Abstract
Objectives. We tested the effectiveness of a culturally tailored, behavioral theory–based community health worker intervention for improving glycemic control.
Methods. We used a randomized, 6-month delayed control group design among 164 African American and Latino adult participants recruited from 2 health systems in Detroit, Michigan. Our study was guided by the principles of community-based participatory research. Hemoglobin A1c (HbA1c) level was the primary outcome measure. Using an empowerment-based approach, community health workers provided participants with diabetes self-management education and regular home visits, and accompanied them to a clinic visit during the 6-month intervention period.
Results. Participants in the intervention group had a mean HbA1c value of 8.6% at baseline, which improved to a value of 7.8% at 6 months, for an adjusted change of -0.8 percentage points (P < .01). There was no change in mean HbA1c among the control group (8.5%). Intervention participants also had significantly greater improvements in self-reported diabetes understanding compared with the control group.
Conclusions. This study contributes to the growing evidence for the effectiveness of community health workers and their role in multidisciplinary teams engaged in culturally appropriate health care delivery.
African American and Latino adults experience a 50% to 100% higher burden of illness and mortality as a result of type 2 diabetes than do White Americans.1–5 Both African American and Latino adults with diabetes have worse glycemic control and report experiencing more barriers to diabetes self-management than do non-Latino White adults.6–9 Community health worker (CHW) interventions have demonstrated promise in improving health behaviors and outcomes, particularly for racial and ethnic minority communities and those who have traditionally lacked access to adequate health care.10–17 CHW interventions enlist and train community members who work as bridges between their ethnic, cultural, or geographic communities and health care providers to promote health.18–20 In chronic disease care, CHWs often educate patients, identify resources, provide case management, coordinate care in partnership with the health care system, and become part of the individual's support network.16
Although initial results from CHW programs are encouraging,11,12,16 many have had methodological limitations, including lack of grounding in behavioral theory and inadequately rigorous evaluation.11,16,20 In particular, as noted in a recent Cochrane review of evaluations of CHW programs,21 most have not used a randomized controlled trial design and thus have not adequately addressed potential threats to their external validity, such as selection bias and unmeasured differences between intervention and control groups. Moreover, in most prior evaluations of diabetes self-management interventions targeting underserved populations, the participating communities were not actively involved in identifying needs or in developing, implementing, and evaluating the interventions, which may have limited their effectiveness and potential sustainability.
The intervention that we describe sought to address these deficiencies. The current intervention is among several diabetes interventions conducted since 2000 by the REACH Detroit Partnership, as part of the Centers for Disease Control and Prevention (CDC)-funded Racial and Ethnic Approaches to Community Health (REACH) Initiative. Using community-based participatory research (CBPR) principles,22–26 community, health system, and academic partners completed a 1-year planning process to develop interventions to improve diabetes prevention and treatment in the participating communities.27 Using a socioecological model, family, health system, and community-level interventions were developed to address sources of diabetes disparities at each level.12,27,28 CHWs were central to each intervention. Interventions were grounded in empowerment theory, which emphasizes a collaborative approach to facilitating the self-directed behavior change of patients.29–36 The empowerment philosophy includes self-determination and autonomy motivation theory, which postulates that an individual will be more successful in a disease-management plan if that individual's goals, objectives, and resources guide the development of that plan.37–39 Empowerment-based approaches have been found to be effective in improving chronic disease self-management among racial and ethnic minority patients.29–36
To date, we have completed 2 cohorts of the study intervention. Our first cohort included 180 African American and Latino participants who received CHW services and were compared with a historical control. Participants in that study had improved hemoglobin A1c (HbA1c) values compared with the control group at 6 months follow-up.12 These encouraging findings led to the approval by our community partners of the 6-month randomized controlled trial design used in the current study. We report the results of a randomized controlled trial that tested whether a culturally tailored CHW intervention for diabetes self-management improved HbA1c levels, blood pressure, lipid levels, diabetes knowledge, diabetes self-management behavior, and diabetes-related distress more than usual care among low-income, inner-city African Americans and Latinos with diabetes.
METHODS
We randomized African American and Latino participants with diabetes into a CHW intervention group or a control group in which the CHW intervention was delayed for 6 months. All participants in the study, whether in the intervention or control group, received information on, and had access to, REACH Detroit community activities that provided free, publicly available healthy eating demonstrations, physical fitness activity (e.g., dance and exercise classes, walking clubs), and a weekly community farmers’ produce market. All participants also received health care at facilities in which health care providers were trained by REACH Detroit in culturally competent diabetes care through our health systems intervention.
Setting
All participants lived in either southwest Detroit, where residents were predominantly Latino of Mexican origin (70%) and had an annual median household income of $11 500, or eastside Detroit, which is largely African American (80%) with a median household income of $25 020.40 Participants from southwest Detroit received medical care at a federally qualified community health center, whereas participants from eastside Detroit received medical care at a major local health system.
Participants
Through medical records we identified eligible participants who were at least 18 years of age, had physician-diagnosed type 2 diabetes, self-identified as African American or Latino/Hispanic, and lived in targeted zip codes. We excluded individuals who already had serious diabetes-related complications, such as blindness, amputated limbs, or kidney failure. We recruited participants from September 2004 to July 2006. Participants were stratified by race/ethnicity and health care site during randomization to ensure that these variables were equally distributed across the 2 arms of the intervention. To account for possible attrition due to our delayed design, we assigned 45% of participants to treatment and 55% to control. Participants assigned to the control group were aware of the randomized structure of the study and were informed that they would receive the intervention after the 6-month control period. Because of the nature of the study design, CHWs and interviewers were not blinded to the group assignment of the participants; however, data analysts were blinded.
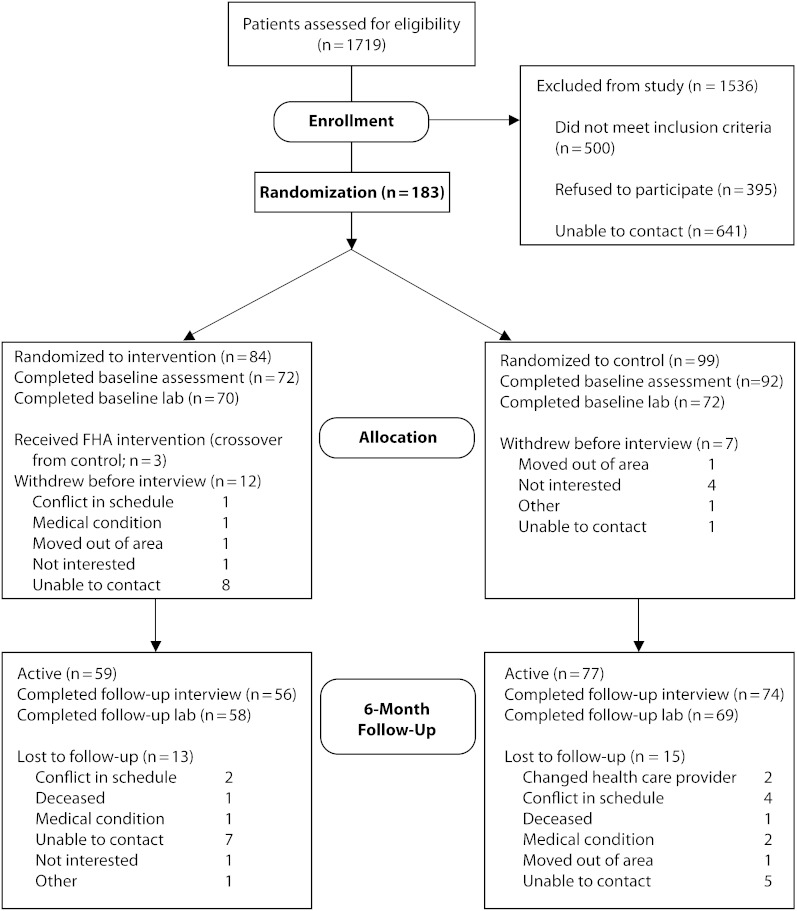
Of 1719 potentially eligible patients, 29% did not meet eligibility criteria, 23% refused to participate, and 37% could not be contacted (Figure 1). Of 183 randomized participants, 164 completed the baseline interview. At the 6-month follow-up, 136 participants completed the study protocols and were analyzed for the primary outcome (attrition rate = 17.7%). Because of severe medical conditions, 3 participants assigned to the control group received the CHW intervention; however, following intention-to-treat principles, these participants remain assigned to the control group in our analyses. Withdrawal from the study was not independently associated with treatment arm, age, gender, education, diabetes duration, baseline HbA1c, low-density lipoprotein (LDL) cholesterol, or blood pressure. However, African American participants were more likely to withdraw from the study and to be missing HbA1c data than were Latino participants. In addition, race/ethnicity was nested within treatment site; we therefore included an indicator of race/ethnicity and site as a covariate in the outcomes models.
FIGURE 1.
Study participant flow diagram of enrollment and loss to follow-up: REACH Detroit, 2004–2006.
Note. FHA = family health advocate.
Intervention
Trained CHWs, known in this study as family health advocates, promoted healthy lifestyle and diabetes self-management activities. We recruited the family health advocates from the 2 participating communities, where they were ethnically matched with their assigned participants, underwent more than 80 hours of training, and conducted 3 primary activities: (1) diabetes education classes, (2) 2 home visits of about 60 minutes each in length per month to address participants’ specific self-management goals, and (3) 1 clinic visit with the participant and his or her primary care provider. The diabetes education classes were culturally tailored group classes in both English and Spanish. Eleven 2-hour group sessions of 8 to 10 participants were held every 2 weeks at community locations. The development, implementation, and evaluation of these curricula are described in depth elsewhere.12,28 In home visits, family health advocates assisted participants in setting patient-specific goals and supporting their progress. In addition, family health advocates helped participants improve their patient–provider communication skills and facilitated necessary referrals to other service systems. Family health advocates also contacted intervention participants by phone once every 2 weeks. Participants in the control group were contacted once per month to update contact information.
Family health advocates were trained in empowerment-based approaches to inform their approach to each component on the intervention.31,32 For example, they received training in approaches based on motivational interviewing, which is used to elicit participants’ goals and help participants formulate their own action plans. They also used empowerment theory in the diabetes education classes by eliciting participants’ experiences and requests for information to be provided during the sessions.
Outcome Measures
We abstracted physiological measures of HbA1c, LDL cholesterol, and blood pressure from medical records. When these measures were not available at baseline or 6 months, we selected the closest values in time. On average, laboratory tests were performed 0.3 months after the baseline interview (range = 5.7 months before to 2.5 months after interview) and, for the 6-month follow-up, 7 months after the baseline interview (range = 3.7–9.5 months after interview). The time between the 2 sets of laboratory tests was always greater than 3 months. We obtained all other measures from a survey conducted in person, in either English or Spanish.
We measured self-management knowledge by participants’ response to the validated question “How well do you understand how to manage your diabetes?”41 and the following 2 items: “I agree that what one eats effects blood sugar control” and “Exercise helps to control blood sugar.”42 We measured diabetes self-management by 5 questions from the Summary of Diabetes Self-Care Activities scale43 on adherence to a healthy eating plan, glucose testing, medication taking, foot checks, and shoe inspections. We measured diabetes-specific psychological distress by the validated Problem Areas in Diabetes scale.44–46 We measured diabetes self-efficacy, one estimate of participant empowerment, with the Perceived Competence for Diabetes scale.47
We assessed physical activity and dietary practices through questions from the CDC's Behavioral Risk Factor Surveillance System.48 We report the percentage of participants who engaged in moderate levels of physical activity 30 minutes per day at least 5 days per week, meeting American Diabetes Association recommendations for physical activity. Specific dietary measures included servings per day of fruits and vegetables, consumption of 2 or more servings per week of fried or fatty foods, and consumption of 2 or more servings per week of soda pop or fruit-flavored drinks.
We assessed the demographic variables gender, age, education, and race/ethnicity. Because 97% of the Latinos spoke Spanish as their primary language, respondents’ primary language was not included in the analyses for Latinos. Health status variables assessed at baseline were self-reported medication regimen and number of years since diabetes diagnosis.
Analysis
We compared baseline characteristics between the intervention and control groups with the Student t test for continuous variables and the Pearson χ2 test for categorical variables. Skewed linear variables were log transformed. To adjust for repeated measures, we evaluated intervention effects on outcomes using linear mixed models for continuous variables and generalized estimating equations for dichotomous variables. We analyzed participant data as part of their original random group assignment, following intention-to-treat principles. For each analysis, we included all available participant data for the particular outcome at baseline and at 6 months. Both linear mixed models and generalized estimating equations use all available data at particular time points for estimates.49–52 We used SAS version 9.2 (SAS Institute, Cary, NC) for all analyses. Because we were missing data for some participants’ HbA1c values, we conducted a second analysis that imputed missing data.53,54 Our results in these analyses were unchanged, so we report the unimputed analyses.
RESULTS
Table 1 presents the baseline characteristics for each group. Because age significantly differed by treatment group, it is included as a covariate in outcomes analyses. Because gender was associated with HbA1c at outcome (data not shown), it is also included as a covariate. Among those completing the baseline assessment, 86.1% attended at least 1 intervention class and 54.2% attended all 11 classes. The mean number of classes attended was 8.1 (SD = 4.2).
TABLE 1.
Baseline Participant Characteristics: REACH Detroit, 2004–2006
| Intervention Group (n = 72) | Control Group (n = 92) | P | |
| Latino adults, no. (%) | 34 (47) | 36 (39) | .3a |
| African American adults, no. (%) | 38 (53) | 56 (61) | |
| Spanish primary language,b no./total no. (%) | 32/34 (94) | 35/36 (97) | .6c |
| Women, no. (%) | 54 (75) | 62 (67) | .29a |
| Age, y mean (95% CI) | 50 (47, 52) | 55 (53, 57) | .02d |
| High school graduate, no. (%) | 43 (60) | 54 (59) | .89a |
| BMI,e kg/m2, mean (95% CI) | 34 (32, 36) | 35 (33, 37) | .64e |
| Diabetes duration,c y, mean (95% CI) | 8 (6, 9) | 9 (7, 11) | .7e |
| Hemoglobin A1c level,c % (95% CI) | 8.6 (8.0, 9.1) | 8.5 (8.0, 8.9) | .88e |
| Self-efficacy score,f mean (95% CI) | 72.7 (69.2, 76.2) | 64.6 (61.1, 68.1) | .03d |
| Taking diabetes medications, no. (%) | .73a | ||
| No medications | 8 (11) | 7 (8) | |
| Oral medications only | 43 (61) | 57 (63) | |
| Insulin | 19 (27) | 26 (29) |
Note. BMI = body mass index; CI = confidence interval. Intervention group received treatment after baseline interview; control received treatment 6 months after baseline interview.
Pearson χ2 test.
Only applicable to Latinos (n = 70).
Fisher's exact test.
Student t test.
Student t test on log transform; untransformed mean and confidence interval displayed.
Perceived Competence for Diabetes scale.47 The scale is based on 4 questions on a 1-5 Likert scale to assess the client's confidence in carrying out the management of type 2 diabetes. The 0-100 score is computed by subtracting 1 from each response and by multiplying the average response to the 4 questions by 25.
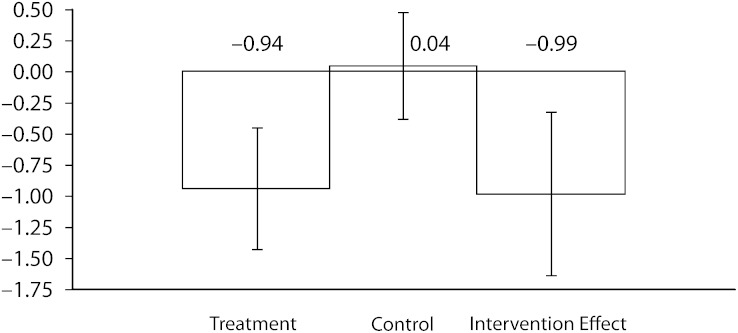
Figure 2 shows the unadjusted mean change in HbA1c values from baseline to 6-month follow-up, which shows a -0.99 intervention effect (P < .01). Table 2 reports the results of linear mixed models of changes in physiological measures in each group, adjusted for age, race/ethnicity and site, and gender. Results of unadjusted and adjusted analyses did not significantly differ between groups or over time. Adjusted mean HbA1c level was 8.6% at baseline in the intervention group; this level improved to 7.8% at 6 months, for a change of -0.8 percentage points (P < .01). Change in HbA1c level for the control group was not significant (mean = 8.5% at baseline and 6-month follow-up). The difference in the change in HbA1c level between the 2 groups was -0.8%. Intervention group participants’ LDL cholesterol level also improved significantly from baseline to 6-month follow-up (mean = 105 mg/dL at baseline and 95 mg/dL at 6-month follow-up; P < .05 within group). The difference in change in LDL level between the 2 groups was not significant. Changes in blood pressure were not significant.
FIGURE 2.
Unadjusted decreases in hemoglobin A1c levels from baseline to 6-month follow-up, by intervention arm with 95% confidence intervals: REACH Detroit, 2004–2006.
TABLE 2.
Changes in Physical Measurements of Study Participants From Baseline to 6-Month Follow-Up: REACH Detroit, 2004–2006
| Intervention Group |
Control Group |
||||||||
| Baseline | 6 Months | Change | No.a | Baseline | 6 Months | Change | No.a | Intervention Effect | |
| Mean HbA1c, % (95% CI) | 8.6 (8.1, 9.2) | 7.8 (7.3, 8.3) | −0.8** (−1.2, −0.4) | 56 | 8.5 (8.0, 9.1) | 8.5 (8.0, 9.1) | 0.0 (−0.4, 0.4) | 57 | −9.7** (−15.9, −3.0) |
| Mean LDL, mg/dL (95% CI) | 105 (95, 115) | 95 (86, 104) | −10* (−17, −2) | 51 | 112 (103, 122) | 108 (99, 118) | −4 (−12, 4) | 55 | −5.8 (−15.6, 5.1) |
| Mean SBP, mm Hg (95% CI) | 131 (127, 134) | 129 (125, 133) | −2 (−6, 2) | 54 | 130 (127, 133) | 127 (124, 130) | −3 (−6, (1) | 65 | 1.0 (−3.1, 5.1) |
| Mean DBP, mm Hg (95% CI) | 77 (74, 80) | 77 (74, 79) | 0 (−3, 3) | 54 | 77 (74, 79) | 75 (72, 77) | −2 (−5, 1) | 65 | 2.2 (−3.1, 7.9) |
| Mean BMI, kg/m2 (95% CI) | 32.7 (30.8, 34.6) | 33.0 (31.1, 35.0) | 0.3 (−0.4, 1.1) | 52 | 34.1 (32.4, 35.9) | 33.7 (32.0, 35.6) | −0.4 (−1.1, 0.3) | 65 | 2.1 (−1.0, 5.3) |
Note. BMI = body mass index; DBP = diastolic blood pressure; HbA1c = hemoglobin A1c; LDL = low-density lipoprotein cholesterol; SBP = systolic blood pressure. Reported data are mean values adjusted for age, gender, race/ethnicity, and site. Estimates and P values are from repeated measures models with log transforms. The estimates of change within the intervention and control arms were computed by inverting the log transformed results. The intervention effect estimates are for percent change between intervention and control groups.
Number with complete data at baseline and 6-month follow-up.
*P < .05; **P < .01.
Table 3 presents the results of linear mixed models of age, race/ethnicity and site, and gender-adjusted changes in self-management knowledge and behavioral variables from baseline to 6-month follow-up. Responses to all 3 self-reported self-management knowledge questions showed statistically significant differences within the intervention group (P < .01 for each), as well as significant differences between the intervention and the control groups (P < .05 between groups for each).
TABLE 3.
Study Participants’ Changes in Knowledge of Diabetes and in Behavioral Variables From Baseline to 6-Month Follow-Up: REACH Detroit, 2004–2006
| Intervention (n = 72) |
Control (n = 92) |
||||||||
| Baseline | 6 Months | Change | No.a | Baseline | 6 Months | Change | No.a | Intervention Effect | |
| Knowledge | |||||||||
| Self-management score,b mean (95% CI) | 3.3 (3.1, 3.6) | 4.0 (3.7, 4.3) | 0.7** (0.4, 1.0) | 56 | 3.3 (3.0, 3.5) | 3.3 (3.1, 3.6) | 0.1 (−0.2, 0.3) | 74 | 0.6** (0.2, 1.0) |
| What you eat affects blood sugar,c % (95% CI) or (OR; 95% CI) | 80.5 (67.7, 89.1) | 98.3 (87.4, 99.8) | (13.8**; 2.0, 94.7) | 55 | 80.0 (69.3, 87.6) | 82.8 (72.2, 90.0) | (1.2; 0.6, 2.6) | 74 | (11.4*; 1.4, 90.4) |
| Exercise helps control blood sugar,d % (95% CI) or (OR; 95% CI) | 54.9 (42.3, 67.0) | 83.0 (70.1, 91.0) | (4.0**; 1.9, 8.6) | 52 | 44.6 (33.5, 56.2) | 42.8 (30.9, 55.7) | (0.9; 0.5, 1.6) | 71 | (4.3**; 1.7, 10.9) |
| Diet and exercise | |||||||||
| Meeting physical activity guidelines,e % (95% CI) or (OR; 95% CI) | 36.6 (25.2, 49.7) | 52.9 (38.2, 67.1) | (1.9*; 1.02, 3.7) | 55 | 31.7 (22.4, 42.8) | 52.8 (40.8, 64.4) | (2.4**; 1.3, 4.3) | 72 | (0.8; 0.3, 1.9) |
| Daily fruit and vegetable servings, mean (95% CI) | 4.3 (3.8, 4.9) | 4.9 (4.3, 5.4) | 0.5 (0.0, 1.1) | 56 | 3.6 (3.2, 4.1) | 4.2 (3.7, 4.7) | 0.6* (0.1, 1.1) | 73 | 0.0 (-0.8, 0.7) |
| Fried, fatty foods ≥ 2/wk, % (95% CI) or (OR; 95% CI) | 37.7 (25.8, 51.2) | 21.7 (12.3, 35.4) | (0.5; 0.19, 1.1) | 56 | 52.4 (40.1, 64.5) | 47.8 (34.9, 61.0) | (0.8; 0.5, 1.5) | 73 | (0.6; 0.2, 1.6) |
| Soda pop or fruit-flavored drinks ≥ 2/wk, % (95% CI) or (OR; 95% CI) | 23.3 (14.1, 36.1) | 12.4 (5.7, 25.1) | (0.5; 0.17, 1.3) | 56 | 37.8 (27.9, 48.8) | 37.8 (27.1, 50.0) | (1.0; 0.6, 1.6) | 74 | (0.5; 0.2, 1.4) |
| Diabetes self-managementf | |||||||||
| Take recommended insulin dose or pills every day, % (95% CI) or (OR; 95% CI) | 88.8 (77.1, 94.9) | 89.6 (78.6, 95.3) | (1.1; 0.53, 2.2) | 52 | 88.0 (78.6, 93.6) | 96.2 (90.5, 98.6) | (3.5**; 1.4, 8.8) | 70 | (0.3; 0.1, 1.0) |
| Follow healthful eating plan every day, % (95% CI) or (OR; 95% CI) | 41.3 (29.1, 54.7) | 43.1 (29.4, 58.0) | (1.1; 0.57, 2.0) | 54 | 37.3 (26.9, 48.9) | 27.7 (18.3, 39.7) | (0.6; 0.4, 1.1) | 72 | (1.7; 0.7, 3.9) |
| Test blood sugar as recommended once/wk, % (95% CI) or (OR; 95% CI) | 73.6 (61.0, 83.2) | 86.7 (74.3, 93.6) | (2.3*; 1.1, 5.0) | 56 | 70.5 (59.6, 79.5) | 78.0 (66.8, 86.2) | (1.5; 0.8, 2.6) | 74 | (1.6; 0.6, 4.0) |
| Check feet every day, % (95% CI) or (OR; 95% CI) | 80.2 (68.3, 88.4) | 86.9 (75.1, 93.6) | (1.6; 0.7, 3.9) | 56 | 75.3 (65.1, 83.3) | 67.1 (55.2, 77.1) | (0.7; 0.4, 1.1) | 73 | (2.5; 0.9, 6.7) |
| Inspect inside of shoes every day, % (95% CI) or (OR; 95% CI) | 49.1 (36.1, 62.2) | 76.7 (62.9, 86.5) | (3.4**; 1.7, 6.8) | 56 | 43.8 (33.2, 55.0) | 44.5 (33.0, 56.7) | (1.0; 0.6, 1.7) | 72 | (3.3**; 1.4, 7.9) |
| Psychological | |||||||||
| Problem Areas in Diabetes (PAID) scale score,gh mean (95% CI) | 11.9 (8.5, 16.6) | 8.7 (6.0, 12.4) | −3.2 (-5.6, 0.1) | 56 | 13.8 (10.2, 18.5) | 12.9 (9.4, 17.6) | −0.9 (−4.0, 3.2) | 74 | −21.9% (−49.0, 19.4) |
| Self-efficacy score,i mean (95% CI) | 74.9 (69.8, 80.1) | 78.3 (72.6, 83.9) | 3.3 (−2.7, 9.3) | 56 | 65.5 (60.9, 70.0) | 70.7 (65.7, 75.7) | 5.2 (0.0, 10.4) | 74 | −1.9 (−9.9, 6.1) |
Note. CI = confidence interval; OR = odds ratio. Data are adjusted for age, gender, race/ethnicity, and site. Estimates and P values are from repeated measures models.
Number with complete data at baseline and 6-month follow-up.
Determined by answer to the question, “How well do you understand how to manage your diabetes?” (1–5; 1 = not at all; 5 = very well).41
Percentage who “Strongly agree that what you eat will make a big difference in your ability to control affect blood sugar levels.”
From Outcome Expectations for Exercise Scale.42 The % and ORs denote clients who strongly agree that exercise helps to control blood sugar.
Moderate activity at least 30 minutes a day and 5 days a week or vigorous activity at least 20 minutes a day and 3 days a week.48
Summary of Diabetes Self-Care Activities Measure.43 Clients who reported practicing the various self-management behaviors every day are represented in the various percentages and odds ratios. For example, if a client reported checking his/her feet every day, s/he would be included in the % or OR for that outcome; anything less than every day would be coded as “No.”
Estimates from log transform. The estimates of change within the intervention and control arms were computed by inverting the log transformed results. The intervention effect estimates are for percentage change, intervention compared with control.
Problem Areas in Diabetes scale (20 items; 0 = not a problem to 4 = serious problem; Cronbach α = 0.94).44,45
Perceived Competence for Diabetes scale (range = 0–100).47
*P < .05; **P < .01.
Both the intervention and the control groups demonstrated a statistically significant increase in meeting guidelines for physical activity: from 37% to 53% for the intervention group (P < .05) and from 32% to 53% for the control group (P < .01). However, there was no significant difference in change in physical activity between the groups. Although the daily servings of fruits and vegetables increased by close to half a serving for both groups, the increase was statistically significant for the control group (P < .05) but not for the intervention group. Changes in the consumption of fried or fatty foods and soda pop or fruit-flavored drinks were not statistically significant. Participants within the intervention group significantly improved adherence to inspecting the inside of their shoes every day at the 6-month follow-up (49% -77%; P < .01), and also improved significantly compared with the control group (P < .01).
The intervention group significantly improved in adherence to testing blood sugar as recommended (from 74% to 87%; P < .05), but there was no significant difference between groups. The control group significantly improved adherence to daily diabetes medications between baseline (88%) and 6-month follow-up (96%; P < .01). Mean diabetes-specific psychological distress improved for the intervention group (3.2 mean decrease from baseline to 6-month follow-up); however, this change was not statistically significant. Finally, there were no significant changes or between-group differences in mean diabetes self-efficacy. No adverse effects were noted among participants as a result of the intervention.
DISCUSSION
In this randomized controlled trial of 164 African American and Latino adults with type 2 diabetes, the average decrease in HbA1c levels from baseline to 6-month follow-up was 0.8% greater for the CHW intervention group than for the delayed-intervention control group. This significant difference provides both statistical and clinical55 evidence for the effectiveness of the intervention in improving glycemic control in a low-income, underserved population. We have contributed to the growing body of literature that suggests that CHWs can have a positive impact on improving racial and ethnic minority participants’ health status (particularly HbA1c levels),56,57 self-reported diabetes knowledge and education,58,59 and some self-care behaviors.59,60 A unique strength of our study is the use of CHWs who were trained in empowerment approaches to promote the self-management of diabetes in their own communities. Additionally, our study joins the small group of rigorous CHW studies that have compared the health outcomes of those receiving CHW services and those receiving usual health care,11,21,60 and it has important implications for further integrating CHWs as an important part of multidisciplinary teams in health care systems.
Our findings support our main hypothesis that the intervention would significantly improve HbA1c levels in the intervention group compared with the control group. When we examined study outcomes in self-management knowledge and behaviors, several possible mechanisms for the intervention effect on HbA1c emerged. Most notable were significant improvements in self-reported knowledge about diabetes self-management and physical activity in the intervention group. The intervention group also showed improvements in healthy eating; however, these changes were not statistically significant. These findings could be a result of sample size limitations in the study. The sample size in the control group (n = 92) was 28% larger than that of the intervention group (n = 72), so some within-group change might be significant in the control group but not in the intervention group. For example, the magnitude of the average increase in fruit and vegetable servings was similar between the 2 groups (0.6 in the control group vs 0.5 in the intervention group), even though this increase was statistically significant only for the larger control group. Also, the overall limited sample size could have led to false negatives in between-group comparisons. In addition, from baseline to 6-month follow-up, the intervention group reduced consumption of fried or fatty foods (from 37.7% to 21.7% mean decrease) and of soda pop or fruit-flavored drinks (from 23.3% to 12.4%), and mean consumption of these unhealthy foods was only slightly changed in the control group.
These qualitatively significant differences in intervention group eating habits may have contributed to reductions in HbA1c levels, even though the between-group differences were not statistically significant. Another statistical concern is the high rate of reported medication adherence in the intervention group at baseline (89%), which may have led to limited ability to detect a statistically significant improvement. Comprehensive exploration of possible mechanisms for the intervention's effect will be an important area for future investigation. In addition, it will be important to assess potential moderators of intervention effects such as baseline literacy levels, as well as the possible dose–response effect of the intervention classes and family health advocate contacts. Because these exposures were not randomly assigned, however, their relationship with outcomes is likely to be confounded by patients’ need for assistance as well as intrinsic motivation for improving their diabetes self-care.
The control group also made significant improvements in fruit and vegetable consumption, physical activity, and medication adherence during the study. These improvements may have been due to the communitywide healthy eating and physical activity programs available to and promoted among both groups as part of REACH Detroit's community-level programs. In addition, all participants’ health care providers were invited to participate in diabetes continuing medical education courses sponsored by REACH Detroit. REACH Detroit's goal is to reduce barriers to healthy lifestyles and self-management in under-resourced communities, beyond individual- and family-level behavior change. Withholding community resources and continuing education for providers of the control group may have improved our ability to detect differences between groups. However, community input through our CBPR process supported our decision to keep community resources and provider education available to all, holding firm to our commitment to a comprehensive approach to reducing diabetes health disparities.
Our findings also confirm the feasibility of conducting rigorous research in disempowered communities using CBPR principles and methods. A CBPR approach affects health disparities in underserved communities in at least 3 ways: through building capacity, through focusing attention on social justice, and through sharing of power and resources.22–26 Our process of developing, implementing, and evaluating the intervention adhered to the principle of partnership on which CBPR is based—that better understanding of the nature and consequences of the disparities, and of how they might be solved, depends on insight from those affected by them. The rigor of the methods we pursued further reinforces the view that it is possible to follow CBPR principles while meeting the highest standards of scientific, randomized controlled trials.61
Limitations
Several limitations should be noted in interpreting our findings. First, our modest sample size may have limited our power to detect significant differences other than in HbA1c levels. Second, we used medical chart reviews to obtain our clinical measures, and the lab tests did not always correspond precisely with our baseline and 6-month interview dates. However, we used only clinical measures that were taken within 3 months of baseline and 2 months of the 6-month assessment dates, which would have had less of an effect on HbA1c data but could have affected other outcomes, such as LDL or blood pressure. Finally, self-reported data also were used for all behavioral measures, possibly resulting in socially desirable responses.
Besides the significant improvement in HbA1c levels in the intervention group compared with the control group, our study demonstrates several accomplishments. First, we have developed an effective CHW diabetes management model that future research can build on, using CBPR principles and methods. Second, we further developed diabetes self-management educational curricula and materials that are culturally and linguistically tailored and replicable and that are rooted in an empowerment-based approach. Finally, we demonstrated the feasibility of conducting a randomized controlled intervention with 2 underrepresented racial/ethnic communities that is attentive to the historical distrust of research by racial/ethnic groups, but rigorous in its consideration of design and methods.
Practice and Policy Implications
A major aim of our study was to provide evidence for the effectiveness of CHWs and their role as members of multidisciplinary teams engaged in culturally appropriate health and social services delivery. Through this evidence, we hope to promote the development and expansion of CHW programs in the United States. However, major challenges need to be addressed before these programs will be more widely accepted throughout the various health sectors, including inadequate and unstable funding and the low value often placed on CHW work, which impedes their recognition as legitimate providers.62–66 The task of addressing these challenges can be accomplished through further research on CHW effectiveness and continued advocacy by health care providers. Our study represents a major step toward demonstrating such effectiveness and can be used in state and national advocacy efforts aimed at increasing public and private funds for supporting programs using CHWs and other lay health workers. As the widening gap in health and social disparities continues to challenge our nation and its strained systems of care, our study lends evidence that CHW interventions provide one possible solution to meeting the needs of disenfranchised communities and are ready and natural allies for health care providers who share the common goals of social equality and culturally appropriate care.
Acknowledgments
This research was supported by the National Institute of Diabetes and Digestive and Kidney Disease (grant R18DK0785501A1), Centers for Disease Control and Prevention (Cooperative Agreement No. U50/CCU417409), the Michigan Diabetes Research and Training Center (NIH grant 5P60-DK20572), and the Robert Wood Johnson Foundation Clinical Scholars Program.
We thank the CHASS/REACH Detroit Partnership staff, the REACH Detroit Partnership Steering Committee (available at: http://www.reachdetroit.org), and the REACH Detroit Family Intervention participants for their involvement in this study. The REACH Detroit Partnership is affiliated with the Detroit Community-Academic Urban Research Center (available at: http://www.sph.umich.edu/URC).
Human Participant Protection
The research was approved by the University of Michigan Health Sciences institutional review board.
References
- 1.Healthy People 2010: Understanding and Improving Health. Washington, DC: US Dept of Health and Human Services; 2000. [Google Scholar]
- 2.Flegal K, Ezzati T, Harris M, et al. Prevalence of diabetes in Mexican Americans, Cubans and Puerto Ricans from the Hispanic Health and Nutrition Examination Survey, 1982–1984. Diabetes Care. 1991;14(7):628–638. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Kaplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22(3):403–408. [DOI] [PubMed] [Google Scholar]
- 5.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–2527. [DOI] [PubMed] [Google Scholar]
- 6.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the Health and Retirement Study. Arch Intern Med. 2007;167(17):1853–1860. [DOI] [PubMed] [Google Scholar]
- 7.Harris MI, Glegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose and impaired glucose tolerance in US adults. Diabetes Care. 1998;21(4):518–524. [DOI] [PubMed] [Google Scholar]
- 8.Kieffer E, Sinco B, Rafferty A, et al. Chronic disease-related behaviors and health among African Americans and Hispanics in the REACH Detroit 2010 Communities, Michigan, and the United States. Health Promot Pract. 2006;7(suppl 3):256S–264S. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez H, Saenz R, Menjivar C. Latinas/os in the United States: Changing the Face of America. New York, NY: Springer; 2008. [Google Scholar]
- 10.American Association of Diabetes Educators Diabetes community health workers. Diabetes Educ. 2003;29(5):818–824. [DOI] [PubMed] [Google Scholar]
- 11.Swider SM. Outcome effectiveness of community health workers: an integrative literature review. Public Health Nurs. 2002;19(1):11–20. [DOI] [PubMed] [Google Scholar]
- 12.Two Feathers J, Kieffer EC, Palmisano G, et al. Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: improving diabetes-related outcomes among African American and Latino adults. Am J Public Health. 2005;95(9):1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng E, Young R. Lay health advisors as community change agents. Fam Community Health. 1992;15(1):24–40. [Google Scholar]
- 14.Israel BA. Social networks and social support: implications for natural helper and community level interventions. Health Educ Q. 1985;12(1):65–80. [DOI] [PubMed] [Google Scholar]
- 15.Navarro AM, Senn KL, McNicholas LJ, Kaplan RM, Roppé B, Campo MC. Por La Vida model intervention enhances use of cancer screening tests among Latinas. Am J Prev Med. 1998;15(1):32–41. [DOI] [PubMed] [Google Scholar]
- 16.Norris SL, Chowdhury FM, Van Let K, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med. 2006;23(5):544–556. [DOI] [PubMed] [Google Scholar]
- 17.Love MB, Gardner K, Legion V. Community health workers: who they are and what they do. Health Educ Behav. 1997;24(4):510–522. [DOI] [PubMed] [Google Scholar]
- 18.Michael YL, Farquhar SA, Wiggins N, Green MK. Findings from a community-based participatory prevention research intervention designed to increase social capital in Latino and African American communities. J Immigr Minor Health. 2008;10(3):281–289. [DOI] [PubMed] [Google Scholar]
- 19.McElmurry BJ, Park CG, Buseh AG. Health policy and systems. The nurse-community health advocate team for urban immigrant primary health care. J Nurs Scholarsh. 2003;35(3):275–281. [DOI] [PubMed] [Google Scholar]
- 20.Satterfield DW, Burd C, Valdez L, Hosey G, Eagle Shield J. The “in-between” people: participation of community health representatives in diabetes prevention and care in American Indian and Alaskan Native communities. Health Promot Pract. 2002;3(2):166–175. [Google Scholar]
- 21.Lewin SA, Dick J, Pond P, et al. Lay Health Workers in Primary and Community Healthcare. The Cochrane Collaboration. Hoboken, NJ: John Wiley and Sons; 2005. [DOI] [PubMed] [Google Scholar]
- 22.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. [DOI] [PubMed] [Google Scholar]
- 23.Strelnick AH, Swiderski D, Fornari A, et al. The residency program in social medicine of Montefiore Medical Center: 37 years of mission-driven, interdisciplinary training in primary care, population health, and social medicine. Acad Med. 2008;83(4):378–389. [DOI] [PubMed] [Google Scholar]
- 24.Pantell RH, Newman TB, Bernzweig J, et al. Management and outcomes of care of fever in early infancy. JAMA. 2004;291(10):1203–1212. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz CR, Arniella A, James S, Bickell NA. Using community-based participatory research to reduce health disparities in East and Central Harlem. Mt Sinai J Med. 2004;71(6):368–374. [PMC free article] [PubMed] [Google Scholar]
- 26.Wallerstein NB, Duran B. Using community-based participatory research to address health disparities. Health Promot Pract. 2006;7(3):312–323. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer EC, Willis SK, Odoms-Young AM, et al. Reducing disparities in diabetes among African American and Latino residents of Detroit: the essential role of community planning focus groups. Ethnic Dis. 2004;14(3 suppl. 1):S27–S37. [PubMed] [Google Scholar]
- 28.Two Feathers J, Kieffer EC, Palmisano G, et al. The development, implementation and process evaluation of the REACH Detroit Partnership's Diabetes Lifestyle Intervention. Diabetes Educ. 2007;33(3):509–520. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RM. Patient empowerment and the traditional medical model. Diabetes Care. 1995;18(3):412–415. [DOI] [PubMed] [Google Scholar]
- 30.Glasgow RE, Anderson RM. In diabetes care, moving from compliance to adherence is not enough: something entirely different is needed. Diabetes Care. 1999;22(12):2090–2091. [DOI] [PubMed] [Google Scholar]
- 31.Anderson RM, Funnell MM. Patient empowerment: reflections on the challenge of fostering the adoption of a new paradigm. Patient Educ Couns. 2005;57(2):153–157. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RM, Funnell MM. Art of Empowerment: Stories and Strategies for Diabetes Educators. 2nd ed Alexandria, VA: American Diabetes Association; 2005. [Google Scholar]
- 33.Funnell MM, Anderson RM. Patient empowerment: a look back, a look ahead. Diabetes Educ. 2003;29(3):454–464. [DOI] [PubMed] [Google Scholar]
- 34.Funnell MM. Patient empowerment. Crit Care Nurs Q. 2004;27(2):201–204. [DOI] [PubMed] [Google Scholar]
- 35.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22(3):123–127. [Google Scholar]
- 36.Funnell MM, Kruger DF, Spencer M. Self-management support for insulin therapy in type 2 diabetes. Diabetes Educ. 2004;30(2):274–280. [DOI] [PubMed] [Google Scholar]
- 37.Deci EL, Eghrari H, Patrick BC, Leone DR. Facilitating internalization: the self-determination theory perspective. J Pers. 1994;62(1):119–142. [DOI] [PubMed] [Google Scholar]
- 38.Williams GC, Grow VM, Freedman Z, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70(1):115–126. [DOI] [PubMed] [Google Scholar]
- 39.Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation: the motivational basis of adherence to medical regimens. Health Psychol. 1998;17(3):269–276. [DOI] [PubMed] [Google Scholar]
- 40. US Census Bureau. American factfinder. 2007. Available at: http://factfinder.census.gov. Accessed December 1, 2010.
- 41.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the diabetes care profile. Eval Health Prof. 1996;19(2):208–230. [DOI] [PubMed] [Google Scholar]
- 42.Resnick B, Luisi D, Vogel A, Junaleepa P. Reliability and validity of the self-efficacy for exercise and outcome expectations for exercise scales with minority older adults. J Nurs Meas. 2004;12(3):235–248. [DOI] [PubMed] [Google Scholar]
- 43.Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities Measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. [DOI] [PubMed] [Google Scholar]
- 44.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–760. [DOI] [PubMed] [Google Scholar]
- 45.Polonsky WH, Welch G. Listening to our patients’ concerns: understanding and addressing diabetes-specific emotional distress. Diabetes Spectrum. 1996;9:8–11. [Google Scholar]
- 46.Spencer MS, Kieffer EC, Sinco BR, et al. Diabetes-specific emotional distress among African Americans and Latinos with type 2 diabetes. J Health Care Poor Underserved. 2006;17:88–105. [DOI] [PubMed] [Google Scholar]
- 47.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. [DOI] [PubMed] [Google Scholar]
- 48.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. [DOI] [PubMed] [Google Scholar]
- 49.Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of Longitudinal Data. 2nd ed New York, NY: Oxford University Press; 2002. [Google Scholar]
- 50.West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide to Using Statistical Software. 1st ed Boca Raton, FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- 51.SAS Institute. The MIXED procedure. Available at: http://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#mixed_toc.htm. Accessed October 29, 2010.
- 52.SAS Institute. The GENMOD procedure. Available at: http://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#genmod_toc.htm. Accessed October 29, 2010.
- 53.Lavori PW, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Stat Med. 1995;14(17):1913–1925. [DOI] [PubMed] [Google Scholar]
- 54.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley Publishing Co; 1987. [Google Scholar]
- 55.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 56.Gary TL, Bone L, Hill M, et al. Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes-related complications in urban African Americans. Prev Med. 2003;37(1):23–32. [DOI] [PubMed] [Google Scholar]
- 57.Fedder DO, Change RJ, Curry S, et al. The effectiveness of a community health worker outreach program in healthcare utilization of west Baltimore City Medicaid patients with diabetes, with or without hypertension. Ethn Dis. 2003;13(1):22–27. [PubMed] [Google Scholar]
- 58.Sixta CS, Ostwald S. Texas–Mexico border intervention by promotores for patients with type 2 diabetes. Diabetes Educ. 2008;34(4):299–309. [DOI] [PubMed] [Google Scholar]
- 59.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: two-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223. [DOI] [PubMed] [Google Scholar]
- 60.Lorig K, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. [DOI] [PubMed] [Google Scholar]
- 61.Altman DG, Schulz KF, Moher D, et al. for the CONSORT Group The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. [DOI] [PubMed] [Google Scholar]
- 62. Community Health Workers: Closing Gaps in Families’ Health Resources. Washington, DC: Family Strengthening Policy Center; March 2006; Policy brief no. 14. [Google Scholar]
- 63.Love MB, Legion V, Shim JK, Tsai C, Quijano V, Davis C. CHWs get credit: a 10-year history of the first college-credit certificate for community health workers in the United States. Health Promot Pract. 2004;5(4):418–428. [DOI] [PubMed] [Google Scholar]
- 64.Ro MJ, Treadwell HM, Northridge M. Community Health Workers and Community Voices: Promoting Good Health. 2nd printing Atlanta, GA: National Center for Primary Care, Morehouse School of Medicine; March 2006. [Google Scholar]
- 65.Keane D, Nielsen C, Dower C. Community Health Workers/Promotores in California. San Francisco: University of California-San Francisco, Center for the Health Professions; March 2006. [Google Scholar]
- 66.Goodwin K, Tobler L. Community Health Workers: Expanding the Scope of the Health Care Delivery System. Denver, CO: National Conference of State Legislatures; April 2008. [Google Scholar]