Abstract
Pediatric high-grade gliomas (World Health Organization grades III and IV astrocytomas) remain tumors with a very poor prognosis for which novel therapeutic strategies are needed. Poly(ADP-ribose) polymerase (PARP) is known to have multiple functions in tumors, including single-strand DNA repair and induction of caspase-independent apoptosis. PARP has been suggested as a therapeutic target in adult malignancies, and this study examines whether it could also be a potential target in pediatric high-grade glioma. Tissue microarrays containing 150 formalin-fixed pediatric high-grade gliomas were examined by immunohistochemistry for levels of PARP and expression of apoptosis inducing factor (AIF). Full retrospective clinical and survival data were available for this cohort. Stratification and statistical analysis was performed to assess the effect of PARP status on prognosis. The level of PARP immunopositivity had a statistically significant inverse correlation (P = .019) with survival in supratentorial pediatric high-grade glioma. AIF staining was notable for its absence in the majority of tumors but with moderate levels of expression in surrounding normal brain. PARP is expressed at high levels in many pediatric high-grade gliomas, and in these tumors, the ability of PARP to activate AIF appears to have been lost. PARP may therefore represent a promising therapeutic target for these lesions and warrants evaluation in clinical trials.
Keywords: apoptosis inducing factor, glioblastoma, high grade glioma, immunohistochemistry, poly(ADP-ribose) polymerase
Tumors of the CNS are the most common group of solid tumors in childhood and are now the leading cause of cancer-related death in children.1 Pediatric high-grade gliomas (HGGs; World Health Organization [WHO] grades III and IV astrocytomas and related tumors) represent approximately 10% of childhood intracranial neoplasms.2 Their prognosis remains extremely poor, with median survival remaining around 14 months, despite maximal resection, radiotherapy, and various chemotherapy regimes.3,4 Glioblastoma multiforme (GBM; WHO grade IV) is the most common primary intracranial tumor in adults and the subject of considerable research efforts. Pediatric HGG, in contrast, has received comparatively little attention. However, recent comparative high-resolution genetic studies have detailed the genetic differences between adult and pediatric tumors of the same histological type.5–8 It is clear that different genetic pathways have differing degrees of importance in this tumor in different age groups. For example, IDH-1 mutation is common in adult secondary GBM but absent from pediatric GBM;6 1q gain is much more common in pediatric compared with adult GBM (30% and 9%, respectively),6 whereas loss of 10q is much less common in pediatric GBM (35% versus 80% in adult GBM).6 It follows that the response to particular therapies may vary according to the abnormalities present in any given tumor. Extrapolating clinical therapy in children from studies of adult GBM may lead to targeting of pathways that are not relevant in the pediatric age group, therefore clinical trials of novel agents in children should be based on the molecular biology of their specific tumors.
Poly(ADP-ribose) polymerases (PARPs) are a family of zinc finger proteins involved in the cellular response to DNA damage, particularly single strand breakages (with PARP1 and PARP2 most active in this respect).9 PARP1 (encoded at 1q 41) is thought to account for the majority (over 80%) of PARP activity in genomically damaged cells. The PARP protein consists of 4 domains (DNA binding, cleavage, automodification, and catalytic). PARP binds to a broken DNA strand via the zinc finger DNA binding domain and catalyses the production of poly(ADP-ribose) (PAR) polymer from cellular nicotinamide adenine dinucleotide (NAD). This process identifies DNA breakages and facilitates their repair by DNA polymerase β and other mechanisms. For DNA repair to proceed successfully, PARP must disengage from the DNA, normally achieved by the accumulation of negative charge on the automodification domain.10
The utilization of substantial quantities of cellular energy reserves in the form of NAD by PARP in the process of poly(ADP-ribosyl)ation is the key mechanism by which PARP acts to prevent genetically damaged cells entering mitosis. In the presence of minor degrees of DNA damage, PARP stimulates repair pathways as described, allowing restoration of genetic integrity and healthy cells to enter the cell cycle. However, if DNA damage is greater than the capacity of the cell to fully repair, then apoptotic pathways are activated to prevent critically damaged cells from reproducing. PARP is known to play roles in both the caspase-dependent and -independent pathways.11 Caspase-3 is known to cleave PARP, removing the automodification domain, so the DNA binding domain is unable to disengage from the DNA breakage (“sugar plug”), thus preventing repair activity and further expenditure of cellular energy reserves that can then be utilized for apoptosis.
It was noted that apoptosis proceeds despite pan-caspase inhibition and that PAR stimulates this process.12 Apoptosis inducing factor (AIF) has been identified as a key mediator of caspase-independent apoptosis,13,14 particularly in response to certain stimuli, such as hypoxia, hypoglycemia, trauma, and excito-toxins.15 AIF has NAD-oxoreductase activity and is normally found in the inner mitochondrial membrane in healthy cells.16 It has important roles in mitochondrial function, with AIF-deficient mice being a model for complex I deficiency mitochondropathy. When stimulated by PAR, AIF translocates from the inner mitochondrial membrane to the nucleus, where it induces DNA fragmentation and chromatin condensation, key features of apoptosis.17 The translocation of AIF from mitochondrion to nucleus can be observed on immunohistochemistry, providing evidence of a cell progressing toward programmed cell death. AIF-deficient mice display greatly reduced levels of apoptosis in response to ischemic or toxic stimuli, whereas PARP-deficient mice have an increased sensitivity to ionizing radiation.
Although it has pro-apoptotic functions in brain tumor cells, PARP has been suggested as a therapeutic target in adult glioblastoma. The extent of PARP expression in pediatric HGG is poorly characterized, so conclusions cannot be drawn as to whether it holds promise in this age group as well. In this study, we examine PARP in our large cohort of childhood HGGs and assess whether it shows any link to survival or treatment response. We also assess whether the AIF pro-apoptotic pathway appears functional, as reducing the amount of tumor cell death by inhibiting a PARP-driven pathway could theoretically be counterproductive.
Methods
Tumor Specimens
This study is based on samples banked by the UK Children's Cancer Leukemia Group (CCLG) through Nottingham Children's Brain Tumor Research Centre. All samples were surgically collected ante mortem at UK pediatric neurosurgical centers (between 1987 and 2007) and are from pediatric HGGs (both supratentorial and brainstem) with diagnosis confirmed by central pathological review. Full consent and ethical approval was obtained for their use in this study from the CCLG and local ethical and Trent Medical Review and Ethics Committee approval (06/MRE04/86). The brainstem tumors were a mixed group but were formed predominantly of the cohorts examined by Cartmill and Punt18 and Barrow et al.8 A full clinical dataset, including demographic and treatment details, was available for each of the samples in this cohort. The length of survival or last known follow-up from initial diagnosis was also known. For AIF staining, several nonneoplastic brain specimens (frontal and temporal lobes) were used as additional control tissues. Following central pathological review in the UK and USA, a tissue microarray was constructed with the formalin-fixed, paraffin-embedded tissue from 150 pediatric HGG tumors. Three cores from representative regions of each tumor were included on the tissue microarray, and only samples with 3 assessable cores were analyzed. Array comparative genomic hybridization (aCGH) and fluorescent in situ hybridization (FISH) were performed as per methods previously described.8
Immunohistochemistry
Immunohistochemistry was performed on the tissue microarrays, with initial deparaffinization being accomplished by immersion in xylene (Sigma-Aldrich) 100% ethanol and 95% ethanol. Antigen retrieval was performed using a bath of sodium citrate (pH 6.0) heated in a steamer at 98°C for 40 min, followed by application of 100 µL peroxidase blocking solution (Dako) per slide. Primary antibodies were then applied to the slides in a humidified chamber. A mouse monoclonal antibody against PARP (AbD Serotec) was utilized at 1:5000 concentration with 100 µL applied to the tissue for 1 hr at room temperature. A rabbit monoclonal antibody against AIF (Abcam) was utilized at 1:500 concentration, and again 100 µL was applied for 1 hr at room temperature. Slides were treated with 100 µL of the secondary antibody (Dako horse radish peroxidase conjugated) for 30 min at room temperature. Slides were then covered with 100 µL of 2 µL of 3,3′-diaminobenzidine chromogen in 98 µL of Dako REAL substrate buffer, which was applied for 5 min. Counterstaining was achieved with Harris hemotoxylin. Human tonsil was used to optimize the method and was included as a positive control material with every batch of slides stained. Substitution of primary antibody for phosphate buffered saline was used as a negative control.
Analysis of Slides
Slides were analyzed under microscopic evaluation, with all cores present on the microarray after staining analyzed. Extent of PARP expression (nuclear stain) was assessed quantitatively utilizing the Cell-B software system (Olympus Soft Imaging Solutions). For each core examined, the total nuclei staining positive for PARP were counted and then divided by the total number of cell nuclei counted in that core, calculating PARP expression as a percentage of the total number of cells. For each tumor, at least 3 cores were assessed, and a mean percentage calculated for each patient.
AIF stained the cytoplasm/mitochondria of cells predominantly, therefore antibody scoring was undertaken for each core as a whole in a semiquantified fashion, assessing and scoring the number of positive cells as follows: 0–1% +/−, 1–5% +, 5–15% ++, and greater than 15% +++. Analysis of the data and correlation with survival and demographic factors were accomplished by SPSS v16 for Windows using appropriate statistical tests, including Cox proportional hazards survival analysis, Student's t-test (normally distributed data), Mann–Whitney U-test (nonparametric data) and Kaplan–Meier log-rank test. Positive results were taken as significant at the P< .05 level for all tests (2-tailed).
Results
Tissue Microarray Demographics
The tissue microarray consisted of 150 pediatric HGGs, ranging in age at diagnosis from 2 days to 20 years old, with a mean age of 7 years and 10 months. The cohort included 86 males, 56 females, and 8 cases in which gender was not recorded. Overall survival was in line with other published series, with a 5-year survival of 20% and a median survival of 15 months from diagnosis. Primary tumors made up 117 of the cohort, with 15 recurrent tumors (second or third operation), 6 secondary tumors (post-ionizing radiation for hematological malignancies), and 12 tumors of unknown status. The cohort included a variety of histological diagnoses (Table 1) demonstrating the majority of tumors to be anaplastic astrocytomas or GBMs. There was a trend to longer survival for patients with anaplastic astrocytomas compared with GBMs, but this did not achieve statistical significance (P= .07).
Table 1.
Cohort of pediatric high-grade gliomas by specific histology
| Diagnosis | Frequency (n) | Percent (%) |
|---|---|---|
| Anaplastic astrocytoma | 29 | 19.3 |
| Anaplastic oligodendroglioma | 8 | 5.3 |
| Anaplastic PXA | 1 | 0.7 |
| High Grade brainstem glioma | 30 | 20.0 |
| GBM | 73 | 48.7 |
| High-grade glioma NOS | 9 | 6.0 |
| Total | 150 | 100.0 |
Abbreviation: Anaplastic PXA, anaplastic pleomorphic xanthoastrocytoma; NOS, not otherwise specified.
Data on extent of resection were available for 133 patients, with 54 undergoing biopsy only, 47 partial resection, and 32 maximal/subtotal resection. Data for adjuvant oncological therapy were available for 114 patients, with 12 patients receiving no further treatment, 21 radiotherapy only, 24 chemotherapy only, and 57 combined chemo- and radiotherapy.
PARP Immunohistochemistry
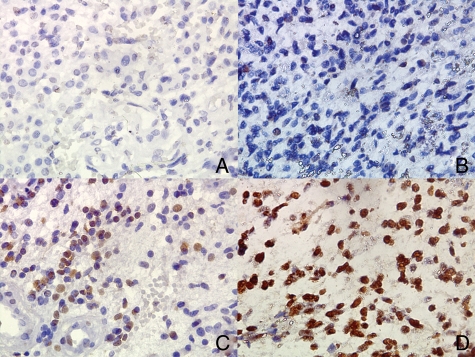
Immunohistochemical staining was successfully achieved for 98 pediatric HGGs, with a mean of 552 cells counted per tumor. Positive tumor cells demonstrated a strong nuclear pattern of staining, with no cytoplasmic staining observed. Mean percentage of tumor cells positive for PARP ranged from 0% to 99.64%, with a mean level overall across the whole cohort of 21.4% cells positive (+/− 1.9%). Heterogeneity of cell populations within tumors was observed with PARP negative cells intermingled with positive cells in the same region of a tumor (Fig. 1). No significant difference was observed in level of PARP staining between supratentorial and brainstem tumors (21.8% vs 18.4%, respectively [P= .514]).
Fig. 1.
Typical appearances of PARP immunohistochemistry demonstrating tumors (all GBM) with absent (A), low (B), moderate (C), and high (D) levels of nuclear PARP staining (brown = positive cells, blue = negative cells). Note presence of negative cells intermingled with PARP positive cells. All views x400 magnification.
On univariate analysis, the level of PARP staining in an individual tumor was not related to gender (P= .525), exact histological diagnosis (P= .742), age (P= .261), or primary/recurrent nature of tumor (P= .617). On analysis of supratentorial tumors, PARP was significant on univariate correlation (P= .019), as were extent of resection and administration of chemo/radiotherapy.
When survival was analyzed by Cox multivariate analysis (including all the variables above, plus resection status, chemo/radiotherapy status, and level of AIF staining), PARP for the tumor population overall was not significantly linked to survival (P= .172). However, if brainstem gliomas were excluded from the analysis (Table 2), then the level of PARP immunopositivity did emerge as a significant correlate with length of survival from diagnosis (P= .044). Other significant correlations with outcome were resection status (P= .006, favorable outcome with more extensive resection), administration of radiotherapy (P= .016), and administration of chemotherapy (P= .016). No factor achieved statistical significance for correlation with survival in brainstem gliomas on multivariate analysis.
Table 2.
Multivariate analysis of survival in pediatric nonbrainstem high-grade glioma
| Variable | P-value | Exp(B) |
|---|---|---|
| Age | .549 | .998 |
| Sex | .060 | .432 |
| Primary vs. recurrent tumors | .394 | 1.310 |
| Resection | .007 | |
| Resection: biopsy versus gross total resection | .006 | 5.294 |
| Resection: partial versus gross total resection | .944 | .963 |
| Diagnosis | .579 | 1.856 |
| Level of PARP immunopositivity | .044 | 1.021 |
| Level of AIF immunopositivity | .837 | 1.055 |
| Administration of radiotherapy | .016 | 3.271 |
| Administration of chemotherapy | .016 | .259 |
P-values in bold for variables significantly correlated with survival.
Upon discrete analysis of tumors with GBM histology, PARP remained a significant variable on multivariate analysis (P= .027). However, if anaplastic astrocytomas were analyzed as a discrete cohort, then level of PARP immunopositivity was no longer significant (P= .870), although only 9 cases remained with a full dataset.
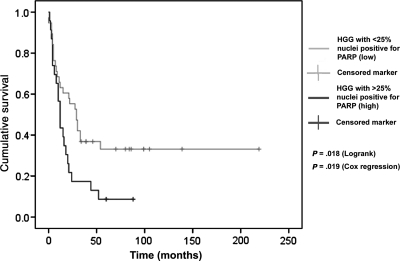
The survival benefit associated with lower levels of PARP staining in nonbrainstem HGGs was confirmed on Kaplan–Meier (log-rank) analysis, with division into low and high staining at 25% cells positive, though the correlation held at various division points (Fig. 2). The benefit of radiotherapy for nonbrainstem HGGs was also apparent on Kaplan–Meier analysis (P= .01), although chemotherapy administration did not achieve statistical significance on Kaplan–Meier for this group (P= .199). Similarly for brainstem HGGs, radiotherapy (P= .002), but not chemotherapy, (P= .247) was associated with increased survival.
Fig. 2.
Kaplan–Meier plot of cumulative survival against time for tumors exhibiting greater or less than 25% of tumor cells positive for PARP with statistically significant differences in survival for the two groups.
AIF Immunohistochemistry
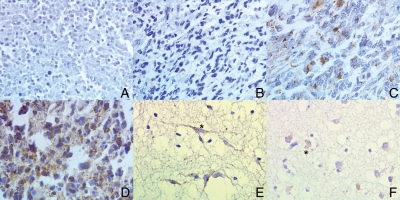
Immunostaining for AIF was successfully achieved for 95 pediatric HGG tumors (Fig. 3 for typical appearances). Expression of AIF followed a different pattern to that of PARP, predominantly staining the cytoplasm/mitochondria of the cells. AIF was expressed at moderate levels in samples of brain from cores taken adjacent to the tumor proper and in 92.5% of tumor cores, was expressed at similar (20%) or lower (72.5%) levels. Tumors stained variably, though usually consistently, for AIF, ranging overall from no staining in any core to +++ in every core. On multivariate analysis, level of AIF staining was not significantly associated with survival (P= .891 for the whole cohort; P= .623 for nonbrainstem HGGs only). Level of AIF (+/− or + versus ++ or +++) was also not significant on Kaplan–Meier analysis (P= .833). There was no significant correlation found between levels of PARP and AIF immunopositivity (Spearman's P= .370).
Fig. 3.
Typical appearances for AIF immunohistochemistry (brown regions staining positive, blue negative) (A–D all GBM). (A) Tumor with absent AIF staining. (B) Low level staining (+). (C) Moderate staining (++). (D) High staining (+++). (E) Nonneoplastic brain from adjacent to tumor demonstrating moderate levels of AIF staining in neurons (*). (F) Nonneoplastic brain demonstrating AIF immunopositivity in glia (*). All views x400 magnification.
Copy Number Changes at 1q
Thirty-three samples in the cohort underwent aCGH, with 6 showing gain of 1q. Mean percentage of cells expressing PARP was higher in samples with 1q gain (28.1% vs 17.4%), but this difference did not achieve statistical significance (t-test P = .181). Forty cases in the current cohort were also analyzed by FISH for the 1q25 locus (Vysis probe). There was no significant difference in PARP expression between tumors with gain (n= 11) and normal copy number (n= 29) (t-test P = .961). Gain of 1q on aCGH and FISH was analyzed with respect to survival. There was a trend for patients with gain of 1q as analyzed by aCGH to do worse (P= .143), but neither this nor gain of 1q on FISH (P= .480) had a statistically significant impact, probably due to the lower numbers involved.
Discussion
This study is the first large-scale examination of PARP and AIF immunostaining in pediatric HGG. We demonstrate that PARP is expressed at high levels in many pediatric HGGs and that there is a statistically significant correlation between the percentage of tumor cells staining positive for PARP and patient survival in nonbrainstem HGG (P= .044 Cox regression). AIF immunostaining did not correlate with survival, but over 70% of tumors had lower levels of AIF immunopositivity than adjacent nonneoplastic brain.
Increased levels of PARP expression in tumor cells have been associated with an adverse outcome in diverse cancers such as melanoma and ovarian serous carcinoma.19,20 One previous study examined levels of PARP staining in 25 cases of adult GBM, finding it to be widespread.21 The authors did not quantify the exact percentage of cells staining positive, though comment was made that negative cells were observed mixed in with those expressing PARP. Barton et al. examined mRNA and protein levels in a variety of childhood brain tumors (including 18 HGGs), finding some PARP expression in all samples and higher levels in HGGs compared with low-grade astrocytomas.22 No link to outcome data was included in either of these studies.
In our analysis by aCGH of 38 pediatric HGGs (including 13 brainstem tumors), gain of 1q was one of the most significant genomic alterations, although this involved the entire chromosome arm and not specifically PARP1 at 1q41.8 A study by Zarghooni et al. based on a single nucleotide polymorphism array found gain of the whole of 1q (including PARP1) in 27% of 11 pediatric brainstem gliomas studied, with 6 out of 11 tumors showing some degree of immunopositivity for PARP.7 These genomic data suggest that aberrations in the copy number of the PARP1 gene may be linked to variable levels of PARP expression in actual pediatric HGGs. However, the lack of correlation of PARP expression levels with 1q gain in the current study suggests that other mechanisms, not driven by copy number, may be involved. Tumors with the higher levels of PARP activity may be more able to repair both spontaneous and treatment-induced single strand DNA breakages, preventing progression of the damage to lethal double strand breakages.
The lack of significant correlation between level of PARP immunopositivity and survival in brainstem tumors in our study could be due to the particular anatomical location of these tumors. The proximity of vital cardiorespiratory and alertness centers means that local cancer cell invasion can quickly produce severe symptoms with only a small increase in tumor size. Alternatively, there may be real biological differences in this group that may provide an alternative DNA repair pathway to PARP and allow the tumors to resist DNA damage (including that caused by treatment) more efficiently.
Increased levels of PARP activity have been associated with activation of the caspase-independent AIF-directed apoptotic pathway. Hence, it may be conjectured that increased levels of PARP activity in a tumor might have the opposite effect on survival to that observed, restricting tumor growth by increasing neoplastic cell death. However, our current study demonstrates that most pediatric HGGs have low levels of AIF on immunohistochemistry (possible mechanisms include decreased expression or increased turnover) and that this mechanism for removing cells with too high a degree of DNA damage may have been lost. Caspase-dependent apoptotic mechanisms may still be available to the cells, but PARP seems to act as an effector rather than a driving component in these pathways.
PARP was first proposed as a therapeutic target in breast cancers stimulated by mutations of the BRCA1 and BRCA2 genes with a deficit of homologous recombination repair pathways. The hypothesis of synthetic lethality suggests that such cells should be more readily stimulated to apoptosis by inhibition of the PARP pathway, as the usual in-built redundancy of DNA repair pathways to cope with significant genome damage is lacking, and recent clinical trials23,24 have shown promising results for the use of PARP inhibitors in ovarian and breast cancer.
Early-stage clinical trials are also under way using PARP inhibitors in combination with other therapy in patients whose malignancy does not have identifiable deficits in DNA repair. For cerebral tumors, the surrounding normal brain is essentially composed of nondividing cells with nondamaged genomes and intact DNA repair mechanisms, which should have enough redundancy to give these cells a relative degree of resistance to PARP inhibition and hence reduce the toxicity of therapy.
Temozolomide therapy (in which PARP inhibition should interfere with the repair of N-methylpurines) was shown to be associated with an increase in PARP activity, and its administration alongside a PARP inhibitor resulted in a significant increase in the number of DNA single-strand breakages observed in tumor biopsies.25 However, in a subsequent phase II trial, unacceptable levels of myelosuppression were encountered, although improved temozolomide dosage regimes may alleviate this problem. No clinical trial has yet reported on a PARP inhibitor in HGG.
Studies on HGG cell lines suggest PARP inhibition could be a valuable therapeutic strategy for this malignancy. Radiosensitization, with an increase in unrepaired double strand DNA breakages, has been shown for a PARP inhibitor on the U251 adult glioblastoma line.26 The combination of PARP inhibition, temozolomide, and radiation significantly delayed tumor growth in a mouse model. In a study using a pediatric HGG cell line, PARP inhibitor monotherapy had modest effects on cell lines in vitro, but in combination with temozolomide a significant effect was achieved both in vitro and on tumors in a mouse xenograft model, with enhanced animal survival.27 Further evidence of the potential importance of PARP inhibitors in pediatric brain tumors is seen in the findings of enhancement of temozolomide activity 20-fold in some childhood medulloblastoma cell lines and significant retardation of tumor growth in mouse models.28 In these studies, the PARP inhibitors also seem to penetrate the blood–brain barrier readily, of crucial importance when considering the therapy of primary brain tumors. Resistance or failure to respond to PARP inhibitors has been observed in a proportion of all the tumors assessed in the studies described. Such lack of efficacy may be due partly to redundancy among the many DNA repair pathways that exist, with other mechanisms taking over the role of PARP when it is inhibited.
The results from our current study support further evaluation of PARP inhibitors in pediatric HGG. Although this is a large cohort, data were missing for some samples (gender for 8, tumor status for 12, extent of resection for 17, adjuvant oncological treatment for 36), and treatment regimes were heterogeneous. Firmer conclusions could be drawn from a prospective cohort treated in a uniform fashion (eg, as part of a defined clinical trial).
However, a clear suggestion does emerge of tumors with low PARP activity having a favorable prognosis. Although nearly all tumors in our study have some cells staining positively for PARP, it is clear that the level of PARP varies through this large cohort of pediatric HGGs. Further clinical evaluation of PARP inhibitors should take this variability into account, as administration of a PARP inhibitor to a patient whose tumor already has low intrinsic PARP activity may not enhance survival benefits and may worsen the myelosuppressive effect of temozolomide or other concurrent therapy.
The dual roles of PARP in DNA repair and stimulation of apoptosis remain to be fully characterized, especially in cancer cells. In the present study, we demonstrate that low intrinsic levels of PARP are linked to a better prognosis. AIF, a key mediator of PARP-induced cell death, is present at reduced levels compared with normal brain in 72.5% of the pediatric HGGs studied. It seems likely that in these tumors, hyperactivation of PARP retains its DNA repair function but has lost its ability to induce apoptosis and that therapeutic targeting of PARP is a promising anti-neoplastic measure. Inhibition of its DNA repair function is likely to enhance the effects of ionizing radiation and temozolomide (both already therapeutic mainstays for GBM), whereas reactivating the AIF pathway would represent a novel field for investigation.
Conflict of interest statement: None declared.
Funding
Royal College of Surgeons of England Surgical Research Fellowship (to S.J.S.); Joseph Foote Trust. This work was supported by the Royal College of Surgeons of England and by the Joseph Foote Trust.
Acknowledgments
We are grateful to Professor J. Lowe and Dr. K. Robson for pathology review and to Dr. R. Rahman for proofreading.
References
- 1.Porter KR, McCarthy BJ, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior and histology. Neuro-Oncol. 2010;12:520–527. doi: 10.1093/neuonc/nop066. doi:10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohrmann GJ, Farwell JR, Flannery JT. Astrocytomas in childhood: a population based study. Surg Neurol. 1985;23:64–68. doi: 10.1016/0090-3019(85)90162-4. doi:10.1016/0090-3019(85)90162-4. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya SJ, Hargrave D, Saran F, et al. Pattern of recurrence in pediatric malignant glioma: an institutional experience. J Neuro-Oncol. 2007;83:279–284. doi: 10.1007/s11060-006-9313-z. doi:10.1007/s11060-006-9313-z. [DOI] [PubMed] [Google Scholar]
- 4.Wisoff JH, Boyett JM, Berger MS, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group Trial no. CCG-945. J Neurosurg. 1998;89:52–59. doi: 10.3171/jns.1998.89.1.0052. doi:10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- 5.Faury D, Nantel A, Dunn SE, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;10:1196–1208. doi: 10.1200/JCO.2006.07.8626. doi:10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 6.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–3068. doi: 10.1200/JCO.2009.26.7252. doi:10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor α and poly(ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. doi:10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 8.Barrow J, Adamowicz-Brice M, Cartmill M, et al. Homozygous loss of ADAM3a revealed by genome wide analysis of pediatric high grade glioma and diffuse intrinsic pontine glioma. Neuro-Oncol. 2011;13(2):212–222. doi: 10.1093/neuonc/noq158. doi:10.1093/neuonc/noq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell C, Mikropoulos C, Kaye SB, et al. Pre-clinical and clinical evaluation of PARP inhibitors as tumor-specific radiosensitizers. Cancer Treat Rev. 2010;36:566–575. doi: 10.1016/j.ctrv.2010.03.003. doi:10.1016/j.ctrv.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers AJ. The potential role and application of PARP inhibitors in cancer treatment. Br Med Bull. 2009;89:23–40. doi: 10.1093/bmb/ldp005. doi:10.1093/bmb/ldp005. [DOI] [PubMed] [Google Scholar]
- 11.Heeres JT, Hergenrother PJ. Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol. 2007;11:644–653. doi: 10.1016/j.cbpa.2007.08.038. doi:10.1016/j.cbpa.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Andrabi SA, Kim NS, Yu SW, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. PNAS. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. doi:10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. doi:10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 14.Yu SW, Andrabi SA, Wang H, et al. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. PNAS. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. doi:10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culmsee C, Zhu C, Landshamer S, et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischaemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. doi:10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. doi:10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 17.Hangen E, Blomgren K, Benit P, et al. Life with or without AIF. Trends Biochem Sci. 2010;35:278–287. doi: 10.1016/j.tibs.2009.12.008. doi:10.1016/j.tibs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Cartmill M, Punt J. Diffuse brain stem glioma: a review of stereotactic biopsies. Child's Nerv Syst. 1999;15:235–237. doi: 10.1007/s003810050379. doi:10.1007/s003810050379. [DOI] [PubMed] [Google Scholar]
- 19.Csete B, Lengyel Z, Kadar Z, Battyani Z. Poly(adenosine diphosphate-ribose) polymerase-1 expression in cutaneous malignant melanomas as a new molecular marker of aggressive tumor. Pathol Oncol Res. 2009;15:47–53. doi: 10.1007/s12253-008-9086-0. doi:10.1007/s12253-008-9086-0. [DOI] [PubMed] [Google Scholar]
- 20.Brustmann H. Poly(adenosine diphosphate-ribose) polymerase expression in serous ovarian carcinoma: correlation with p53, MIB-1, and outcome. Int J Gynecol Pathol. 2007;26(2):147–153. doi: 10.1097/01.pgp.0000235064.93182.ec. [DOI] [PubMed] [Google Scholar]
- 21.Wharton SB, McNelis U, Bell HS, Whittle IR. Expression of poly(ADP-ribose) polymerase and distribution of poly(ADP-ribosyl)ation in glioblastoma and in a glioma multicellular tumor spheroid model. Neuropathol Appl Neurobiol. 2000;26:528–535. doi: 10.1046/j.0305-1846.2000.00288.x. doi:10.1046/j.0305-1846.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- 22.Barton VN, Donson AM, Kleinschmidt-DeMasters BK, et al. PARP1 expression in pediatric central nervous system tumors. Pediatr Blood Cancer. 2009;53:1227–1230. doi: 10.1002/pbc.22141. doi:10.1002/pbc.22141. [DOI] [PubMed] [Google Scholar]
- 23.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. doi:10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 24.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. doi:10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 25.Plummer R, Middleton M, Jones C, et al. Temozolomide pharmacodynamics in patients with metastatic melanoma: dna damage and activity of repair enzymes O6-alkylguanine alkyltransferase and poly(ADP-ribose) polymerase-1. Clin Cancer Res. 2005;11:3402–3409. doi: 10.1158/1078-0432.CCR-04-2353. doi:10.1158/1078-0432.CCR-04-2353. [DOI] [PubMed] [Google Scholar]
- 26.Russo AL, Kwon HC, Burgan WE, et al. In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-ribose) polymerase inhibitor E7016. Clin Cancer Res. 2009;15:607–612. doi: 10.1158/1078-0432.CCR-08-2079. doi:10.1158/1078-0432.CCR-08-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tentori L, Leonetti C, Scarsella M, et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003;9:5370–5379. [PubMed] [Google Scholar]
- 28.Daniel RA, Rozanska AL, Mulligan EA, et al. Central nervous system penetration and enhancement of temozolomide activity in childhood medulloblastoma models by poly(ADP-ribose) polymerase inhibitor AG-014699. Br J Cancer. 2010;103(10):1588–1596. doi: 10.1038/sj.bjc.6605946. [DOI] [PMC free article] [PubMed] [Google Scholar]