Abstract
Rationale
Spinal cord plasticity can be assessed in spinal rats using an instrumental learning paradigm in which subjects learn an instrumental response, hindlimb flexion, to minimize shock exposure. Prior exposure to uncontrollable intermittent stimulation blocks learning in spinal rats but has no effect if given before spinal transection, suggesting that supraspinal systems modulate nociceptive input to the spinal cord, rendering it less susceptible to the detrimental consequences of uncontrollable stimulation.
Objective
The present study examines whether disrupting brain function with pentobarbital blocks descending inhibitory systems that normally modulate nociceptive input, making the spinal cord more sensitive to the adverse effect of uncontrollable intermittent stimulation.
Materials and methods
Male Sprague–Dawley rats received uncontrollable intermittent stimulation during pentobarbital anesthesia after (experiment 1) or before (experiment 2) spinal cord transection. They were then tested for instrumental learning at a later time point. Experiment 3 examined whether these manipulations affected nociceptive (thermal) thresholds.
Results
Experiment 1 showed that pentobarbital had no effect on the induction of the learning deficit after spinal cord transection. Experiment 2 showed that intact rats anesthetized during uncontrollable intermittent stimulation failed to learn when later transected and tested for instrumental learning. Experiment 3 found that uncontrollable intermittent stimulation induced an antinociception in intact subjects that was blocked by pentobarbital.
Conclusions
The results suggest a surgical dose of pentobarbital (50 mg/kg) suppresses supraspinal (experiment 2) but not spinal (experiment 1) systems that modulate nociceptive input to the spinal cord by blocking the antinociception that is induced by this input (experiment 3).
Keywords: Anesthesia, Spinal cord, Plasticity, Pain, Conditioning, Operant, Barbiturate
Introduction
Traditionally, the spinal cord was viewed primarily as a conduit for afferent and efferent neural impulses with the limited capacity for organizing simple responses (spinal reflexes). Recent studies have challenged this perspective, demonstrating that spinal cord neurons can support some complex behaviors (e.g., stepping) and simple forms of learning (Fitzgerald and Thompson 1967; Buerger and Fennessy 1970; Patterson et al. 1973; Chopin and Buerger 1976; Beggs et al. 1983; Edgerton et al. 1992; Grau et al. 1998). An example of spinal learning is observed in the pain system, where stimuli that engage pain (nociceptive) fibers can sensitize spinal neurons, a phenomenon known as central sensitization (Woolf 1983). Central sensitization produces a lasting increase in behavioral reactivity that has been linked to the development of neuropathic pain (Treede et al. 1992). Other studies showed that spinal cord neurons are sensitive to stimulus (Pavlovian) relations (Fitzgerald and Thompson 1967; Patterson et al. 1973; Beggs et al. 1983) and that the vigor of a spinal reflex is modified by its consequences (instrumental learning; Buerger and Fennessy 1970; Chopin and Buerger 1976; Grau et al. 1998). These effects appear to be mediated by some of the same neurochemical mechanisms implicated in neurobiological models of learning and memory within the brain (Harris et al. 1984; Morris et al. 1986). For example, all depend on a form of NMDA-receptor (NMDAR)-mediated plasticity (Durkovic and Prokowich 1998; Ji et al. 2003; Joynes et al. 2004; Ferguson et al. 2006; Woolf and Thompson 1991).
Studies in our laboratory have focused on a form of learning known as instrumental conditioning. In this type of learning, instituting a relationship between a response and outcome (a.k.a., reinforcer) brings about a change in behavior (Grau et al. 1998, 2006). In a typical experiment, transected (spinalized) rats are placed into a tube that allows the hindlimbs to hang freely. Whenever one hindlimb falls below a preset criterion, a shock is delivered to the tibialis anterior muscle, causing an upward flexion of the hindlimb that terminates the shock (controllable shock). Over the 30-min training period, rats gradually learn to maintain the hindlimb in a flexed position, effectively minimizing shock exposure. Rats that receive uncontrollable intermittent stimulation independent of leg position do not exhibit an increase in flexion duration and later fail to learn when tested with controllable shock (Grau et al. 1998). This loss of learning capacity (behavioral deficit) lasts up to 24 h and appears to be NMDAR-mediated (Crown et al. 2002; Ferguson et al. 2006).
It is interesting to note that instrumental learning is not impaired in subjects given uncontrollable intermittent stimulation before spinal transection, suggesting that descending supraspinal systems normally modulate nociceptive input to the spinal cord, preventing the deleterious effects of shock (Crown and Grau 2005). This effect appears to depend on supraspinal systems that influence spinal function by means of serotonergic fibers that project through the dorsolateral funiculus (DLF). We showed that intrathecal injections of serotonin (5 HT) and 8-OH DPAT (a 5-HT1A receptor agonist) before uncontrollable intermittent stimulation in spinal rats restores the modulatory effects typically observed in intact subjects (Crown and Grau 2005). In addition, intrathecal administration of a 5-HT1A receptor antagonist (WAY 100635) in intact rats blocked descending inhibitory influences that preserve spinal cord plasticity. Collectively, these results suggest that intact subjects do not develop a behavioral deficit because serotonergic fibers that traverse through the DLF preserve spinal cord plasticity by blocking the adverse effects of uncontrollable intermittent stimulation.
The brain-dependent protection of spinal cord circuitry could be tied to mechanisms that inhibit nociceptive neurons within the spinal cord, a phenomenon known as antinociception. It is well established that electrical stimulation of portions of the medial brainstem can inhibit both vocalization to nociceptive stimuli and spinally mediated nociceptive reflexes (Reynolds 1969; Mayer et al. 1971; Oliveras et al. 1974; Akil and Liebeskind 1975; Basbaum et al. 1977; Basbaum and Fields 1979). The inhibitory signal, is believed to originate in the periaqueductal gray, travel to the rostroventral medulla, including the nucleus raphe magnus (NRM), and then to the spinal cord through serotonergic fibers that descend through the DLF to suppress firing of neurons within the dorsal horns (Mayer et al. 1971; Oliveras et al. 1974; Basbaum and Fields 1979, 1984). This antinociceptive system can be engaged by aversive environmental stimuli, such as shock and restraint (Madden et al. 1977; Terman et al. 1984; Tricklebank and Curzon 1984). In the case of shock and other localized noxious stimuli, the antinociception is correlated with the inhibition of nociceptive neurons within the spinal cord and occurs in areas distant from the stimulation site (Morgan and Whitney 1996). In intact animals, uncontrollable intermittent stimulation may activate these inhibitory systems and thereby preserve spinal cord circuitry by preventing the adverse consequences of prolonged nociceptive stimulation.
Pentobarbital has been shown to depress activity of descending inhibitory systems, such as those contained within the DLF. Indeed, a pentobarbital-induced depression in brain activity can affect the electrophysiological properties of spinal neurons in a manner that parallels the effect of a spinal transection (Taub 1964; Mori et al. 1981). If pentobarbital disrupts descending inhibition, and thereby mimics the effect of spinal cord transection, treatment with pentobarbital should make intact rats more sensitive to the adverse consequences of uncontrollable intermittent stimulation. The novel prediction is that a level of uncontrollable intermittent stimulation that has no effect on spinal cord function in awake intact rats may adversely affect spinal cord neurons in intact anesthetized rats.
Alternatively, a high dose of pentobarbital may directly impact spinal neurons. This is an important consideration because uncontrollable intermittent stimulation could undermine spinal cord function by inducing a form of overexcitation akin to central sensitization (Ferguson et al. 2006). It is known that a high dose of pentobarbital can engage a gamma-aminobutyric acid (GABA)-mediated inhibition of spinal neurons and block the induction of central sensitization (Stein et al. 1987; Cleland et al. 1994). Given this, an anesthetic dose of pentobarbital could have a direct effect on spinal cord neurons that diminishes the impact of uncontrollable intermittent stimulation and inhibits the induction of the behavioral deficit. To discount this alternative, we first established whether an anesthetic dose of pentobarbital (50 mg/kg, i.p.) affects the induction of the behavioral deficit when input from the brain was removed by transecting the spinal cord (experiment 1). Experiment 2 then evaluated the effect of pentobarbital treatment on the induction of a spinal deficit in intact rats. Experiment 3 examined whether uncontrollable intermittent stimulation induces a pentobarbital-sensitive antinociception in intact rats.
Materials and methods
Animals
All protocols were approved by the Animal Care and Use committee at Texas A&M University. Male Sprague–Dawley rats obtained from Harlan (Houston, TX, USA) served as subjects. These animals were approximately 100–120 days old and weighed between 360 and 460 g. Subjects were maintained on a 12-h light–dark schedule and housed individually. Food and water were available ad libitum, and behavioral testing was performed during the light portion of the cycle.
Surgical procedure
All subjects in experiment 1 underwent surgery 24 (±2) h before experimental treatment, whereas animals in experiment 2 received surgery 24 (±2) h after experimental treatment. In both experiments, subjects were anesthetized with pentobarbital (50 mg/kg, i.p.), and the area surrounding the shoulders was shaved and sterilized with iodine. An anteroposterior incision approximately 1.5 cm long was made over the second thoracic vertebra (T2). The tissue immediately anterior to T2 was then cleared, and the exposed spinal cord was transected using cauterization. The resulting space was filled with Gelfoam (Harvard Apparatus, Holliston, MA, USA), and the wound was closed with Michel Clips (Fine Science Tools, Foster City, CA, USA). The hindlimbs were shaved and marked for electrode placement at this time.
Rats were injected with 0.9% saline (2.5 ml, i.p.) immediately after surgery, and the hindlimbs were secured in a natural flexed position with a piece of porous tape (Ortholetic 1.3 cm wide) wrapped once around the body and legs to prevent muscular damage due to unnatural extension during recovery. Subjects were allowed to recover in a temperature-controlled room (26.7¡C). Bladder expression took place twice a day and immediately before any behavioral procedures were conducted. At the end of testing, animals were euthanized with pentobarbital (100 mg/kg).
Transections were confirmed by (1) visually inspecting the cord during surgery to verify that the tissue had retracted and that no fibers bridged the injury, (2) observing behavior after recovery to ensure complete paralysis below the forelimbs and no vocalization when exposed to leg shock, and (3) examining the cord postmortem in a randomly selected subset (n=8) of subjects. The last step was taken to verify that there were no changes in the surgical procedure that could lead to the accidental sparing of neural tissue. Across dozens of experiments, and hundreds of subjects, postmortem analyses have failed to reveal any spared tissue.
Administration of anesthesia
All pentobarbital-treated groups received either a 25- or 50-mg/kg (i.p.) dose of pentobarbital 15 min before shock treatment. To minimize the impact of extraneous environmental variables, and to help assure a stable plane of anesthesia was achieved, subjects were acclimated to the transport bin and were handled by an experimenter (blind to the subject’s treatment condition) before testing. We also verified that animals that received an anesthetic dose of pentobarbital (50 mg/kg) did not react to a pinch of the front paw (experiment 1) or tail (experiments 2 and 3). All subjects met these criteria.
Behavioral procedures
Treatment conditions
Table 1 illustrates treatment order for experiments 1–3. In all cases, subjects were randomly assigned to groups with six subjects per condition. In experiment 1, spinally transected rats were given saline or pentobarbital (50 mg/kg) and, 15 min later, received intermittent shock or nothing. The capacity for instrumental learning was tested 24 h later. In experiment 2, intact rats received pentobarbital at a dose of 0, 25, or 50 mg/kg and approximately 15 min later given uncontrollable intermittent stimulation or nothing. The next day, the spinal cord was transected and instrumental learning was assessed 24 h later.
Table 1.
Time course of experiments 1–3
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| Experiment 1: Impact of pentobarbital anesthesia during uncontrollable shock in transected rats | Surgery Transection of the spinal cord under pentobarbital anesthesia | Experimental Treatment Anesthetized with pentobarbital (50 mg/kg) and given uncontrollable shock | Test for Instrumental Learning with controllable shock. |
| Experiment 2: Impact of pentobarbital anesthesia during uncontrollable shock in intact animals | Experimental Treatment Anesthetized with pentobarbital (25 mg/kg or 50 mg/kg) and given uncontrollable shock | Surgery Transection of the spinal cord under pentobarbital anesthesia | Test for Instrumental Learning with controllable shock. |
| Experiment 3: Impact of pentobarbital anesthesia on shock-induced antinociception |
Experimental Treatment: Anesthetized with pentobarbital (50 mg/kg) and given uncontrollable shock Test for antinociception |
__________ | __________ |
The table includes a day-by-day description of surgery, experimental treatment, and testing for each experiment. The primary difference between experiments 1 and 2 is the time at which experimental treatment took place. Subjects received the treatment after spinal transection in experiment 1 and before spinal transection in experiment 2.
Uncontrollable intermittent stimulation
Shock treatment occurred while subjects were loosely restrained in Plexiglas tubes as previously described in Crown et al. (2002). Intermittent constant current AC (60 Hz) shock was applied through electrodes taped to the tail. Shocked rats received 80-ms tail shocks on a variable time schedule with a mean of 2 s (range 0.2–3.8 s) for 6 min (approximately 180 shocks). We have previously established that this shock regimen induces a strong behavioral deficit in spinal, but not intact, rats (Crown and Grau 2005). Unshocked controls were placed in the restraining tubes, had the electrodes attached, but did not receive shock.
Instrumental learning testing procedure
Testing for instrumental learning occurred after spinal cord transection in experiments 1 and 2 as previously described (Grau et al. 1998). Briefly, on the day of testing, a wire electrode was inserted through the skin over the distal portion of the tibialis anterior (1.5 cm from the plantar surface of the foot), and one lead from the generator was attached to this wire. A contact electrode was secured to the foot between the second and third digits with a piece of porous tape. The shock generator was set to deliver a 0.4-mA shock, and the proximal portion of the tibialis anterior (approximately 1.7 cm proximal to the wire electrode) was probed with stainless steel pin attached to a shock lead to find a robust flexion response. The pin was then inserted into the muscle and a strain gauge was used to verify that a single, intense (1.6 mA, 0.3 s), test shock elicited at least a 0.8 N flexion force. Next, shock intensity was set at a level that elicited a flexion force of 0.4 N.
To minimize lateral leg movements, a piece of porous tape was wrapped around the leg and was attached to a bar extending across the apparatus directly under the front panel of the restraining tube. The tape was adjusted so that it was taut enough to slightly extend the knee. Finally, three short (0.15 s) shock pulses were applied, and the level of the salt solution was adjusted so that the tip of the contact electrode (attached to the rat’s foot) was submerged 4.0 mm below the surface. A rat’s capacity to perform the instrumental response was then tested with exposure to 30 min of controllable shock. Whenever the rat’s leg fell below the level of the salt solution, the electrodes delivered a shock to the tibialis anterior muscle, causing flexion of the hindlimb.
Three behavioral measures, response number, response duration, and time in solution, were used to assess a subject’s capacity to perform the instrumental response as previously described (Grau et al. 1998). Performance was measured over time in 30 1-min time bins. The computer monitoring leg position recorded an increase in response number whenever the contact electrode left the salt solution. Response duration was derived from time in solution and response number using the following equation: response durationi = (60 s − time in solutioni)/(response numberi+1), where i is the current time bin.
Tail flick test
Nociceptive thresholds were assessed using a radiant heat tail flick device, which consisted of a 375-W movie light (Sylvania, Type EBR) and a condenser lens that was positioned 8 cm below the light to focus light onto the subject’s tail. The tail was placed into a shallow grove cut into an aluminum block that was positioned 4.7 cm below the condenser lens. A photocell located under this aluminum block was used to detect lateral tail movements. Tail movements exceeding 0.5 cm automatically terminated the light and the time at which this occurred was recorded to the nearest 0.01 s. The intensity of the light source was controlled by an AC potentiometer (Leviton, no. 6681-W) that illuminated approximately 2 cm of the tail.
Subjects in experiment 3 were placed in the restraining tubes 10 min after pentobarbital treatment (50 mg/kg, i.p.). They were acclimated for 5 min to the test room, which was maintained at 26.7¡C. Two baseline measures of thermal reactivity were then taken approximately 2 min apart for each subject. After baseline testing, subjects received uncontrollable intermittent stimulation or nothing, followed by 2 additional tests of thermal reactivity spaced 2 min apart.
Statistical analysis
The effects of experimental treatment over time were analyzed using a repeated measures analysis of variance (ANOVA). Group differences were further evaluated using Duncan’ new multiple range test. Differences were considered significant at p values <0.05.
Results
Experiment 1: anesthesia does not affect the induction of the deficit in spinal rats
To examine whether the anesthesia affected reactivity to leg shock, the shock intensities required to produce a 0.4-N change in flexion force were analyzed. Mean intensities (±SEM) ranged from 0.40 (±0.04) to 0.42 (±0.06) mA. These differences were not statistically significant (all F< 2.42, p>0.05).
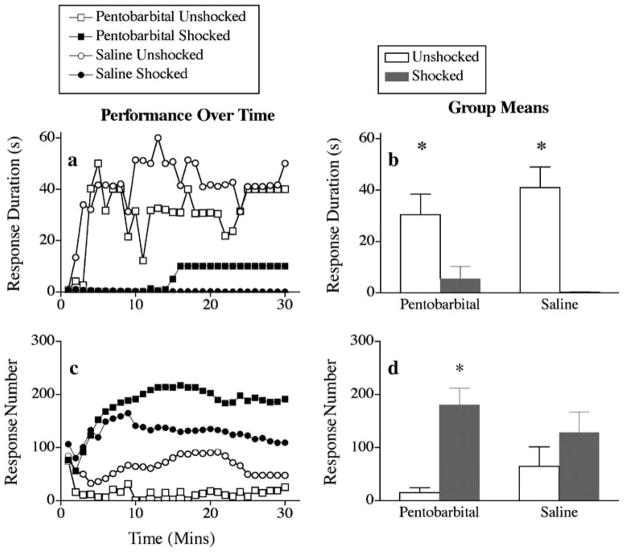
The effect of pentobarbital on response duration is depicted in Fig. 1a. As previously shown, unshocked subjects treated with saline exhibited an increase in response durations over the 30-min testing period, whereas saline-treated subjects previously exposed to uncontrollable intermittent stimulation did not. Pentobarbital treatment had no impact on this pattern of results. An ANOVA revealed significant main effects of time and shock and a significant time × shock interaction (all F>3.03, p<0.05). Post hoc comparisons of the group means (Fig. 1b) confirmed that subjects that received uncontrollable intermittent stimulation differed from the unshocked groups (p<0.05). No other differences were significant.
Fig. 1.
Effect of pentobarbital on mean response duration (a, b) and response number (c, d) in animals exposed to uncontrollable intermittent stimulation (shocked) or tube restraint (unshocked) after spinal cord transection. Subpanels a and c depict the data over the 30-min test period. Each point represents the average observed over a 60-s time bin. The right panels (b, d) represent mean performance collapsed across time (±SEM; n=6)
The failure to learn did not reflect an inability to perform the flexion withdrawal response. Indeed, the rats that failed to learn exhibited more responses (Fig. 1c,d). Rats in the unshocked groups exhibited a progressive increase in flexion duration that led to a decrease in the number of responses made over the 30-min test period. An ANOVA revealed significant main effects of shock [F(1, 20) = 11.22, p<0.05] and time [F(29, 580) = 2.24, p<0.05]. The time × shock interaction and pentobarbital × shock × time three-way interaction were also significant (F>1.57, p<0.05). These interactions emerged because shocked rats, which failed to learn, progressively exhibited more responses over the 30-min test session.
Experiment 2: pentobarbital anesthesia allows for the development of the deficit in intact rats exposed to uncontrollable intermittent stimulation
Experiment 1 showed that pentobarbital anesthesia does not protect the spinal cord against the deleterious effects of uncontrollable intermittent stimulation in spinal rats. Experiment 2 examined whether the same pentobarbital treatment would impact the induction of the learning deficit in intact rats. The novel prediction was that pentobarbital anesthesia would block brain-mediated preservation of spinal circuitry, making spinal neurons more susceptible to the adverse effects of nociceptive stimulation. If this is true, intact rats shocked under anesthesia should exhibit a failure to learn when tested after spinal transection.
The shock intensity needed to elicit a 0.4-N change in flexion force ranged from 0.42 (± 0.02) to 0.57 (±0.04) mA. These group differences were not statistically significant (F<2.59, p>0.05).
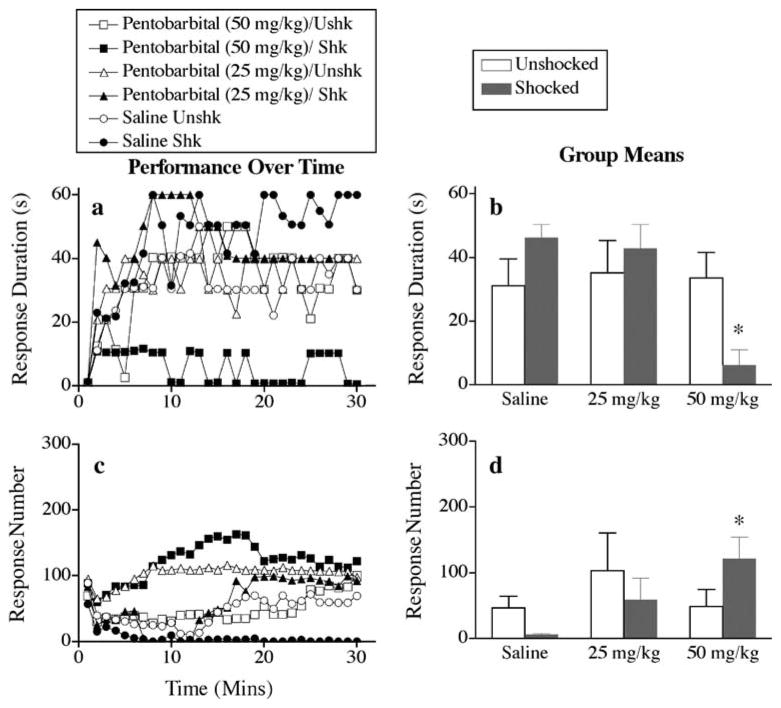
Saline-treated rats given uncontrollable intermittent stimulation before transection did not exhibit a learning deficit (Fig. 2a). However, animals that received the highest dose of pentobarbital (50 mg/kg) before delivery of uncontrollable intermittent stimulation failed to learn, suggesting that pentobarbital removes the brain-mediated preservation of spinal cord systems. A repeated measures ANOVA on response duration revealed significant main effects of pentobarbital and time (F>3.55, p<0.05). Both the pentobarbital × shock and pentobarbital × shock × time interactions reached significance (F>1.93, p<0.05). Post hoc comparisons of the group means (Fig. 2b) revealed that subjects that received the highest dose of pentobarbital (50 mg/kg) before uncontrollable intermittent stimulation differed from the other groups (p<0.05). No other group differences were significant (p>0.05).
Fig. 2.
Impact of pentobarbital on mean response duration (a, b) and response number (c, d) in rats given uncontrollable intermittent stimulation (shocked) or restraint (unshocked) before spinal cord transection. Subpanels a and c depict the data over the 30-min test period. Each point represents the average observed over a 60-s time bin. The right panels (b, d) represent mean performance collapsed across time (±SEM; n=6)
All subjects, except those given the highest dose of pentobarbital (50 mg/kg) before uncontrollable intermittent stimulation, displayed a decrease in response number as they acquired the instrumental response (Fig. 2c). Rats given uncontrollable intermittent stimulation and the highest dose of pentobarbital (50 mg/kg) failed to learn even though shock continued to drive a high level of responding, which habituated over the course of the test session. An ANOVA revealed a significant main effect of time [F(29, 870) = 3.01, p<0.05] and a significant pentobarbital × shock × time interaction [F(58, 870) = 1.80, p<0.05].
Experiment 3: anesthesia blocks shock-induced antinociception in intact rats
Prior studies have shown that uncontrollable intermittent stimulation can induce an antinociception that inhibits nociceptive systems within the spinal cord by means of descending pathways. The mechanisms mediating this antinociception can vary depending upon a variety of physical parameters, including the duration of exposure, whether shock is continuous or intermittent, and intensity (Grau et al. 1996). In some cases, the antinociception is blocked by pentobarbital treatment, but in others, it is not. Experiment 3 examined whether the uncontrollable intermittent stimulation procedure used in experiments 1 and 2 produced a pentobarbital-sensitive antinociception in intact rats.
Baseline tail flick latencies ranged from 4.60 (±0.30) to 5.39 (±0.62) s. These differences did not reach significance (all F<2.10, p>0.05). Shock treatment induced an anti-nociception in saline treated, but not pentobarbital treated rats (Fig. 3). An analysis of covariance, using the second baseline tail flick measure as the covariate, confirmed that the effect of shock treatment depended on pentobarbital condition, yielding a significant shock × pentobarbital interaction [F(1, 19) = 6.44, p<0.05]. Post hoc comparisons showed that the saline-treated shocked group differed from the saline-treated unshocked controls and the pentobarbital-treated shocked group (p<0.05). No other comparisons were significant.
Fig. 3.
Impact of pentobarbital treatment on tail withdrawal from radiant heat in anesthetized (Pento) and awake (Saline) rats given uncontrollable intermittent stimulation (shocked) or nothing (unshocked). The error bars indicate the SEM (n=6)
General discussion
We have previously shown that uncontrollable intermittent stimulation inhibits adaptation to new response–outcome relations in spinal, but not intact, rats (Crown and Grau 2005). The present study examined the effect of uncontrollable intermittent stimulation in rats anesthetized with pentobarbital. A dose of pentobarbital (50 mg/kg) that induced anesthesia did not affect the induction of the learning deficit in spinally transected rats (experiment 1). Experiment 2 examined whether pentobarbital would block the brain-dependent preservation of spinal neurons. As previously reported in Crown and Grau (2005), uncontrollable intermittent stimulation did not have a lasting effect when given to awake rats before spinal transection. Similar results were observed in rats given a subanesthetic dose (25 mg/kg) of pentobarbital. In contrast, rats that received uncontrollable intermittent stimulation while anesthetized with pentobarbital (50 mg/kg) later exhibited a spinal learning deficit when tested after a spinal transection. This suggests that a dose of pentobarbital sufficient to induce anesthesia inhibits the process that normally preserves spinal cord circuitry in intact subjects. We reasoned that this preservation could be linked to a brain-dependent antinociception that is sensitive to pentobarbital treatment. Supporting this, experiment 3 showed that uncontrollable intermittent stimulation induced a robust antinociception on the tail flick test in awake, but not anesthetized, rats.
Prior research suggests that the learning deficit lasts 24–48 h (Crown et al. 2002). Because the design of experiment 2 required a surgical procedure (spinal transection) between treatment and testing, shock treatment and instrumental testing were separated by 48 h. The fact that a robust deficit was observed in rats that received an anesthetic dose of pentobarbital and uncontrollable intermittent stimulation under these conditions suggests that shock treatment also has a lasting effect in intact rats. Moreover, because the transection was performed 24 h after treatment, long after the initial period of anesthesia had worn off, it is clear that the recovery of normal brain function does not “erase” the adverse consequences of uncontrollable intermittent stimulation given under anesthesia.
Research by Mori et al. (1981) suggests that pentobarbital may facilitate the development of the behavioral deficit because it induces a physiological state similar to that of spinal transection, releasing nociceptive processing within the spinal cord from supraspinal control. They, and others, have reported that descending inhibitory systems are suppressed by barbiturates (Frank and Ohta 1971; Collins et al. 1990). Since these reports, others showed that pentobarbital blocks shock-induced analgesia (Terman et al. 1984; Grau 1987) and other forms of anesthesia were shown to diminish the inhibition of dorsal horn neuron firing seen after noxious stimulation (Tomlinson et al. 1983). The exact mechanism through which this occurs remains unknown, although the inhibitory neurotransmitter GABA appears to play a major role (Asana and Ogasawara 1981; Olsen and Snowman 1982; Sivam et al. 1982). For example, activation of GABA receptors in the NRM was shown to increase responses to noxious stimuli (Drower and Hammond 1988). Pentobarbital may bind to GABA receptors in the NRM, resulting in a disinhibition of systems necessary for pain modulation. Released from this source of inhibition, nociceptive input that normally has little impact may now overactivate spinal cord neurons. In addition, the absence of descending inhibition could unmask a descending facilitation that augments this overexcitation.
In the absence of noxious input, a pentobarbital-induced modification in nociceptive processing may have little or no functional consequences. However, in the presence of nociceptive stimulation, the evidence reviewed above suggests that anesthesia can have an adverse effect by removing a form of negative feedback that normally suppresses the development of spinal central sensitization, a phenomenon that has been linked to both the development of neuropathic pain and the inhibition of adaptive plasticity (Woolf 1983; Treede et al. 1992; Ferguson et al. 2006). Central sensitization is thought to depend on a form of NMDAR-mediated plasticity and has been tied to the development of spinal long-term potentiation (LTP; Woolf and Thompson 1991; Ji et al. 2003). Prior work indicates that the induction of spinal LTP is suppressed by descending inhibition (Sandkuhler and Liu 1998); therefore, any manipulation that interferes with this inhibition could foster the development of central sensitization. By suppressing descending inhibitory systems, pentobarbital may facilitate central sensitization and the development of pain after nociceptive input. Supporting this, others showed that barbiturates can induce enhanced pain (hyperalgesia) on a number of measures (Franklin and Abbott 1993; Yokoro et al. 2001). This hyperalgesia may result from changes in dorsal horn neurons, which change response profiles to noxious stimuli from low threshold to wide dynamic range neurons after pentobarbital administration in intact animals (Collins and Ren 1987). This change is thought to result from a decrease in the release of serotonin and norepinephrine, effectively reducing the descending inhibition that helps to regulate neural activity in the dorsal horn. It is interesting to note that serotonergic fibers that project through the DLF appear to play a major role in preserving the function of spinal cord neurons that receive nociceptive input resulting from uncontrollable intermittent stimulation in our intact subjects (Crown and Grau 2005).
These findings imply that the learning deficit and central sensitization depend on similar mechanisms. Supporting this, we showed that manipulations that induce central sensitization (e.g., peripheral inflammation) undermine spinal learning (Ferguson et al. 2006). Conversely, exposure to intermittent shock heightens mechanical reactivity (allodynia), a common behavioral index of central sensitization (Ferguson et al. 2006). Further, like central sensitization, the long-term consequences of uncontrollable intermittent stimulation can be blocked by pretreatment with a NMDAR antagonist (Ferguson et al. 2006).
Paradoxically, instrumental learning is also blocked by administration of an NMDA antagonist (Joynes et al. 2004; Ferguson et al. 2006). This could mean that the deficit and learning depend on opposing NMDAR-dependent processes (e.g., LTP vs depression). Alternatively, uncontrollable intermittent stimulation and peripheral inflammation may inhibit the acquisition of selective adaptive responses (instrumental learning) because both manipulations diffusely saturate NMDAR-mediated plasticity (Moser et al. 1998; Moser and Moser 1999).
Our suggestion that pentobarbital adversely affects spinal function by allowing a form of overexcitation may seem counterintuitive because pentobarbital is often regarded as an inhibitory substance with antinociceptive properties. Indeed, others showed that pentobarbital can reverse the potentiated tail flick response observed after naloxone treatment (Doi and Jurna 1982) and reduce C-fiber-evoked activity in spinalized rats (Stein et al. 1987; Cleland et al. 1994). Based on these observations, we would expect pentobarbital to dampen overexcitation within the spinal cord and thereby exert a protective effect. However, experiment 1 found no evidence that systemic pentobarbital, at the dose employed, affects the induction of the deficit in spinally transected rats. Furthermore, systemic pentobarbital did not have a significant effect on tail flick latencies in the unshocked controls in experiment 3. Taken together, we found little evidence that systemic pentobarbital, at a dose that produces surgical anesthesia, has a direct effect on spinal nociceptive processing or motor reactivity. This apparent discrepancy with earlier reports likely reflects a difference in the molar concentration of pentobarbital within the spinal cord. Studies demonstrating a spinally mediated antinociceptive effect of pentobarbital treatment have typically used intrathecal administration or a higher systemic dose, both of which should lead to higher molar concentrations within the cord. Moreover, because motor neurons are more susceptible to the depressant effects of pentobarbital than dorsal horn neurons, levels of pentobarbital that inhibit hindlimb withdrawal may not affect dorsal horn neurons (Paik et al. 1989; Carstens and Campell 1992). This is important because both uncontrollable intermittent stimulation and peripheral inflammation could affect spinal function by sensitizing neurons within the dorsal horn. As a result, doses of pentobarbital that inhibit motor responses may not necessarily diminish the adverse consequences of uncontrollable intermittent stimulation.
Our demonstration that uncontrollable intermittent stimulation has a lasting effect on instrumental learning in pentobarbital-anesthetized rats has important clinical implications. It suggests that noxious stimuli that occur during surgery, or similar pharmacological states, may induce intraspinal processes that disrupt spinal cord function. Furthermore, if the consequences of uncontrollable intermittent stimulation are related to the development of central sensitization and neuropathic pain, as previous data suggest (Ferguson et al. 2006), these intraspinal changes could facilitate the development of postoperative pain. Our findings further support the use of preemptive measures to limit nociceptive-induced overexcitation during periods of anesthesia.
Acknowledgments
This work was funded by the National Institute of Neurological Disorders and Stroke grant no. R01 NS41548-01 to J. W.G.
References
- Akil H, Liebeskind JC. Monoaminergic mechanisms of stimulation-produced analgesia. Brain Res. 1975;94:279–296. doi: 10.1016/0006-8993(75)90062-1. [DOI] [PubMed] [Google Scholar]
- Asana T, Ogasawara N. Chloride-dependent stimulation of GABA and benzodiazepine binding by pentobarbital. Brain Res. 1981;225:212–216. doi: 10.1016/0006-8993(81)90333-4. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–532. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control system: brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309–398. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Marley NJE, O’Keefe J, Clanton CH. Reversal of morphine and stimulus-produced analgesia by subtotal spinal cord lesions. Pain. 1977;3:43–56. doi: 10.1016/0304-3959(77)90034-3. [DOI] [PubMed] [Google Scholar]
- Beggs AL, Steinmetz JE, Romano AG, Patterson MM. Extinction and retention of a classically conditioned flexor nerve response in acute spinal cat. Behav Neurosci. 1983;97:530–540. doi: 10.1037//0735-7044.97.4.530. [DOI] [PubMed] [Google Scholar]
- Buerger AA, Fennessy A. Long-term alteration of leg position due to shock avoidance by spinal rats. Nature. 1970;225:751–752. doi: 10.1016/s0014-4886(71)80001-8. [DOI] [PubMed] [Google Scholar]
- Carstens E, Campell IG. Responses of motor units during the hind limb flexion withdrawal reflex evoked by noxious skin heating: phasic and prolonged suppression by midbrain stimulation and comparisons with simultaneous recorded dorsal horn units. Pain. 1992;48:15–226. doi: 10.1016/0304-3959(92)90061-F. [DOI] [PubMed] [Google Scholar]
- Chopin SF, Buerger AA. Instrumental avoidance conditioning in the spinal rat. Brain Res Bull. 1976;1:177–183. doi: 10.1016/0361-9230(76)90067-8. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Lim FY, Gebhart GF. Pentobarbital prevents the development of C-fiber-induced hyperalgesia in the rat. Pain. 1994;57:31–43. doi: 10.1016/0304-3959(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Collins JG, Ren K. WDR response profiles of spinal dorsal horn neurons may be unmasked by barbiturate anesthesia. Pain. 1987;28:369–378. doi: 10.1016/0304-3959(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Collins JG, Ren K, Saito Y, Iwasaki H, Tang J. Plasticity of some spinal dorsal horn neurons as revealed by pentobarbital-induced disinhibition. Brain Res. 1990;525:189–197. doi: 10.1016/0006-8993(90)90863-7. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Evidence that descending serotonergic systems protect spinal cord plasticity against the disruptive effect of uncontrollable stimulation. Exp Neurol. 2005;197:164–176. doi: 10.1016/j.expneurol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Crown ED, Joynes RL, Ferguson AR, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav Neurosci. 2002;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Doi T, Jurna I. Intrathecal pentobarbital prevents naloxone-induced facilitation of the tail-flick response in the rat. Neurosci Lett. 1982;32:81–84. doi: 10.1016/0304-3940(82)90233-6. [DOI] [PubMed] [Google Scholar]
- Drower EJ, Hammond DL. GABAergic modulation of nociceptive threshold: effects of THIP and bicuculline micro-injected in the ventral medulla of the rat. Brain Res. 1988;450:316–324. doi: 10.1016/0006-8993(88)91570-3. [DOI] [PubMed] [Google Scholar]
- Durkovic RG, Prokowich LJ. D-2-amino-5-phosphonovalerate, and NMDA receptor antagonist, blocks induction of associative long-term potentiation of the flexion reflex in spinal cat. Neurosci Lett. 1998;257:162–164. doi: 10.1016/s0304-3940(98)00820-9. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Hodgson JA, Prober RJ, de Guzman CP, de Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J Neurotrauma. 1992;9(Suppl 1):S119–S128. [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LA, Thompson RF. Classical conditioning of the hindlimb flexion reflex in the acute spinal cat. Psychon Sci. 1967;47:345–351. [Google Scholar]
- Frank GB, Ohta M. Blockade of the reticulospinal inhibitory pathway by anaesthetic agents. Br J Pharmacol. 1971;42:328–342. doi: 10.1111/j.1476-5381.1971.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Abbott FV. Pentobarbital, diazepam, and ethanol abolish the interphase diminution of pain in the formalin test: evidence for pain modulation by GABAA receptors. Pharmacol Biochem Behavior. 1993;46:661–666. doi: 10.1016/0091-3057(93)90558-b. [DOI] [PubMed] [Google Scholar]
- Grau JW. The central representation of an aversive event maintains the opioid and nonopioid forms of analgesia. Behav Neurosci. 1987;101:272–288. doi: 10.1037//0735-7044.101.2.272. [DOI] [PubMed] [Google Scholar]
- Grau JW, Burks K, Kallina CF, King TE, Meagher MW. Activation of the opioid and nonopioid antinociceptive systems in pentobarbital anesthetized rats: assessing the role of shock severity. Psychobiology. 1996;24:71–84. [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behav Neurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: Underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Cotman CW. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984;323:132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms. Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts acquisition and maintenance of an acquired flexion response. Behav Brain Res. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Madden J, Akil H, Patrick RL, Barchas JD. Stress-induced parallel changes in central opioid levels and pain responsiveness. Nature. 1977;265:358–360. doi: 10.1038/265358a0. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Wolfe TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brain stem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK. Behavioral analysis of diffuse noxious inhibitory controls (DNIC): antinociception and escape reactions. Pain. 1996;66:307–312. doi: 10.1016/0304-3959(96)03061-8. [DOI] [PubMed] [Google Scholar]
- Mori K, Komatsu T, Tomemori N, Shingu K, Urabe N, Seo N, Hatano Y. Pentobarbital-anesthetized and decerebrate cats reveal different neurological responses in anesthetic-induced analgesia. Acta Anaesthesiol Scand. 1981;25:349–354. doi: 10.1111/j.1399-6576.1981.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. Is learning blocked by saturation of synaptic weights in the hippocampus? Neurosci Biobehav Rev. 1999;23:661–672. doi: 10.1016/s0149-7634(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Oliveras JL, Besson JM, Guilbaud G, Liebeskind JC. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974;20:32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Snowman AM. Chloride-dependent enhancement by barbiturates of GABA receptor binding. J Neurosci. 1982;2:1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik KS, Nam SC, Chung JM. Different classes of cat spinal neurons display differential sensitivity to sodium pentobarbital. J Neurosci Res. 1989;23:107–115. doi: 10.1002/jnr.490230114. [DOI] [PubMed] [Google Scholar]
- Patterson MM, Cegavske CF, Thompson RF. Effects of a classical conditioning paradigm on hind-limb flexion nerve response in the immobilized cat. J Comp Physiol Psychol. 1973;84:88–97. doi: 10.1037/h0035021. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Sivam SP, Nabeshima T, Ho IK. Acute and chronic effects of pentobarbital in relation to postsynaptic GABA receptors: a study with muscimol. J Neurosci Res. 1982;7:37–47. doi: 10.1002/jnr.490070105. [DOI] [PubMed] [Google Scholar]
- Stein C, Morgan MM, Liebeskind JC. Barbiturate-induced inhibition of a spinal nociceptive reflex: role of GABA mechanisms and descending modulation. Brain Res. 1987;407:307–311. doi: 10.1016/0006-8993(87)91108-5. [DOI] [PubMed] [Google Scholar]
- Taub A. Local, segmental and supraspinal interaction with a dorsolateral spinal cutaneous afferent system. Exp Neurol. 1964;10:357–374. doi: 10.1016/0014-4886(64)90006-8. [DOI] [PubMed] [Google Scholar]
- Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- Tomlinson RW, Gray BG, Dostrovsky JO. Inhibition of rat spinal cord dorsal horn neurons by non-segmental, noxious cutaneous stimuli. Brain Res. 1983;279:291–294. doi: 10.1016/0006-8993(83)90195-6. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Curzon G. Stress-induced analgesia. Wiley; New York: 1984. [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;305:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yokoro CM, Pesquero SM, Turchetti-Maia RMM, Francischi JN, Tatsuo MAKF. Acute Phenobarbital administration induces hyperalgesia: pharmacological evidence for the involvement of GABA-A receptors. Braz J Med Biol Res. 2001;34:397–405. doi: 10.1590/s0100-879x2001000300015. [DOI] [PubMed] [Google Scholar]