries with self-reported data, female life expectancy would improve by an average of 0.73 years (95% CI = 0.63, 1.13) and male life expectancy by an average of 0.98 years (95% CI = 0.86, 1.16) if obesity were eliminated. When measured BMI was used, the estimated gains in US life expectancy were greater by an additional 0.24 to 0.26 years. No other country is estimated to gain as much from the elimination of obesity as the United States.
TABLE 3.
Life Expectancy at Age 50 years With and Without All-Cause Mortality Attributable to Obesity, by Country and Sex: 2006
| e50 for Women |
e50 for Men |
|||||
| Country | Actual | Without Obesity | Difference (95% CI) | Actual | Without Obesity | Difference (95% CI) |
| Estimates based on self-reported height and weight | ||||||
| Comparison countries | ||||||
| Austria | 33.96 | 34.67 | 0.71 (0.59, 1.07) | 29.39 | 30.39 | 1.00 (0.86, 1.23) |
| Belgium | 33.70 | 34.42 | 0.73 (0.61, 1.16) | 29.03 | 30.01 | 0.98 (0.82, 1.18) |
| Czech Republic | 31.24 | 32.25 | 1.01 (0.85, 1.40) | 26.04 | 27.38 | 1.34 (1.12, 1.57) |
| Denmark | 31.90 | 32.52 | 0.62 (0.52, 1.02) | 28.22 | 29.05 | 0.82 (0.68, 1.02) |
| France | 35.68 | 36.20 | 0.52 (0.43, 0.90) | 29.86 | 30.85 | 0.99 (0.82, 1.20) |
| Germany | 33.60 | 34.31 | 0.70 (0.60, 1.07) | 29.07 | 30.12 | 1.05 (0.85, 1.27) |
| Israel | 33.61 | 34.40 | 0.79 (0.61, 1.18) | 30.64 | 31.56 | 0.92 (0.71, 1.22) |
| Italy | 35.24 | 35.81 | 0.57 (0.49, 0.96) | 30.57 | 31.47 | 0.90 (0.73, 1.12) |
| Netherlands | 33.31 | 34.00 | 0.69 (0.59, 1.03) | 29.45 | 30.18 | 0.73 (0.61, 0.92) |
| Poland | 31.39 | 32.58 | 1.19 (1.02, 1.60) | 24.73 | 26.09 | 1.37 (1.21, 1.61) |
| Spain | 35.40 | 36.27 | 0.87 (0.72, 1.23) | 29.94 | 31.09 | 1.15 (0.95, 1.39) |
| Sweden | 34.10 | 34.73 | 0.63 (0.53, 1.01) | 30.45 | 31.17 | 0.72 (0.59, 0.92) |
| Switzerland | 35.33 | 35.83 | 0.50 (0.41, 0.84) | 31.14 | 31.93 | 0.79 (0.63, 0.99) |
| Average | 33.73 | 34.46 | 0.73 (0.63, 1.13) | 29.12 | 30.10 | 0.98 (0.86, 1.16) |
| United States | 32.95 | 34.23 | 1.28 (1.14, 1.70) | 29.20 | 30.81 | 1.61 (1.44, 1.82) |
| Estimates based on measured height and weight | ||||||
| Comparison countries | ||||||
| Canada | 34.50 | 35.66 | 1.15 (1.00, 1.51) | 30.72 | 32.09 | 1.37 (1.18, 1.59) |
| England | 33.31 | 34.54 | 1.23 (1.07, 1.60) | 29.84 | 31.18 | 1.34 (1.13, 1.53) |
| United States | 32.95 | 34.49 | 1.54 (1.37, 1.93) | 29.20 | 31.05 | 1.85 (1.62, 2.10) |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); CI = confidence interval; e50 = life expectancy at age 50 years.
Source. Sources and years of data are as follows: United States, National Health and Nutrition Examination Survey, cycles 2003–2004, 2005–2006, and 2007–2008; Canada, Canadian Community Health Survey, cycle 3.1 (2005); European countries excluding England, Survey of Health, Ageing and Retirement in Europe, wave 1 (2004) and refresher sample from wave 2 (2006–2007); England, English Longitudinal Study of Ageing, wave 2 (2004–2005).
Table A (available as a supplement to the online version of this article at http://www.ajph.org) presents the US shortfall in life expectancy at age 50 years (relative to countries with higher life expectancies) and the estimated change in that shortfall if obesity were eliminated. Since life expectancy at age 50 years in the United States would increase substantially more than in other countries through the hypothetical elimination of obesity, the US shortfall would be reduced and in some cases eliminated. US life expectancy for women was 1.37 years lower than the mean for the 12 other countries. It would be an estimated 0.80 years (95% CI = 0.70, 0.87) lower without obesity, so that obesity accounted for an average of 42% (95% CI = 36, 48) of the gap. For men, the equivalent fraction of the difference in life expectancy accounted for by obesity, relative to 10 countries with higher life expectancies, was 67% (95% CI = 57, 76). For women, after the elimination of obesity, the difference in life expectancy between the United States and England, Germany, and Israel became statistically indistinguishable from zero and US life expectancy surpassed that of the Netherlands. For men, the difference in life expectancy between the United States and France was eliminated and US life expectancy surpassed that of Austria and the Netherlands. These estimates suggest that obesity is contributing very substantially to the low US ranking in longevity.
Results of using the alternative risk factors are presented in Table 4. In every country for both sexes, the use of the alternative risk factors reduced the estimated gain in life expectancy from eliminating obesity. As determined through self-reported data, for countries other than the United States, women's mean gain in life expectancy was only 42% as large with the risk factors identified by Adams et al.17 as with those identified by the PSC5; for men, it was only 21% as large. Proportionate reductions were smaller in the United States than in other countries because a much higher fraction of the US population is in obesity classes II or III, for which risks remain considerable even under the alternative sets of risks.
TABLE 4.
Estimated Gain in Life Expectancy at Age 50 Years From Hypothetically Redistributing Obese to Optimal Body Mass Index Categories, Using 2 Sets of Risk Factors: 2006
| Women |
Men |
|||
| Country | PSC Risk Factors,a Years (95% CI) | Adams Risk Factors,a Years (95% CI) | PSC Risk Factors,a Years (95% CI) | Adams Risk Factors,a Years (95% CI) |
| Estimates based on self-reported height and weight | ||||
| Comparison countries | ||||
| Austria | 0.71 (0.59, 1.07) | 0.30 (0.23, 0.40) | 1.00 (0.86, 1.23) | 0.23 (0.16, 0.32) |
| Belgium | 0.73 (0.61, 1.16) | 0.32 (0.24, 0.42) | 0.98 (0.82, 1.18) | 0.20 (0.14, 0.27) |
| Czech Republic | 1.01 (0.85, 1.40) | 0.44 (0.32, 0.61) | 1.34 (1.12, 1.57) | 0.30 (0.20, 0.41) |
| Denmark | 0.62 (0.52, 1.02) | 0.28 (0.19, 0.38) | 0.82 (0.68, 1.02) | 0.16 (0.10, 0.23) |
| France | 0.52 (0.43, 0.90) | 0.22 (0.16, 0.29) | 0.99 (0.82, 1.20) | 0.22 (0.15, 0.31) |
| Germany | 0.70 (0.60, 1.07) | 0.29 (0.22, 0.38) | 1.05 (0.85, 1.27) | 0.22 (0.16, 0.29) |
| Israel | 0.79 (0.61, 1.18) | 0.30 (0.20, 0.45) | 0.92 (0.71, 1.22) | 0.19 (0.10, 0.31) |
| Italy | 0.57 (0.49, 0.96) | 0.22 (0.17, 0.31) | 0.90 (0.73, 1.12) | 0.17 (0.12, 0.23) |
| Netherlands | 0.69 (0.59, 1.03) | 0.31 (0.23, 0.41) | 0.73 (0.61, 0.92) | 0.15 (0.10, 0.20) |
| Poland | 1.19 (1.02, 1.60) | 0.58 (0.45, 0.75) | 1.37 (1.21, 1.61) | 0.38 (0.26, 0.53) |
| Spain | 0.87 (0.72, 1.23) | 0.38 (0.28, 0.50) | 1.15 (0.95, 1.39) | 0.24 (0.16, 0.33) |
| Sweden | 0.63 (0.53, 1.01) | 0.26 (0.20, 0.35) | 0.72 (0.59, 0.92) | 0.17 (0.10, 0.25) |
| Switzerland | 0.50 (0.41, 0.84) | 0.19 (0.14, 0.28) | 0.79 (0.63, 0.99) | 0.15 (0.10, 0.20) |
| Average | 0.73 (0.63, 1.13) | 0.31 (0.25, 0.40) | 0.98 (0.86, 1.16) | 0.21 (0.16, 0.27) |
| United States | 1.28 (1.14, 1.70) | 0.71 (0.59, 0.86) | 1.61 (1.44, 1.82) | 0.52 (0.40, 0.64) |
| Estimates based on measured height and weight | ||||
| Comparison countries | ||||
| Canada | 1.15 (1.00, 1.51) | 0.65 (0.51, 0.80) | 1.37 (1.18, 1.59) | 0.37 (0.25, 0.49) |
| England | 1.23 (1.07, 1.60) | 0.61 (0.50, 0.74) | 1.34 (1.13, 1.53) | 0.33 (0.25, 0.42) |
| United States | 1.54 (1.37, 1.93) | 0.88 (0.74, 1.04) | 1.85 (1.62, 2.10) | 0.62 (0.50, 0.76) |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); CI = confidence interval; PSC = Prospective Studies Collaboration.
Source. Sources and years of data are as follows: United States, National Health and Nutrition Examination Survey, cycles 2003–2004, 2005–2006, and 2007–2008; Canada, Canadian Community Health Survey, cycle 3.1 (2005); European countries excluding England, Survey of Health, Ageing and Retirement in Europe, wave 1 (2004) and refresher sample from wave 2 (2006–2007); England, English Longitudinal Study of Ageing, wave 2 (2004–2005).
The 2 sets of risk factors used in the calculations are drawn from the PSC5 and Adams et al.17
Confining comparisons in Table 4 to countries with higher life expectancies than in the United States, as in Table A, we recalculated the proportion of the life expectancy gap explained by obesity. When we used the risk factors of Adams et al.,17 obesity accounted for 29% of the US shortfall for women and 32% of that for men. Obesity continued to account for a substantial part of the US shortfall in life expectancy even when lower risks were assumed.
We also applied a second alternative set of risk factors derived from NHANES III by Mehta and Chang.20 They identified a national probability sample of 4375 individuals enrolled at ages 50 to 69 years between 1988 and 1994 and followed into the National Death Index through 2006. Advantages of the study included recent data, a probability sample of the US population, and a relatively long follow-up period. Relative risks were adjusted for smoking and socioeconomic status. The results (not shown) were very similar to those produced when we used the risk factors of Adams et al.17: obesity accounted for 22% of the shortfall in life expectancy for US women and 29% of that for men.
Effects of Misreported Height and Weight
After adjustment of self-reported height and weight data for misreporting, the difference between actual life expectancy at age 50 years and life expectancy if obesity were eliminated increased by 0.23 years for US women and by 0.20 years for US men (results not shown). The estimated effect of eliminating obesity also increased in other countries, although by less than in the United States. As a result, correcting for misreporting positively affected the magnitude of the life expectancy gap attributable to obesity between the United States and other countries. The greatest difference occurred between the United States and Spain, amounting to 0.10 years for women and 0.17 years for men. No other differences in the table reached a level of a tenth of a year of life expectancy. We conclude that errors in self-reported BMI have produced underestimates of the impact of obesity on life expectancy, and that the underestimate is somewhat greater in the United States than in most other countries. In this sense, obesity explains more of the gap in life expectancy between the United States and other countries than is indicated by self-reports. The bias is modest, however, amounting in only 1 case to more than 0.10 years of the life expectancy gap between the United States and other countries.
DISCUSSION
In our analysis of the effects of obesity on longevity in 16 countries, we have estimated that obesity reduced longevity in all countries ranging from half a year for females in Switzerland to more than a year and a half for US males. These effects have been more severe in the United States than in other countries. Two key features of the US distribution of BMI that distinguish it from comparison countries include an unusually high rate of obesity in younger age groups and significantly higher rates of severe obesity. When obesity is hypothetically eliminated, gains to life expectancy are 25% to 40% higher in the United States than in Canada and England, the 2 countries with the next-highest rates of obesity. As a result of its greater impact there, obesity has contributed substantially to the US longevity disadvantage, which would be significantly reduced and in some cases eliminated in the absence of obesity.
Olshansky et al. have also produced estimates of the effect of obesity on US life expectancy using NHANES III risk factors.7 They did not use the full BMI distribution but rather experimented with various binary specifications of risk, producing estimated effects on US life expectancy that ranged widely, from 0.28 years to 0.88 years. Our estimates (Table 4) were at the high end of that range when we used the risk factors from Adams et al.17 and far above it when we used the risk factors from the PSC.5
It is clear that the estimated effect of obesity on levels of life expectancy is sensitive to the set of obesity risk factors that is used. The risk factors derived from the studies of Adams et al. have the advantage of pertaining to a period closer to the time when the levels of both obesity and mortality were recorded in the various countries and when the PAFs were modeled. This study also controls for social class in its analyses, an important confounding factor of the relationship between obesity and mortality.21
The choice of the proper set of risk factors probably depends most heavily on whether the mortality risks of obesity have declined. A large study begun in 1982 by the American Cancer Society, with follow-up of healthy nonsmokers through 2002, found no decline in the mortality risk from obesity.22 However, Flegal et al. found such a decline using successive waves of NHANES.16 Mehta and Chang also identified a reduction in obesity risks in the United States in 3 different data sets, including NHANES, the Framingham Heart Study, and a National Health Interview Survey follow-up study.20
A decline in the mortality risks of obesity may have occurred for a number of reasons. Gregg et al. noted that the use of lipid-lowering and antihypertensive medications increased rapidly from the period 1988 to 1994 to the years 1999 to 2000 with the largest gains among obese individuals.23 In addition, deaths from cardiovascular disease are a diminishing proportion of all mortality.24 Combined with greater obesity risks from cardiovascular diseases than from the aggregate of other causes of death, such a decline also implies that the all-cause mortality risk from obesity should be declining.
An additional factor that may have reduced relative risks among the obese is the rapid inflow of people into the obese category. A rapid increase such as occurred in the United States12 may produce a decline in the average duration of obesity for an obese person. To the extent that there are duration effects of obesity—risks that accumulate with length of time spent in the obese state—the risk of obesity per se may have declined when duration is not accounted for in the research design. The fact that childhood or early adulthood obesity is highly predictive of adult mortality implies that duration effects may be important for obesity.25,26
If there were a clear-cut trend in the mortality risk of obesity, there would be a strong reason to prefer estimates derived from the 2 most recent studies. But evidence of a trend is suggestive rather than definitive because it has not appeared in all analyses where its presence has been investigated, and it has not always been statistically significant when it has appeared. As a result, we believe that our results should be interpreted as providing a plausible range of estimates of the impact of obesity on the shortfall in American longevity.
Strengths and Limitations
Our analysis has a number of strengths. We used nationally representative data from 16 countries to measure distributions of BMI that together capture a large fraction of the variation in obesity rates among high-income countries. We incorporated detailed information on the mortality risks of obesity, differentiated by age, sex, and narrow BMI intervals using high-quality data from a large meta-analysis of prospective cohort studies. We characterized uncertainty in our estimates from multiple sources and conducted numerous analyses of the sensitivity of our results to alternative procedures.
Our analysis is also subject to limitations. We assumed that the same set of individual-level mortality risks of obesity was applicable to all countries although these risks may differ somewhat across contexts. Our analysis would have been strengthened by the availability of measured BMI data in all sample countries and inclusion of data from high-income countries outside North America and Europe, such as Japan and Australia, where conditions may differ from those of the countries included in the sample. Although the risk factors that we used were adjusted for smoking behavior, they were not adjusted for all other factors with which obesity may be correlated.
Conclusions
On the basis of our results, the high prevalence of obesity in the United States has reduced life expectancy at age 50 years by 0.88 to 1.54 years for women and by 0.62 to 1.85 years for men. To study the impact of obesity on international differences in longevity, we also estimated the effects of obesity on longevity in 15 other countries. We conclude that even when relatively low mortality risks associated with obesity are used, the high levels of obesity in the United States contribute substantially—in the neighborhood of 30%—to the lower level of longevity in the United States. If the risk factors from the PSC are used, the impact of obesity is substantially larger, accounting for 42% of the longevity shortfall for US women and 67% of that for US men.
High levels of obesity in the United States appear to be strongly implicated in its relatively low level of longevity. We believe that this demonstration should add urgency to public health efforts aimed at achieving healthier weights for Americans.
Acknowledgments
This research is supported by the US Social Security Administration (SSA) through a grant to the National Bureau of Economic Research as part of the SSA Retirement Research Consortium.
We are grateful to Gary Whitlock, Virginia Chang, Eileen Crimmins, Irma Elo, Douglas Ewbank, and Neil Mehta for comments and suggestions.
Note. The findings and conclusions presented here are solely those of the authors and do not represent the views of any agency of the federal government or the National Bureau of Economic Research.
Human Participant Protection
No protocol approval was necessary because data were obtained from secondary sources.
References
- 1.World Health Organization Global Health Observatory. Available at: http://apps.who.int/ghodata. Accessed September 1, 2010
- 2.Obama B. President's speech before the Annual Meeting of the American Medical Association House of Delegates; June 15, 2009; Chicago, IL [Google Scholar]
- 3.Crimmins EM, Preston SH, Cohen B, International Differences in Mortality at Older Ages: Dimensions and Sources. Washington, DC: Panel on Understanding Divergent Trends in Longevity in High-Income Countries, National Research Council; 2010 [PubMed] [Google Scholar]
- 4.The SuRF Report 2: Surveillance of Chronic Disease Risk Factors. Geneva, Switzerland: World Health Organization; 2005. Available at: https://apps.who.int/infobase/Publicfiles/SuRF2.pdf. Accessed June 1, 2010 [Google Scholar]
- 5.Prospective Studies Collaboration, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–193 [DOI] [PubMed] [Google Scholar]
- 7.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145 [DOI] [PubMed] [Google Scholar]
- 8.Arias E, Rostron B, Tejada-Vera B. United States Life Tables, 2005. Natl Vital Stat Rep. 2010;58(10). [PubMed] [Google Scholar]
- 9.Ho J, Preston SH. US mortality in an international context: age variations. Popul Dev Rev. 2010;36(4):749–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am J Clin Nutr. 2009;89(4):1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison DB, Fontaine KR, Manson JAE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–1538 [DOI] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241 [DOI] [PubMed] [Google Scholar]
- 13.Wilmoth JR, Shkolnikov V. The Human Mortality Database. University of California at Berkeley and Max Planck Institute for Demographic Research. Available at: http://www.mortality.org. Accessed June 1, 2010
- 14.Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Population Processes. Oxford, England: Wiley-Blackwell; 2001 [Google Scholar]
- 15.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75 [Google Scholar]
- 16.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867 [DOI] [PubMed] [Google Scholar]
- 17.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778 [DOI] [PubMed] [Google Scholar]
- 18.Ezzati M, Martin H, Skjold S, Hoorn SV, Murray CJL. Trends in national and state-level obesity in the USA after correction for self-report bias: analysis of health surveys. J R Soc Med. 2006;99(5):250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhauser RV, Cawley J. Beyond BMI: the value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008;27(2):519–529 [DOI] [PubMed] [Google Scholar]
- 20.Mehta NK, Chang VW. Secular declines in the association between obesity and mortality in the United States. Popul Dev Rev. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta NK, Chang VW. Mortality attributable to obesity among middle-aged adults in the United States. Demography. 2009;46(4):851–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353(20):2197–2199 [DOI] [PubMed] [Google Scholar]
- 23.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874 [DOI] [PubMed] [Google Scholar]
- 24.Beltrán-Sánchez H, Preston SH, Canudas-Romo V. An integrated approach to cause-of-death analysis: cause-deleted life tables and decompositions of life expectancy. Demogr Res. 2008;19:1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrilova NS, Gavrilov LA. Search for mechanisms of exceptional human longevity. Rejuvenation Res. 2010;13(2–3):262–264 [DOI] [PMC free article] [PubMed] [Google Scholar]


