Abstract
Adenosine 3',5'-cyclic monophosphate (cAMP)-mediated signal transduction is common in both prokaryotes and eukaryotes, and several bacterial pathogens modulate cAMP signaling pathways with their mammalian hosts during infection. In this study, cAMP levels associated with M. tuberculosis and M. bovis BCG were measured during macrophage infection. cAMP levels within both bacteria increased approximately 50-fold during infection of J774.16 macrophages, relative to the cAMP levels within bacteria incubated in tissue culture media alone. cAMP levels also increased within the macrophage cytoplasm upon uptake of live, but not dead, mycobacteria. The presence of albumin in the absence of oleic acid significantly decreased cAMP secretion and production by both M. tuberculosis and M. bovis BCG. These results suggest that cAMP signaling plays a role in the interaction of TB-complex mycobacteria with macrophages during infection, and that albumin may be a physiological indicator differentiating host environments during infection.
Keywords: Mycobacterium tuberculosis, Mycobacterium bovis BCG, cyclic AMP, macrophage, albumin
Introduction
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is responsible for an annual burden of 8 million new cases of disease, and 2 million deaths (WHO Report 2007, http://www.who.int/tb/en/). Limited understanding of M. tuberculosis biology and pathogenesis, particularly with respect to its interaction with the host during infection, reduces our ability to control TB infection.
Cyclic AMP (cAMP) is an important signaling molecule in many bacterial and eukaryotic cells (Botsford, 1981; Botsford and Harman, 1992; Daniel et al., 1998). However, little is known about the role of cAMP in mycobacteria, despite its potential importance to M. tuberculosis metabolism and pathogenesis (Bai et al., 2005; Bai et al., 2007; Gazdik and McDonough, 2005; Lowrie et al., 1975; Rickman et al., 2005; Spreadbury et al., 2005). The M. tuberculosis genome encodes 15 adenylyl cyclases (McCue et al., 2000), most of which have been shown to be functional using in vitro biochemical approaches (Shenoy and Visweswariah, 2006). Mycobacteria produce high levels of endogenous cAMP, compared to other bacteria (Botsford, 1981; Lee, 1979; Padh and Venkitasubramanian, 1976a, b), consistent with this abundance of adenylyl cyclases. Exogenously added cAMP affects gene expression in TB complex mycobacteria in response to environmental conditions such as hypoxia (Gazdik and McDonough, 2005). A CRP - like transcriptional regulator, CRPMt, encoded by Rv3676, has recently been described (Bai et al., 2005; Bai et al., 2007; Hunt et al., 2008; Rickman et al., 2005; Spreadbury et al., 2005). The CRPMt regulon includes ~114 genes (Bai et al., 2005), and its disruption causes impaired growth in laboratory medium, in bone marrow - derived macrophages and in a murine model of tuberculosis (Rickman et al., 2005).
A previous study reported that infection with live, but not dead, M. microti increased cAMP levels in the infected macrophages, and that these elevated cAMP levels correlated with impaired phagosome - lysosome fusion (Lowrie et al., 1975). Increased cAMP levels were also observed within the macrophages infected by M. bovis BCG, but not by M. lepraemurium (Lowrie et al., 1979). However, information is not available on the cAMP levels associated with M. tuberculosis during macrophage infection, particularly with respect to the effect of the macrophage environment on cAMP levels within the bacteria during infection. In this study, we measured cAMP levels of M. tuberculosis and M. bovis BCG within macrophages and different growth media, and found that cAMP levels within the bacteria are dramatically increased upon macrophage infection.
Materials and Methods
Growth of bacteria and macrophages
M. tuberculosis H37Rv and recombinant M. bovis BCG (Pasteur strain, Trudeau Institute) were grown in mycomedium (Middlebrook 7H9 medium supplemented with 0.5% glycerol, 10% oleic acid-albumin-dextrose-catalase (OADC), 0.05% Tween-80), as previously described (Florczyk et al., 2003). Fresh cultures were inoculated from frozen stocks for every experiment. Bacteria were typically used in late log phase, at 7 days of growth. Sauton’s medium was supplemented with 0.05% Tween 80. Additional supplements were used for some experiments, as specified in the text.
J774.16 murine macrophage cells were maintained and passaged twice weekly in J774 medium containing Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 20% fetal bovine serum (Gibco), 5% NCTC109 medium (Gibco), and 1% nonessential amino acids (Gibco) as described previously (McDonough et al., 1993).
Infection of macrophages
Macrophages were infected with M. tuberculosis or M. bovis BCG at a MOI of 200 bacteria/macrophage in 6-well plates. Heat-killed bacteria were used for comparison. Parallel infections were performed using macrophages on coverslips in 6-well plates to allow monitoring of infection levels by microscopy using the Kinyoun acid-fast stain (Kinyoun, 1915).
After 2 h of infection, cells were rinsed three times with PBS before lysing with 0.5% triton X-100 and 0.5% sodium deoxycholate (DOC) for 2–3 min, as described (McDonough and Kress, 1995). Lysates were then diluted with 7H9 broth, followed by centrifugation, to recover the pelleted bacteria. Supernatants were reserved for measurement of cAMP levels from the macrophage cytoplasm. Pellets containing the intracellularly passaged bacteria were resuspended in a volume of 7H9 broth equal to that of the recovered supernatants, and a small portion of each sample was plated for colony formation units (CFUs) to determine the number of bacteria recovered. The remaining bacteria were lysed by boiling for 5 minutes and used to determine the amount of cAMP within the bacteria (Imamura et al., 1996).
Comparison of media components
Sauton’s medium was compared with mycomedium, which contains supplements of 0.2% glucose, 0.5% albumin and 0.005% oleic acid that are lacking in standard Sauton's (2.9 mM dipotassium phosphate, 4.2 mM magnesium sulfate, 30.5 mM asparagines, 0.2 mM ferric ammonium citrate, 10.4 mM citric acid, and 6% glycerol (v/v), adjust pH to 6.8–7.2). Sauton’s medium was supplemented with the same amount of glucose, albumin, ADC or OADC as found in mycomedium. Equal numbers of bacteria were pelleted and resuspended in 2 ml of fresh mycomedium, Sauton’s medium or Sauton’s medium with supplements in a 6-well plate for 2 h at 37°C. cAMP concentrations of supernatants and bacterial pellets were measured for each sample, as described above.
cAMP assays
cAMP levels were measured by radioimmunoassay (RIA) using a rabbit anti-cAMP antibody (Calbiochem) and [125I]cAMP tyrosine methyl ester, as previously reported (Steiner et al., 1972; Swift and Dias, 1987). Assays were performed in disposable glass tubes, each containing anti-cAMP antibody, [125I]cAMP tyrosine methyl ester, and acetylated cAMP standard (0 – 512 fmol) or unknown samples, as described (Steiner et al., 1972; Swift and Dias, 1987). These mixtures were incubated for 18 h at 4°C, followed by co-precipitation of cAMP-antibody complexes with γ-globulin using 60% saturated (NH4)2SO4. For each reaction, supernatant was aspirated and the pellet was counted in a Wallac 1470 Wizard gamma counter (PerkinElmer Life Sciences). Data were analyzed using the RIA program (NIH-RIA) (Yanagishita and Rodbard, 1978), and results were normalized by bacterial numbers (determined by plating CFUs) or OD600 readings. Samples were measured in duplicate or triplicate for all the assays, and at least three independent repeats were performed for each experiment.
Results and Discussion
cAMP levels increase within M. tuberculosis and M. bovis BCG upon macrophage infection
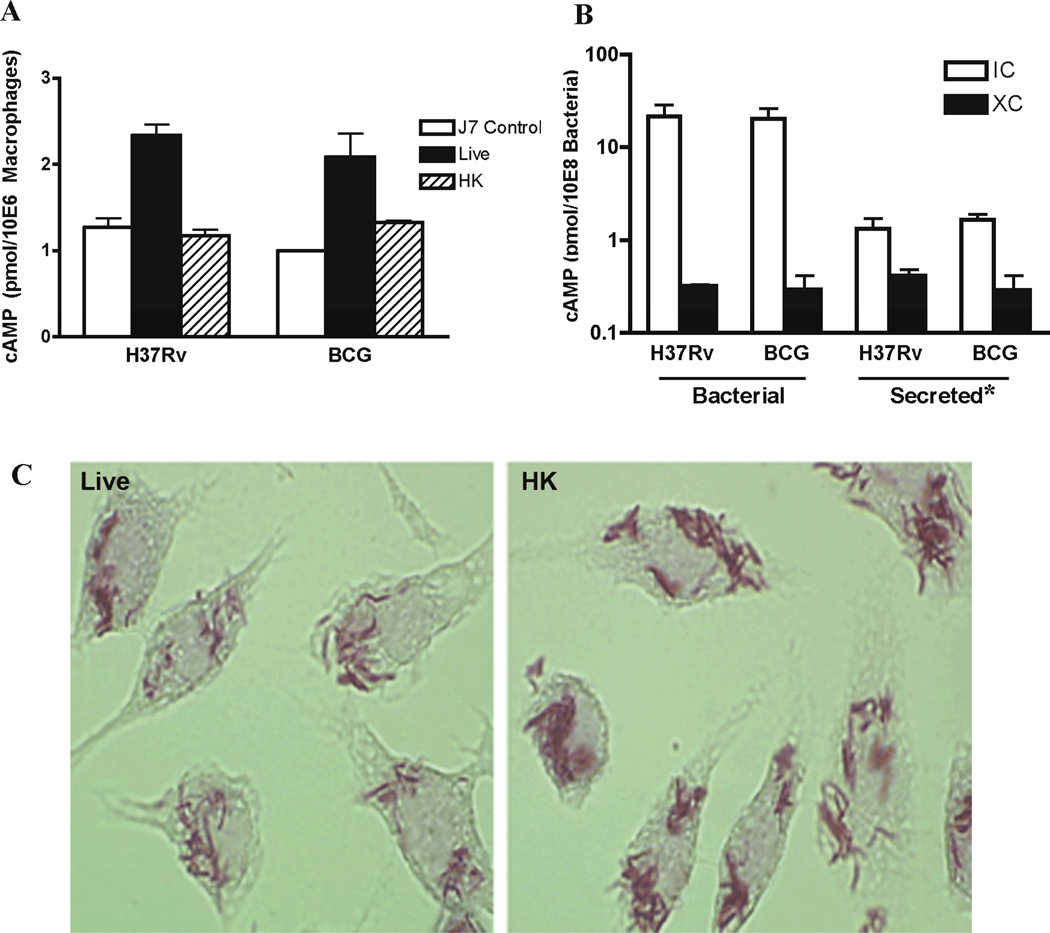
A previous study showed that phagocytosis of M. microti increased cAMP levels within the host macrophages, but M. tuberculosis was not tested (Lowrie et al., 1975). We measured cAMP levels within macrophages following infection with live M. tuberculosis or M. bovis BCG at levels similar to the previous study. We found that cAMP levels within the infected macrophages increased approximately two fold relative to uninfected macrophages or macrophages infected with heat-killed bacteria, in which cAMP levels did not change (Fig. 1A). These results indicate that the increased cAMP levels observed within the macrophages were specifically due to uptake of viable bacteria. This is consistent with results from the previous M. microti study, which used a similar MOI with different macrophages (Lowrie et al., 1975).
Fig. 1.
cAMP production of M. tuberculosis and M. bovis BCG within J774.16 macrophages infected at MOI of 200 (IC) versus tissue culture media (XC). (A) cAMP levels in supernatants of lysed macrophages that had been infected with either live (Live) or heat-killed (HK) M. tuberculosis and M. bovis BCG. Measurements were made after removal of intact, unlysed bacteria. J7 control represents uninfected macrophages. cAMP levels were normalized for the number of macrophages in each sample. Each data point represents average of duplicate samples. The results are representative of three independent experiments. Error bars denote SD of duplicate samples. (B) 'Bacterial' refers to cAMP levels of pelleted bacteria recovered from infected macrophages (IC) 2 h post infection or in macrophage tissue culture medium (XC) 2 h after incubation. 'Secreted' refers to the cAMP levels from the supernatants of unlysed IC or XC bacteria. *In the case of IC bacteria, this corresponds to the cAMP measured in the cytoplasm of infected macrophages minus the cAMP from equal number of uninfected macrophages. cAMP levels were normalized for bacterial numbers using CFU counts. Note that the y axis is expressed as log scale in this panel. The result shown is mean of three biological repeats. Error bars denote SD. (C) Acid-fast stain of macrophages infected with live (Live) and heat-killed (HK) M. bovis BCG at MOI of 200 for 2 h at 37°C, showing similar relative infection levels.
Recent studies showed that cAMP levels affect gene expression within TB-complex mycobacteria, and these include genes that are induced upon macrophage infection (Gazdik and McDonough, 2005). We addressed the role of macrophage infection on bacterial cAMP levels by comparing cAMP levels within M. tuberculosis or M. bovis BCG bacteria purified from macrophages (intracellular) versus those subjected only to tissue culture media (extracellular) for the duration of the infection period. cAMP levels in the intracellular bacteria were more than 50 fold higher than those of the corresponding extracellular bacteria (Fig. 1B). This dramatic increase in bacterial cAMP production upon infection of macrophages has not been reported previously, and has important implications for M. tuberculosis gene regulation and metabolism while within a host. cAMP levels within the intracellular bacteria (Fig. 1B) were also approximately 3 to 4 fold higher than those measured in bacteria maintained in mycomedium (Fig. 2A), which is associated with generally high levels of cAMP. This finding is consistent with results of another study that showed the ATP content of M. tuberculosis derived from infected mice was 2–4 fold lower than that of in vitro-grown bacteria (Dhople and Ryon, 2000). Accelerated conversion of ATP into cAMP could cause such a depletion in ATP levels, and future studies to address this possibility are warranted.
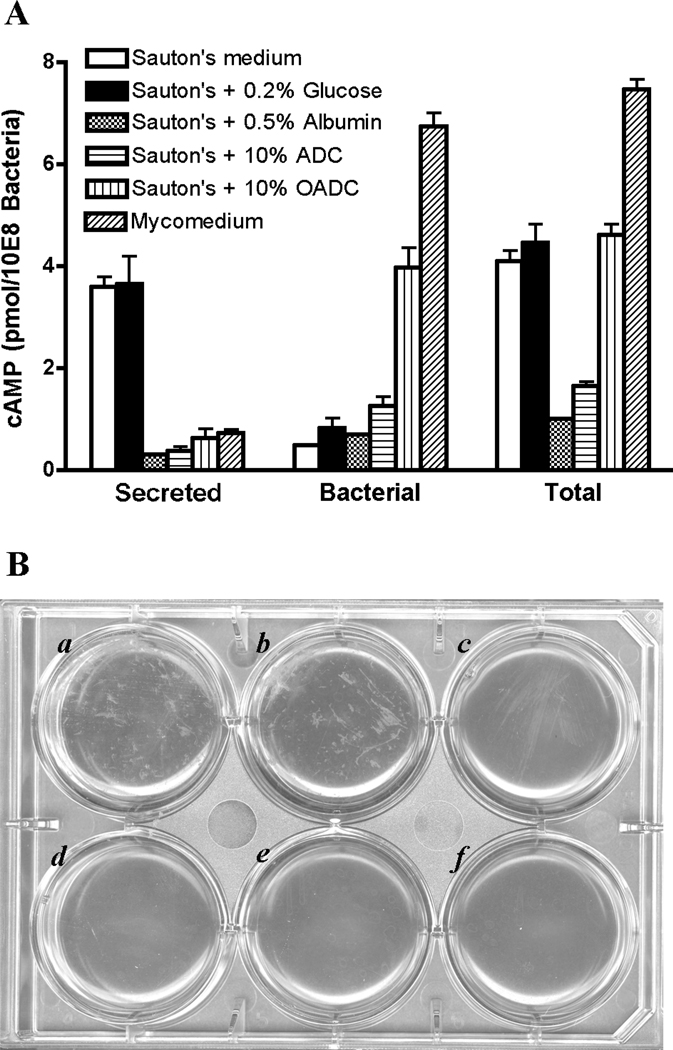
Fig. 2.
Comparison of cAMP production of M. bovis BCG in mycomedium, Sauton’s medium, and modified Sauton’s media. (A) Bacteria were incubated with each medium as listed in the figure for 2 h at 37°C. Secreted and bacterial cAMP was compared, as described in the text. Total refers combination of secreted and bacterial cAMP. Each data point represents average of duplicate samples. The results are representative of three independent experiments. Error bars denote SD of duplicate samples. (B) Incubation of M. bovis BCG in different media. Wells a to f correspond to the media listed from the top to the bottom in panel A. Clumps of bacteria presented in Sauton’s medium (a) and Sauton’s medium supplemented with 0.2% glucose (b), but not in other media (c–f) after 2 h incubation at 37 °C. The results are representative of three independent experiments.
These studies used a high MOI of 200 to allow cross-study comparison with Lowrie et al (Lowrie et al., 1975). We repeated these studies at a much reduced (and more physiological) MOI of 10, and again found that cAMP levels increased significantly in intracellular M. tuberculosis or M. bovis BCG relative to extracellular control bacteria (Fig. S1). cAMP levels within the macrophages were not measurably affected by M. tuberculosis or M. bovis BCG infection at this lower dose. However, potentially significant local increases in cAMP in the vicinity of internalized bacteria, as proposed by Kalamidas et al, (Kalamidas et al., 2006), would be greatly diluted by the vast excess of macrophage cytoplasm relative to the bacteria, and probably not be detected by the methods used.
The cAMP levels of heat-killed bacteria recovered from macrophages were barely detectable above background associated with the macrophage debris. Samples were visually examined to confirm that the levels of heat-killed bacteria associated with the macrophages were comparable to those in the live bacteria samples (Fig. 1C). Heat - killing reduced cAMP levels in the extracellular bacteria, which had cAMP levels approximately 20 – 50% of the viable extracellular bacteria in tissue culture medium (not shown). This reduction was most likely caused by leakage of cAMP through heat damaged cell envelopes, as cAMP is heat stable.
cAMP secretion levels from intracellular bacteria were sufficient to account for most of the observed change in macrophage cAMP levels (Fig. 1), and it is likely that at least a portion of the additional cAMP within the infected macrophages was produced and secreted by the intracellular bacteria. Nonetheless, enhanced production of cAMP by macrophages in response to a component within the live bacteria, in addition to increased production within the bacteria, cannot be ruled out. Regardless of its source, the increased cAMP levels have the potential for modulating macrophage responses to M. tuberculosis infection. This is supported by a recent study, in which addition of cAMP to macrophages inhibited phagosome-lysosome fusion and increased intraphagosomal growth of both M. tuberculosis and the non-pathogenic Mycobacterium smegmatis within macrophages (Kalamidas et al., 2006).
Transfer of cAMP from the macrophage cytoplasm to the bacteria during the limited infection period in this study is unlikely. The cAMP concentration within the intracellular bacteria greatly exceeded that of the macrophages, so uptake from the macrophages would have required cAMP transport to occur against a gradient. Moreover, cAMP uptake from the environment by TB-complex mycobacteria requires a very extended time course, even with hydrophobic derivatives such as dibutyrl cAMP that are transported through the lipid rich bacterial cell wall more readily than cAMP (Gazdik and McDonough, 2005). In contrast, cAMP secretion from TB-complex bacteria is easily observed, and likely to be mediated by an environmentally regulated transport system (Figs 1 and 2).
Albumin affects mycobacterial cAMP production
We noticed that the levels of cAMP secreted by the bacteria differed depending on the growth media, and we reasoned that specific components in the media might affect cAMP production and/or secretion. cAMP levels were measured in bacteria subjected to several different media conditions to identify components that affect mycobacterial cAMP production and/or secretion.
M. bovis BCG in Sauton’s medium secreted more cAMP into the media, and retained less cAMP within their cytoplasm, than bacteria in mycomedium (Fig. 2A). Sauton’s medium supplemented with the same amount of glucose, albumin, ADC or OADC as found in mycomedium was also tested to measure the effects of these components on M. bovis BCG’s cAMP production and secretion. A previous study reported a drop in cAMP levels following addition of 2% glucose (Padh and Venkitasubramanian, 1976b), however, addition of 0.2% glucose did not change the levels of either secreted or retained bacterial cAMP in the present study (Fig. 2A). We also saw no effect on cAMP levels in M. bovis BCG with the addition of 2% glucose (not shown). The reason for the difference between studies is not clear, although the M. tuberculosis CRP regulon differs from that of E. coli and may not play the same role in catabolite repression (Bai et al., 2005; Bai et al., 2007; Hunt et al., 2008; Rickman et al., 2005). In contrast, the addition of 0.5% albumin significantly inhibited cAMP secretion, and reduced the overall levels of cAMP in treated samples (Fig. 2A). A similar albumin effect was detected with M. bovis BCG in PBS (not shown). The effect of adding ADC was similar to that of albumin alone, indicating that albumin was the cause of the reduced cAMP levels in M. bovis BCG.
This albumin effect on cAMP levels may have physiological relevance during M. tuberculosis host infection, as bacteria will be exposed to varying levels of serum albumin depending on their location within the host. Human serum contains approximately 3.5 – 5.5% albumin (Sardesai, 2003). Albumin levels are much lower in extravascular compartments, where they are affected by proximity to venules and the nutritional status of the host (Coward and Sawyer, 1977). It is possible that a change in albumin levels signals the bacterium's transition to different environments within the host.
This albumin effect is also consistent with a report that albumin non-specifically inhibits cAMP production in eukaryotic cells (Fischer et al., 1992). The sheer number and variety of adenylyl cyclases and cNMP binding proteins in M. tuberculosis indicate that their cAMP signal transduction cascade is likely to be more complex than the classical prokaryotic paradigm established in E. coli (McCue et al., 2000). Interestingly, two M. tuberculosis adenylyl cyclases are phylogenetically classified as eukaryotic rather than prokaryotic (McCue et al., 2000), and it is possible that cAMP signaling in M. tuberculosis will ultimately be found to represent a blend of eukaryotic and prokaryotic paradigms.
Surprisingly, overall cAMP production, but not secretion, was restored with the addition of OADC rather than ADC alone to Sauton's medium (Fig. 2A). This indicates that oleic acid can reverse or compensate for the albumin-associated inhibition of cAMP production, but does not affect cAMP secretion. These results indicate that mycobacterial cAMP production and secretion are regulated independently by different environmental conditions. cAMP production in bacteria in mycomedium (which includes OADC) was also consistently higher than it was in Sauton's medium supplemented with OADC, so it is likely that factors other than oleic acid affect cAMP production. Activities of several M. tuberculosis adenylyl cyclases are affected by lipid and/or pH (Abdel Motaal et al., 2006; Findeisen et al., 2007; Tews et al., 2005), making it likely that they respond to changing host conditions by modulating cAMP levels during host infection. cAMP produced during infection has the potential to affect the physiology of either M. tuberculosis or its host, depending on whether or not it is secreted, and this topic warrants further investigation.
The mechanism of cAMP secretion by M. tuberculosis has not been explored, although it seems likely that regulated membrane transporters are required. We noted that increased cAMP secretion correlated with bacterial aggregation, despite identical levels of Tween-80 in each of the media. Bacteria became clumpy after 2 h incubation in Sauton’s medium or Sauton’s medium supplemented with 0.2% glucose, but not in Sauton’s medium supplemented with albumin, ADC or OADC (Fig. 2B). This clumping may reflect changes in the bacterial surface, and this connection between albumin, bacterial clumping, and cAMP secretion, warrants additional study.
Further investigation of the biological role of cAMP signaling during M. tuberculosis infection of macrophages, along with study of the environmental factors that independently regulate cAMP production and secretion by TB-complex bacteria, will also provide new insights into M. tuberculosis' interaction with the host during infection.
Supplementary Material
Acknowledgements
We thank Dr. James Dias, Dr. Brian Cohen and Richard Thomas for technical support with the cAMP assays. This work was supported by grants AI4565801 and AI063499 from the National Institutes of Health.
Abbreviations
- TB
tuberculosis
- cAMP
cyclic AMP
- MOI
multiple of infection
- CFU
colony forming unit
- OADC
oleic acid albumin dextrose complex
- ADC
albumin dextrose complex
References
- Abdel Motaal A, Tews I, Schultz JE, Linder JU. Fatty acid regulation of adenylyl cyclase Rv2212 from Mycobacterium tuberculosis H37Rv. Febs J. 2006;273:4219–4228. doi: 10.1111/j.1742-4658.2006.05420.x. [DOI] [PubMed] [Google Scholar]
- Bai G, McCue LA, McDonough KA. Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a Cyclic AMP Receptor Protein-Like DNA Binding Protein. J Bacteriol. 2005;187:7795–7804. doi: 10.1128/JB.187.22.7795-7804.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G, Gazdik MA, Schaak DD, McDonough KA. The Mycobacterium bovis BCG Cyclic AMP Receptor-Like Protein Is a Functional DNA Binding Protein In Vitro and In Vivo, but Its Activity Differs from That of Its M tuberculosis Ortholog, Rv3676. Infect Immun. 2007;75:5509–5517. doi: 10.1128/IAI.00658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford JL. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981;45:620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward WA, Sawyer MB. Whole-body albumin mass and distribution in rats fed on low-protein diets. Br J Nutr. 1977;37:127–134. doi: 10.1079/bjn19770012. [DOI] [PubMed] [Google Scholar]
- Daniel PB, Walker WH, Habener JF. Cyclic AMP signaling and gene regulation. Annu Rev Nutr. 1998;18:353–383. doi: 10.1146/annurev.nutr.18.1.353. [DOI] [PubMed] [Google Scholar]
- Dhople AM, Ryon DL. ATP content of Mycobacterium tuberculosis grown in vivo and in vitro. Microbios. 2000;101:81–88. [PubMed] [Google Scholar]
- Findeisen F, Linder JU, Schultz A, Schultz JE, Brugger B, Wieland F, Sinning I, Tews I. The structure of the regulatory domain of the adenylyl cyclase Rv1264 from Mycobacterium tuberculosis with bound oleic acid. J Mol Biol. 2007;369:1282–1295. doi: 10.1016/j.jmb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Fischer MJ, van Oosterhout AJ, Janssen LH, Nijkamp FP. Effect of albumin on adenylate cyclase receptor-related signal transduction of human peripheral blood mononuclear cells. Biochem Pharmacol. 1992;44:351–358. doi: 10.1016/0006-2952(92)90019-f. [DOI] [PubMed] [Google Scholar]
- Florczyk MA, McCue LA, Purkayastha A, Currenti E, Wolin MJ, McDonough KA. A family of acr-coregulated Mycobacterium tuberculosis genes shares a common DNA motif and requires Rv3133c (dosR or devR) for expression. Infect Immun. 2003;71:5332–5343. doi: 10.1128/IAI.71.9.5332-5343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdik MA, McDonough KA. Identification of cyclic AMP-regulated genes in Mycobacterium tuberculosis complex bacteria under low-oxygen conditions. J Bacteriol. 2005;187:2681–2692. doi: 10.1128/JB.187.8.2681-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DM, Saldanha JW, Brennan JF, Benjamin P, Strom M, Cole JA, Spreadbury CL, Buxton RS. Single nucleotide polymorphisms that cause structural changes in the cyclic AMP receptor protein transcriptional regulator of the tuberculosis vaccine strain Mycobacterium bovis BCG alter global gene expression without attenuating growth. Infect Immun. 2008;76:2227–2234. doi: 10.1128/IAI.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura R, Yamanaka K, Ogura T, Hiraga S, Fujita N, Ishihama A, Niki H. Identification of the cpdA gene encoding cyclic 3',5'-adenosine monophosphate phosphodiesterase in Escherichia coli. J Biol Chem. 1996;271:25423–25429. doi: 10.1074/jbc.271.41.25423. [DOI] [PubMed] [Google Scholar]
- Kalamidas SA, Kuehnel MP, Peyron P, Rybin V, Rauch S, Kotoulas OB, Houslay M, Hemmings BA, Gutierrez MG, Anes E, Griffiths G. cAMP synthesis and degradation by phagosomes regulate actin assembly and fusion events: consequences for mycobacteria. J Cell Sci. 2006;119:3686–3694. doi: 10.1242/jcs.03091. [DOI] [PubMed] [Google Scholar]
- Kinyoun JJ. A note on Uhlenhuth's method for sputum examination for tubercle bacilli. Am J Pub Health. 1915;5:867–870. doi: 10.2105/ajph.5.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH. Metabolism of cyclic AMP in non-pathogenic Mycobacterium smegmatis. Arch Microbiol. 1979;120:35–37. doi: 10.1007/BF00413269. [DOI] [PubMed] [Google Scholar]
- Lowrie DB, Jackett PS, Ratcliffe NA. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975;254:600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- Lowrie DB, Aber VR, Jackett PS. Phagosome-lysosome fusion and cyclic adenosine 3':5'-monophosphate in macrophages infected with Mycobacterium microti, Mycobacterium bovis BCG or Mycobacterium lepraemurium. J Gen Microbiol. 1979;110:431–441. doi: 10.1099/00221287-110-2-431. [DOI] [PubMed] [Google Scholar]
- McCue LA, McDonough KA, Lawrence CE. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 2000;10:204–219. doi: 10.1101/gr.10.2.204. [DOI] [PubMed] [Google Scholar]
- McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough KA, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padh H, Venkitasubramanian TA. Cyclic adenosine 3':5'-monophosphate in mycobacteria. Indian J Biochem Biophys. 1976a;13:413–414. [PubMed] [Google Scholar]
- Padh H, Venkitasubramanian TA. Adenosine 3',5'-monophosphate in Mycobacterium phlei and Mycobacterium tuberculosis H37Ra. Microbios. 1976b;16:183–189. [PubMed] [Google Scholar]
- Rickman L, Scott C, Hunt DM, Hutchinson T, Menendez MC, Whalan R, Hinds J, Colston MJ, Green J, Buxton RS. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol. 2005;56:1274–1286. doi: 10.1111/j.1365-2958.2005.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai VM. Introduction to Clinical Nutrition. New York: Marcel Dekker Inc; 2003. [Google Scholar]
- Shenoy AR, Visweswariah SS. New messages from old messengers: cAMP and mycobacteria. Trends Microbiol. 2006;14:543–550. doi: 10.1016/j.tim.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Spreadbury CL, Pallen MJ, Overton T, Behr MA, Mostowy S, Spiro S, Busby SJ, Cole JA. Point mutations in the DNA- and cNMP-binding domains of the homologue of the cAMP receptor protein (CRP) in Mycobacterium bovis BCG: implications for the inactivation of a global regulator and strain attenuation. Microbiology. 2005;151:547–556. doi: 10.1099/mic.0.27444-0. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Parker CW, Kipnis DM. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- Swift TA, Dias JA. Stimulation of polyamine biosynthesis by follicle-stimulating hormone in serum-free cultures of rat Sertoli cells. Endocrinology. 1987;120:394–400. doi: 10.1210/endo-120-1-394. [DOI] [PubMed] [Google Scholar]
- Tews I, Findeisen F, Sinning I, Schultz A, Schultz JE, Linder JU. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005;308:1020–1023. doi: 10.1126/science.1107642. [DOI] [PubMed] [Google Scholar]
- Yanagishita M, Rodbard D. Computerized optimization of radioimmunoassays for hCG and estradiol: an experimental evaluation. Anal Biochem. 1978;88:1–19. doi: 10.1016/0003-2697(78)90393-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.