Abstract
Objectives. We evaluated smoking-cessation efficacy of an extended course of sustained-release bupropion (bupropion SR) and cognitive-behavioral treatment (CBT).
Methods. Participants who smoked at least 10 cigarettes per day and who smoked within 30 minutes of arising (n = 406) completed a 12-week smoking-cessation treatment including group counseling, nicotine-replacement therapy, and bupropion SR. Participants were then randomly assigned to 1 of 5 conditions: (1) no further treatment, (2) active bupropion SR for 40 weeks, (3) placebo for 40 weeks, (4) active bupropion SR and 11 sessions of CBT for 40 weeks (A-CBT), or (5) placebo and 11 sessions of CBT for 40 weeks. Participants were assessed at baseline and at weeks 12, 24, 52, 64, and 104.
Results. A-CBT was not superior to the other 3 extended treatments. From weeks 12 through 104, all extended treatment conditions were superior to standard treatment. At weeks 64 and 104, the 2 CBT conditions produced significantly higher abstinence rates than did the other 3 conditions.
Conclusions. Brief contact with providers can increase abstinence during treatment. CBT may increase long-term abstinence after extended treatment is terminated.
Researchers and clinicians have come to expect low rates of long-term cigarette abstinence subsequent to tobacco-dependence treatment—usually 25% or less at 1 year, even with combination therapy.1,2 These low rates may be attributable to a failure to conceputalize tobacco dependence as an addiction with a chronic, relapsing course. The implications of a chronic-disease model suggest that longer, more extended courses of tobacco-dependence treatment may result in higher long-term cigarette abstinence rates.
Three studies have examined the efficacy of extended administration of sustained-release bupropion (bupropion SR). Hays et al. treated participants for 7 weeks with open-label bupropion SR and then randomly assigned only cigarette-abstinent participants (59% of the sample) to active or placebo bupropion SR for an additional 45 weeks. Cigarette abstinence was significantly higher in the active drug condition (55.1%) than in the placebo drug condition (42.3%) after 1 year of therapy, but the conditions did not differ at 2-year follow-up (41.6% for active drug; 40.0% for placebo).3 In a second study, smokers were treated with nicotine patches calibrated to individual cigarette intake. Abstinent participants (31% of the sample) were then randomly assigned to either active or placebo bupropion SR for 6 months. Abstinence rates did not differ between conditions at 6 months (25% for placebo; 28% for active drug).4 Cox et al.5 randomized abstinent smokers who had been treated with 7 weeks of bupropion SR to either continued bupropion SR for the remainder of 1 year or to placebo. Active drug produced greater cigarette abstinence at the end of treatment when compared with placebo (55.89% vs 43.58%), but there were no differences at 1-year follow-up (42.34% vs 42.95%). Thus, it appears that extended bupropion SR provides an increase in abstinence rates while being administered, but this effect is lost after medication termination.
Extended administration of varenicline has also been studied. Williams et al. administered either varenicline or placebo over 1 year and found that varenicline was superior to placebo at both 12 weeks (76.5% to 72.2%) and 52 weeks (37.8% to 34.1%).6 Tonstad et al.7 randomized abstinent smokers who had been treated with 12 weeks of varenicline to either continued varenicline or to placebo for an additional 12 weeks. Continuous cigarette abstinence rates were higher for the varenicline group than for the placebo group for weeks 13 through 24 (70.5% to 49.6%) and for weeks 13 through 52 (43.6% to 36.9%). Thus, the therapeutic effects of extended varenicline may last past the period of administration.
In earlier work, our group studied extended administration of nortriptyline. We assigned smokers to 1 of 4 treatment conditions in a 2 × 2 factorial design (nortriptyline vs placebo by brief treatment vs extended treatment). Participants in extended treatment continued taking drug or placebo and received monthly individual counseling sessions through week 52. At week 52, abstinence rates were 56% for extended nortriptyline and 57% for extended placebo. Both conditions produced abstinence rates that exceeded those of short-term treatment.
Three studies have investigated the effects of extended cognitive-behavioral treatment (CBT). Killen et al.8 treated smokers for 12 weeks with open-label bupropion SR, nicotine patch, and weekly relapse-prevention training. All participants, independent of smoking status, were then offered 4 relapse-prevention sessions and continued on either active or placebo drug for an additional 14 weeks. There were no differences in abstinence rates between conditions at 1 year. In a second study9 participants received bupropion SR, nicotine patch, and CBT for 8 weeks and were then randomly assigned to receive either 12 weeks of CBT plus voicemail monitoring and telephone counseling or telephone-based general support. The investigators reported significant differences at 20 weeks (45% vs 29%) but not at 52 weeks (31% vs 27%).
Recently, we studied 402 people who smoked at least 10 cigarettes per day and who were 50 years old or older.10 All completed a 12-week treatment that included group counseling, nicotine gum, and bupropion SR, and all were then randomly assigned to 1 of 4 follow-up conditions: (1) standard treatment (no further treatment), (2) extended nicotine-replacement therapy (NRT) with 40 weeks of nicotine gum availability, (3) extended CBT (11 cognitive behavioral sessions over a 40-week period), or (4) extended CBT plus extended NRT (11 CBT sessions plus 40 weeks of nicotine gum availability). The extended CBT condition produced high cigarette-abstinence rates that were maintained throughout the 2-year study period (week 24 = 58.3%; week 52 = 55.0%; week 64 = 54.6%; week 104 = 54.8%). The extended CBT condition was significantly more effective than extended NRT and standard treatment across that period. No other treatment condition was significantly different from standard treatment. These findings suggest that extended CBT can produce high and stable cigarette abstinence rates. Medication does not appear to play a major role in maintaining abstinence when combined with CBT.
In the current study, we evaluated a CBT intervention similar to that described by Hall et al.10 We also evaluated the efficacy of long-term bupropion SR versus placebo. We proposed the following hypotheses: (1) at all assessments after baseline, the active bupropion extended CBT (A-CBT) condition would produce higher point prevalence abstinence rates than placebo with extended CBT (P-CBT), placebo alone, active bupropion alone, or standard treatment (our primary hypothesis); and (2) at all assessments after the end of extended treatment, the 2 conditions that included CBT (combined with active or placebo bupropion) would produce abstinence rates superior to those produced by the 3 conditions that did not include CBT.
METHODS
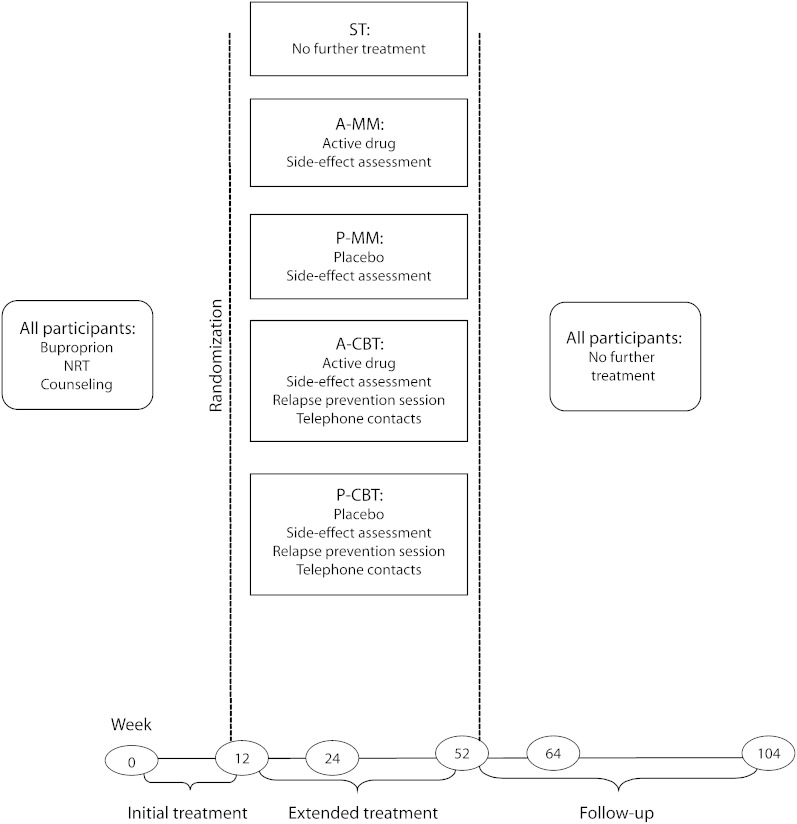
Active versus placebo bupropion SR was crossed with extended CBT versus medical management in a 2 × 2 factorial design. These 4 conditions were compared with each other and with standard 12-week treatment. Assessments were conducted at baseline and at weeks 12, 24, 52, 64, and 104. The design is summarized in Figure 1.
FIGURE 1.
Design of study evaluating smoking-cessation treatment: San Francisco, CA, February 2003–November 2007.
Note. A-CBT = active cognitive-behavioral treatment; A-MM = active medical management; NRT = nicotine-replacement therapy; P-CBT = placebo cognitive-behavioral treatment; P-MM = placebo medical management; ST = standard treatment.
Participants
Participants were recruited by advertising, public service announcements, and flyers. Recruitment began in February 2003 and ended in November 2005. After telephone screening, participants were invited to an orientation meeting at which they signed written statements of informed consent. They were then invited to a baseline assessment that included a physical examination, EKG, and blood draws for basic blood chemistry analyses. Each participant then completed the depression, alcohol, and nicotine sections of the Computerized Diagnostic Interview Schedule for DSM-IV,11 administered by research staff. Research activities took place at the University of California, San Francisco.
Individuals were eligible to participate if they were aged at least 18 years, smoked at least 10 cigarettes per day, and smoked within 30 minutes of arising. Exclusionary criteria included cardiovascular disease, history of seizure, severe allergies, life-threatening disease, lifetime bipolar disorder, current major depression disorder, current use of any psychiatric medication, suicidal or psychotic symptoms, treatment of drug or alcohol use within the prior 6 months, psychiatric hospitalization within the prior year, and pregnancy or lactation.
A total of 407 participants consented to participate and completed all tests and screenings. One participant died before randomization into treatment conditions; the remaining 406 participants were randomly assigned to 1 of 5 experimental conditions. Stratification variables were cigarettes per day, gender, and smoking status at week 12.
Assessments
Data were collected at baseline and weeks 12, 24, 52, 64, and 104. All participants were contacted for each assessment, whether or not they continued in treatment. Participants were paid $25 each for assessment they completed at weeks 12, 24, 52, 64, and 104. If they completed all assessments, they received a bonus of $50 at week 104.
The primary dependent variable was biochemically verified 7-day point prevalence abstinence from cigarettes, indicated by self-reported abstinence, expired-air carbon monoxide levels of 10 ppm or lower, and urinary cotinine levels of 60 ng/ml or lower.12
We also administered a standard demographics questionnaire; the Profile of Mood States (POMS)13; the Fagerström Test for Nicotine Dependence; measures of drug and alcohol use developed by the authors; the Thoughts About Abstinence Questionnaire14,15; the Social Participation Index, which indicates the size of the respondent's social network; the Center for Epidemiological Studies Depression Scale16; and the Minnesota Nicotine Withdrawal Questionnaire.17,18 We used these measures to identify possible covariants.
We assessed treatment attendance, use of study medication, use of nonstudy prescription medications approved for the treatment of nicotine dependence, use of nonstudy NRT, and use of other tobacco products (e.g., cigars, snus, smokeless tobacco). We also analyzed the effectiveness of the blinding procedure by asking participants whether they thought they had received bupropion or placebo. The 6 response options were:
“absolutely certain” they had received bupropion;
“pretty good idea” they had received bupropion;
“not sure, but I think I may have” received bupropion;
“absolutely certain” they had received placebo;
“pretty good idea” they had received placebo;
“not sure, but I think I may have” received placebo.
Standard Treatment Condition
All participants were provided with 12 weeks worth of bupropion SR and 10 weeks worth of nicotine patch, and they received counseling based on the standard treatment used by the authors in earlier studies.1 The bupropion SR dose was 150 milligrams per day for days 1 through 3 and 300 milligrams per day for days 4 through 7 of the first week of treatment. If there were no adverse effects, the patient continued on 300 milligrams per day for the remainder of the 12-week period. If adverse effects occurred during the second week, the dose was reduced to 150 milligrams per day for the remainder of the 12-week period. All participants were provided with 10 weeks worth of nicotine patches beginning at the quit date during week 3. A group counselor instructed participants on the use of the nicotine patch. Participants who smoked at least 25 cigarettes per day received 6 weeks of a 21-milligram patch, followed by 4 weeks of a 14-milligram patch. Participants who smoked fewer than 25 cigarettes per day received 10 weeks of a 7-milligram patch. At the week 11 meeting, participants were instructed to discontinue use of the patch (if they had not already done so) by week 12. All participants received 5 group-counseling sessions held at weeks 1, 3 (2 sessions were held during week 3, 1 before the quit date and 1 immediately after), 5, and 11. Participants assigned to the standard treatment (ST) condition received no further treatment after week 12.
Extended Treatment Conditions
Drug (active vs placebo bupropion).
Following the 12-week standard treatment, participants randomized to the active drug or placebo extended treatment conditions could receive either active or placebo drug up to week 52. In both the active and placebo conditions, participants took 2 identical-appearing capsules twice per day. Participants met monthly with a nurse practitioner at the study clinic for approximately 5 to 10 minutes to check for side effects and report smoking status. Active drug dose was 300 milligrams per day for most patients for the extended treatment period. If dosage had been reduced to 150 milligrams per day during the initial 12 weeks, it remained at that dose for the duration of the study. In both conditions, participants could terminate drug use for any reason, including lack of proven efficacy or the participant's perception that stable abstinence had been achieved and that the drug was no longer needed.
Cognitive-behavioral treatment.
Eleven individual extended CBT sessions were provided during weeks 12 through 52. Spacing was more frequent earlier in the extended treatment period (2 weeks apart during weeks 12–20; 4 weeks apart during weeks 20–52). Sessions were 20 to 40 minutes long. The first 5 sessions introduced new content; the remaining 6 sessions were used to reinforce the material learned and to provide support appropriate to the participant's smoking status. Counselors completed brief telephone check-ins and counseling sessions with participants midway between each face-to-face meeting.
The CBT content areas were evidence-based and were taken from the recommendations for relapse prevention contained in the US Public Health Service's 2000 practice guidelines for treating tobacco use and dependence.1 The content areas were: motivation, social support, dysphoria, dependence and withdrawal, and weight gain. A detailed description of the CBT intervention is presented in Hall et al.10 Briefly, the motivational component of the intervention focused on providing cues to elicit motivations for smokers by using a decisional balance chart that emphasized the positive effects of quitting and the costs of smoking. Participants were reminded to continually commit to abstinence and to make that commitment to themselves and to significant others. The social-support component had 2 parts—management of current support network, and attempts to build a larger nonsmoking network—through suggestions to participate in activities that might include a larger population of nonsmokers.
We also provided a self-directed intervention for depressed mood, developed on the basis of earlier work,19,20 that used a self-administered Mood Management Guide. Participants were instructed to increase pleasant activities, to note the correspondence of pleasant activities with mood, and to note the correspondence of mood with the number of cigarettes smoked. Ideas for increasing pleasant activities were provided. With respect to withdrawal symptoms, counselors worked with participants to develop strategies to deal with emerging symptoms. We addressed weight control through increased physical activity, because attempts to restrict calories during smoking-cessation intake have been shown to increase the probability of relapse.21 The goal of the activity program was to have participants complete the equivalent of 30 minutes of moderate exercise most days of the week. Most participants used a study-provided pedometer, and their goal was 10 000 steps per day, which corresponds to the public health activity guideline of at least 30 minutes of moderate physical activity per day.
Medical management.
Participants in the medical management (MM) conditions did not receive any behavioral interventions during the extended treatment period. As described above, they did meet monthly with the nurse practitioner to report side effects and smoking status. No counseling was offered at that visit.
Statistical Methods
The sample size was determined on the basis of requirements implied by the primary hypothesis: testing differences in 7-day point prevalence abstinence rates across weeks 12 through 104. Power was set at 80%, with a type I error rate of 0.05. The estimated effect size was taken from Hall et al.22 and factored in the anticipated attrition rate. We used generalized estimating equations (GEE)—a generalization of the classic linear model that uses quasi-likelihood estimation—to test hypotheses about 7-day point prevalence abstinence at weeks 12, 24, 52, 64, and 104, and we based our power analyses on this method of analysis. We used SAS version 9.1.3 (SAS Institute, Cary, NC) for all analyses.23 Analyses included all available data.
To identify potential covariates, we correlated these baseline variables with point prevalence abstinence at weeks 12, 24, 52, 64, and 104, using point-biserial correlations. We included in preliminary hypothesis-testing models those variables that had significant correlations with abstinence. These variables were eliminated if they did not contribute to the final model. To ensure that randomization had not been compromised, we examined baseline variables in the 5 experimental conditions; we used analysis of variance (ANOVA) for continuous variables and Pearson's χ2 tests for categorical variables. These were no significant differences among conditions in any of the 28 variables.
We used a 1-way ANOVA to test differences in the number of weeks of bupropion or placebo drug use among the extended treatment conditions. Differences among the 5 conditions in the use of nonstudy prescription medications and the use of nonstudy NRT (use vs no use) at each assessment were determined by a Pearson's χ2 analysis for categorical variables. Belief about drug received was tested by a 6 × 2 χ2 test, where beliefs about drug categories were crossed with actual drug. All tests for all analyses were 2-tailed.
RESULTS
Demographic, smoking, and psychiatric characteristics of the participants at baseline by experimental condition are shown in Table 1.
TABLE 1.
Baseline Variables for Study Population: Adult Smokers, San Francisco, CA, February 2003–November 2007
| Variables | Mean ±SD or No. (%) |
| Continuous | |
| No. of participants | 406 |
| Age, y | 40.7 ±9.8 |
| Age started smoking regularly, y | 17.8 ±4.5 |
| Years of regular smoking | 22.9 ±10.1 |
| No. of cigarettes smoked per d | 19.0 ±7.4 |
| Carbon monoxide, ppm | 21.6 ±11.3 |
| Fagerström total score | 4.9 ±2.1 |
| Center for Epidemiological Studies Depression Scale | 7.7 ±6.1 |
| Profile of Mood States total score | 21.9 ±29.2 |
| MOS SF-36—Physical component score | 50.6 ±7.9 |
| MOS SF-36—Mental component score | 48.8 ±8.9 |
| Perceived Stress Scale | 20.7 ±7.6 |
| Minnesota Nicotine Withdrawal Scale | |
| Craving score | 2.7 ±0.9 |
| Withdrawal score | 6.0 ±5.4 |
| Michigan Nicotine Reinforcement Questionnaire | |
| Positive reinforcement score | 7.9 ±2.9 |
| Negative reinforcement score | 12.5 ±4.8 |
| Categorical | |
| Positive DSM-IV diagnoses | |
| History of MDE | 113 (28.0) |
| Nicotine dependence | 324 (80.4) |
| Nicotine withdrawal | 208 (51.7) |
| Alcohol dependence | 89 (22.1) |
| Femalea | 159 (39.2) |
| White, non-Hispanic | 281 (69.7) |
| Educational level achieved | |
| High school graduate or less | 64 (16.0) |
| Some college | 156 (39.1) |
| College graduate/some graduate school | 135 (31.3) |
| Graduate degree | 54 (13.5) |
| Married/lives with partner | 152 (37.9) |
Note. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; MDE = major depressive episode; MOS SF-36 = Medical Outcomes Study short form 36-item questionnaire. Percentages may not total 100% because of rounding.
Four participants self-reported as transgender; 2 requested to be randomized as male, and 2 requested to be randomized as female.
Attrition
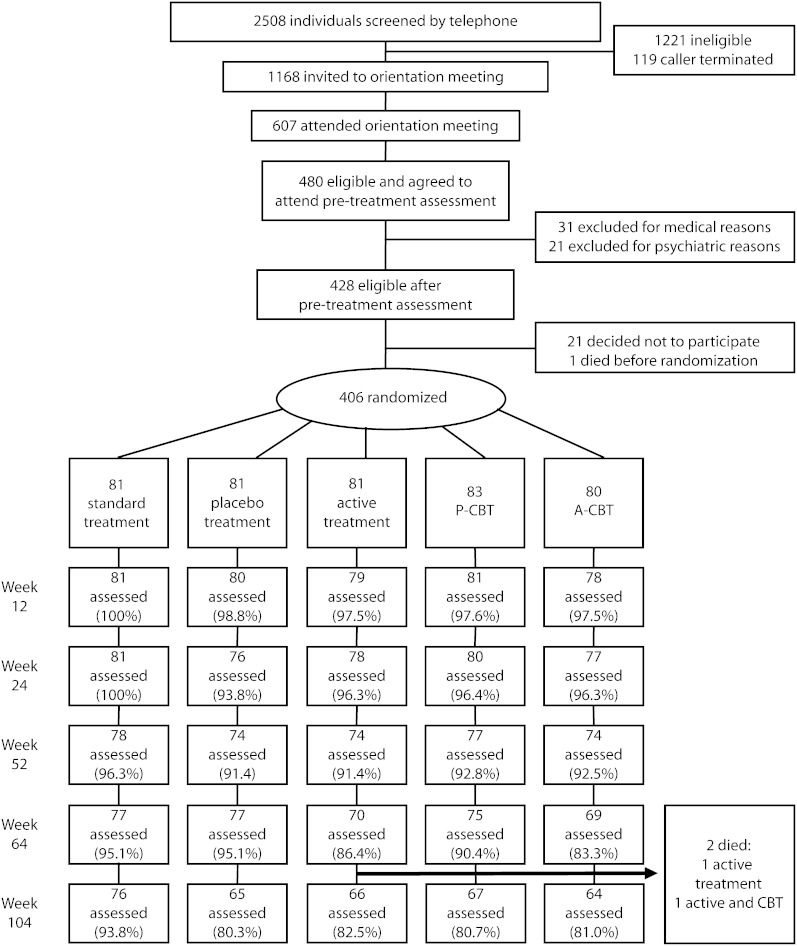
The CONSORT flow diagram in Figure 2 shows participant recruitment and attrition from the first contact with the program to the week-104 assessment. The percentage of participants from whom we successfully collected smoking data at weeks 12, 24, 52, 64, and 104 did not differ significantly by treatment condition.
FIGURE 2.
Recruitment and follow-up for evaluation of smoking-cessation treatment: San Francisco, CA, February 2003–November 2007.
Note. A-CBT = active cognitive-behavioral treatment; A-MM = active medical management; P-CBT = placebo cognitive-behavioral treatment; P-MM = placebo medical management.
At every assessment but week 104, attrition was sufficiently small (week 12 = 1.7%; week 24 = 3.4%; week 52 = 7.1%; week 64 = 9.3%; week 104 = 16.2%) that creation of a pattern-mixture model was not feasible. At week 104, there were no significant differences in attrition rates by treatment condition. For the week-104 data, variables that were significantly correlated with attendance were not correlated with abstinence status at that assessment.
Abstinence
Hypothesis 1:The active bupropion, extended CBT condition would have higher abstinence rates than each of the other 4 conditions at all assessments.
This hypothesis was not supported. Initial analyses of possible covariates indicated that POMS total score, number of cigarettes smoked per day, gender, and ethnicity at baseline were significantly correlated with the main abstinence outcome for weeks 12 through 104. Therefore, these variables were entered as covariates in the GEE model containing the variables of treatment condition, time, and the treatment × time interaction. The interactions of gender with treatment and of ethnicity with treatment did not contribute significantly and were dropped from the final model. Cigarettes per day and POMS total score were retained. The final model thus included cigarettes per day and POMS total score as covariates, treatment condition, time, and the interaction of treatment condition with the covariates. Significant effects were found for treatment condition (χ2 [4, n = 406] = 9.69; P = .046), time (odds ratio [OR] = 0.764 per week; 95% confidence interval [CI] = 0.715, 0.817); P < .001), cigarettes smoked per day (OR = 0.951), and the POMS total score by treatment interaction (OR = 0.983 for 1-point increase; 95% CI = 0.967, 0.999; P = .037).
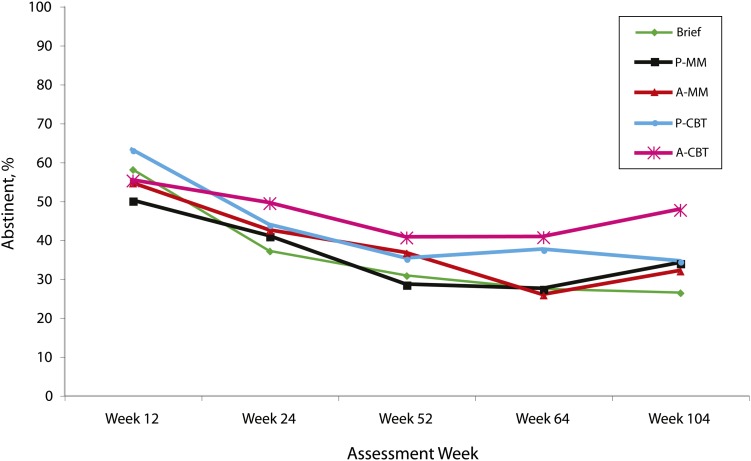
The A-CBT condition had significantly higher abstinence rates than did the ST condition over time (OR = 13.97; 95% CI = 3.03, 64.37; P < .001) but was not significantly different when compared with the remaining 3 treatment conditions. The other 3 extended conditions also produced higher abstinence rates over time than did the ST condition: placebo CBT (P-CBT) OR = 7.22 (95% CI = 1.80, 29.00; P = .005), active MM (A-MM) OR = 10.30 (95% CI = 2.56, 41.45; P = .018), and placebo MM (P-MM) OR = 7.06 (95% CI = 1.40, 35.62; P = .001). The A-CBT condition produced consistently high abstinence rates at weeks 24 (49.4%), 52 (40.5%), 64 (40.6%), and 104 (47.6%). Seven-day biochemically corrected point prevalence abstinence rates by condition are shown in Figure 3.
FIGURE 3.
Participants’ biochemically verified cigarette-abstinence rates, by treatment condition: San Francisco, CA, February 2003–November 2007.
Note. A-CBT = active cognitive-behavioral treatment; A-MM = active medical management; P-CBT = placebo cognitive-behavioral treatment; P-MM = placebo medical management.
Fewer cigarettes per day predicted abstinence. High POMS total mood disturbance scores in the ST condition correlated with abstinence at week 52, with abstinence at week 12 in P-MM, and with abstinence at week 104 in A-CBT.
Hypothesis 2: After the end of extended treatment, the 2 conditions that included CBT would produce superior abstinence rates to those produced by the 3 conditions that did not include CBT.
This hypothesis was supported. For hypothesis 2, after the elimination of covariates that did not contribute to the model, only the social participation score was retained. Thus, the final model included social participation score, time, treatment condition, and the social participation score × treatment condition interaction. For the GEE model of point prevalence abstinence for weeks 64 and 104, significant effects were found for CBT versus no CBT (OR = 1.54; 95% CI = 1.05, 2.26; P = .029) and social participation score at baseline (OR = 1.15 for 1-point increase; 95% CI = 1.02, 1.30; P = .023). Receiving extended CBT during weeks 12 through 52 was associated with a higher probability of abstinence at weeks 64 through 104, as was a higher social participation score.
Counseling Sessions and Use of Bupropion
The 2 CBT conditions did not significantly differ in the mean number of counseling sessions attended (P-CBT = 4.2, SD = 3.8; A-CBT = 5.0, SD = 3.7; F[1, n = 163] = 1.84; P = .176). Attendance at counseling sessions correlated significantly with abstinence status at weeks 52 (r = 0.384; P < .001) and 104 (r = 0.397; P = .001) for the A-CBT condition, and at week 52 only for the P-CBT condition (r = 0.303; P = .007). The CBT content was provided in 5 sessions; all additional sessions in extended treatment reinforced this content and supported abstinence. Of 83 participants in the P-CBT condition 34 (41.0%) attended at least 5 sessions, and 39 of 80 (48.8%) in the A-CBT condition did so. This difference was not significant (χ2 [1, n = 163] = 1.00; P = .318).
There also was no difference between treatment conditions in the number of weeks that study drugs were prescribed (A-CBT = 20.4, SD = 15.0; P-CBT = 18.3, SD = 17.0; A-MM = 18.8, SD = 13.7; P-MM = 17.2, SD = 13.6), both when all 4 conditions were considered separately on ANOVA (F [1, n = 323] = 0.65; P = .586) and when comparing participants receiving active drug or placebo drug during extended treatment (active = 19.6, SD = 14.4; placebo = 17.7, SD = 15.4) on ANOVA (F [1, n = 324] = 1.25; P = .264).
Other Nonstudy Medications
There was no significant difference between treatment groups in use of nonstudy medications known for their smoking-cessation properties (i.e., bupropion, nortriptyline, or varenicline) at any assessment, and rates of reported use were low (week 12 = 0.6%; week 24 = 2.8%; week 52 = 6.5%; week 64 = 10.5%; week 104 = 11.0%).
There also was no significant difference in nonstudy NRT use between treatment groups at any point. The P-CBT participants reported the highest percentage for week 24 (8.8%), week 52 (12.7%), and week 64 (19.3%).
Effectiveness of the Blinding Procedure
At week 104, participants who received either active or placebo drug did not differ in their beliefs about which drug they had received as a function of actual drug assignment (χ2 [5, n = 152] = 2.35; P = .812). Of those who received placebo, 65.3% believed they had received bupropion; of those who had received bupropion, 64.9% believed they had received bupropion (χ2 < 1). Perceived drug assignment was significant in predicting the perceived helpfulness of medication in the participants’ efforts to quit smoking (χ2 [3, n = 150] = 23.7; P < .001). Actual drug assignment was not a significant predictor of perceived helpfulness (χ2 [3, n = 149] = 1.34; P = .727).
DISCUSSION
Our first hypothesis—that the A-CBT condition would produce the highest abstinence rates during weeks 24 through 104—was not supported. A-CBT did result in significantly higher abstinence rates than did ST, but it did not produce significantly higher rates than P-CBT, A-MM, or P-MM.
Our second hypothesis—that during weeks 52 through 104 the 2 extended treatment conditions would produce higher abstinence rates than the remaining 3 conditions—was supported. These results present an interesting picture of long-term maintenance strategies. On the one hand, all extended treatments produced better long-term abstinence rates than standard treatment, but they did not differ from one another. The implications of this finding are intriguing. For example, A-CBT and P-MM did not differ, suggesting that simple periodic check-ins are as effective as an active drug and counseling, despite the fact that the differential cost of these 2 interventions must be considerable.
However, availability of a placebo drug complicates the interpretation of these results, because if a drug had not been offered, we do not know how many participants would have attended the sessions, nor whether the results would have differed from those obtained. This is especially important because the blind in this study appears to have been maintained. It does appear, however, that CBT is more efficacious in maintaining abstinence after treatment is terminated. This finding is tempered by the fact that this effect was more evident in the A-CBT condition than in the P-CBT condition, especially at week 104.
It is difficult to explain why the addition of medication to CBT enhanced abstinence in the current study but did not do so in our earlier study (with participants older than 50 years), because the 2 studies differed in both sample characteristics and class of drug provided. We examined abstinence data for smokers older than 50 years in the current study; for that subsample, CBT was more effective than medical management or brief treatment, regardless of whether it was combined with an active drug or a placebo. This finding more closely parallels our earlier findings. It may be that participants older than 50 years are sufficiently focused or motivated to quit smoking, making them more likely to expend the effort to learn the skills presented in CBT sessions and hence more likely to have a favorable outcome from CBT.
Once treatment was terminated, extended CBT was superior to interventions that did not include CBT. This suggests that a primary premise of CBT—that it provides participants with tools to manage their smoking on their own—is correct. Inspection of the data suggests that this effect was a result primarily of the effectiveness of the active CBT condition, however.
There is now a fairly sizable literature on the efficacy of extended treatments. As noted earlier, the results are mixed. The most favorable results appear to occur in studies in which all participants are included in the extended treatment, not just those who have achieved abstinence at the end of the initial or brief treatment period. This finding suggests that extended treatments work by “re-recruiting” individuals who have lapsed (or who are lapsing) back into abstinence, rather than by maintaining abstinence. On the other hand, the studies that have found effects for extended treatments have provided both counseling and at least 2 pharmacological interventions during the initial treatment period. This finding suggests that extended treatments are only efficacious when they are provided on a strong foundation of abstinence interventions.
Use of CBT sessions and medications was modest. Approximately 40% of the participants attended enough sessions to have received all the CBT content, and participants requested medication prescriptions for about half the time period for which they were available. This underutilization may indicate participants’ ability to determine when they no longer need an intervention and can successfully terminate their active involvement.
The question of how best to offer long-term treatments for cigarette smokers is an increasingly important one as the proportion of the population that smokes shrinks, becoming potentially more chronic and dependent. Interventions differ in both cost and effectiveness, and studies that can resolve this issue will contribute to the public's health. One important next step is to develop modalities to provide the interventions that would be feasible for a larger proportion of the smoking population. One way to do so is to adapt the content to an Internet intervention that could be completed entirely online. Another is to combine Internet modalities with other modalities, such as telephone interventions, that require less travel time on the part of the participant.
Another important next step is to consider how best to determine what subpopulation of smokers would provide a cost-effective match for extended, multimodal interventions. For example, such an intervention possibly should be used only for participants who have failed to achieve prolonged abstinence with shorter, unimodal treatment interventions. In the current study, we accepted only participants who smoked at least 10 cigarettes per day and who reported smoking within 30 minutes of arising. We used these criteria to establish a working definition of dependent smoking. Still, it is possible that the intervention would be equally beneficial for lighter smokers and those who do not have evidence of physical dependence. These questions might best be resolved by analyses of larger data sets than those accrued by a single study; that is, secondary analyses of data from a variety of sources.
Acknowledgments
This study was supported by the National Institute on Drug Abuse (awards R01 DA02538, K05 DA016752, K23 DA018691, and P50 DA09253).
The authors wish to thank Kevin Delucchi for statistical consultation, Jennifer Morris for article preparation, Ronald Pilato for his clinical contributions, and Melissa Bartman for help with statistical analysis.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Human Participant Protection
This study protocol was approved by the University of California, San Francisco institutional review board. Participants provided written informed consent.
References
- 1.The Tobacco Use and Dependence Clinical Practice Guideline panel, staff, and consortium representatives. A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report. JAMA. 2000;283(24):3244–3254. [PubMed] [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Dept of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 3.Hays JT, Hurt RD, Rigotti N, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation: a randomized, controlled trial. Ann Intern Med. 2001;135(6):423–433. [DOI] [PubMed] [Google Scholar]
- 4.Hurt RD, Krook JE, Croghan IT, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21(5):914–920. [DOI] [PubMed] [Google Scholar]
- 5.Cox LS, Patten CA, Niaura RS, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. J Gen Intern Med. 2004;19(8):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin. 2007;23(4):793–801. [DOI] [PubMed] [Google Scholar]
- 7.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):64–71. [DOI] [PubMed] [Google Scholar]
- 8.Killen JD, Fortmann SP, Murphy GM, et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol. 2006;74(2):286–294. [DOI] [PubMed] [Google Scholar]
- 9.Killen JD, Fortmann SP, Schatzberg AF, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103(8):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction. 2009;104(6):1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. [DOI] [PubMed] [Google Scholar]
- 12.Jacob P, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1668–1673. [PubMed] [Google Scholar]
- 13.McNair DM, Lorr M, Droppleman LF. Manual: Profile of Mood States (POMS). San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 14.Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psychol. 1991;59(4):526–532. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 17.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 19.Hall SM, Muñoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo controlled study. J Consult Clin Psychol. 1996;64(5):1003–1009. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz RF, Marín BV, Posner SF, Pérez-Stable EJ. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. Am J Community Psychol. 1997;25(3):325–343. [DOI] [PubMed] [Google Scholar]
- 21.Hall SM, Tunstall CD, Vila KL, Duffy J. Weight gain prevention and smoking cessation: cautionary findings. Am J Public Health. 1992;82(6):799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161(11):2100–2107. [DOI] [PubMed] [Google Scholar]
- 23.Ossip-Klein DJ, Carosella AM, Krusch DA. Self-help interventions for older smokers. Tob Control. 1997;6(3):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]