Abstract
Objectives. I examined the relationship between insurance coverage, which may influence physician incentives and maternal choices, and cesarean delivery before labor.
Methods. I analyzed hospital discharge data for mothers without previous cesarean deliveries in New Jersey between 2004 and 2007, with adjustment for maternal age, race, marital status, and maternal, fetal, and placental conditions.
Results. Nearly 1 in 7 women (13.9%) had a cesarean delivery without laboring. Insurance status was strongly associated with cesarean birth. Women insured by Medicaid (adjusted relative risk [ARR] = 0.88; 95% confidence interval [CI] = 0.84, 0.91) or self-paying (ARR = 0.81; 95% CI = 0.78, 0.85) had a significantly lower likelihood, and women insured by BlueCross (ARR = 1.06; 95% CI = 1.03, 1.09) or standard commercial plans (ARR = 1.06; 95% CI = 1.02, 1.10) had a significantly higher likelihood of cesarean delivery than did women insured by commercial health maintenance organizations. These associations persisted in subsets restricted to lower-risk women and in qualitative sensitivity analyses for a hypothetical single, binary, unmeasured confounder.
Conclusions. Insurance status has a small, independent impact on whether a woman without a previous cesarean delivery proceeds to labor or has a cesarean delivery without labor.
The rising rate of cesarean deliveries is an important public health and clinical medicine issue because current US rates are more than double the World Health Organization consensus optimal cesarean delivery rate (10%–15% of births).1 Because most women with a previous cesarean delivery will deliver subsequent infants the same way, initiatives to reduce cesarean deliveries might usefully focus on women who have never had a cesarean delivery.2 Although most of these women will deliver vaginally, their likelihood of undergoing cesarean delivery has also been rising.3
The majority of primary cesarean deliveries are clinically indicated. However, concern exists globally that some, especially when performed without a woman having begun to labor, are not medically indicated.4–6 Whether primary prelabor cesarean delivery is indicated matters for health policy.2,7 If a substantial number of these surgeries are not medically indicated, then education, financial incentives for vaginal deliveries, and other management tools could reduce the rate of elective cesarean delivery procedures.8 If they are indicated, a rising rate implies either that maternal health is worsening or that changes in obstetric practice are broadening the set of clinical indications, or both.3
Some elective primary prelabor cesarean deliveries may be a result of maternal choice. Anecdotal evidence suggests that a woman may trade off more convenient scheduling against greater morbidity later,9 may be concerned with vaginal tone or pelvic floor preservation,10,11 may fear delivery pain or have had a previous negative birth experience,12 or, especially in the case of less fertile women, may believe that a cesarean delivery is safer for her baby.13 Although the evidence for such mechanisms is at best scant, they are plausible in insured women from higher socioeconomic strata.
Other such elective procedures could result from physician choice,14 for example, in light of legal liability.15,16 Physician choice may also be driven by financial pressure,17–19 which may be more acute when patients have generous fee-for-service insurance than when they are insured by a health maintenance organization (HMO), underinsured, uninsured, or indigent. Conversely, when dealing with poorly insured women (e.g., Medicaid recipients), physicians may perform fewer cesarean deliveries.20
Finally, elective cesarean deliveries may reflect hospital preferences for faster, less variable birthing schedules and better utilization of operating rooms and other hospital resources. It is plausible that an insured woman is more able than is an uninsured woman to afford higher charges; hence these procedures might be more likely at the margin among the insured.
Past research examined prelabor primary cesarean delivery with administrative discharge data and sought to understand which coded diagnoses represented high-risk deliveries in which a cesarean delivery was indicated.2,21,22 Few studies have considered the relationship of insurance with delivery type.17,20 I therefore examined the independent relationship between prelabor cesarean delivery and maternal insurance status, with controls for validated maternal, fetal, and placental conditions and maternal demographics. I examined hospital discharge data from 2004 to 2007 in New Jersey, which had the second-highest cesarean delivery rate among 19 states releasing all-payer data and the fourth-highest rate of cesarean delivery without apparent medical indication, according to HealthGrades.23
I hypothesized that payer status, after adjustment for maternal, fetal, and placental diagnoses, would have an independent effect on the odds of receiving cesarean delivery without at least a trial of labor. I also expected to find a dose–response relationship in which more comprehensive insurance would be more strongly associated with prelabor cesarean delivery. I further expected that this effect would be similar in subsets of women defined by the presence or absence of common maternal, fetal, or placental diagnoses that are associated with higher-risk pregnancies.
METHODS
I obtained data on all in-patient admissions for delivery to New Jersey state-regulated hospitals in 2004 to 2007 from the State Inpatient Database data sets of the Agency for Healthcare Research and Quality's Healthcare Cost and Utilization Project group. I validated and restricted this data to the 362 611 women without previous cesarean delivery whose primary payers were private commercial HMO plans, private BlueCross plans, or private commercial plans; who were insured by Medicaid; or who reported as self-payers. (Details on validation are shown in Table A, available as a supplement to the online version of this article at http://www.ajph.org.)
I used validated administrative data–coding schemes to understand whether women had evidence of labor in the focal admission and whether they had previously had a cesarean delivery.2 I distinguished the presence or absence of a previous cesarean delivery by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)24 diagnostic code 654.2XX in any of 9 diagnosis fields. I identified the presence of labor and its complications during this admission (ICD-9-CM procedure codes for the presence of vaginal delivery, cephalic version from breech [652.1XX], disproportion [653.XX], obstructed labor [660.XX], abnormality of forces of labor [661.XX], long labor [662.XX], failed induction of labor [659.0XX and 659.1XX], fetal distress [656.3], and cord prolapse [663.0]). I also identified a cesarean delivery procedure during this admission (ICD-9-CM procedure code 74, 74.0, 74.1, 74.2, 74.4, 74.9, or 74.99) in any of 7 procedure fields.
I coded control covariates of maternal demographics (age, race/ethnicity, marital status, and nonmetropolitan location) as well as a set of maternal, placental, and fetal conditions that were coded as diagnoses during the admission. These diagnoses (Table B, available as a supplement to the online version of this article at http://www.ajph.org) may characterize a delivery as high risk22,25 or may be associated with a medically indicated prelabor cesarean delivery.2,21,26 I coded 26 such covariates with ICD-9-CM codes (Table C, available as a supplement to the online version of this article at http://www.ajph.org). I also coded month and year of admission, an encrypted identifier representing the treating specialist physician, and identifiers of the admitting hospital.
For baseline differences in characteristics between series of differently insured women, I calculated proportions for categorical variables and medians with interquartile ranges for nonnormally distributed continuous variables. I tested the significance of differences in categorical variables with χ2 tests and used the Kruskal–Wallis equality-of-populations test for changes in continuous variables. I report the 2-tailed P value for these tests of the significance of trend across differently insured women.
My objective was to assess the independent impact of insurance status on the likelihood that a mother without previous cesarean delivery would receive a cesarean delivery without recorded evidence of having proceeded to labor. I used multivariate adjusted logistic regression in these analyses, adjusting for the control covariates and calculating robust standard errors throughout. I report the multivariate adjusted odds ratios (AORs) and 95% confidence intervals (CIs) for the impact of varying insurance status, with women insured by a commercial HMO plan serving as the referent group.
I also computed the relative risk, adjusting the OR to correct for differing event rates by a well-known approximation.27 To take into account the likelihood that patient outcomes were likely clustered within hospitals or geographical locations or within the practices of individual physicians, I specified standard errors clustered accordingly in several robustness analyses (data not shown).
In other robustness analyses I estimated new multivariate adjusted logistic regressions, restricted to more clinically homogeneous subsets of women with fewer or none of the most common maternal, placental, and fetal conditions associated with cesarean delivery. I made no adjustments for multiple comparisons.28 This method used Greenland's approach, which cannot mitigate such covert biases but can provide reassurance that the risk of such biases is qualitatively small.29 I used SAS version 9.2 (SAS Institute Inc, Cary, NC) for data management and Stata version 10 (StataCorp LP, College Station, TX) for all analyses.
RESULTS
Over the 4 years of pooled data, 50 417 of the 362 611 women who had not had a previous surgical delivery and who met the study's payer restrictions and data validation underwent a cesarean delivery without laboring. This pooled ratio of 13.9% rose steadily over the study period (Table D, available as a supplement to the online version of this article at http://www.ajph.org). In the first quarter of 2004, the rate of prelabor cesarean delivery was 12.0% of all women without previous cesarean delivery; this rate rose to a high of 15.2% in the final quarter of 2007. Prelabor cesarean delivery occurred—as expected—most often in women with 1 or more of the 26 conditions constituting a high-risk pregnancy (Table E, available as a supplement to the online version of this article at http://www.ajph.org). This rate rose from 11.5% to 14.2% over the study period (Table D). The rate of prelabor cesarean delivery among women without at least 1 of the indications for prelabor cesarean delivery was far lower, rising from 0.6% to 0.9% over the study period.
Table 1 shows the maternal, fetal, and placental risk factors that were most prevalent in New Jersey women without previous cesarean delivery over the study period. The table also shows the key outcomes of interest: prelabor cesarean delivery, labor with vaginal delivery, and labor followed by cesarean delivery. Payer types were associated with highly significant differences in the crude rates of these outcomes. Self-paying women and Medicaid recipients had the lowest rates of prelabor primary cesarean delivery (10.8% and 11.5%, respectively); women who were privately insured with BlueCross plans had the highest rate (16.0%).
TABLE 1.
Characteristics of Mothers and Deliveries Without Previous Cesarean Delivery, by Mother's Insurance Status: New Jersey, 2004–2007
| Characteristic | HMO (n = 195 928), % or Median (IQR) | BlueCross (n = 69 762), % or Median (IQR) | Commercial (n = 27 507), % or Median (IQR) | Medicaid (n = 38 083), % or Median (IQR) | Self-Pay (n = 31 331), % or Median (IQR) | Total (n = 362 611), % or Median (IQR) | P |
| Age, y | 29 (24–33) | 31 (27–34) | 30 (27–34) | 25 (21–29) | 26 (22–31) | 29 (24–33) | <.001 |
| Race/ethnicity | <.001 | ||||||
| White | 53.2 | 67.2 | 56.6 | 15.8 | 20.8 | 49.4 | |
| Asian | 6.4 | 5.7 | 9.5 | 2.1 | 3.0 | 5.8 | |
| Hispanic | 14.3 | 8.4 | 12.8 | 50.3 | 52.9 | 20.2 | |
| Black | 16.4 | 9.8 | 9.3 | 16.0 | 10.3 | 14.0 | |
| Other | 9.7 | 8.9 | 11.8 | 15.8 | 13.0 | 10.7 | |
| Married | 63.9 | 82.8 | 78.2 | 24.5 | 32.1 | 61.7 | <.001 |
| Nonmetropolitan core | 7.6 | 8.3 | 11.6 | 3.2 | 8.5 | 7.7 | <.001 |
| Fetal heart rate abnormal | 13.0 | 13.3 | 11.8 | 13.1 | 10.3 | 12.7 | <.001 |
| Preterm gestation | 7.9 | 8.1 | 7.8 | 8.5 | 9.3 | 8.1 | <.001 |
| Malpresentation | 6.9 | 8.1 | 7.3 | 5.8 | 6.0 | 7.0 | <.001 |
| Hypertension, other | 6.8 | 7.0 | 6.9 | 6.7 | 5.5 | 6.8 | <.001 |
| Abnormal glucose tolerance | 5.8 | 6.0 | 6.7 | 5.1 | 5.0 | 5.7 | <.001 |
| Oligohydramnios | 3.9 | 3.5 | 4.1 | 5.0 | 4.9 | 4.0 | <.001 |
| Macrosomia | 3.2 | 3.9 | 3.5 | 1.7 | 2.1 | 3.1 | <.001 |
| Mental illness | 3.1 | 2.3 | 2.0 | 4.0 | 4.0 | 3.0 | <.001 |
| Maternal soft tissue | 3.0 | 3.7 | 3.3 | 1.9 | 1.9 | 2.9 | <.001 |
| Rhesus isoimmunization | 2.8 | 2.9 | 3.0 | 1.6 | 1.9 | 2.7 | <.001 |
| Asthma | 2.9 | 2.7 | 2.2 | 2.0 | 1.4 | 2.6 | <.001 |
| Thyroid disorder | 2.6 | 3.2 | 3.0 | 0.8 | 1.1 | 2.4 | <.001 |
| Antepartum bleed | 1.8 | 1.9 | 2.0 | 1.7 | 1.9 | 1.8 | .003 |
| Unengaged fetal head | 1.8 | 2.1 | 1.9 | 1.4 | 1.2 | 1.8 | <.001 |
| Chorioamnionitis | 1.7 | 1.3 | 1.5 | 3.1 | 2.2 | 1.8 | <.001 |
| Multiple gestation | 1.8 | 2.3 | 1.8 | 0.9 | 0.9 | 1.7 | <.001 |
| Intrauterine growth retardation | 1.7 | 1.7 | 1.6 | 1.6 | 1.7 | 1.7 | .26 |
| Heart disease | 1.8 | 2.4 | 2.0 | 0.6 | 0.7 | 1.7 | <.001 |
| Herpes | 1.6 | 1.4 | 1.3 | 1.8 | 1.5 | 1.6 | <.001 |
| Severe hypertension | 0.9 | 1.1 | 1.0 | 1.3 | 1.1 | 1.0 | <.001 |
| Other uterine scar | 0.3 | 0.3 | 0.3 | 0.2 | 0.1 | 0.3 | <.001 |
| Substance use | 0.2 | 0.1 | 0.1 | 0.7 | 1.0 | 0.3 | <.001 |
| Kidney disorder | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | .3 |
| Liver disorders | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | .001 |
| Fetal congenital anomaly | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | .004 |
| Prelabor primary cesarean delivery | 14.0 | 16.0 | 14.4 | 11.5 | 10.8 | 13.9 | <.001 |
| Labored, vaginal delivery | 74.1 | 71.0 | 73.0 | 77.5 | 79.7 | 74.3 | <.001 |
| Labored, primary cesarean delivery | 11.8 | 13.0 | 12.6 | 11.0 | 9.5 | 11.8 | <.001 |
Note. HMO = health maintenance organization; IQR = interquartile range. Intracranial hemorrhage omitted because rate was < 0.1%.
I also observed substantial and highly significant differences in maternal characteristics across the payer types. Self-paying and Medicaid-insured women tended to be 5 to 6 years younger than were the privately insured and belonged overwhelmingly to minority races: 63% to 66% were Black and Hispanic; only 18% of women with BlueCross and 22% of women with commercial plans belonged to racial/ethnic minorities.
Table 1 shows the incidence of the diagnosed conditions associated with medical indications for primary cesarean delivery without a trial of labor. In order of frequency, the most common was the presence of a broadly defined fetal heart rate abnormality (ICD-9-CM diagnostic code 659.71). This poorly defined cardiotocography diagnosis was previously identified as associated with primary cesarean delivery.26 It may be applied to both fetal bradycardia and tachycardia and to increased or decreased heart rate variability, and it may be associated with uterine contractions.30
For almost all of these diagnoses, the distribution was significantly skewed toward privately insured patients, who also tended to have the highest rates of prelabor cesarean delivery. For example, multiple gestations were more common among privately insured women, whose older average age was possibly associated with a greater prevalence of fertility treatments. The only high-risk conditions that were more prevalent among Medicaid recipients and self-paying women were a history of genital herpes and a background of mental illness. The distribution of maternal diagnoses highlighted the importance of appropriate risk adjustment in detecting the independent impact of payer status on prelabor cesarean delivery.
Table 2 shows the multivariate AORs for the 4 payer types relative to women insured by commercial HMO plans, the largest subgroup in the study (54%). I suppressed the estimates of the other covariates (available on request). Self-paying women were significantly less likely (AOR = 0.80; 95% CI = 0.76, 0.84), and women insured by BlueCross or commercial indemnity plans were significantly more likely (AOR = 1.07; 95% CI = 1.04, 1.11 and AOR = 1.07; 95% CI = 1.02, 1.12, respectively) than were those in the HMO group to experience a prelabor primary cesarean delivery. Relative risks adjusted for varying event rates did not materially differ: for self-pay, 0.81 (95% CI = 0.78, 0.85); for Medicaid, 0.88 (95% CI = 0.84, 0.91); for commercial plans, 1.06 (95% CI = 1.02, 1.10); and for BlueCross, 1.06 (95% CI = 1.03, 1.09).
TABLE 2.
Association Between Insurance Status and Prelabor Cesarean Delivery in Women Without Previous Cesarean Delivery: New Jersey, 2004–2007
| Risk Factor Subsets and Insurance Type | AORa (95% CI) |
| All women without previous cesarean delivery (n = 362 611) | |
| HMO (Ref) | 1.00 |
| Self-pay | 0.80 (0.76, 0.84) |
| Medicaid | 0.86 (0.83, 0.90) |
| Commercial plan | 1.07 (1.02, 1.12) |
| BlueCross | 1.07 (1.04, 1.11) |
| Women without any of the 5 most common maternal, fetal, and placental high-risk conditions (n = 238 833) | |
| HMO (Ref) | 1.00 |
| Self-pay | 0.68 (0.62, 0.75) |
| Medicaid | 0.76 (0.70, 0.83) |
| Commercial plan | 1.12 (1.04, 1.22) |
| BlueCross | 1.12 (1.06, 1.18) |
| Women without any of the 10 most common maternal, fetal, and placental high-risk conditions (n = 207 661) | |
| HMO (Ref) | 1.00 |
| Self-pay | 0.60 (0.53, 0.68) |
| Medicaid | 0.72 (0.65, 0.80) |
| Commercial plan | 1.14 (1.03, 1.26) |
| BlueCross | 1.11 (1.04, 1.19) |
| Women without any of the 26 most common maternal, fetal and placental high-risk conditions (n = 182 108) | |
| HMO (Ref) | 1.00 |
| Self-pay | 0.51 (0.42, 0.61) |
| Medicaid | 0.65 (0.55, 0.76) |
| Commercial plan | 1.22 (1.06, 1.40) |
| BlueCross | 1.21 (1.10, 1.33) |
Note. AOR = adjusted odds ratio; CI = confidence interval; HMO = health maintenance organization.
Adjusted for maternal age, marital status, race/ethnicity, weekend admission, nonmetropolitan location, county of residence, month of year, year, and, according to subset, maternal, fetal, and placental conditions.
In several sensitivity analyses I used models that took the clustering of patients within the practices of individual physicians, hospitals, or locations into account (data not shown). Results were very similar in the estimated effect size and in significance levels for all payer types in all models, except that the estimates for commercial indemnity plans lost significance at conventional levels (P > .05 but < .1).
To assess whether payer type had similar effects in lower-risk subgroups of women without some or any of the 26 maternal, fetal, or placental conditions that defined high-risk deliveries, I conducted additional analyses in progressively restricted subsets. Restricting inclusion to women without the 5 or 10 most common high-risk conditions did not substantially change the estimated AORs (Table 2).
Table 2 also shows estimates of the multivariate AORs of prelabor cesarean delivery in women without a previous cesarean delivery and without any of the 26 high-risk maternal, fetal, or placental conditions listed in Table 1. To the extent that observed and unobserved higher-risk conditions were correlated with insurance status, this approach reduced such confounding by rendering the series of differently insured women more clinically homogeneous. Estimated AORs by insurance status remained similar although somewhat stronger in this analysis.
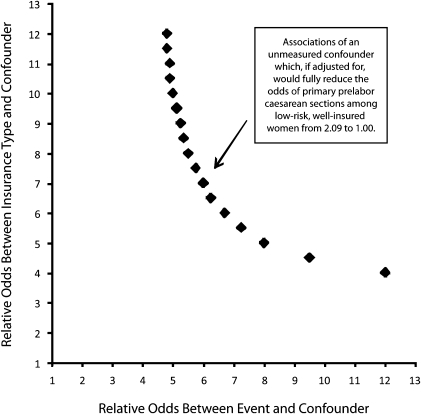
An additional approach to gauging the sensitivity of our results to omitted variables was to simulate a hypothetical unmeasured confounder with varying strengths of association between confounder and outcome and between confounder and insurance type.29 This method used Greenland's approach and did not control for such covert biases, but could provide reassurance that the risk of such biases was qualitatively small. I modeled how strong a hypothetical binary unmeasured confounder would have to be to completely explicate my observed results. I implemented this in women without previous cesarean delivery and without any of the 26 high-risk obstetrical conditions listed in Table 1, summarizing the results in Figure 1.
FIGURE 1.
Sensitivity analysis for the strength of unmeasured confounding sufficient to fully explain observed association between insurance type and prelabor cesarean delivery in lowest-risk women: New Jersey, 2004–2007.
Note. HMO = health maintenance organization. The analysis assumed hypothetical binary unmeasured confounder with prevalence of 40% among well-insured women and plotted combinations of strengths of association (between the confounder and the outcome of prelabor cesarean delivery and between the confounder and the exposure of commercial, HMO, and BlueCross insurance) that would be sufficient to fully explain the observed association between insurance and prelabor cesarean delivery among 185 828 women without prior cesarean delivery and without any of 26 maternal, fetal, and placental conditions coded as diagnoses during hospital admission. The analysis aggregated 38 400 women with Medicaid or self-pay primary insurance, of whom 326 (0.85%) underwent a prelabor cesarean delivery, and 147 428 women with commercial HMO, BlueCross, or commercial primary insurance, of whom 2591 (1.76%) underwent a prelabor cesarean delivery, yielding an odds ratio of 2.09 for the event.
The y-axis in Figure 1 displays varying strengths of association between a hypothetical clinical confounder and the presence of commercial insurance, and the x-axis displays varying strengths of association between the clinical confounder and prelabor cesarean delivery. The plotted points form an isobar that represents combinations of associations that would bring the observed AOR of 2.09 for prelabor cesarean delivery among commercially insured women (aggregating indemnity, HMO, and BlueCross) without previous cesarean delivery down to 1.00, relative to poorly insured women (aggregating Medicaid and self-pay). It is apparent that large and implausible AORs of 6.00 or more, between such an unmeasured clinical confounder and both the outcome and the presence of commercial insurance, would be required to fully explain the observed association.
DISCUSSION
The existing observational evidence suggests that routine elective cesarean delivery is not supported from a maternal and neonatal mortality or morbidity perspective10 but that it can ethically be performed when requested by an informed patient.11 Nevertheless, this practice raises obvious health care resource allocation and cost issues and creates a need for subsequent cesarean deliveries, with further increased neonatal morbidity.31 The rising rate of cesarean delivery is an important public health issue; Healthy People 2020 set an objective of a cesarean delivery rate lower than 23.9% for low-risk pregnancies (full term, singleton, vertex presentation) among women giving birth for the first time.32
I examined cesarean delivery in women without previous cesarean delivery for 2 reasons. Most pregnant women have never had a previous cesarean delivery (in this data, approximately 5 times as many as those who had), and pregnant women with a previous cesarean delivery are far more likely to have another cesarean delivery (in this data, approximately 3.5 times as likely) rather than delivering vaginally. As a public health priority, focusing cesarean delivery reduction efforts on such women is thus important.
I focused on women receiving such a primary cesarean delivery without apparent labor or its complications because these may include so-called elective cesarean deliveries that may not be medically necessary. I modeled the likelihood of a woman without previous cesarean delivery undergoing a cesarean delivery without laboring as a function of maternal demographics and maternal, fetal, and placental conditions as well as maternal primary insurance status.
I observed a slightly higher rate of such elective cesarean deliveries among insured women than among women without insurance. In this large, well-powered data set, this difference in outcomes was very precisely estimated. Controlling for observable factors that are associated with such prelabor cesarean delivery did not reduce the observed differences, which also persisted in more clinically homogeneous subsets. Although these data were very likely to mismeasure or omit clinically important confounders such as obesity, the results appeared robust to sensitivity analyses that simulated such unmeasured confounders.
Worsening maternal health over time could contribute to the rise in cesarean deliveries, although the national data does not support this explanation.3,33 Suggestive—although anecdotal—examples of such unmeasured maternal health characteristics are obesity and antenatal weight gain. These may render vaginal delivery more difficult and may simultaneously be more prevalent among poorer women. Scant evidence has been found for a causal relationship between obesity and prelabor cesarean delivery in women without previous cesarean delivery. Obesity increases maternity care costs,34 but the overall cesarean delivery rate has not conclusively been shown to be associated with weight gain35 or obesity.36
Limitations
No clinical consensus exists regarding which maternal, fetal, and placental conditions are valid indications for prelabor cesarean delivery and which are not.37 The conditions that have been identified in the literature are derived from analysis of existing practice rather than from randomized clinical trial evidence.38,39 Substantial physician- and hospital-level variation has also been observed in primary prelabor cesarean delivery rates.40 My use of a superset of such conditions derived from the specialist literature is not a completely satisfactory approach to controlling for conditions that might be associated with prelabor cesarean delivery. Practically, this means that my results were most likely biased toward the null hypothesis of finding no differences between differently insured women.
My analysis focused on relative differences in procedure rates between series of women and I was thus unable to confirm whether one rate is too high or another too low. It is, for example, possible that the rate of prelabor cesarean deliveries is reasonable among better-insured women, but too low among those without insurance, but the reverse is also possible. Or it could even be that rates are both too high among well-insured women and too low among the under- or unisured.
I restricted my analysis to women in 1 state, albeit a large state with high rates of cesarean delivery. How this limitation affected the external validity of my conclusions is not clear, although I have no reason to expect that the behavior of women and their physicians in New Jersey differed systematically from the practice in other states.
I used administrative claims data, in which the coding of comorbidities and clinically important conditions was unlikely to be consistent, accurate, or complete.37 Similar studies that used administrative data in the context of vaginal birth after cesarean delivery found accuracy and completeness issues, with approximately 10% of sample records having undocumented indications for cesarean delivery.41 I assumed that any such data issues did not differ across insurance types. A related data limitation was that the primary payer of record might not precisely identify the actual payer or the relative level of reimbursement. This would cause a conservative bias toward the null hypothesis of finding no differences.
A clinically important but unmeasured confounder could predispose to the outcome of interest (prelabor cesarean delivery) and be spuriously correlated with the exposure of interest (insurance type). I gauged the sensitivity of my results by simulating a hypothetical single, binary unmeasured confounder with varying strengths of association between confounder and outcome and between confounder and insurance type.29 However, this approach was not robust to multiple unobserved confounders nor to continuous as opposed to binary confounders.
Conclusions
Almost all primary prelabor cesarean deliveries are performed in women with at least 1 high-risk condition (94% in my data). Combined with the lack of consensus on what constitute reasonable indications and the resulting variations in practice, further research is needed to better understand which conditions genuinely represent medically necessary indications for prelabor cesarean delivery.
Although I identified small but consistent increases in the likelihood that women with more comprehensive insurance will have a prelabor cesarean delivery, I was unable to attribute this to a particular mechanism. Several possible mechanisms could explain these findings, with different policy implications.
If the findings were attributable to physician-induced demand driven by financial self-interest, then reengineering the fee-for-service scale or price incentives would be most important. The movement toward accountable care organizations42 and payments for episodes of care may provide some balance to such unwarranted variation.
If, instead, women with more comprehensive insurance coverage prefer elective cesarean deliveries because of possibly inaccurate perceptions of postdelivery morbidity or neonatal health advantages of cesarean delivery, then better antenatal education would be more important. Previous research showed that pregnant women and their physicians can react quickly to new medical findings.43 More research is needed on how women perceive the benefits, costs, and risks of cesarean delivery. Further research into the influence of a woman's obstetrician is also needed. When US obstetricians were surveyed about cesarean delivery in general, about half of the respondents acknowledged having performed at least 1 cesarean delivery on maternal request.12
Finally, if the observed increased rate of prelabor cesarean delivery was attributable to hospital or physician preferences for scheduling or staffing or to women's preferences for delivery timing, then policy solutions may not be easy to find. A clearer understanding of these potential mechanisms is important for mothers, neonates, their providers, and payers alike.
Acknowledgments
An abstract of this article was presented at AcademyHealth's annual research meeting, Seattle, WA, June 2011. An earlier version of this article was also presented at the Disparities Interest Group adjunct meeting, Seattle, June 2011.
Human Participant Protection
This study was approved by the Duke University Health System's institutional review board.
References
- 1.Appropriate technology for birth. Lancet. 1985;2(8452):436–437 [PubMed] [Google Scholar]
- 2.Gregory KD, Korst LM, Gornbein JA, Platt LD. Using administrative data to identify indications for elective primary cesarean delivery. Health Serv Res. 2002;37(5):1387–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Declercq E, Menacker F, Macdorman M. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health. 2006;96(5):867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health state-of-the-science conference statement: cesarean delivery on maternal request March 27–29, 2006. Obstet Gynecol. 2006;107(6):1386–1397 [PubMed] [Google Scholar]
- 5.Kringeland T, Daltveit AK, Moller A. What characterizes women in Norway who wish to have a caesarean section? Scand J Public Health. 2009;37(4):364–371 [DOI] [PubMed] [Google Scholar]
- 6.Coleman VH, Lawrence H, Schulkin J. Rising cesarean delivery rates: the impact of cesarean delivery on maternal request. Obstet Gynecol Surv. 2009;64(2):115–119 [DOI] [PubMed] [Google Scholar]
- 7.Korst LM, Gornbein JA, Gregory KD. Rethinking the cesarean rate: how pregnancy complications may affect inter-hospital comparisons. Med Care. 2005;43(3):237–245 [DOI] [PubMed] [Google Scholar]
- 8.Mawson AR. Reducing cesarean delivery rates in managed care organizations. Am J Manag Care. 2002;8(8):730–740 [PubMed] [Google Scholar]
- 9.Keeler EB, Brodie M. Economic incentives in the choice between vaginal delivery and cesarean section. Milbank Q. 1993;71(3):365–404 [PubMed] [Google Scholar]
- 10.Wagner M. Choosing caesarean section. Lancet. 2000;356(9242):1677–1680 [DOI] [PubMed] [Google Scholar]
- 11.Minkoff H, Chervenak FA. Elective primary cesarean delivery. N Engl J Med. 2003;348(10):946–950 [DOI] [PubMed] [Google Scholar]
- 12.Bettes BA, Coleman VH, Zinberg S, et al. Cesarean delivery on maternal request: obstetrician–gynecologists' knowledge, perception, and practice patterns. Obstet Gynecol. 2007;109(1):57–66 [DOI] [PubMed] [Google Scholar]
- 13.Ma K-ZM, Norton EC, Lee S- YD. Declining fertility and the use of cesarean delivery: evidence from a population-based study in Taiwan. Health Serv Res. 2010;45(5 Pt 1):1360–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spetz J, Smith MW, Ennis SF. Physician incentives and the timing of cesarean sections: evidence from California. Med Care. 2001;39(6):536–550 [DOI] [PubMed] [Google Scholar]
- 15.Brown HS., 3rd Lawsuit activity, defensive medicine, and small area variation: the case of cesarean sections revisited. Health Econ Policy Law. 2007;2(Pt 3):285–296 [DOI] [PubMed] [Google Scholar]
- 16.Yang YT, Mello MM, Subramanian SV, Studdert DS. Relationship between malpractice litigation pressure and rates of cesarean section and vaginal birth after cesarean section. Med Care. 2009;47(2):234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nathanael Johnson. For-profit hospitals performing more C-sections. California Watch. September 11, 2010. Available at: http://californiawatch.org/health-and-welfare/profit-hospitals-performing-more-c-sections-4069. Accessed November 14, 2010.
- 18.Finkler MD, Wirtschafter DD. Why pay extra for cesarean deliveries? Inquiry. 1993;30(2):208–215 [PubMed] [Google Scholar]
- 19.Gruber J, Kim J, Mayzlin D. Physician fees and procedure intensity: the case of cesarean delivery. J Health Econ. 1999;18(4):473–490 [DOI] [PubMed] [Google Scholar]
- 20.Misra A. Impact of the HealthChoice program on cesarean section and vaginal birth after C-section deliveries: a retrospective analysis. Matern Child Health J. 2008;12(2):266–274 [DOI] [PubMed] [Google Scholar]
- 21.Henry OA, Gregory KD, Hobel CJ, Platt LD. Using ICD-9 codes to identify indications for primary and repeat cesarean sections: agreement with clinical records. Am J Public Health. 1995;85(8 Pt 1):1143–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory KD, Korst LM, Fridman M, et al. Vaginal birth after cesarean: clinical risk factors associated with adverse outcome. Am J Obstet Gynecol. 2008;198(4):452.e1–452.e12 [DOI] [PubMed] [Google Scholar]
- 23.HealthGrades Sixth Annual Women's Health in American Hospitals Study. June 30, 2009 Available at: http://www.healthgrades.com/cms/ratings-and-awards/2009-10-Maternity-Care-Excellence-Announcementaspx Accessed November 14, 2010
- 24.International Classification of Diseases, Ninth Revision, Clinical Modification. Hyattsville, MD: National Center for Health Statistics; 1980. DHHS publication PHS 80-1260 [Google Scholar]
- 25.Gregory KD, Korst LM. Age and racial/ethnic differences in maternal, fetal, and placental conditions in laboring patients. Am J Obstet Gynecol. 2003;188(6):1602–1608 [DOI] [PubMed] [Google Scholar]
- 26.Meikle SF, Steiner CA, Zhang J, Lawrence WL. A national estimate of the elective primary cesarean delivery rate. Obstet Gynecol. 2005;105(4):751–756 [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691 [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46 [PubMed] [Google Scholar]
- 29.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25(6):1107–1116 [PubMed] [Google Scholar]
- 30.Hadar A, Sheiner E, Hallak M, Katz M, Mazor M, Shoham-Vardi I. Abnormal fetal heart rate tracing patterns during the first stage of labor: effect on perinatal outcome. Am J Obstet Gynecol. 2001;185(4):863–868 [DOI] [PubMed] [Google Scholar]
- 31.Kamath BD, Todd JK, Glazner JE, Lezotte D, Lynch AM. Neonatal outcomes after repeat cesarean delivery. Obstet Gynecol. 2009;113(6):1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healthy People 2020 Maternal, infant, and child health. Objective MICH-7.1, women giving birth for the first time. Available at: http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=26. Accessed May 8, 2011
- 33.Joesch JM, Gossman GL, Tanfer K. Primary cesarean deliveries prior to labor in the United States, 1979–2004. Matern Child Health J. 2008;12(3):323–331 [DOI] [PubMed] [Google Scholar]
- 34.Trasande L, Lee M, Liu Y, Weitzman M, Savitz D. Incremental charges, costs, and length of stay associated with obesity as a secondary diagnosis among pregnant women. Med Care. 2009;47(10):1046–1052 [DOI] [PubMed] [Google Scholar]
- 35.Rhodes JC, Schoendorf KC, Parker JD. Contribution of excess weight gain during pregnancy and macrosomia to the cesarean delivery rate, 1990–2000. Pediatrics. 2003;111(5 Pt 2):1181–1186 [PubMed] [Google Scholar]
- 36.Kaiser PS, Kirby RS. Obesity as a risk factor for cesarean in a low-risk population. Obstet Gynecol. 2001;97(1):39–43 [DOI] [PubMed] [Google Scholar]
- 37.Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108(6):1506–1516 [DOI] [PubMed] [Google Scholar]
- 38.Lavender T, Hofmeyer GJ, Neilson JP, Kingdon C, Gyte GM. Caesarean section for non-medical reasons at term. Cochrane Database Syst Rev. 2006;3:CD004660. Available at: http://dx.doi.org/doi:10.1002/14651858.CD004660.pub2. Accessed November 14, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Miesnik SR, Reale BJ. A review of issues surrounding medically elective cesarean delivery. J Obstet Gynecol Neonatal Nurs. 2007;36(6):605–615 [DOI] [PubMed] [Google Scholar]
- 40.Gregory KD, Korst LM, Platt LD. Variation in elective primary cesarean delivery by patient and hospital factors. Am J Obstet Gynecol. 2001;184(7):1521–1532 [DOI] [PubMed] [Google Scholar]
- 41.Lydon-Rochelle MT, Gardella C, Cardenas V, Easterling TR. Repeat cesarean delivery: what indications are recorded in the medical chart? Birth. 2006;33(1):4–11 [DOI] [PubMed] [Google Scholar]
- 42.Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in Medicare. Health Aff (Millwood). 2009;28(2):w219–w231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price J, Simon K. Patient education and the impact of new medical research. J Health Econ. 2009;28(6):1166–1174 [DOI] [PubMed] [Google Scholar]