Abstract
Risk assessment determines pathways, and exposures that lead to poor health. For exposures that fall disproportionately on urban low-income communities, minorities, and Native Americans, these pathways are often more common than in the general population.
Although risk assessors often evaluate these pathways on an ad hoc basis, a more formal way of addressing these nonstandard pathways is needed to adequately inform public health policy.
A conceptual model is presented for evaluating nonstandard, unique, or excessive exposures, particularly for environmental justice communities that have an exposure matrix of inhalation, dermal, ingestion, and injection. Risk assessment can be improved by including nonstandard and unique exposure pathways as described in this conceptual model.
Environmental hazards, exposures, and risks are not uniformly distributed across populations, and multiple biological and social factors, including age, poverty, and minority status, intersect to create unique exposures that place some individuals at disproportionately high risk of environmental disease. The interplay of poverty, race/ethnicity, life stages, and health (including pregnancy status) is pervasive, complex, and well documented.1–11 However, it is easier to find data on disparities in health outcomes12 than on the environmental exposure disparities associated with health disparities.
Many circumstances expose individuals to hazardous substances or conditions at levels above those accounted for in standard risk assessment paradigms.13 These high-end exposures (above 95th or 99th percentile) to common agents or unique pathways are usually not encountered in the general population. Some groups have nonstandard exposures because of where and how they live. For some groups, uniqueness lies in the multiplicity of exposure pathways, mixed exposures, or the interplay of cultural–psychosocial and economic factors with toxicants.14 The importance of exposure to multiple stressors has been recognized for 30 years but remains difficult to study, although study of the interplay of nonchemical stressors and chemical toxicology is developing rapidly.15 Conventional methods of risk assessment often exclude outliers or log-transform them into submission; these methods must be supplemented with identification of unique pathways.
We propose a conceptual framework for identifying important but unique pathways that risk assessors, public health personnel, and the public can use to adequately incorporate the exposures of minority, low-income, and tribal population groups in risk and health assessments, and to examine how these exposures contribute to health disparities. Although measuring chemicals in human tissues is the gold standard for assessing exposure,16 examining risk factors, nonstandard vulnerabilities, unique pathways, and behaviors that lead to excessive exposures is also critical. Exposure assessment is becoming increasingly complex, from consideration of single exposure pathways with single contaminants, to complex evaluations of cumulative chemical exposures occurring in different environmental settings.17,18 We examined exposure sources that result in disproportionate environmental burdens on low-income populations, minorities, children, Native Americans, and Native Alaskans, and combinations thereof.
References were identified through Medline using the keywords environmental justice, vulnerable populations, and exposure pathways, as well as through table of contents searches of environmental exposure, risk, and public health journals, and US Environmental Protection Agency (EPA) documents.19 We also searched for exposure pathways and specific environmental justice communities (those exposed disproportionately), such as Native Americans, low-income populations, and minorities. After identifying specific exposure pathways leading to high exposure, we searched for key words such as fish and wildlife consumption, cosmetics, and Asian medicine. Our synthesis was based on literature review, study of environmental health and exposure for 35 years, and constructing an exposure route and pathway model to synthesize possible pathways for unique exposures.
UNIQUE EXPOSURES AND INJUSTICE
Disproportionate environmental health risks, impacts, and burdens refer to findings that some populations systematically experience higher levels of exposure and risk than the general population.20–25 These disparities in exposure and risk may be related to race/ethnicity, age, sex, nutrition, exercise, and residence, which influence the probability of remaining healthy or becoming sick. However, the common denominator for environmental justice communities seems to be low income. For example, air pollution levels in Washington, DC, are higher in poorer areas where African American populations live,26 a situation that also exists in Chicago, Illinois; Denver, Colorado; Los Angeles, California; San Francisco California; and New York, New York.27 A broad geographic examination revealed a greater risk of accidents from chemical facilities in heavily populated African American counties than in others.28 Similarly, eating self-caught fish often led to disproportionate exposures of minorities29,30 or Native Americans.29–37 In the United Kingdom, minority communities were disproportionately located near nuclear plants.38 Notably, however, recent research suggested that when income was removed from environmental inequality, ethnicity remained an important variable.39,40
Multiple factors, including cultural, social, religious, psychosocial, economic, physical, and chemical and biological determinants, contribute to disproportionately high and adverse exposures or impacts. Children often bear the burden of environmental risk disparities.41–43 Minority status and youth, as well as other categories (language, underemployment), intersect with low income to create vulnerability. These population-level disparities in exposures, risks, and health impacts may be attributable to various combinations of inequities related to harmful exposures, the ability to withstand or mitigate harm, and limited access to preventive or therapeutic actions.
Not all populations that experience unique exposure pathways are traditionally identified as “environmental justice” groups; other groups also experience disproportionate occupational exposures by working in undocumented, nonunionized, and nontraditional sectors. Although harmful exposure avoidance can reduce risk, avoidance may be difficult for certain occupational, cultural, religious, or superstitious practices, as well as food consumption.44
Risk and Exposure Assessments
Data on unusual pathways are essential to address questions regarding environmental justice and unique exposures that form the basis for exposure assessment, risk assessment,45–48 risk management,49 reparations,50–52 cultural or religious exposures, and interactions of culture and ecology.33,53 Risk assessment continually evolves to improve toxicology, exposure assessment, and quantitative approaches,54 with increasing attention to mixtures and cumulative exposures.55
Risk arises at the intersection of vulnerability and susceptibility compounded by lack of information and inadequate access to health care. “Vulnerability” refers to heightened opportunities for hazardous exposure. “Susceptibility” refers to intrinsic individual factors that render some people more likely to get ill from exposure. As noted, “place makes the poison” as much as dose does.56
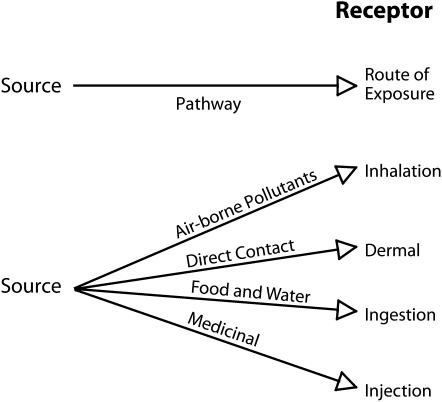
An exposure pathway, that is the route a substance takes from source to endpoint and how people get exposed, has 5 parts: (1) a source of contamination, (2) an environmental media and transport mechanism, (3) a point of exposure, (4) a route of exposure, and (5) a receptor population.57 Many toxic substances have established or typical exposure pathways. An exposure pathway becomes nonstandard when it differs from a known norm in any of the 5 parts. Standard pathways of exposure are well adopted by risk assessors and managers, and are incorporated into EPA risk assessment paradigms.48,58–60 Atypical or nonstandard pathways of exposure are of interest in exposure assessments for minority, low-income, and tribal populations.61
Nonstandard exposure pathways occur under 4 circumstances: (1) qualitatively nonstandard exposures (e.g., dietary, medicinal, or cosmetic use of unusual plants), (2) quantitatively nonstandard exposure (i.e., high consumption rates, children eating dirt, a very large meal [feast of fish], high exposure relative to other foods, body size, or age), (3) both nonstandard and excessive exposure (i.e., applying a chemical or cosmetic to skin, potential exposure to chemicals through cultural activities such as sweat baths), and (4) inadvertent exposure as byproducts of other consumptive, social, or cultural practices (i.e., mercury exposure from cultural practices). Each circumstance can be addressed by risk managers. For example, high mercury exposure from fish can be reduced by reducing mercury in fish (source reduction), preventing exposure through laws, regulations, or commerce restrictions, and preventing exposure by risk communication to the public.
The EPA method of examining exposure includes examining central tendencies (means, medians), high-end exposure (e.g., 95th percentile), and maximally exposed individuals.62 Probabilistic risk assessments use distribution parameters and techniques, such as Monte Carlo simulation, to estimate risks.63,64 However, necessary exposure distribution data may be impossible to obtain for small or isolated populations, and for environmental justice communities where assessment is difficult because of distrust or access. Although some studies have dealt with inequities in health assessments and health outcomes,65,66 particularly for minorities and Native Americans,67–69 less explicit attention has been given to nonstandard, excessive, and unique exposure pathways that may underlie health disparities. High exposures (above 99th percentile) are not well addressed in conventional risk assessments. Unique exposure pathways that are atypical for most communities are often unrecognized in the risk assessment process. Some of these unique exposures are inadvertently omitted in the course of standard risk assessments because they are not addressed by current default values or guidelines.
Temporal and Spatial Patterns of Exposure Pathways
Exposures may occur once, multiple times, or continually; be of short or long duration; and show short or long latencies to response. Exposures may occur during a critical developmental period. Receptor activity and locations modify the timing of exposure. Fetal and neonatal exposures may be a basis of childhood or adult diseases70; for example, prenatal exposure to polychlorinated biphenyls resulted in later cognitive impairment.70,71 Disproportionate exposure related to race, color, national origin, or income during gestation, infancy, or childhood could predispose people to disproportionate diseases later in life, and the cause of these diseases might not be ascribed to environmental justice issues because of geographic or socioeconomic mobility.
Spatial issues include identification of disproportionately exposed populations who are not colocated. People who face environmental inequities may be identified in national exposure databases, but may not be located in discrete spatial communities. Such databases might identify Hispanics, Native Americans, Blacks, or others who face a disproportionate adverse health outcome, but unless they live in a community that is spatially identified, it is difficult to address common exposures utilizing conventional risk assessment approaches. For example, if 1000 individuals are dispersed within a larger group of 10 000, they cannot be easily identified or addressed, in contrast to a community of 1000 people in a contiguous area with a disproportionate burden from environmental exposures.
Broad-scale surveys, site specific surveys, and national databases are beneficial and can be used to identify environmental inequities among minorities, ethnic groups, and Native Americans that are not spatially related. For example, the National Health and Nutrition Examination Survey (NHANES) showed that 17% of women who self-identified as Asian, Pacific Islander, Native American, or multiracial had blood mercury levels over 5.8 micrograms per liter, compared with 5% for others in the survey.72 These elevated mercury levels were attributed to high levels of fish consumption.72 Similarly, using NHANES data, it was reported that Asian women had higher blood mercury than did others in the data set.73 These data point toward a common cultural exposure pathway that should be investigated.
CONCEPTUAL FRAMEWORK FOR UNIQUE EXPOSURE PATHWAYS
Building conceptual models to understand and encompass unique exposures is critical for the EPA and other organizations for risk assessment to become more realistic. An overarching framework involves (1) understanding how traditional assessment pathways and routes of exposure intersect unique or nonstandard pathways, (2) identifying nonstandard but important pathways and routes of exposure, and (3) diagramming unique exposure pathways that might occur, whether in environmental justice communities or others with nonstandard or high exposures.
The value of conceptual frameworks (or exposure trees) identifies important exposure pathways that might not be immediately obvious. Building on the traditional routes of exposure (Table 1), we developed a conceptual model for examining unique and nonstandard exposure pathways that have particular relevance for environmental justice communities, as well as others. Although many of these concepts were included in an EPA document,61 they are not readily available to public health professionals, risk communicators, advocacy groups, or the general public. Our model provides a checklist to examine the range of possible exposure pathways that are pertinent in assessing exposure in environmental justice. These pathways can be cumulative, additive, and perhaps synergistic, with resultant disproportionately high exposures. This framework can be used by risk assessors, public health officials, and the general public in health impact and risk assessments, and for the development of health protection policies for communities and populations.
TABLE 1.
Exposure Matrix for Major and Minor Exposure Pathways
| Exposure Pathways | ||||
| Means of Intake | Air | Water | Soil or Dust | Food |
| Inhalation | Major route | Showering (volatiles) | Major route | |
| Ingestion | Deposition on food | Major route | Major route; toddlers also gardeners, farmers | Major route |
| Dermal | Some organics through showers or swimming | Some organics from muds and slurries, farms | ||
Source. Adapted from Gochfeld, M. An exposure matrix for multimedia environmental exposures. Environmental and Occupational Health Sciences Institute, Piscataway, New Jersey; 1996.
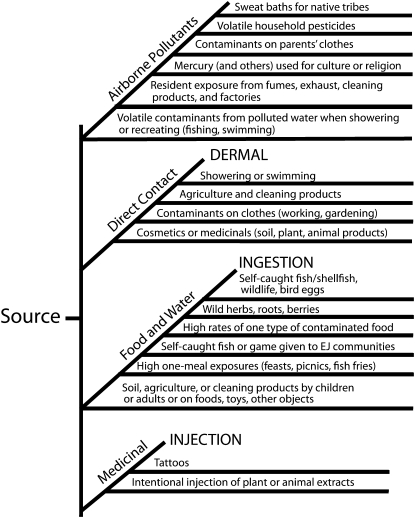
The exposure pathways in Figure 1 involve excesses in recognized pathways (e.g., consumption of fish, game, or other wildlife; soil ingestion by children), nonstandard pathways (dermal exposure from cosmetics or religious or cultural ceremonies), or unusually high or prolonged exposures (sweat baths, excessive meals at fish fries or feasts). We conclude that risk assessment methods for the EPA and other agencies should encompass a formal recognition of the pathways illustrated in Figure 2, providing a checklist for risk assessors to ascertain that all potentially significant pathways are assessed before proceeding to estimate exposure and risk. It is partly a matter of recognizing that although there may be a primary pathway (e.g., ingestion of wild-caught fish), others (inhalation, dermal) may be additive or otherwise significant.
FIGURE 1.
Main pathways of exposure for human risk assessment.
Source. Environmental and Occupational Health Sciences Institute, Piscataway, New Jersey.
FIGURE 2.
Expanded conceptual approach to exposure pathways for human risk assessment, particularly for environmental justice (EJ) communities.
Source. Environmental and Occupational Health Sciences Institute, Piscataway, New Jersey.
PATHWAYS THAT MAY RESULT IN DISPROPORTIONATE EXPOSURE
Nonstandard exposures typically result from activities that fall into categories such as consumptive and nonconsumptive resource use; maintenance and cosmetics; medicinal, religious, and cultural; inadvertent; and eco-dependency webs and eco-cultural attributes (relationship of culture and ecology). Consumptive resource use refers to activities that extract resources from ecosystems (e.g., fish, game, plants), and nonconsumptive refers to activities that do not (e.g., hiking, bird-watching, vision quests).74 Maintenance and cosmetics refer to exposures that result from applying cosmetics or maintaining the body.75,76 Inadvertent exposures occur without intent and do not fit into the other categories (e.g., workers bringing home contaminants on their clothes), or occur as an interaction between ecology and culture.33,53 Regardless of the exposure type, scenario-specific data are essential to estimate exposure.77–81 Risk assessors must be aware that evaluating environmental justice communities means researching site- and activity-specific data for these groups.
Consumptive Resource Use
Consumptive resource use refers to removal of resources by hunting, fishing, or harvesting food, fiber, medicines, or other products, which leads to ingestion of fish, fowl, meat or vegetables that may contain harmful chemicals. The term subsistence often implies that persons must gather or hunt foods because they cannot obtain or afford commercial food. However, for Native tribes such traditional uses often reflect preference rather than need, although many recreationists also have high exposure levels. Because these exposures are related to nutritional, cultural, or religious needs, they may go unnoticed or underestimated in a normal exposure assessment.
Information needed for exposure assessment includes (1) target population identification; (2) where they are exposed; (3) daily and seasonal consumption patterns (biota species, parts, preparation method); (4) sporadic peak consumption events (feasts and socials) that can result in potentially harmful peak exposures, particularly during pregnancy82 or critical developmental periods; and (5) inadvertent consumption (e.g., children ingesting soil). Pica, the deliberate consumption of objects considered inedible (e.g., soil, paint chips, chalk, soap), is an important component of consumptive exposure scenarios. Geophagy (eating clay or soil) is a type of pica that can be caused by nutritional deficiency among poor pregnant women, but cultural determinants also exist (clay ingestion among pregnant women is a norm in Africa and African American communities).83
Although exposure to commercial foods is not usually considered a unique exposure, it is not uncommon for people to consume canned tuna fish daily and regularly feed it to children. This is a significant source of mercury exposure.84 Although excessive consumption of canned tuna may be a function of low-income, eating fresh, predatory fish may be related to high income.85 Fish consumption has been identified as the most common pathway for human exposure to hazardous substances.86 The balance between traditional foods and commercial foods, an issue faced by many Native Americans, remains complex and results in risk balancing (one food type vs another) among different subsistence and commercial foods.87–89
Unpredictable delivery of food may affect consumption patterns, thus leading to unique or excessive exposures in isolated communities where commercial foods are not regularly available. For example, Aleuts living on remote islands in the Aleutian chain (some are 1500 km from mainland Alaska) have irregular and unreliable deliveries of commercial foods, which increases dependence on subsistence fish and game that may be contaminated.
Ingestion Pathways for Food and Drink
The risk from consumption of food and drink has received the most attention, particularly for minorities, low-income families, Native Americans, and others. The major difficulty is that site- or community-specific data on consumption are not usually available for most groups of interest. Data on the trade-offs between traditional foods and commercial items is seldom examined in detail, but many Native American families desire more wild-caught fish and meat (up to 94% of families for Cree),87 and many gardeners value home or locally grown produce over store-bought food. Organic and “locavore” movements have increased this preference. Risk assessors also need to consider that consumption rates may increase as waters are less polluted.89 The Boldt decision90 cited 500 pounds per capita as the Treaty rate for fish consumption from the Columbia River.37 This equals a consumption rate of 620 grams per day, much higher than current consumption (or risk assessment assumptions). Tribes argued that anthropogenic contamination of their fish should have been reduced to a level that allowed resumption of this historic consumption rate. At a hypothetical preindustrial level of 0.01 micrograms per gram of mercury in fish tissue, a 70-kilogram person consuming 620 grams per day would have ingested 0.08 micrograms per kilogram per day, thereby not exceeding the current reference dose.
A framework presented for determining how to select appropriate exposure rates for fish consumption concluded that individual intake rates were lower for (1) individual bodies than for multiple bodies of water, (2) sport-caught marine fish relative to sport-caught freshwater fish, and (3) moving waters than from still waters.91 Determining consumption for high-end fish consumers (or other self-caught or gathered food) is the most important factor, rather than determining consumption rates for an entire population (many of whom may not consume wild foods), if the goal is to set standards that are protective for all. However, intake information for these populations is not readily available. Determining dietary exposure for any population is not a simple procedure,92,93 because many studies rely on recall. A cohort study for New York State anglers, however, found that food frequency recall methods were a viable approach.94
Populations Uniquely Exposed Through Consumptive Resource Use
Ideally, to determine risk from consumption, the following information is required: meal frequency and size, fish species and parts, seasonal changes in availability or preference, exposure duration, cooking method, and body weight of the consumer (see the box on the previous page).53,78,95–98 There are 2 basic sources of information on ingestion: national and site-specific surveys. The former provide information for the general population, but may seriously underestimate consumption categories for special communities. One of the major difficulties with assessing exposure from consumption is the lack of uniformity in reporting information, even from questionnaires or surveys; often the number of fish meals per week is reported, without providing information on meal size or type.99 Usually children eat the same number of meals as their parents, but in less quantity.99
The number of fish meals eaten varies by age, gender, season, and fish species.99,101 For example, Vietnamese immigrant women consumed 3 times as much in spring and summer compared with fall and winter.102 Similarly, the Ojibwa in Northern Ontario had 3 times higher fishing rates in October and November than in February, April, and September,103 yet the Ojibwa from the Great Lakes consumed the most fish from April through July.104 The Chippewa Indians consumed fish nearly 5 times as often in April and May, compared with October.105 This finding suggests that site-specific information on seasonal patterns of consumption is critical to assessing exposure.
The information varies depending on the recall period,106 making comparison among groups difficult. Number of fish meals per time period, however, has been the measure of fish consumption used in many studies; meal size is either assumed, or less often, estimated from fish models. Meal size for risk assessments is often considered to be 4 to 6 ounces (114–170 g). However, this is generally an underestimate for Native Americans and many others,89 and varies depending upon the type of fish.107 For example, for a general population living in New Orleans, average meal size varied from 16.3 to 32.6 grams for shrimp, from 16.3 to 32.6 grams for tuna, from 12 to 19.5 grams for crawfish, and from 10.4 to 30.8 grams for other fish per meal.107
To be maximally useful, consumptive rates (grams per day) should be site-specific. Even with such data, the rates used in the risk assessment must be selected by the risk assessor. For example, a general population survey of 1000 New Jersey residents estimated that the arithmetic mean was 50.2 grams per day, geometric mean 36.6 grams per day, and 90th percentile 107.4 grams per day.108 Also, data for the entire distribution should have been made available, but this is not common practice. Most studies give only means (or medians) with variances, although some provide consumption patterns for different age classes and for means, 75th, 90th, 95th, and 99th percentiles, and maximums (or some combination).109–111 These data are useful for examining the most highly exposed individuals within a population.
Exposure is a product of consumption rate and contaminant concentrations in the foods consumed. The difficulties with determining contaminant concentrations in food involve lack of information about specific fish consumed (species, size), as well as the conversion from whole fish tissue to fillets (the portion usually eaten),112 which could be solved by having updated, clear EPA guidelines. Also, distinctions need to be made between self-caught or self-grown food, commercial foods, and those obtained from other sources.92 People with the highest consumption levels may have the highest proportion of self-caught fish in their diet.77
Nonconsumptive Exposures, and Eco-cultural Dependency Webs and Attributes
Although gathering, fishing, or hunting can result in high or unique exposures from ingestion, nonconsumptive uses of environments can also expose communities to risk from contaminants. Nonconsumptive use refers to walking, hiking, biking, watching birds, photographing, visiting sacred or ceremonial grounds in natural habitats, or otherwise using a habitat without removing resources. This exposure pathway can result in exposure from inhalation (dust or volatiles), unintentional ingestion (swallowing water while swimming or fishing), or dermal exposure (getting contaminated soil or water on the body). Traditional risk assessment includes inhalation of dust or volatiles and inadvertent ingestion of soil as pathways, but does not incorporate other atypical activities (camping, sweat baths), which may predispose some individuals to high exposures.
One class of unique exposures that is not usually considered in traditional risk assessment59 is the cumulative and collective risks from contaminants involved in eco-cultural dependency webs and eco-cultural attributes.33,53 These concepts were developed using the values and ecological, social, and religious approaches of American Indians. Eco-cultural attributes refer to the ecological factors that affect human health and well-being (in addition to goods and services), including perceived exposure and degradation of cultural and religious activities or events that depend upon intact ecosystems. Eco-cultural dependency webs tie together goods, services, resources, aesthetic, and other cultural aspects of on- and off-site systems to form interconnections that are important to Native Americans.33
Both eco-cultural dependency webs and eco-cultural attributes involve complex interactions between ecological resources and services, the ecological basis for cultural, medicinal and religious activities, and sacred events, and a worldview of the integration of all aspects of life. This approach involves not only equity within this generation, but between generations.32,35 Native Americans have a holistic view of pollution, exposure pathways, routes, and scenarios encompassing social and psychological effects, as well as traditional knowledge.113
Maintenance and Cosmetics
Many cosmetics undergo toxicological and dermatological testing before being placed on the market. However, a wide range of imported products (and some domestic products) are not tested, and those that are self-collected or self-produced may also result in unintentional exposures. Herbs, remedies, and cosmetics from Asia have been associated with potentially serious toxic effects.114 Women from India may put a circular red mark (bindi or kumkum) on their forehead, and traditionally these contain a lead pigment, as well as coal tar, toluidine, erythrosine, and red calcium salt that causes discoloration of the skin with long-term exposure.76,115 Lead poisoning can occur from Asian tongue powder,116 and although these reports were about products purchased outside the United States, these products can be obtained in the United States117 N-Nitrosodiethanolamine, a compound that produces liver tumors in rats, has been detected in cosmetics, hand lotions, and hair shampoos in concentrations as high as 48 micrograms per gram,75 although presumably these concentrations are now lower. This chemical can damage the skin.118
For exposure assessors, the task is to determine the exposed populations (e.g., Chinese, Indian), spatial distribution and availability of these products, information on toxic concentrations, and rate of use. Questions surrounding exposure include: how often is the substance used, for what part of the day, for how many years, and in what demographics (e.g., age, gender, socioeconomic status, ethnic background). Some forms of eye makeup, for example, may be used mainly at night, or during ceremonies or holidays. These practices need to be quantified by risk assessors. Some other practices, such as Native American sweat baths, may expose practitioners to both dermal and inhalation risks if the water is contaminated, particularly given their extended use.32,33 Although these activities clearly have cultural and religious significance, they also serve to cleanse the body. Sweat baths are of interest because frequency and duration can be extensive and can involve inhalation exposure for many hours and several days.
Medicinal, Religious, and Cultural
Medicinal, religious, and cultural practices can involve many exposure routes. Exposure can occur through consumption of plants, animals, or other products (even soil), through dermal exposure (paints or other decorations for ceremonies), or through herbs or other supplements taken for health reasons. These exposures are combined here because they are unregulated. Kelp herbal supplements have been implicated in arsenic toxicosis.119 This finding is of broader interest because some Native Americans, Asian immigrants, and others use kelp as food and may consume great quantities,119 particularly at times when other foods are less available.
Some herbal medicines used by Indian and Chinese populations contain toxic heavy metals or unidentified or labeled drugs otherwise requiring prescriptions. Inclusion of heavy metals can be intentional or accidental.121,122 Asian Indian medical systems (e.g., Ayurveda and Unani) have a history of herbal medicines; heavy metals are regular constituents of remedies,121,122 and these herbal medicines are distributed to many countries (including the United States). Lead poisoning may occur from some Ayurvedic medicines.123 A study of these remedies from India showed that a significant proportion contained lead (64%), mercury (64%), arsenic (41%), and cadmium (9%). Some reports noted adverse effects, even in the United States.122 Indian scientists who examined 31 Ayurvedic formulations and found that all exceeded 1 part per million mercury and 16 exceeded 100 parts per million.121 It was found that 20% of Ayurvedic herbal medicine products sold in Boston contained heavy metal levels that, if taken as directed, would exceed the published regulatory standards for each metal.117 These adulterations have resulted in adverse health effects and illnesses,121,122,124 as well as adverse effects from their interactions with prescribed drugs.125,126 The levels of lead and cadmium were above World Health Organization permissible limits in samples of herbal drugs from India.127 Cases of lead, arsenic, and mercury poisonings related to these products have been recorded.114
Similarly, Chinese traditional medicines are associated with heavy metal poisoning from mercury, cadmium, arsenic, copper, and thallium.128 A study from California found that 18 of 251 traditional Chinese medicines contained undeclared pharmaceuticals, including high levels of lead, mercury, and arsenic.129 These herbal medicines have the potential to cause adverse health effects, and prevalence of use in the United States needs to be examined, particularly in Asian communities, health-conscious Americans trying alternative remedies, and recent immigrants. These reported adverse effects129 from herbal remedies indicate that exposure assessors need to examine medicinal, herbal, and cosmetic pathways.
Asian patent medicines have gained widespread popularity in the United States but escape regulation, despite containing heavy metals and other toxic substances.124 Botanicals, Asian medicines, vitamins, and minerals are regulated under the Dietary Supplement Health and Education Act, which requires proof of danger, in contrast to pharmaceutical regulation, which requires proof of safety.130 Risk assessors and clinicians should be aware that supplements and traditional medications may be an important pathway for toxic exposure, particularly given the new globalization of herbal medicines, cosmetics, and foods.131 Ayurvedic medicines obtained on the Internet had similar levels of metal contamination to those manufactured in the United States and in India.117
Remedies used by Mexican families include azarcon and greta, lead oxide powders, containing up to 95% lead used for vomiting and colic in children,132 and 7% to 12% of samples of Mexican families in Los Angeles acknowledged using these medications.133 Hmong children are often treated with “pay-loo-hah” for rash or fever, which contains up to 90% lead as well as arsenic, and has caused clinical poisoning.133 Several types of information should be assessed for high-risk groups, some of which are low-income populations or minorities in urban environments (see box on page S67 and Figure 2).
Outline of potentially unique exposure pathways we developed for environmental justice communities.
| Consumptive uses (ingestion) |
| Daily and seasonal consumption patterns of wild-caught or gathered foods |
| Daily and seasonal consumption of unusual commercial foods or herbs |
| Daily and seasonal consumption patterns of wild foods by biota species and parts |
| Daily and seasonal preparation patterns for wild foods or unusual commercial foods |
| Unusual consumption patterns by group, age, gender, or season |
| Presence of high 1 meal per day consumption (feasts, fish fries, socials) |
| Geophagy (e.g., children intentionally eating dirt, southern pregnant women [pica]) |
| Species, types, seasonality, and exposure from commercial foods versus wild foods |
| Maintenance and cosmetic uses (inhalation, ingestion, dermal) |
| Occurrence of tribal sweat baths |
| Use of sand or soil or plant material for maintenance or cosmetics |
| Use of usual commercial materials for cosmetics |
| Use of unique substances for religious/cultural practices (such as cultural use of mercury) |
| Temporal patterns: daily and seasonal, frequency, duration |
| Medicinal, religious and cultural uses (ingestion, inhalation, dermal) |
| Types of medicine and healing practices (allopathic and others) |
| Species, types, seasonality, and exposure from herbs or other medicines |
| Potential role of commercial medicinals in relation to self-gathered |
| Types and frequencies of religious events or ceremonies (individual or communal) |
| Types of on-site, nonconsumptive uses, such as vision quests or dream quests |
| Folk or cultural medicines (e.g., Ayurvedic, mercury) |
| Temporal patterns: daily and seasonal, frequency, duration |
| Lifestyle exposures: alcohol, tobacco, pharmaceutical |
| Contribution of commercial versus self-gathered medicinals |
| Eco-cultural dependency webs and eco-cultural attributes as exposure pathways (all forms) |
| Occupational exposures and inadvertent exposures (inhalation, ingestion, dermal) |
| Unique exposures and coexposures |
| Take-home exposures from any occupation |
| Nonpoint source exposures (inhalation) |
| Air pollution and exhaust fumes |
| Traffic |
| Hazardous waste sites or landfills |
| Building-related exposures (inhalation, ingestion) |
| Housing stock age and condition |
| School age and condition |
| Pesticides, lead, mold |
| Residences above or next to small industrial sources |
RECOMMENDATIONS
Nonstandard or unique exposure pathways can lead to disproportionate exposures for minority, low income, Native American, and other populations that should be taken into account by risk assessors and public health officials. Some of these pathways are accounted for by risk assessors on an ad hoc basis, but unique exposure pathways may not be included. We believe the impact of these unusual pathways is greater for racial/ethnic minorities, low-income populations, and Native Americans because of cultural and traditional practices, language barriers, and lack of access to health protective information.
Without a formal framework for evaluating the importance of these nonstandard or unique pathways, the EPA may miss important risks to the most highly exposed and disproportionately impacted populations, and therefore not develop sufficiently protective actions. A concerted effort is required to capture important data, highlighted in this conceptual model, and to translate this information into guidelines for risk assessors. Sometimes individuals, or small populations, are dispersed within a larger population that might make it difficult to identify the pathways shown in Figure 2.
Recognizing that there are populations with poorly quantified exposures is the first step in improving risk methodology and protecting public health. We suggest several tiers of actions and research that are needed to move risk assessment forward to identify and assess unique and nonstandard exposure pathways. We recommend the EPA start with the conceptual model shown in Figure 2, and a list of potentially important, nonstandard exposure pathways as shown in the box on page S67 and develop a more comprehensive list of pathways. These should be developed as guidance on how to evaluate the importance of these pathways for specific types of assessments. This guidance might include checklists for types of pathways to include for assessments regarding Native American populations, low-income urban populations, or other types of environmental justice populations.
Additional recommendations include (1) updating fish consumption guidance to reflect the needs of ethnic minorities, low-income populations, and Native Americans; (2) encouraging researchers to report the distributions of their exposure data, highlighting distributions in the 95th and 99th percentiles; (3) collecting data on site-specific contaminants in foods; (4) targeting data collection on populations that depend on self-caught versus commercial food; and (5) collecting and synthesizing robust site- and population-specific information, including patterns of use. Such efforts can be achieved through ethnic studies that identify highly exposed populations and elucidate cultural and traditional practices, behaviors, foods, medicines, meal sizes, foods, fish and wildlife species, and consumption rates for medicines and foods for these population groups. Although such information is not easily incorporated into national-scale risk assessments, they are informative in the development of policies to protect human health. Policies that protect highly exposed populations are likely to be more protective than policies not based on such groups. In protecting these highly exposed populations, standards have to be strict, which should potentially narrow the inequality gap between groups with high or unique exposures and those with much lower exposures.
Generating these data will enable the EPA and risk assessors to evaluate risk for ethnic/racial minorities, low-income populations, and Native Americans. If protecting public health includes protecting those who are disproportionally impacted, consideration of nonstandard or unique exposure pathways that lead to that disproportional impact must be part of the solution.
Acknowledgments
This review was partially funded by the Environmental Protection Agency (EPA; GS-00F-0001S), Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the Department of Energy (DE-FC01-06EW07053), National Institutes of Environmental Health Sciences (P30ES005022), and EOHSI.
We particularly thank M. Callahan and O. Nweke for organizing the EPA conference, and for critically reading this article. We also thank M. Greenberg, C. W. Powers, Jim Johnson, and C. Chess for helpful information and discussions about environmental justice communities, Native Americans, and the complexities of environmental evaluation in relation to exposure, resource use and future land use.
Note. The conclusions and interpretations reported herein are the sole responsibility of the authors and should not in any way be interpreted as representing the views of the funding agencies.
Human Participant Protection
This is a review paper, and no original research with human participants was conducted, only syntheses of other papers.
References
- 1.Bullard RD, Wright BH. The politics of pollution: implications for the black community. Phylon. 2009;47:71–78 [Google Scholar]
- 2.Bullard RD, Wright BH. Environmentalism and the politics of equity: emergent trends in the black community. Mid Am Rev Sociol. 1987;12(2):21–39 [Google Scholar]
- 3.Bullard RD. Dumping in Dixie: Race, Class, and Environmental Quality. Boulder, CO: Westview Press; 1990 [Google Scholar]
- 4.Bullard RD. Overcoming racism in environmental decision making. Environment. 1994;36(4):10–20, 39–4412290153 [Google Scholar]
- 5.Environmental Equity: Reducing the Risk for All Communities. Washington, DC: Environmental Protection Agency; 1992a. Report No.: EPA230-R92-008 [Google Scholar]
- 6.Environmental Justice Initiatives 1993. Washington, DC: Environmental Protection Agency; 1994. Report No.: EPA 2000-R-93–001 [Google Scholar]
- 7.National Environmental Justice Advisory Council: Fish Consumption and Environmental Justice; Washington DC: Environmental Protection Agency 2002a [Google Scholar]
- 8.Mohai P, Bryant B. Environmental injustice: weighing race and class as factors in the distribution of environmental hazards. Univ Colo Law Rev. 1992;63(4) :921–932 [Google Scholar]
- 9.Sexton K, Adgate JL. Looking at environmental justice from an environmental health perspective. J Expo Anal Environ Epidemiol. 1999;9(1):3–8 [DOI] [PubMed] [Google Scholar]
- 10.Brugge D, Hynes PH. Community Research in Environmental Health: Studies in Science, Advocacy, and Ethics. Burlington, VT: Ashgate Press; 2005 [Google Scholar]
- 11.Lynch JW, Smith GD, Kaplan GA, House JS. Income inequality and mortality: importance to health of individual income, psychosocial environment, or material conditions. BMJ. 2000;320(7243):1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003 [PubMed] [Google Scholar]
- 13.National Research Council Science and Decisions: Advancing Risk Assessment. Washington, DC: National Academies Press; 2009 [PubMed] [Google Scholar]
- 14.Cory-Slechta DA, Weiss B, Cranmer J. The environmental etiologies of neurobehavioral deficits and disorders: weaving complex outcomes and risk modifiers into the equation. Neurotoxicology. 2008;29(5):759–760 [DOI] [PubMed] [Google Scholar]
- 15.Virgolini MB, Rossi-George A, Lisek R, et al. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29(5):812–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sexton K, Needham LL, Pirkle JL. Human biomonitoring of environmental chemicals. Am Sci. 2004;92:38–45 [Google Scholar]
- 17.Boyce CP, Garry MR. Review of information resources to support human exposure assessment models. Hum Ecol Risk Assess. 2002;8:1445–1487 [Google Scholar]
- 18.Callahan MA, Sexton K. If cumulative risk assessment is the answer, what is the question? Environ Health Perspect. 2007;115:799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sociodemographic Data Used for Identifying Potentially Highly Exposed Populations. Washington, DC: Environmental Protection Agency; 1999. Report No.: 600/5–99/060 [Google Scholar]
- 20.House JS, Williams DR. Understanding and reducing socioeconomic and racial-ethnic disparities in health. In: Smedley B D, Syme SL, eds. Promoting Health: Intervention Strategies from Social and Behavioral Research. Washington, DC: National Academies Press; 2008:81–124 [PubMed] [Google Scholar]
- 21.Lantz PM, Lynch JW, House JS, et al. Socioeconomic disparities in health change in a longitudinal study of US adults: the role of health-risk behaviors. Soc Sci Med. 2001;53(1):29–40 [DOI] [PubMed] [Google Scholar]
- 22.Ettner SL, Grzywacz JG. Socioeconomic status and health among Californians: an examination of multiple pathways. Am J Public Health. 2003;93:441–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.President's Cancer Panel Facing Cancer in Indian Country: the Yakama Nation and Pacific Northwest Tribes 2002 Annual Report. Bethesda, MD: US Dept of Health and Human Services; 2003 [Google Scholar]
- 24.Judd NL, Griffith WC, Faustman EM. Consideration of cultural and lifestyle factors in defining susceptible populations for environmental disease. Toxicology. 2004;198:121–133 [DOI] [PubMed] [Google Scholar]
- 25.Bellinger DC, Bellinger AM. Childhood lead poisoning: the torturous path from science to policy. J Clin Invest. 2006;116:853–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo R. Unheard voices: environmental equity. Electronic Green J. 2003;1(18). Available at: http://escholarship.org/uc/item/2qv220g6# Accessed December 15, 2009 [Google Scholar]
- 27.Gelopter M. The distribution of air pollution by income and race. Paper presented at: Second Symposium on Social Science in Resource Management; June 6–10, 1988; Urbana, IL. [Google Scholar]
- 28.Elliott MR, Wang Y, Lowe RA, Kleindorfer PR. Environmental justice: frequency and severity of US chemical industry accidents and socioeconomic status surrounding communities. J Epidemiol Community Health. 2004;58:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger J, Stephens W, Boring CS, et al. Ethnicity and risk: fishing and consumption in people fishing along the Savannah River. Risk Anal. 1999a;19:427–438 [DOI] [PubMed] [Google Scholar]
- 30.Burger J, Kirk-Pflugh K, Lurig L, et al. Fishing in urban New Jersey: II. ethnicity affects information sources, perception and compliance. Risk Anal. 1999b;19:217–229 [DOI] [PubMed] [Google Scholar]
- 31.Toth JF, Brown RB. Racial and gender meanings of why people participate in recreational fishing. Leis Sci. 1997;19:129–146 [Google Scholar]
- 32.Harris SG, Harper BL. A Native American exposure scenario. Risk Anal. 1997;17(6):789–795 [DOI] [PubMed] [Google Scholar]
- 33.Harris SG, Harper BL. Using eco-cultural dependency webs in risk assessment and characterization of risks to tribal health and cultures. Environ Sci Poll Res. 2000;2:91–100 [Google Scholar]
- 34.Harris SG, Harper BL. How Incorporating Tribal Information Will Enhance Waste Management Decisions. Pendleton, OR: Confederated Tribes of the Umatilla Indian Reservation; 2008 [Google Scholar]
- 35.Harris SG. Risk analysis: changes needed from a Native American perspective. Hum Ecol Risk Assess. 2000;6:529–535 [Google Scholar]
- 36.Burger J, Gochfeld M, Jeitner C, et al. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci Total Environ. 2007a;384:93–105 [DOI] [PubMed] [Google Scholar]
- 37.Donatuto J, Harper BL. Issues in evaluating fish consumption rates for Native American tribes. Risk Anal. 2008;28:1497–1505 [DOI] [PubMed] [Google Scholar]
- 38.Morton A, Airoldi M, Phillips LD. Nuclear risk management on stage: a decision analysis perspective on the UK's Committee on Radioactive Waste Management. Risk Anal. 2009;29(5):764–779 [DOI] [PubMed] [Google Scholar]
- 39.Marshall AD. Environmental inequality: air pollution exposures in California's South Coast air basin. Atmos Environ. 2008;42:5499–5503 [Google Scholar]
- 40.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequity: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Needham LL, Sexton K. Assessing children's exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J Expo Anal Environ Epidemiol. 2000;10:611–629 [DOI] [PubMed] [Google Scholar]
- 42.Malhotra P, Saksena S, Joshi V. Time budgets of infants for exposure assessment: a methodological study. J Expo Anal Environ Epidemiol. 2000;10(3):267–284 [DOI] [PubMed] [Google Scholar]
- 43.Suk WA, Murray K, Avakian MD. Environmental hazards to children's health in the modern world. Mutat Res. 2003;544(2-3):235–242 [DOI] [PubMed] [Google Scholar]
- 44.O'Neill CA. Risk avoidance, cultural discrimination, and environmental justice for indigenous peoples. Ecol Law Q. 2003;30:1–57 [Google Scholar]
- 45.National Research Council Risk Assessment in the Federal Government, Managing the Process. Washington, DC: National Academy Press; 1983 [PubMed] [Google Scholar]
- 46.National Research Council Ecological Knowledge and Environmental Problem-Solving. Washington: DC National Academy Press; 1986 [Google Scholar]
- 47.National Research Council Pesticides in the Diets of Infants and Children. Washington, DC: National Academy Press; 1993a [PubMed] [Google Scholar]
- 48.Guidelines for Exposure Assessment. Washington, DC: Environmental Protection Agency; 1992b. Report No.: 600Z-92/001 [Google Scholar]
- 49.PCCRAM Presidential/Congressional Commission on Risk Assessment and Management. U.S. Government Printing Office, Washington, DC; 1997 [Google Scholar]
- 50.Helvey M, Magoon OT, Converse H, Tippie V, Tobin IT, Clark D. Restoration planning for natural resource damage assessment cases. Coastal Zone. 1991;2:1436–1445 [Google Scholar]
- 51.Sheehy DJ, Vik SF. Natural resource damage claims: potential DOD liabilities and mitigation opportunities. Fed Facil Environ J. 2003;14:17–28 [Google Scholar]
- 52.Environmental Justice. Budapest, Hungary: Center for Environmental Policy and Law; 2009 [Google Scholar]
- 53.Burger J. Perceptions as indicators of potential risk from fish consumption and health of fish populations. Environ. Bioindicat. 2008;3:90–105 [Google Scholar]
- 54.Gochfeld M, Burger J. Environmental and ecological risk assessment. In: Wallace RB, Kohatsu N, eds. Maxcy-Rosenau-Last Public Health and Preventive Medicine. 15th ed New York: McGraw Hill; 2008:545–562 [Google Scholar]
- 55.Weiss B, Cory-Slechta D, Gilbert SG, et al. The new tapestry of risk assessment. Neurotoxicology. 2008;29(5):883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith KR. Place makes the poison: Wesolowski Award Lecture—1999. J Expo Anal Environ Epidemiol. 2002;12(3):167–171 [DOI] [PubMed] [Google Scholar]
- 57.Toxicological Profile for Mercury. Atlanta, Georgia: Agency for Toxic Substances and Disease Registry; 1999 [Google Scholar]
- 58.Guidance on Cumulative Risk Assessment. Part 1. Planning and Scoping. Washington DC: Environmental Protection Agency; 1997. Available at http://www.epa.gov/osa/spc/pdfs/comrisk2pdf. Accessed December 20, 2009. [Google Scholar]
- 59.Environmental Protection Agency EPA Research and Development: Risk Paradigm. 2009b. Available at: http://www.epa.gov/ord/htm/risk.htm. Accessed July 16, 2009. [Google Scholar]
- 60.Environmental Protection Agency The NRC Risk Assessment Paradigm; 2009c. Available at http://www.epa.gov/ttn/atw/toxsource/paradigm.html. Accessed December 10, 2009. [Google Scholar]
- 61.Sociodemographic Data Used for Identifying Potentially Highly Exposed Populations. Washington DC: Environmental Protection Agency; 1999. Report No.: EPA/600/5–99/060 [Google Scholar]
- 62.Callahan MA, Clickner RP, Whitmore RW, et al. Overview of important design issues for a national human exposure assessment survey. J Expo Anal Environ Epidemiol. 1995;5:257–282 [PubMed] [Google Scholar]
- 63.Stern AH. A revised probabilistic estimate of the maternal methyl mercury intake dose corresponding to a measured cord blood mercury concentration. Environ Health Perspect. 2005;113(2):155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnston JJ, Snow JL. Population-based consumption survey and probabilistic methylmercury risk assessment. Hum Ecol Risk Assess. 2007;13:1214–1227 [Google Scholar]
- 65.American Public health Association. Racism and health. Am J Public Health. 2003;93(2). 185–348 [Google Scholar]
- 66.Sexton K. (ed). Environmental Justice. J Expos Anal Environ Epidemiol. 1999;9(1).3–78 [DOI] [PubMed] [Google Scholar]
- 67.American Public Health Association. Disparities in health communication Am J Public Health. 2004;94(12):2047–221215569946 [Google Scholar]
- 68.American Public Health Association. American Indian/Alaska native health policy Am J Public Health. 2005;95(5):757–91715855446 [Google Scholar]
- 69.American Public Health Association. Diabetes Am J Public Health. 2005;95(9):1493–164916051926 [Google Scholar]
- 70.Boucher N, Koren G, Beaulac-Baillargeon L. Maternal use of venlafaxine near term: correlation between neonatal effects and plasma. Ther Drug Monit. 2009;31(3):404–409 [DOI] [PubMed] [Google Scholar]
- 71.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–789 [DOI] [PubMed] [Google Scholar]
- 72.Hightower JM, O'Hare A, Hernandez GT. Blood mercury reporting in NHANES: identifying Asian, Pacific Islander, Native American, and multiracial groups. Environ Health Perspect. 2006;114(2):173–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahaffey KR, Clickner RP, Jeffries RA. Adult women's blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999-2004). Environ Health Perspect. 2009;117(1):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeGroot RS, Wilson MA, Boumans RMJ. A typology for the classification, description and valuation of ecosystem functions, goods and services. Ecol Econ. 2002;41:393–408 [Google Scholar]
- 75.Fan TY, Goff U, Song L, Fine DH. N-Nitrosodiethanolamine in cosmetics, lotions and shampoos. Food Cosmet Toxicol. 1977;15:423–430 [DOI] [PubMed] [Google Scholar]
- 76.Mehta SS, Reddy BSN. Cosmetic dermatitis—current perspectives. Int J Dermatol. 2003;42(7):533–542 [DOI] [PubMed] [Google Scholar]
- 77.Burger J. Gender differences in meal patterns: role of self-caught fish and wild game in meat and fish diets. Environ Res. 2000a;83:140–149 [DOI] [PubMed] [Google Scholar]
- 78.Burger J. Consumption patterns and why people fish. Environ Res. 2002a;90:125–135 [DOI] [PubMed] [Google Scholar]
- 79.Burger J. Daily consumption of wild fish and game: exposures of high end recreationists. Int J Environ Health Res. 2002b;12:343–354 [DOI] [PubMed] [Google Scholar]
- 80.Sunderland EM. Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environ Health Perspect. 2007;115:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kinnell JC, Bingham MF, Hastings EA, et al. A survey methodology for collecting fish consumption data in urban and industrial water bodies (part 1). J Toxicol Environ Health A. 2007;70(6):477–495 [DOI] [PubMed] [Google Scholar]
- 82.Ginsberg GL, Toal BF. Development of a single-meal fish consumption advisory for methylmercury. Risk Anal. 2000;20:41–47 [DOI] [PubMed] [Google Scholar]
- 83.Hunter JM. Geophagy in Africa and in the United States. Geogr Rev. 1973;63(2):173–195 [Google Scholar]
- 84.Burger J, Gochfeld M. Mercury in canned tuna: white versus light and temporal variation. Environ Res. 2004;96:239–249 [DOI] [PubMed] [Google Scholar]
- 85.Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111(4):604–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ebert ES, Harrington NW, Boyle KJ, et al. Estimating consumption of freshwater fish among Maine anglers. N Am J Fish Manage. 1993;13:737–745 [Google Scholar]
- 87.Campbell ML, Diamant RMF, Macpherson BD, Hallaaday JL. The contemporary food supply of three northern Manitoba Cree communities. Can J Public Health. 1997;88:105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gochfeld M, Burger J. Good fish/bad fish: a composite benefit-risk by dose curve. Neurotoxicology. 2005;26:511–520 [DOI] [PubMed] [Google Scholar]
- 89.Harper BL, Harding AD, Waterhous T, Harris SG. Traditional Tribal Subsistence Exposure Scenario and Risk Assessment Guidance Manual. Washington, DC: Environmental Protection Agency; 2008. Report No.: EPA-STAR-J1-R831-46 [Google Scholar]
- 90.US vs Washington, 1974. 384 F, Suppl. 312, 380
- 91.Ebert ES, Price PS, Keenan RE. Selection of fish consumption estimates for use in the regulatory process. J Expo Anal Environ Epidemiol. 1994;4:373–393 [Google Scholar]
- 92.Price PS, Su SH, Gray MN. The effect of sampling bias on estimates of angler consumption rates in creel surveys. J Expo Anal Environ Epidemiol. 1994;4:355–372 [Google Scholar]
- 93.Berry MR. Dietary exposure and total human exposure assessment: an overview. J Expo Anal Environ Epidemiol. 1997;7:1–2 [Google Scholar]
- 94.Li Q, Vena JE, Swanson MK. Reliability of sport fish consumption in New York state angler cohort study. Environ Res. 2005;97:142–148 [DOI] [PubMed] [Google Scholar]
- 95.Paradis S, Wheatley B, Boswell-Purdy J, et al. Mercury contamination through fish consumption: a model for predicting and preventing hazardous behaviour on a community level. Water Air Soil Pollut. 1997;97:147–185 [Google Scholar]
- 96.Strauss H. Sportsfish consumption surveys: a risk assessment practitioner's wish list. Hum Ecol Risk Assess. 2004;10:1213–1225 [Google Scholar]
- 97.Moya J. Overview of fish consumption rates in the United States. Hum Ecol Risk Assess. 2004;10:1195–1211 [Google Scholar]
- 98.Burger J, Gochfeld M. A framework and information needs for the management of the risks from consumption of self-caught fish. Environ Res. 2006;101:275–285 [DOI] [PubMed] [Google Scholar]
- 99.Fitzgerald EF, Hwang S, Brix KA, Bush B, Cook D, Worswick P. Fish PCB concentrations and consumption patterns among Mohawk women at Akwesasne. J Expo Anal Environ Epidemiol. 1995;5:1–10 [PubMed] [Google Scholar]
- 100.Beehler GP, Weiner JM, McCann SE, et al. Identification of sport fish consumption patterns in families of recreational anglers through factor analysis. Environ Res. 2002;89:19–28 [DOI] [PubMed] [Google Scholar]
- 101.West PC, Fly JM, Larkin F, Marans RW. Minority anglers and toxic fish consumption: evidence from a statewide survey of Michigan. In: Bunyan R, Mohar P, eds. Race and the Incidences of Environmental Hazards. Boulder, Colorado: Westview Press; 1992:100–113 [Google Scholar]
- 102.Cavan KR, Gibson BL, Cole DC, Riedel D. Fish consumption by Vietnamese women immigrants: a comparison of methods. Arch Environ Health. 1996;51(6):452–457 [DOI] [PubMed] [Google Scholar]
- 103.Hopper M, Power G. The fisheries of an Ojibwa community in Northern Ontario. Arctic. 1991;44(4):267–274 [Google Scholar]
- 104.Gerstenberger SL, Travis DR, Hansen LK, et al. Concentration of blood and hair mercury and serum PCBs in an Ojibwa population that consumes Great Lakes fish. J Toxicol Clin Toxicol. 1997;35:377–386 [DOI] [PubMed] [Google Scholar]
- 105.Peterson DE, Kanarek MS, Kuykendall MA, et al. Fish consumption patterns and blood mercury levels in Wisconsin Chippewa Indians. Arch Environ Health. 1994;49(1):53–58 [DOI] [PubMed] [Google Scholar]
- 106.Tran NL, Barraj L, Smith K, et al. Combining food frequency and survey data to quantify long-term dietary exposure: a methyl mercury case study. Risk Anal. 2004;24(1):19–30 [DOI] [PubMed] [Google Scholar]
- 107.Anderson AC, Rice JC. Survey of fish and shellfish consumption by residents of the greater New Orleans area. Bull Environ Contam Toxicol. 1993;51:508–514 [DOI] [PubMed] [Google Scholar]
- 108.Stern AH, Korn LR, Ruppel BE. Estimation of fish consumption and methylmercury intake in the New Jersey population. J Expo Anal Environ Epidemiol. 1996;6(4):503–525 [PubMed] [Google Scholar]
- 109.Murray DM, Burmaster DE. Estimated distributions for average daily consumption of total and self-caught fish for adults in Michigan Angler households. Risk Anal. 1994;14:513–519 [Google Scholar]
- 110.Ruffle B, Burmaster DE, Anderson PD, Gordon HD. Lognormal distributions for fish consumption by the general U.S. population. Risk Anal. 1994;14:395–404 [Google Scholar]
- 111.Jacobs HL, Kahn HD, Stralka KA, Phan DB. Estimates of per capita fish consumption in the U.S. based on the continuing survey of food intake by individuals (CSFII). Risk Anal. 1998;18(3):283–291 [DOI] [PubMed] [Google Scholar]
- 112.Roseberry AM, Burmaster DE. A note: estimating exposure concentrations of lipophilic organic chemicals to humans via raw finfish fillets. J Expo Anal Environ Epidemiol. 1991;1(4):513–521 [PubMed] [Google Scholar]
- 113.Arquette M, Cole M, Cook K, et al. Holistic risk-based environmental decision making: a native perspective. Environ Health Perspect. 2002;110:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shaw D, Leon C, Kolev S, Murray V. Traditional remedies and food supplements: a 5-year toxicological study (1991-1995). Drug Saf. 1997;17:342–356 [DOI] [PubMed] [Google Scholar]
- 115.Reddy BSN, Ramesh V. Occupational contact dermatitis in India. Newsletter Occupat Health Saf. 1998;5:145–148 [Google Scholar]
- 116.Woolf AD, Hussain J, McCullough L, et al. Infantile lead poisoning from an Asian tongue powder: a case report & subsequent public health inquiry. Clin Toxicol. 2008;46(9):841–844 [DOI] [PubMed] [Google Scholar]
- 117.Saper RB, Kales SN, Paquin J, et al. Heavy metal content of Ayurvedic herbal medicine products. JAMA. 2004;292:2868–2873 [DOI] [PubMed] [Google Scholar]
- 118.Franz TJ, Lehman PA, Franz SF, et al. Percutaneous penetration of N-Nitrosodiethanolamine through human skin (in vitro): consequences of finite and infinite dose applications from cosmetic vehicles. Fundam Appl Toxicol. 1993;21:213–231 [DOI] [PubMed] [Google Scholar]
- 119.Amster E, Tiwary A, Scheneker MB. Case report: potential arsenic toxicosis secondary to herbal kelp supplements. Environ Health Perspect. 2007;115:606–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burger J, Gochfeld M, Jeitner C, et al. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci Total Environ. 2007;384:93–105 [DOI] [PubMed] [Google Scholar]
- 121.Ernst E. Heavy metals in traditional Indian remedies. Eur J Clin Pharmacol. 2002a;57:891–896 [DOI] [PubMed] [Google Scholar]
- 122.Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002b;23:136–139 [DOI] [PubMed] [Google Scholar]
- 123.Prpic-Majic D, Pizent A, Jurasovic J, et al. Lead poisoning associated with the use of Ayurvedic metal-mineral tonics. Clin Toxicol. 1996;34(4):417–423 [DOI] [PubMed] [Google Scholar]
- 124.Adulterants in Asian Patent Medicine. Sacramento, CA: California Department of Public Health; 2009 [Google Scholar]
- 125.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61(15):2163–2175 [DOI] [PubMed] [Google Scholar]
- 126.Fugh-Berman A, Ernst E. Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rai V, Kakar P, Khatoon S, et al. Heavy metal accumulation in some herbal drugs. Pharm. Biol. 2001;39:384–387 [Google Scholar]
- 128.Ernst E, Thompson Coon J. Heavy metals in traditional Chinese medicines: a systematic review. Clin Pharmacol Ther. 2001;70(6):497–504 [DOI] [PubMed] [Google Scholar]
- 129.Ko RJ. Adulterations in Asian patent medicines. N Engl J Med. 1998;339(12):847. [DOI] [PubMed] [Google Scholar]
- 130.Slifman NR, Obermeyer WR, Aloi BK, et al. Contamination of botanical dietary supplements by Digitalis lanata. N Engl J Med. 1998;339(12):806–811 [DOI] [PubMed] [Google Scholar]
- 131.Watson JL, Caldwell ML. Introduction. In: Watson JL, Caldwell ML, eds. The Cultural Politics of Food and Eating: A Reader. Malden, MA: Blackwell Publishing; 2005:1–10 [Google Scholar]
- 132.Bose A, Vashistha K, O'Loughlin BJ. Azarcón por Empacho—another cause of lead toxicity. Pediatrics. 1983;72:106–108 [PubMed] [Google Scholar]
- 133.Leads from the MMWR Folk remedy-Associated lead Poisoning in Hmong Children. JAMA. 1983;250(23)3149–3150 [PubMed] [Google Scholar]