Abstract
Emerging evidence suggests that psychosocial stress and toxicants may interact to modify health risks. Stress–toxicant interactions could be important in chemical risk assessment, but these interactions are poorly understood and additional research is necessary to advance their application.
Environmental health research can increase knowledge of these interactions by exploring hypotheses on allostatic load, which measures the cumulative impacts of stress across multiple physiological pathways, using knowledge about physiological pathways for stress-related health effects, and evidence of common target pathways for both stress and toxicants.
In this article, critical physiological pathways for stress-related health effects are discussed, with specific attention to allostatic load and stress–toxicant interactions, concluding with research suggestions for potential applications of such research in chemical risk assessment.
IN RECENT RECOMMENDATIONS to the US Environmental Protection Agency (EPA), the National Academies of Science (NAS) stated that nonchemical stressors should be addressed in risk assessment and management when permitted by data. Specifically, the NAS suggested a focus on nonchemical stressors that may influence risk estimates, or that are influenced differentially by potential risk management options.1 The NAS recommendations coincide with the growing scholarship on the role of psychosocial stress, a nonchemical stressor, in environmental health risk. Psychosocial stress has been examined as a risk factor in environmentally related diseases,2–5 and as a risk modifying factor for chemical stressors in epidemiological studies6–8 and toxicological studies.9–11 It is also emerging as an important explanatory variable in theoretical research/analytical framework proposals to examine disparities, including racial/ethnic and income disparities, in environmental health risk and for diseases with environmental origins.12–16
The framework proposed by Morello-Frosch and Shenassa14 is noteworthy because it explicitly integrates the stress-related concept of “allostatic load” into the traditional environmental exposure–disease paradigm. Allostasis is defined as a dynamic regulatory process wherein homeostatic control is maintained by an active process of adaptation during exposure to physical and behavioral stressors. Allostatic load is defined as the wear and tear on brain and body resulting from allodynamic overactivity as well as dysregulation of the mediators of allostasis. Morello-Frosch and Shenassa14 posited that allostatic load interferes with normal functioning of protective toxicokinetic and toxicodynamic processes in ways that impair individual resilience and ability to recover from toxic insults; in other words, allostatic load confers some vulnerability to toxic exposures. How allostatic load may confer vulnerability to toxic exposures is poorly understood. Nevertheless, it is a fairly established concept in neuroscience, health psychology, and epidemiology, reflecting both co-occurring risk across multiple physiological systems and cumulation of such risk across time at the individual level after exposure to stressful circumstances.17,18 To advance knowledge of stress–toxicant interactions and, therefore, the subsequent consideration of stress in chemical risk assessment, the next generation of environmental health research should explore hypotheses on allostatic load using knowledge about established critical physiological pathways for stress-related health effects, and evidence of common target physiological systems and pathways for both toxicants and stress. With a metric that captures multiple impacted systems, the integration of psychosocial stress in environmental health research and risk assessment can focus less on pathway-specific interactions and more on overall physiological or organ system vulnerability. Overall, this macro level focus on stress-induced vulnerability advances the adoption of emerging concepts in chemical risk assessment, such as the use of distributions of background vulnerability in dose–response assessment1 to better inform risk-based decision-making.
In this article, these physiological pathways are discussed, concluding with suggestions for research to advance the consideration of stress in chemical risk assessment based on the concept of allostatic load. Specifically, this article (1) provides an overview of the neurobiology of stress in the context of pathways through which it contributes to adverse health effects, and with specific attention to the concept of allostatic load; and (2) highlights areas of cross-disciplinary research collaboration to advance knowledge about stress–toxicant interactions within the context of the potential applications of such research in chemical risk assessment.
PSYCHOSOCIAL STRESS AND CHEMICAL-RELATED HEALTH RISK
The experience of stress can vary considerably by race/ethnicity and income,19–21 and certain types of stress experiences such as poverty seem to be of longer duration through the life course and across generations for groups such as African Americans.22 Individuals that experience excessive stress in their lives, as measured by multiple periods of poverty level income, are associated with earlier aging, more depression, and an earlier decline of both physical and mental functioning.23 Timing of exposure to stress also may be an important consideration. Individuals who were abused as children experience an increased risk for depression, suicide, substance abuse, and earlier mortality and morbidity from a wide range of diseases.23–25 Neighborhood conditions may also influence stress,26,27 directly impact health,28,29 or modify the effects of stress on health.29 A more detailed discussion of this scholarship is beyond the scope of this article.
Psychosocial stress contributes to adverse physical health effects, including cardiovascular effects such as increased blood pressure and triggering of acute myocardial infarctions (MIs) and reversible cardiomyopathies,30–34 immune system effects such as inflammation,35 psychological and social effects such as increased postdisaster depression and anxiety disorders,36 and even premature death.37 These same physiological systems are adversely affected by exposure to chemical stressors. For example, particulate matter exposure is associated with cardiovascular morbidity and mortality,36–45 and exposure to lead has been linked to increased risk of blood pressure and hypertension.46–48
Psychosocial stress may interact with chemical stressors to modify risks of adverse health effects. Co-exposure to psychosocial stress and lead has been associated with impaired cognition7,49 and higher risk of hypertension50 in adults. Higher levels of chronic family stress have been associated with high inflammatory markers in asthmatic children at low levels of traffic-related pollution, leading investigators to hypothesize that the “role of chronic stress may be to lower the threshold at which physical exposures affect biological and clinical outcomes.”6 Increased susceptibility to the effects of traffic-related pollution and in utero tobacco exposure have been observed among children from chronically stressed households,8 and statistically significant positive associations have been reported between measures of noise disturbance at night and doctor-diagnosed asthma in female children.51
Research findings in animal studies lend further support to the concept of chemical exposure–stress interactions. Concurrent exposure of dams to both lead and stress produced a pattern of hypothalamic-pituitary-adrenal (HPA) axis dysfunction with slightly different effects in male and female offspring.9–11,52–55 Chronic stress caused by social conflict and defeat in rats enhanced the adverse respiratory effects of breathing air containing diesel exhaust and other fine particulates and irritants, and also potentiated white blood cell counts.56 Reduced fetal weight and increased fetal toxicity were observed after joint exposure to perfluorooctane sulfonate and stress in pregnant mice, relative to previously reported studies of perfluorooctane sulfonate exposure alone.57
CRITICAL PHYSIOLOGICAL PATHWAYS FOR STRESS-RELATED EFFECTS
There is cumulative evidence that disparities in income, education, occupation, and other dimensions of socioeconomic status (SES) account statistically for appreciable variance in all-cause and disease-specific morbidity and mortality rates, as well as the prevalence of risk factors for chronic medical conditions58–60 and prevalent psychopathologies of mood and substance abuse.61,62 That health and longevity track a socioeconomic gradient cannot be explained entirely by material deprivation, illiteracy, or restricted availability of quality health care among individuals of lower SES.59,63,64 Hence, several conceptual models of SES-related health disparities posited that additional life experiences inherent to socioeconomic position at the individual, familial, and community levels could influence well-being and disease risk through stress-related pathways.59,63,65,66 For example, the chronic experience of low SES at the individual level could involve a number of issues causing stress, including enduring financial hardships, a sense of insecurity regarding future prosperity, and the possible demoralizing feelings of marginalization or social exclusion attributable to comparative social, occupational, or material disadvantage. Further, an individual's negative perception of her or his relative standing or ranking in a social hierarchy, formally termed subjective social status, might affect an individual's pattern of emotional, behavioral, and physiological reactivity to and recovery from life stressors, consequently impacting risk for ill health.67–71
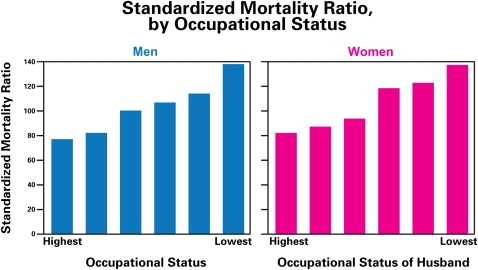
Low SES is associated with shorter lifespan and increased incidence of a variety of diseases (Figure 1).
FIGURE 1.
Inverse relationship between SES and mortality ratio in Whitehall Study.63,72,73
Note. SES = socioeconomic status. The standardized mortality ratio is the ratio of actual deaths to expected deaths. Note that there is an almost linear gradient with occupational status in the British Civil Service in which all persons have jobs and access to health care. The gradient indicates that there are aspects of income and education related to stress and lifestyle that are related to health and mortality.
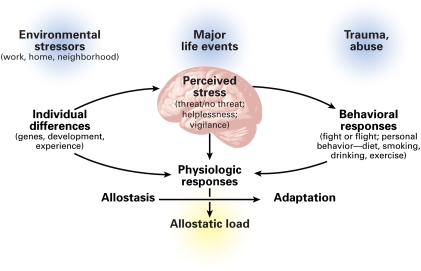
SES is thought to “get under the skin” via the brain (Figure 2), impacting its ability to regulate peripheral physiology, engage in adaptive social and health behaviors, experience and control emotions, and support cognitive functioning. Hence, a person who develops, matures, and ages in a household of low SES could become vulnerable to impairments in the functionality of stress regulatory systems of the brain and body, systems important for health.
FIGURE 2.
Critically, such stress-related processes may unfold not only at the individual level, but also at the level of families and residential areas. For example, children who develop in lower SES households, in addition to being exposed to toxic substances and excessive noise and temperature variations, are more likely to live in unfavorable housing conditions and to be exposed to what have been termed “risky family” dynamics.74 Such dynamics are characterized by conflict-laden relationships, aggressive and harsh parenting, and other forms of early life stress that may alter risk trajectories for ill health in later life.74 Finally, individuals living in low SES neighborhoods may be more frequently exposed to stressful life events75,76 in association with higher concerns over community crime, pollution, and crowding,77 as well as unstable, effortful, and unrewarding employment opportunities related to persistent economic hardship.78
As the key target organ for stress and the effects of inequality, deprivation, and discrimination18,72,78,79 (Figure 2), the brain not only processes inputs from the external environment, but it also controls adjustments of the body engendered by behavioral states like waking, sleeping, lying, standing, and exercising. These bodily adjustments promote adaptive activities, such as locomotion, and coping with aversive situations and discrete stimuli, such as noise, crowding, hunger, excessive heat or cold, and other threats to safety. Brain regions such as the hippocampus (memory), amygdala (fear, anxiety), and prefrontal cortex (decision-making, impulse, and mood control) are all affected by stress.79
The body has a set of mediators of stress and adaptation strategies that are activated by physical and psychological stressors and their interactions with each other. These mediators include not only hormones of the HPA axis, but also the sympathetic and parasympathetic nervous systems, and the pro- and anti-inflammatory cytokines. Each class of mediators regulates activity of other mediators and thus operates in a nonlinear network.80 This network of mediators affects all tissue and organ systems, including the brain.79 For most diseases, from diabetes to cardiovascular disease to cancer, inflammation is a key factor. Production of inflammatory mediators is stimulated by physical pollutants (e.g., from diesel exhaust), as well as by psychological stress.81,82 Thus, psychological and physical stressors could potentiate each other through common physiological pathways such as inflammation.79,81,83
To further understand these regulatory processes, the key concepts of allostasis and allostatic load that complement the concept of homeostasis are introduced. Although it is true that physiological parameters like blood oxygen and pH are maintained in a narrow range (homeostasis), the cardiovascular system, metabolic machinery, immune system, and central nervous system all show a large range of activity as a function of the time of day and in response to external and internal demands (allostasis). Allostatic load affects the brain and the body and promotes ill health, involving not only the consequences of stressful experiences themselves, but also the alterations in lifestyle that result from a state of chronic anxiety and stress (e.g., eating too much of the wrong things, smoking, drinking, and sleeping badly).
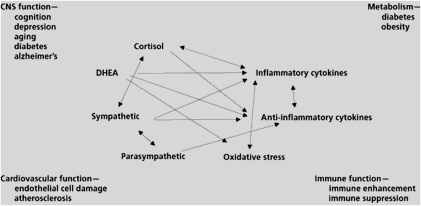
Systems of allostasis that promote adaptation include the HPA axis, the autonomic nervous system, the metabolic system (including the thyroid axis, insulin, and other metabolic hormones), the gut, the kidneys, and the immune system (including the regulated network of cytokine producing cells throughout the body). The biological mediators of these systems (e.g., cortisol, sympathetic and parasympathetic transmitters, cytokines, and metabolic hormones) operate as a nonlinear, interactive network to maintain allostasis (Figure 3), in which mediators down- and up-regulate each other, depending on such factors as concentration, location in the body, and sequential temporal patterning.80 Importantly, the activity of these mediating systems and mediators is closely coupled to psychological and genetic makeup, developmental history, and behavioral state of the individual.
FIGURE 3.
The nonlinearity of the mediators of stress and adaptation.80
Note. CNS = central nervous system; DHEA = dehydroepiandrosterone.
STRESS-RELATED DYSREGULATION AND ADVERSE OUTCOMES
Adversity, including interpersonal conflicts, social instability, and other stressful experiences, can accelerate pathophysiological processes through adaptive systems of the body, increasing vulnerability for higher morbidity and mortality rates at the population level. The cardiovascular system is one of the most susceptible systems to stress. For example, blood pressure can increase because of job stress in factory workers, particularly in employees with repetitive jobs and time pressures,84 and in British civil servants of departments undergoing privatization.85 As further evidence, the stressful social collapse after the fall of communism in Eastern Europe led to cardiovascular disease being a primary cause for the increased death rate.86 It is noteworthy that otherwise adaptive and brain-mediated stressor-evoked blood pressure surges have been linked to accelerated atherosclerosis,87 as well as increased risk for MI.88,89 Besides the adverse effects on the cardiovascular system, there are indications that metabolic disorders and abdominal obesity—contributors to cardiovascular disease—are increased at the stressful lower end of the socioeconomic gradient in Swedish men90 and in the British Civil Service63 (Figure 3). Finally, there is growing epidemiological evidence that impaired immune system function is also a likely target of stress processes within the context of socioeconomic position.2,91–96
Stress-related processes impacting health within the context of SES can be viewed and understood by appreciating the marked differences that individuals show in response to adverse acute and chronic stressors.97 In other words, individuals respond in different ways to adversity and threats (real or implied) to their safety and homeostasis. Mediators of allostasis, therefore, facilitate adaptation, whereas the parameters associated with homeostasis do not vary as a means of promoting adaptation. Importantly, variation in mediators associated with adaptation has long been appreciated, particularly beginning with the early work of Walter Cannon98 on the human body. Allostatic systems are involved in coping and adaptation, and generally, they are most useful when they can be rapidly mobilized and then shut off when not needed. It is when their activity is prolonged or not terminated promptly that these systems undermine health. Moreover, the inability to engage allostatic systems when needed also produces a load on the body, because the normal protection afforded by these systems is lacking.
An important aspect of allostasis is the notion of anticipation, which can add to allostatic load. Although originally introduced in relation to explaining the reflex that prevents us from blacking out when we get out of bed in the morning,99 anticipation also implies psychological states, such as apprehension, worry, and anxiety, as well as cognitive preparation for a coming event. Because anticipation can drive the output of allostatic biomediators (this is particularly true of hormones like adrenocorticotropic hormone, cortisol and adrenalin), it is likely that states of prolonged anxiety from anticipation can result in allostatic load.100
Other important aspects of individual responses in relation to allostasis and allostatic load are health damaging and health promoting behaviors, such as smoking, drinking, sleeping, eating a prudent diet, and regularly exercising, collectively called “lifestyle” behaviors. These may be embodied within the overall notion of allostasis (i.e., how individuals cope with a challenge) and they also contribute in some ways to allostatic load (e.g., a Western [high-fat] diet accelerates atherosclerosis and progression to type 2 diabetes; smoking accelerates atherogenesis; exercise and restorative sleep promote cognitive functioning and health).80
PHYSIOLOGICAL RESPONSES TO ALLOSTATIC LOAD
There are 4 types of physiological response that may contribute to and reflect allostatic load. The first type is related to frequent stressors, for example, blood pressure surges that not only trigger MI in susceptible individuals, but also accelerate atherosclerosis and prime the risk for MI when they are repeatedly expressed over the lifespan. Here, it is the frequency and intensity of the “hits” or events (e.g., high blood pressure surges) that determine the level of allostatic load engendered by this type. Frequent stress may lead to the other types of allostatic load described as the body responds to repeated events by either failing to terminate neural and endocrine responses or failing to respond adequately.
The second type of allostatic load is the failure to habituate to repetition of the same stressor, leading to a persistent elevation of mediators like cortisol. This was first described in a subset of individuals who failed to habituate their cortisol response in a repeated public speaking challenge.101 Later studies showed that such individuals had a low sense of self confidence, low self-esteem, and a smaller hippocampus, leading to stress-related behavioral and neurobiological processes.102,103
The third is the failure to terminate adaptive autonomic and neuroendocrine responses. Consider, for example, blood pressure elevations in repetitive, time pressured work104 and that chronic, elevated levels of glucocorticoids accelerate obesity and type 2 diabetes. Moreover, persistent glucocorticoid elevation and/or excitatory activity in brain systems that regulate glucocorticoid secretion cause dendritic remodeling and neuronal death in the hippocampus and in other limbic brain areas. When these conditions persist over months and years, chronic overactivity of cortisol as well as other mediators of stress and adaptation through allostasis (see Figure 2) leads to allostatic load and promotes cumulative changes that lead to disease.
The fourth type of allostatic load is the failure to respond adequately to a challenge. Consider, for example, autoimmunity and inflammation that are associated with inadequate endogenous glucocorticoid responses, as in the Lewis rat105 and possibly also in chronic fatigue syndrome and fibromyalgia.106,107 Here, other biomediators of allostatic systems, such as inflammatory cytokines, show elevated activity, and this elevation may increase allostatic load because of inadequate HPA regulation, which normally “constrains” the activity of these biomediators. Posttraumatic stress is a form of psychopathology; it is yet another example of how an acute, but traumatic event leads to dysregulated HPA axis activity that may not respond adequately to acute challenge and may therefore promote comorbid physical disease.108
Measures of allostatic load hold promise for identifying populations that are already vulnerable because of psychosocial stress, and also for conducting the necessary population studies to elucidate the interactions between chemical stressors and psychosocial stress. The measurement of allostatic load involves tests that are normally used in physical examinations. Up to 14 different measures are collected and a point is awarded when an individual's value in a given test is in an extreme quartile for the population under study. The total points determine the overall allostatic load score.109 As an example of how allostatic load scores are developed, the current allostatic load battery in the National Institute of Health's sponsored study called Coronary Artery Disease Development in Young Adults taps into autonomic nervous system, HPA, inflammatory, and acute phase measures as well as metabolic and cardiovascular parameters,110 as shown in Table 1.
TABLE 1.
Coronary Artery Risk Development in Young Adults Allostatic Load Measurement Battery110
| Measure (biological medium) | Assayed for (change in biological marker) |
| Urine (12 h overnight) | Norepinephrine, epinephrine, cortisol |
| Saliva (6/d to map circadian variation) | Cortisol |
| Blood | Total and HDL cholesterol, glycosylated hemoglobin, IL-6, CRP, fibrinogen |
| Other measures | Waist/hip ratio, systolic and diastolic blood pressure, heart rate variability |
Note. CRP = C-reactive protein; HDL = high-density lipoproteins; IL = interleukin.
The allostatic load score has been very useful in predicting mortality over 7 years in the MacArthur Successful Aging study; it has also been useful in predicting decline of physical and cognitive functioning.109 High allostatic load is also related to having few social ties and being isolated.111 Racial differences are found in allostatic load scores between Black and White men and women, with individual biological markers showing different importance between Blacks and Whites and between men and women.112 The racial differences may reflect, in part, effects of discrimination.110
DISCUSSION
From a neurobiological viewpoint, it is most important to recognize that there is a response network for stress—the network of allostasis that responds to psychological stressors—generated through the brain, the central organ of stress and adaptation. This network, or at least parts of it, respond to toxic agents (e.g., air pollution leads to inflammation, which, in turn, activates cortisol responses). Lead exposure may do the same; it certainly seems to alter cortisol113 and is probably proinflammatory as are most toxicants (including radiation). Both cortisol and parasympathetic activity “attempt” to contain the inflammation, but sympathetic activity related to acute stress, sleep deprivation, and other stress enhances inflammation. Imbalances in the network because of chronic psychological stress and lifestyle (e.g., poor sleep, excess calories and obesity/diabetes, alcohol) cause the network to respond differently to toxic agents, and evidence so far (which is in need of more documentation) indicates that there is synergy and enhancement of, for example, the inflammatory response and further imbalance in the network. Over time, the imbalance in the network leads to allostatic load/overload that accelerates disease processes.
These points lead to the general conclusion that one cannot study toxic agents in a vacuum without considering psychological stressors and their impact on body physiology. Several opportunities exist to advance current knowledge of chemical–stress interactions in ways that are useful to chemical risk managers. For example, more data on differential dose–response relationships as a function of psychosocial stress levels can inform the selection of regulatory options to limit the concentration of chemicals in ambient media. This type of information can be generated through population studies in which toxic effect modification, because of allostatic load as a measure of the cumulative impact of stress, is a key hypothesis. This field of research can be advanced in present time and with limited resources using exploratory cross-sectional studies to investigate how allostatic load may change known relationships between exposure to environmental contaminants and adverse health outcomes, and using existing databases such as the National Health and Nutrition Examination Survey. The use of National Health and Nutrition Examination Survey data for allostatic load considerations has been illustrated by several authors.112,114–116 This type of research can provide the basis for more in-depth confirmatory population studies. Also, knowledge that allostatic load confers enhanced vulnerability to chemical exposures can lead to the development of distributions of baseline vulnerability because of psychosocial stress using allostatic load as the measure. Data on baseline vulnerability can also be applied in dose–response assessments in risk assessment.1
Given that allostatic load requires measurement of biological parameters in the population, its wide deployment in policymaking may be limited by lack of data. For this reason, we advocate cross-disciplinary research mostly between the social and biological sciences to identify community level characteristics that correlate with and accurately predict allostatic load. As mentioned in the section Critical Physiological Pathways for Stress-Related Health Effects, SES may be a useful proxy measure of allostatic load because there is greater vulnerability to illness in lower SES populations. Additionally, there is evidence that children exposed to low levels of environmental toxins in higher SES, more well educated households may not experience the same health effects as children in more risky, adverse households.117 The protective effects of a less adverse childhood on child development after toxic exposure is an area that could be explored. Community level data are easier to collect through the census and local scale surveys. These data are a potentially useful layer in screening and targeting tools designed to identify vulnerable or environmental justice communities. Several of these tools have already been developed or are being developed by environmental protection agencies and researchers.118–121 Also, the ability to identify vulnerable communities or places can advance the evaluation of the impacts of regulatory policy options on these populations, and therefore aid the selection of protective regulatory policy.
How stress from fear of exposure to environmental hazards changes the allostatic load of populations living within the vicinity of sources or exposed to hazards is an unexplored yet important area of research. The prevalence and concentration of sources of environmental hazards are more common in racial minority and low income neighborhoods.121,122 Populations that are chronically faced with these “technological disasters,” which include contamination sites, are more likely to experience higher levels of chronic psychosocial stress.26 Coping with such stress has to deal with threat perception, which is greater in racial and ethnic minorities in the United States123; minorities may experience a greater threat level because of issues such as greater proximity to location of a perceived threat, attachment to that location, and economic ties to the industry involved in the technological disaster.26 Given that certain racial/ethnic minorities tend to have higher baseline allostatic load,111 the effect of incremental psychosocial stress triggered by proximity to environmental pollution may mean that these groups are in the extreme right of the allostatic load distribution. Atypically high levels of allostatic load may not be captured in general population surveys. Therefore, the extent to which conditions of unusually high allostatic load impact health may not be adequately reflected in studies that use general population surveys. Assuming that the presence of sources of environmental hazards consistently predicts very high allostatic load, such a community vulnerability characteristic can serve as a useful layer in the types of risk screening tools alluded to in the previous paragraph.
Mechanistic studies are also necessary to advance understanding of chemical–psychosocial stress interactions in ways that can directly inform chemical risk management. One reasonable model would be to evaluate how psychosocial stress, measured by allostatic load, independently affects key biomarkers of the effects of a specific chemical. This research can inform assumptions that feed into the dose–response assessment for that chemical. With information from this type of study, risk assessors would have access to ample evidence to apply the concept of differential vulnerability to the process of identifying an exposure level that is without appreciable risk of harm. Finally, the nature of the interactions between various chemicals and stress is not fully elucidated and merits additional research in both the fields of epidemiology and toxicology.
Conclusions
In summary, bringing together the worlds of neurobiology, social sciences, epidemiology, and toxicology is the next frontier in terms of generating the necessary data to support suggested theoretical frameworks for considering psychosocial stress in risk assessment or management aimed at addressing environmental health disparities. Some of this work is already in progress in the form of emerging epidemiology and toxicology studies that explore the interactions between psychosocial stress and exposure to chemical stressors. We envision that the new directions proposed herein will lead to an influx of readily applicable data in a 3 to 5 year period. Specifically, we anticipate the generation of several types of pertinent data to advance the field, such as modified dose–response curves as a function of allostatic load and/or external measures of stress such as psychosocial hazards at the community level. Given that risk assessments do not currently account for effects of psychosocial stress in addition to toxic exposures that people experience in their communities, the inclusion of such data are likely to produce risk management decisions that are more informed and more health protective.
Finally, we recognize the need for more cross-disciplinary training and interaction for regulators, risk assessors, public health scientists, and other types of scientists whose research is obviously relevant to environmental health protection. This requires paradigm shifts in how training is structured in institutions of higher learning, and more importantly highlights the need for regulatory agencies to increase their technical capacity in areas originally considered nontraditional, such as the social sciences.
Acknowledgments
This article was developed with support provided by the US Environmental Protection Agency's Office of Environmental Justice under GSA contract no GS-00F-0001S, Task Order No. 16.
The authors thank Onyemaechi Nweke of the US EPA and Michael A. Callahan of MDB, Inc. for their comments and editorial support.
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the Agency for Toxic Substances and Disease Registry.
Human Participant Protection
This article did not involve any research involving human subjects; institutional review board approval was not required.
References
- 1.NRC (National Research Council) Science and Decisions: Advancing Risk Assessment. Committee on Improving Risk Analysis Approaches Used by the U.S. EPA, National Research Council. Washington, DC: National Academies Press; 2009 [PubMed] [Google Scholar]
- 2.Chen E, Hanson MD, Paterson LQ, et al. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J Allergy Clin Immunol. 2006;117(5):1014–1020 [DOI] [PubMed] [Google Scholar]
- 3.Augustin T, Glass TA, James BD, Schwartz BS. Neighborhood psychosocial hazards and cardiovascular disease: the Baltimore Memory Study. Am J Public Health. 2008;98:1664–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf JM, Miller GE, Chen E. Parent psychological states predict changes in inflammatory markers in children with asthma and healthy children. Brain Behav Immun. 2008;22:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosom Med. 2009;71:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen E, Schreier HMC, Strunk RE, Brauer M. Chronic traffic related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008;116(7):970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass TA, Bandeen-Roche K, McAtee M, et al. Neighborhood psychosocial hazards and the association of cumulative lead dose with cognitive function in older adults. Am J Epidemiol. 2009;169:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankardass K, McConnell R, Jerrett M, et al. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A. 2009;106(30):12406–12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, et al. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112(6):717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virgolini MB, Rossi-George A, Lisek R, et al. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29(5):812–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal PB exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology. 2008;29:928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environ Health Perspect. 2005;113:1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132:757S–769S [DOI] [PubMed] [Google Scholar]
- 16.Clougherty JE, Kubzansky LD. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ Health Perspect. 2009;117:1351–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer BH, Ryff CD. New Horizons in Health: An Integrative Approach. Committee on Future Directions for Behavioral and Social Sciences Research at the National Institutes of Health, Board on Behavioral, Cognitive and Sensory Sciences. Washington, DC: National Research Council; 2001 [Google Scholar]
- 18.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179 [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, Schwartz JE, Epel E, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2006;68:41–50 [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeman T, Epel E, Gruenewald T, et al. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–239 [DOI] [PubMed] [Google Scholar]
- 22.Wagmiller RL, Adelman RM. Childhood Poverty and Intergenerational Poverty: The Long-Term Consequences of Growing Up Poor. New York: National Center for Children in Poverty, Mailman School of Public Health, Columbia University; 2009 [Google Scholar]
- 23.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124 [DOI] [PubMed] [Google Scholar]
- 24.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258 [DOI] [PubMed] [Google Scholar]
- 25.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA. 2009;301:2252–2259 [DOI] [PubMed] [Google Scholar]
- 26.Couch SR, Coles CJ. Community stress, psychosocial hazards and EPA decision-making in communities impacted by chronic technical disasters. Am J Public Health. 2011; this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med. 2001;23:177–185 [DOI] [PubMed] [Google Scholar]
- 28.Boardman JD. Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Soc Sci Med. 2004;58:2473–2483 [DOI] [PubMed] [Google Scholar]
- 29.Matthews SA, Yang TC. Exploring the role of the built and social neighborhood environment in moderating stress and health. Ann Behav Med. 2010;39:170–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum A, Fleming I. Implications of psychological research on stress and technological accidents. Am Psychol. 1993;48(6):665–672 [DOI] [PubMed] [Google Scholar]
- 31.Baum A, Fleming I, Israel A, O'Keefe MK. Symptoms of chronic stress following a natural disaster and discovery of a human-made hazard. Environ Behav. 1992;24(3):347–365 [Google Scholar]
- 32.Baum A, Gatchel RJ, Schaeffer MA. Emotional, behavioral, and physiological effects of chronic stress at Three Mile Island. J Consult Clin Psychol. 1983;54(4):565–572 [DOI] [PubMed] [Google Scholar]
- 33.Lipsky SI, Pickering TG, Gerin E. World Trade Center disaster effect on blood pressure. Blood Press Monit. 2002;7:249. [DOI] [PubMed] [Google Scholar]
- 34.Sharkey SW, Lesser JR, Zenowich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479 [DOI] [PubMed] [Google Scholar]
- 35.Pace TW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633 [DOI] [PubMed] [Google Scholar]
- 36.Norris FH, Friedman MJ, Watson PJ. 60,000 disaster victims speak: part II. Summary and implications of the disaster mental health research. Psychiatry. 2002;65(3):240–260 [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687 [DOI] [PubMed] [Google Scholar]
- 38.Braga AL, Zanobetti A, Schwartz J. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J Occup Environ Med. 2001;43:927–933 [DOI] [PubMed] [Google Scholar]
- 39.D'Ippoliti D, Forastiere F, Ancona C, et al. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology. 2003;14:528–535 [DOI] [PubMed] [Google Scholar]
- 40.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanki T, Pekkanen J, Aalto P, et al. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: the HEAPSS study. Occup Environ Med. 2006;63:844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759 [DOI] [PubMed] [Google Scholar]
- 43.Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Perspect. 2001;109(Suppl 4):483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117:898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.USEPA Integrated Science Assessment for Particulate Matter. Research Triangle Park, NC: National Center for Environmental Assessment. US Environmental Protection Agency, EPA/600/R-08/139F; 2009 [Google Scholar]
- 46.Nash D, Magder L, Lustberg M, et al. Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA. 2003;289:1523–1532 [DOI] [PubMed] [Google Scholar]
- 47.Martin D, Glass TA, Bandeen-Roche K, et al. Association of blood lead and tibia lead with blood pressure and hypertension in a community sample of older adults. Am J Epidemiol. 2006;163:467–478 [DOI] [PubMed] [Google Scholar]
- 48.Navas-Acien A, Schwartz BS, Rothenberg SJ, et al. Bone lead levels and blood pressure endpoints: a meta-analysis. Epidemiology. 2008;19:496–504 [DOI] [PubMed] [Google Scholar]
- 49.Peters JL, Weisskopf MG, Spiro A, et al. Interaction of stress, lead burden, and age on cognition in older men: the VA Normative Aging study. Environ Health Perspect. 2010;118(4):505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters JL, Kubzansky L, McNeely E, et al. Stress as a potential modifier of the impact of lead levels on blood pressure: The Normative Aging Study. Environ Health Perspect. 2007;115:1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bockelbrink A, Willich SN, Dirzus I, et al. Environmental noise and asthma in children: sex-specific differences. J Asthma. 2008;45:770–773 [DOI] [PubMed] [Google Scholar]
- 52.Cory-Slechta DA, Virgolini MB, Rossi-George A, et al. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227 [DOI] [PubMed] [Google Scholar]
- 53.Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA. Alternations in glucocorticoid feedback following maternal PB, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicol Appl Pharmacol. 2009;234:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virgolini MB, Chen K, Weston DD, et al. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87(2):469–482 [DOI] [PubMed] [Google Scholar]
- 55.Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicology. 2006;27:11–21 [DOI] [PubMed] [Google Scholar]
- 56.Clougherty JE, Rossi CA, Lawrence J, et al. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ Health Perspect. 2010;118(6):769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuentes S, Colomina MT, Rodriguez J, Vicens P, Domingo JL. Interactions in developmental toxicology: concurrent exposure to perfluorooctane sulfonate (PFOS) and stress in pregnant mice. Toxicol Lett. 2006;164:81–89 [DOI] [PubMed] [Google Scholar]
- 58.Adler NE, Boyce WT, Chesney MA, et al. Socioeconomic inequalities in health. No easy solution. JAMA. 1993;269:3140–3145 [PubMed] [Google Scholar]
- 59.Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health: the challenge of the gradient. Am Psychol. 1994;49:15–24 [DOI] [PubMed] [Google Scholar]
- 60.Adler NE, Marmot M, McEwen B, Stewart J, Socioeconomic Status and Health in Industrialized Nations: Social, Psychological, and Biological Pathways. Vol 896 New York: Academy of Sciences; 1999 [Google Scholar]
- 61.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19 [DOI] [PubMed] [Google Scholar]
- 62.Lorant V, Deliege D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157:98–112 [DOI] [PubMed] [Google Scholar]
- 63.Marmot M. The Status Syndrome: How Social Standing Affects Our Health and Longevity. New York: Henry Holt and Company; 2004 [Google Scholar]
- 64.Sapolsky R. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418 [Google Scholar]
- 65.Dohrenwend BP. The role of adversity and stress in psychopathology: some evidence and its implications for theory and research. J Health Soc Behav. 2000;41:1–19 [PubMed] [Google Scholar]
- 66.Kelly S, Hertzman C, Daniels M. Searching for the biological pathways between stress and health. Annu Rev Public Health. 1997;18:437–462 [DOI] [PubMed] [Google Scholar]
- 67.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592 [DOI] [PubMed] [Google Scholar]
- 68.Goodman E, Adler NE, Daniels SR, et al. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obes Res. 2003;11:1018–1026 [DOI] [PubMed] [Google Scholar]
- 69.Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychol. 2000;19:613–618 [DOI] [PubMed] [Google Scholar]
- 70.Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc Sci Med. 2003;56:1321–1333 [DOI] [PubMed] [Google Scholar]
- 71.Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–861 [DOI] [PubMed] [Google Scholar]
- 72.McEwen BS, Gianaroe PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. : Adler N, Steward J, The Biology of Disadvantage: Socioeconomic Status and Health (Annals of the New York Academy of Sciences). Hoboken, NJ: Wiley-Blackwell: 2010:190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adler N, Stewart J, Cohen S, et al. Reaching for a Healthier Life: Facts on Socioeconomic Status and Health in the U.S. Chicago, IL: The John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status and Health; 2007 [Google Scholar]
- 74.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366 [PubMed] [Google Scholar]
- 75.Dohrenwend BS. Social status and stressful life events. J Pers Soc Psychol. 1973;28:225–235 [DOI] [PubMed] [Google Scholar]
- 76.McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. J Health Soc Behav. 1990;31:162–172 [PubMed] [Google Scholar]
- 77.Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. J Health Soc Behav. 1996;37(4):293–310 [PubMed] [Google Scholar]
- 78.Phelan JC, Link BG, Diez-Roux A, et al. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav. 2004;45:265–285 [DOI] [PubMed] [Google Scholar]
- 79.McEwen BS. The physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904 [DOI] [PubMed] [Google Scholar]
- 80.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosc. 2006;8(4):367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bierhaus A, Wolf J, Andrassy M, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saxon A, Diaz-Sanchez D. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology. 2000;48:325–327 [DOI] [PubMed] [Google Scholar]
- 83.Bierhaus A, Humpert PM, Nawroth PP. Linking stress to inflammation. Anesthesiol Clin North Am. 2006;24:325–340 [DOI] [PubMed] [Google Scholar]
- 84.Melin B, Lundberg U, Soderlund J, Granqvist M. Psychological and physiological stress reactions of male and female assembly workers: a comparison between two different forms of work organization. J Organ Behav. 1999;20:47–61 [Google Scholar]
- 85.Ferrie JE, Shipley MJ, Stansfeld SA, Marmot MG. Effects of chronic job insecurity and change in job security on self reported health, minor psychiatric morbidity, physiological measures, and health related behaviours in British civil servants: the Whitehall II study. J Epidemiol Community Health. 2002;56:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bobak M, Marmot M. East-West mortality divide and its potential explanations: proposed research agenda. BMJ. 1996;312:421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaplan J, Pettersson K, Manuck S, Olsson G. Role of sympathoadrenal medullary activation in the initiation and progression of atherosclerosis. Circulation. 1991;84(Suppl 6):V123–V132 [PubMed] [Google Scholar]
- 88.Muller J, Tofler G, Edelman E. Probable triggers of onset of acute myocardial infarction. Clin Cardiol. 1989;12:473–475 [DOI] [PubMed] [Google Scholar]
- 89.Muller JE, Tofler G, Stone P. Circadian variation and triggers of onset of acute caridovascular disease. Circulation. 1989;79:733–743 [DOI] [PubMed] [Google Scholar]
- 90.Larsson B, Seidell J, Svärdsudd K, et al. Obesity, adipose tissue distribution and health in men—The Study of Men Born in 1913. Appetite. 1989;13:37–44 [DOI] [PubMed] [Google Scholar]
- 91.Cohen S. Keynote Presentation at the Eighth International Congress of Behavioral Medicine: the Pittsburgh common cold studies: psychosocial predictors of susceptibility to respiratory infectious illness. Int J Behav Med. 2005;12(3):123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen S, Doyle WJ, Turner RB, et al. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66(4):553–558 [DOI] [PubMed] [Google Scholar]
- 93.Cohen S, Alper CM, Doyle WJ, et al. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–274 [DOI] [PubMed] [Google Scholar]
- 94.Dowd JB, Goldman N. Do biomarkers of stress mediate the relation between socioeconomic status and health? J Epidemiol Community Health. 2006;60(7):633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanson MD, Chen E. Socioeconomic status, race, and body mass index: the mediating role of physical activity and sedentary behaviors during adolescence. J Pediatr Psychol. 2007;32(3):250–259 [DOI] [PubMed] [Google Scholar]
- 96.Owen N, Poulton T, Hay FC, et al. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17:286–295 [DOI] [PubMed] [Google Scholar]
- 97.Seeman T, Epel E, Gruenewald T, et al. Socio-economic differentials in peripheral biology: cumulative allostatic load. : Adler N, Stewart J, The Biology of Disadvantage: Socioeconomic Status and Health (Annals of the New York Academy of Sciences). Hoboken, NJ; Wiley-Blackwell; 2010:223–239 [DOI] [PubMed] [Google Scholar]
- 98.Cannon WB. The Wisdom of the Body. New York: WW Norton & Company; 1932 [Google Scholar]
- 99.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. : Fisher S, Reason J, Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988:629–649 [Google Scholar]
- 100.Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev. 1994;18:385–396 [DOI] [PubMed] [Google Scholar]
- 101.Kirschbaum C, Prussner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–474 [DOI] [PubMed] [Google Scholar]
- 102.Pruessner JC, Baldwin MW, Dedovic K, et al. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28:815–826 [DOI] [PubMed] [Google Scholar]
- 103.Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Pers Individ Dif. 1999;27:477–489 [Google Scholar]
- 104.Lundberg U, Granqvist M, Hansson T, et al. Psychological and physiological stress responses during repetitive work at an assembly line. Available at: http://www.tandfonline.com/doi/abs/10.1080/02678378908256940#preview. Accessed September 15, 2010
- 105.Sternberg EM, Young WS, III, Bernardini R, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989;86:4771–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crofford LJ, Pillemer SR, Kalogeras K, et al. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37:1583–1592 [DOI] [PubMed] [Google Scholar]
- 107.Griep EN, Boersma JW, De Kloet ER. Altered reactivity of the hypothalamic-pituitary-adrenal axis in the primary fibromyalgia syndrome. J Rheumatol. 1993;20:469–474 [PubMed] [Google Scholar]
- 108.Yehuda R, Giller E, Southwick S, et al. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry. 1991;30:1031–1048 [DOI] [PubMed] [Google Scholar]
- 109.Seeman TE, Crimmins E, Huang M-H, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997 [DOI] [PubMed] [Google Scholar]
- 110.Seeman T, Gruenewald T, Karlamangla A, et al. Modeling multisystem biological risk in young adults: the Coronary Artery Risk development in Young Adults Study. Am J Hum Biol. 2010;22:463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seeman TE, Singer BH, Ryff CD, et al. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64:395–406 [DOI] [PubMed] [Google Scholar]
- 112.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gump BB, Stewart P, Reihman J, et al. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environ Health Perspect. 2008;116:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crimmins EM, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol. 2003;38:731–734 [DOI] [PubMed] [Google Scholar]
- 115.Allsworth JE, Weitzen S, Boardman LA. Early age at menarche and allostatic load: data from the third National Health and Nutrition Examination Survey. Ann Epidemiol. 2005;15:438–444 [DOI] [PubMed] [Google Scholar]
- 116.Nelson KM, Reiber G, Kohler T, Boyko EJ. Peripheral arterial disease in a multiethnic national sample: the role of conventional risk factors and allostatic load. Ethn Dis. 2007;17:669–675 [PubMed] [Google Scholar]
- 117.Vreugdenhil HJ, Lanting CI, Mulder PGH, et al. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr. 2002;140:48–56 [DOI] [PubMed] [Google Scholar]
- 118.Alexeef G, Faust J, August LM, et al. Cumulative Impacts: Building a Scientific Foundation. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; 2010. Available at: http://www.oehha.ca.gov/ej/pdf/CIReport123110.pdf. Accessed September 15, 2010
- 119.Zartarian VG, Schultz BD, Barzyk TM, et al. C-FERST: Community-focused exposure and risk screening tool: questions and answers. Available at: http://www.epa.gov/heasd/c-ferst/C-FERST_Qs%26As_1-5-2011.pdf. Accessed July 13, 2011 [DOI] [PMC free article] [PubMed]
- 120.Su JG, Morello-Frosch R, Jesdale BM, Kyle AD, Shamasunder B, Jerrett M. An index for assessing demographic inequalities in cumulative environmental hazards with application to Los Angeles, California. Environ Sci Technol. 2009;43:7626–7634 [DOI] [PubMed] [Google Scholar]
- 121.United Church of Christ Toxic Waste and Race in the United States: A National Report on the Racial and Socio-Economic Characteristics of Communities with Hazardous Waste Sites. New York, NY: United Church of Christ Commission for Racial Justice; 1987 [Google Scholar]
- 122.Bullard RD. Dumping in Dixie: Race, Class, and Environmental Quality. Boulder, CO: Westview Press; 1990 [Google Scholar]
- 123.Brown P. Race, class, and environmental health: a review and systematization of the literature. Environ Res. 1995;69:15–30 [DOI] [PubMed] [Google Scholar]