Abstract
Intrauterine gene transfer (IUGT) offers ontological advantages including immune naiveté mediating tolerance to the vector and transgenic products, and effecting a cure before development of irreversible pathology. Despite proof-of-principle in rodent models, expression efficacy with a therapeutic transgene has yet to be demonstrated in a preclinical nonhuman primate (NHP) model. We aimed to determine the efficacy of human Factor IX (hFIX) expression after adeno-associated-viral (AAV)-mediated IUGT in NHP. We injected 1.0–1.95 × 1013 vector genomes (vg)/kg of self-complementary (sc) AAV5 and 8 with a LP1-driven hFIX transgene intravenously in 0.9G late gestation NHP fetuses, leading to widespread transduction with liver tropism. Liver-specific hFIX expression was stably maintained between 8 and 112% of normal activity in injected offspring followed up for 2–22 months. AAV8 induced higher hFIX expression (P = 0.005) and milder immune response than AAV5. Random hepatocellular integration was found with no hotspots. Transplacental spread led to low-level maternal tissue transduction, without evidence of immunotoxicity or germline transduction in maternal oocytes. A single intravenous injection of scAAV-LP1-hFIXco to NHP fetuses in late-gestation produced sustained clinically-relevant levels of hFIX with liver-specific expression and a non-neutralizing immune response. These data are encouraging for conditions where gene transfer has the potential to avert perinatal death and long-term irreversible sequelae.
Introduction
Clinically important outcomes of intrauterine gene therapy (IUGT) using a therapeutic transgene have not been comprehensively studied in a nonhuman primate (NHP) model. This approach is relevant for monogenic diseases which cause irreversible damage to the developing fetus, such as α-thalassaemia and lysosomal storage disorders, offering an opportunity to intervene early and limit pathogenesis.1 Gene therapeutic agents, when delivered in a timely manner, may circumvent development of irreversible pathology. Although correction of several diseases has been convincingly demonstrated with adeno-associated-viral vector (AAV)-mediated postnatal gene therapy in small and large animal models,2 clinical translation has been hampered by the significant immune barrier.3 Gene delivery early in gestation may overcome this obstacle by exploiting the “preimmune” state during the critical window of thymic antigen processing (12–14 weeks' gestation in humans)4 facilitating acquisition of tolerance to foreign proteins. IUGT in late gestation may still be more effective than postnatal gene transfer considering the relative immaturity of the fetal immune system. Another advantage when using integrating vectors is the accessibility of rapidly-cycling stem and progenitor cells during fetal development which can be targeted.5 Additionally the size of the developing fetus (30–60 grams at 12–14 weeks) facilitates attainment of a higher vector-to-cell ratio to achieve the desired therapeutic effect more readily than can be realized postnatally.6 That IUGT can cure human disease has been demonstrated in proof-of-principle experiments using rodent models of Crigler–Najjar disease type I,7 Leber Congenital Amaurosis8 and hemophilia B.9 NHP are a critical pretranslational step; they not only share close immunological and developmental homology with human fetuses, but their similar placentation enables maternal safety to be explored.10 These features facilitate longitudinal assessment of treatment and safety outcomes from IUGT in a clinically-relevant model. While IUGT has been extensively studied in rodents and sheep,5,11 evaluation in NHP has been limited to delivery of marker transgenes driven by universal promoters following local and regional routes of delivery.12,13,14,15,16 As a number of candidate diseases may benefit from systemic delivery or a liver-targeting approach it is important to evaluate the outcomes of this approach in the NHP.
Recombinant AAV (rAAV) vectors are favored for gene therapy because they demonstrate wide tissue-tropism, are able to transduce postmitotic tissues efficiently, rarely integrate in the host genome, and are not known to cause human disease.17 AAV2 can be pseudotyped with different capsid proteins, the use of which can evade naturally-acquired host immunity to wild-type AAV to allow transgene expression.18 The usefulness of rAAV for mediating gene transfer has been demonstrated with both postnatal19 and intrauterine delivery.20,21,22 IUGT in fetal macaques using single-stranded AAV2 with a marker transgene resulted in multiple organ transduction, including brain and peripheral blood13 although neither duration nor effectiveness of transgene activity were examined. We have previously shown that self-complementary (sc) AAV8-human Factor IX (hFIX) produced therapeutic hFIX levels in adult macaques with far fewer vector particles than was required with single-strand AAV.23 Yet long term outcomes of scAAV-IUGT have not been evaluated in NHP.
To address this, we injected recombinant scAAV8-LP1-hFIXco23 into the intrahepatic vein of the Macaca fascicularis fetus at 0.9 gestation and evaluated tissue transduction and transgene expression. Fetuses whose mothers had pre-existing immunity to AAV8 received AAV5. We addressed clinically-relevant issues not assessed in prior NHP studies, including attainment of long-term therapeutic targets and safety aspects concerning injected offspring and mothers.
Results
IUGT and follow-up of treated offspring
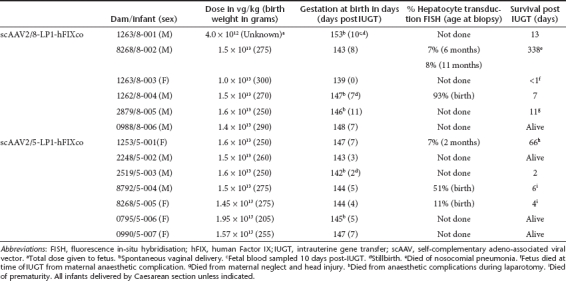
Thirteen fetuses received 4 × 1012 vector genomes (vg) (1.0–1.95 × 1013 vg/kg) by intrahepatic venous injection without transgressing the placenta. In 12 procedures reassuring fetal status was documented over 3 days post-IUGT. To analyze the temporal pattern of vector biodistribution and hFIX expression, blood and tissue samples were harvested from animals that expired in the early perinatal period; long-term monitoring was possible for six treated offspring (Table 1). Liver biopsies were performed every 6 months and no hepatic tumors were revealed. No solid tumors were found in other organs.
Table 1. Offspring biodata and outcomes following IUGT.
hFIX expression
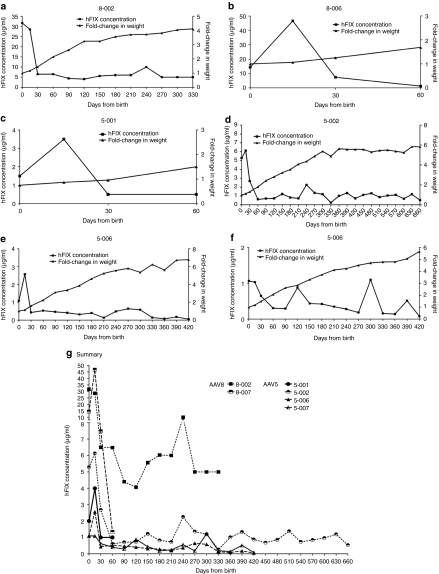
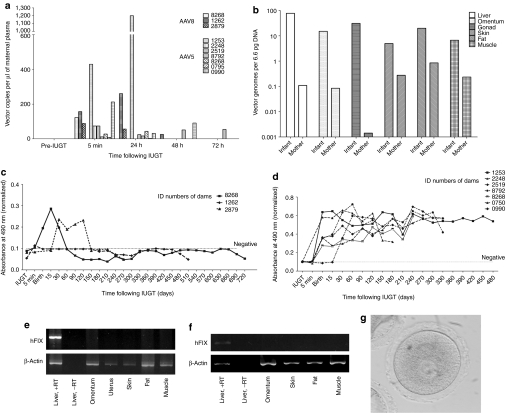
Among infants injected with scAAV2/8-LP1-hFIXco, perinatal plasma concentrations of hFIX ranged from 2.5 to 39.8 µg/ml (50–796% of normal factor IX activity) 7 to 11 days following IUGT. Among infants inoculated with scAAV2/5-LP1-hFIXco, perinatal transgene expression was lower, at 0.1 to 5.3 µg/ml (2–106%) 2 to 7 days following IUGT (P = 0.005) (Supplementary Table S1). hFIX expression was quantified longitudinally in six offspring (8-002, 8-006, 5-001, 5-002, 5-006, and 5-007). In 8-002, hFIX was 31.7 µg/ml (633%) at birth, decreased rapidly to 6.5 µg/ml (130%) at day 30, and remained between 4.1 and 10.0 µg/ml (81–200%) until 11 months of age. Remarkably, hFIX levels were stable between 1 and 11 months despite a 2.8-fold increase in body weight during this period (Figure 1a). In 8-006 hFIX was 14.3 µg/ml (286%) at birth, peaking at 46.5 µg/ml (930%) at day 15 and decreasing to 1.2 µg/ml (24%) by day 60 (Figure 1b). Offspring injected with AAV5 demonstrated a similar pattern of transgene expression as 8-002. hFIX levels in 5-001 peaked at 3.5 µg/ml on day 15 (70%) and eventually declined to and stabilized at 0.5 µg/ml (10%) between days 30 and 60 despite a 1.6-fold weight gain (Figure 1c). In 5-002 hFIX peaked at 6.1 µg/ml (122%) at 15 days, decreased to 2.7 µg/ml (54%) at 1 month, and remained stable at a median of 0.9 µg/ml (range 0.3–2.7 µg/ml) or 18%, until 22 months despite a 4.5-fold weight gain during this steady-state (Figure 1d). In 5-006 hFIX decreased from 2.6 to 0.4 µg/ml between days 15 and 30 (52% to 8%) and remained stable at a median of 0.4 µg/ml (range 0.05–1.1 µg/ml) or 8% until 14 months of age following a 4.2-fold weight gain (Figure 1e). hFIX peaked at 1.1 µg/ml at birth (22%) in 5-007 and stabilized at a median of 0.4 µg/ml (range 0.1 to 1.2 µg/ml) or 8% at 11 months, during which time weight increased 3.8-fold (Figure 1f), summarized in Figure 1g.
Figure 1.
Transgene expression in treated offspring. IUGT of 4 × 1012 scAAV2/8-LP1-hFIXco in (a) 8-002 and (b) 8-006 led to supraphysiological hFIX levels at birth (31.6 and 14.3 µg/ml respectively). This stabilized at 5.6 µg/ml in 8-002 (112%) from 1 to 11 months (duration of experiment). Therapeutic levels were maintained despite a 2.8-fold increase in body weight during this time. IUGT of 4 × 1012 vector genomes of scAAV2/5-LP1-hFIXco in 5-001 resulted in hFIX of 1.5 µg/ml (30%) at birth (c), which increased transiently to 3.5 µg/ml (70%) at 2 weeks before declining to 0.5 µg/ml (10%) between 1 and 2 months despite a 50% increase in body weight in this period. IUGT of the same AAV2/5 dose in (d) 5-002, (e) 5-006, and (f) 5-007 resulted in steady-state median hFIX expression of between 0.4 and 1.1 µg/ml (8 and 22%). hFIX expression levels from all five animals are compared in (g). hFIX, human Factor IX; IUGT, intrauterine gene transfer; scAAV, self-complementary adeno-associated viral vector.
Vector biodistribution in treated offspring
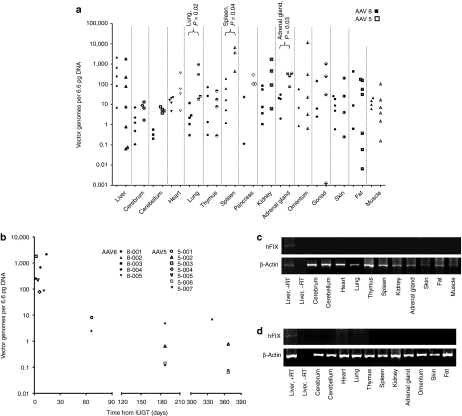
Widespread biodistribution was observed in injected infants. Both AAV5 and AAV8 demonstrated liver-tropism, and higher transduction in the lung (85-fold), spleen (65-fold), and adrenal gland (14-fold) was observed with AAV5 (P < 0.05). Among AAV8-treated offspring there was significantly higher hepatic vector copy number (VCN) compared to the cerebrum, cerebellum, heart, and lung (P ≤ 0.03). Among AAV5-treated infants VCN was higher in the spleen than in liver, skin, fat, and muscle (P ≤ 0.03) (Figure 2a). Vector copies in the liver dropped with increasing time from IUGT, reaching seven copies at 11 months in 8-002, and 0.8, 0.06, and 0.08 copies at 12 months in 5-002, 5-006, 5-007 respectively (Figure 2b). The LP1 promoter resulted in liver-specific expression of hFIX (representative reverse transcription PCR (RT-PCR), Figure 2c), as previously reported in postnatal recipients.23 In animals 8-002, 5-002, 5-006, and 5-007 liver biopsies were performed serially. Hepatic VCN remained stable over the first year with median VCN 0.4 copies at both 6 months (range 0.1–5.0) and 12 months (range 0.1–7.2) (Figure 3a).
Figure 2.
Other outcomes in treated offspring. Biodistribution of the vector of all animals (AAV8, n = 5; AAV5, n = 7) taken at necropsy or at 12 months survival surgery (5-002, 5-006, 5-007) showed comparable liver transduction with either vector (a), while AAV5 treated animals showed higher vector load in the lung, spleen, and adrenal glands (P < 0.03, nonparametric Mann–Whitney U test). Among the AAV8-treated animals there was higher VCN in the liver compared with the cerebrum, cerebellum, heart, and lung (P ≤ 0.03). Within the AAV5 group there was higher VCN in the spleen compared with the liver, skin, fat, and muscle (P ≤ 0.03). Hepatic vector biodistribution as a function of time from IUGT (b). Vector copy number in the liver decreased over four-log fold from birth to 12 months after IUGT. Delivery of either scAAV2/8-LP1-FIXco or scAAV2/5-LP1-FIXco resulted in liver-specific transgene expression as shown by RT-PCR (c,d). Shown below are representative data from offspring injected with AAV8 (at 11 months, c) and AAV5 (at 12 months, d). Gel images were captured with the AlphaDigiDoc gel documentation system (Alpha Innotech/Cell Biosciences, Santa Clara, California), AlphaEase FC/AlphaDigiDoc 1200 software and a 4 megapixel C4000 Zoom digital camera providing three times optical zoom (Olympus). AAV, adeno-associated viral vector; hFIX, human Factor IX; IUGT, intrauterine gene transfer; RT-PCR; reverse transcription PCR; VCN, vector copy number.
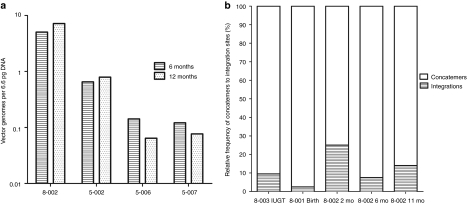
Figure 3.
Integration analysis. (a) Stable hepatic vector copy number at 6 and 12 months in the four offspring monitored during this period. (b) The retrieval frequency of all integration sites was calculated and compared with the retrieval frequency of concatemers. The frequency of concatemers (most likely originated from episomes) was variable in all analysed samples and was always greater than the frequency of integration sites. IUGT, intrauterine gene transfer.
Fluorescence in-situ hybridization and integration analysis
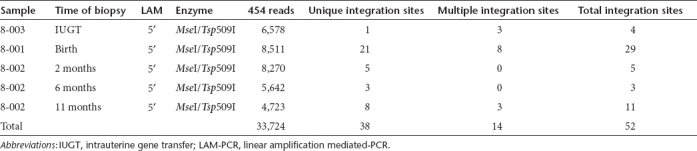
To understand the mechanism of sustained transgene expression and stable hepatic VCN, we performed fluorescence in-situ hybridization for vgs in the livers of treated offspring. The AAV genome was identified in 11–93% of hepatocytes at birth (Table 1). In 8-002 where longitudinal analysis was performed, vg was detected in 7% and 8% of hepatocytes at 6 and 11 months respectively consistent with stable VCN on qPCR (Figure 3a). Next, we investigated the presence of stably-integrated AAV genomes by searching for vector–genomic junctions which could be responsible for the observed long-term hFIX expression. We performed linear amplification mediated (LAM)-PCR followed by 454 pyrosequencing on DNA obtained from the liver of animals 8-001, 8-002, and 8-003. Indeed, out of more than 30,000 raw LAM-PCR sequence reads we identified 38 unique and 14 multiple mappable AAV integration sites from 75–250 ng of genomic DNA of each sample (Table 2, Supplementary Table S2). Though the number of retrieved integration sites does not allow statistically significant genomic distribution analyses, the detected integration loci indicate random AAV integration without obvious hotspots. Examination of liver biopsies (from different sites) taken at 2, 6, and 11 months from animal 8-002 revealed the absence of identical integrations over time (Supplementary Figure S1). In addition to the identified vector-cellular junctions, up to 97% of sequences which passed our validation criteria originated from a complex set of concatemeric rearrangements typically observed in AAV-transduced tissue.24 The retrieval frequency of sequenced LAM-PCR amplicons suggests that the number of sequenced concatemers was substantially higher than vector-cellular junctions (Figure 3b).
Table 2. LAM-PCR integration site analysis after IUGT.
Immune response in AAV-injected offspring
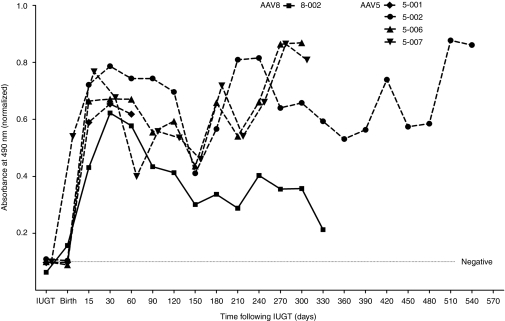
Offspring demonstrated an immune response to the viral capsid by day 15, which peaked on day 30 postnatal, more pronounced in AAV5-treated offspring as evidenced by sustained anti-AAV5 IgG for up to 19 months, in contrast to anti-AAV8 IgG for which a decline was observed from day 90 (Figure 4). Anti-hFIX antibodies were not detected and in vitro neutralizing antibody assays were negative (data not shown). hFIX expression remained within a range of 8–130% during peak anti-capsid response and hepatic transaminases were normal.
Figure 4.
Immune response in treated offspring. Late gestation IUGT with scAAV2/8-LP1-hFIXco resulted in a robust immune response against the viral capsid 8-002 which gradually declined over time. In comparison IUGT with scAAV2/5-LP1-hFIXco resulted in more robust and prolonged immune responses. AAV, adeno-associated viral vector; hFIX, human Factor IX; IUGT, intrauterine gene transfer.
Transplacental trafficking and transduction of maternal tissues
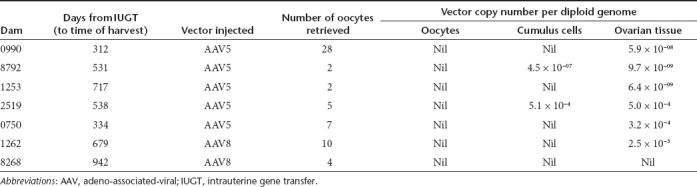
To determine the safety of AAV-mediated IUGT for dams, we evaluated maternal viral load by qPCR and found evidence of transplacental trafficking of both vectors. AAV5 viraemia was evident 72 hours postprocedure, while AAV8 viraemia was undetected beyond 48 hours (Figure 5a). Transplacental viral vector passage resulted in transduction of maternal liver, ovary, and other peripheral tissues. The median VCN of paired maternal-infant tissue samples biopsied at birth were compared in 11 mothers and their corresponding infants (Figure 5b). Hepatic, omental, and gonadal VCN were 82-fold, 141-fold, and 20-fold lower respectively in mothers compared to infants. All mothers mounted an immune response towards the respective vector capsids, with anti-AAV8 IgG peaking between 15 and 30 days postnatal before returning to pre-IUGT levels between 60 and 150 days. In contrast anti-AAV5 IgG peaked between 30 and 120 days after delivery, but did not return to pre-IUGT levels even by day 480 (Figure 5c,d). RT-PCR showed hepatic hFIX transcription but plasma hFIX was undetectable in both groups of dams (Figure 5e,f). This was not due to the development of anti-hFIX or anti-AAV neutralizing antibodies, which were absent in these animals (data not shown). To investigate maternal germ-line transmission, seven mothers underwent ovarian hyperstimulation to facilitate oocyte harvest for molecular analysis (AAV5 n = 5, AAV8 n = 2; median number of oocytes retrieved = 5, range 2–28; median time of harvest 534 days after IUGT, range 312–942). After dissociation of the oocyte-cumulus complex and teasing out pure oocytes under direct vision, qPCR confirmed vector sequences in cumulus cells and bulk ovarian tissues, but not in oocytes (qPCR sensitivity 1 vector copy in 455 diploid genomes) (Table 3, Figure 5g).
Figure 5.
Transplacental viral trafficking and maternal outcomes. Maternal viraemia available from 10 mothers (a, AAV5 n = 7, AAV8 n = 3) followed IUGT with both vectors, detectable 5 minutes after injection and resolving 72 hours later (qPCR sensitivity down to 1.5 copies). Median VCN of paired tissue biopsies taken at birth were compared in all 11 mother-infant pairs (b, AAV5 n = 7, AAV8 n = 4 with the exception of 1263/8-001). Median VCN in maternal tissues was between one and four-log fold lower than median VCN in corresponding infant tissues. (c,d) Mothers developed an immune response toward viral capsids; (d) the response was more sustained after AAV5 (c) compared to AAV8. Transgene expression was liver-specific but hFIX was undetectable in maternal plasma for both (e) AAV8 and (f) AAV5. Gel images were recorded as described. (g) Cumulus cell-free oocytes were found to be free of vector sequences whereas cumulus cells themselves contained minute quantities of proviral DNA. AAV, adeno-associated viral vector; hFIX, human Factor IX; IUGT, intrauterine gene transfer; VCN, vector copy number.
Table 3. Oocyte harvest from dams.
Discussion
The utility of AAV-directed IUGT in transducing muscle,22 retina,20 liver, myocardium, and brain21,25 is demonstrated in mice. Experience with AAV-directed IUGT in NHP is more limited. Lai et al. reported successful transduction of fetal macaques after hepatic parenchymal injection of AAV2 at 0.5–0.9 gestation, with GFP expressed up to 120 days post-IUGT.13 They observed evidence of transplacental trafficking (viraemia and peripheral monocyte transduction) in two mothers. More recently, Tarantal and Lee demonstrated long-term expression of luciferase delivered by AAV5, AAV9 and AAV10 via intrathoracic and intramyocardial injection in mid-pregnancy.26 In both studies consequent maternal effects were not discussed. Here we evaluated systemic delivery of rAAV5 and AAV8 to the late-gestation macaque fetus via percutaneous ultrasound-guided intravenous injection, using a widely-practiced clinical technique. Intravenous vector delivery should be advantageous for rare coagulopathies which benefit from an approach targeting the liver and diseases affecting multiple organs over a large tissue distribution. Considerable vector spillage into maternal circulation may be anticipated because of the proximity of fetal and maternal intraplacental circulation. Accordingly, we not only examined maternal viraemia but also maternal tissue transduction, germline transmission, and immune response, all of which are essential preclinical endpoints to support phase I trials.
IUGT was performed in late gestation when the fetal immune system remains functionally immature even at birth, albeit still capable of antibody production.27,28 The immune response was anticipated to be less vigorous than that expected in older infant and adult recipients and more permissive to transduction. Injected offspring developed anti-AAV capsid IgG but not anti-hFIX antibodies. In view of sustained transgene expression and in the absence of transaminitis this suggests the lack of an inhibitory or cell-mediated immune response, and is encouraging for future human studies. We acknowledge that late gestation intervention as used herein is temporally not vastly different from the early neonatal period, and that earlier gestation studies in the macaque are now warranted. Nevertheless, late gestation intervention may still be preferred clinically over neonatal vector delivery for certain disorders in which it is advantageous to time transgene expression to peak close to birth, such as in congenital Factor VII and Factor X deficiencies which predispose to perinatal lethality shortly after birth29 or where certain cell types are more transducible in the fetus, presenting an ontological advantage.
In our study, injected animals demonstrated hFIX levels that stabilized above 7% for at least 11 months despite a greater than threefold weight gain, which clinically is adequate to ameliorate the severe phenotype in Hemophilia B. The wide range of hFIX expression observed within the group here may be due to variability in vector delivery, batch-to-batch vector quality and individual biological response. In the attempt to understand the mechanisms behind stability of FIX expression, we inspected the relationship between VCN and FIX expression at early and late time-points. Taken as a whole without distinguishing animals injected with either serotype, hepatic analysis revealed a trend in VCN ranging from just under a hundred to over a thousand copies at or shortly following birth (n = 7), to under 10 copies at 2 months (n = 2) and a stable VCN between ~0.1 and 7 copies per cell at 6 and 12 months (n = 4), a greater than four log-fold decrease between birth and 6 months. Without the benefit of frequent serial liver biopsies on very young infants, we interpret these data to be consistent with loss of episomal copies of the AAV proviral DNA from injected animals who (with the exception of 5-006) received similar doses of between 1.0 and 1.6 × 1013 vg/kg of AAV. The trend towards rapid decline in VCN before plateauing by 2 months of age mirrors that of hFIX expression, which similarly peaks shortly after birth and reaches steady-state by 1 month postnatal age. Both patterns are observed on a background of rapid growth in infancy, and broadly comparable to mouse studies by Cunningham et al. of a 40-fold decrease in VCN 2 weeks after neonatal gene transfer.30 Although underlying mechanisms of hepatocyte transduction are not fully understood, this observed decline is likely to be due to episomal loss through hepatocellular proliferation and vector dilution as suggested by murine studies.31 Our data also concur with the above mouse studies in suggesting that while the majority of AAV reside as episomes, there is a level of vector integration which remains stable with time. We found hepatic VCN in the longitudinal analysis of 8-002, 5-002, 5-006, and 5-007 to be largely constant with minor fluctuations possibly attributable to different biopsy sites. The detection of 52 unique integration loci and the absence of hotspots in the temporal analysis of 8-002 agree with the random integration pattern observed with rAAV.32 The frequency of concatemer and integration site retrieval revealed a substantially greater presence of the former (median ca. 14-fold) at all time-points examined. Given that concatemers may originate from persisting episomes24 it seems reasonable that both stably-integrated and episomal forms of AAV contribute to long-term transgene expression.
We compared the level of transgene expression achieved with fetal treatment to that obtained by adult AAV delivery. We previously showed that systemic intravenous delivery of scAAV2/8-LP1-hFIXco to adult macaques led to hFIX expression at 14–28% of normal activity, achieved with 40–47 copies per hepatocyte33 or ca. 0.024 µg/ml of hFIX per vector copy. Our data suggest that a higher level of gene expression per vector copy is achieved with IUGT, as 8-002, 5-002, 5-006, and 5-007 in the current study expressed between 0.7 and 2.5 µg/ml per vector copy (or a mean of 1.5 µg/ml per vector copy), a 62-fold higher expression. We posit that this may be due to ontologically-acquired differences in the methylation status of the promoter/transgene in intrauterine and postnatally treated animals. An alternative postulation is that a higher VCN may result in “repeat-induced gene silencing”.34 Nevertheless, our findings suggest that a single intravenous AAV injection of a suitable vector dose in late gestation may be adequate to provide long-term protein expression at a therapeutic level without needing to re-administer vector in early infancy. Given reports of rAAV integration35,36 and of hepatocellular carcinoma after intravenous delivery in neonatal mice,37 we report no liver dysfunction or hepatic tumors in treated offspring monitored up to 22 months (median 14 months, range 2–22), despite a high vector dose of ca. 1.5 × 1013 vg/kg administered during fetal life. Similarly, no tumors were revealed in mothers monitored for a median of 18 months post-IUGT (range 10–31 months). While these reassuring data mirror studies indicating the safety of AAV gene transfer in fetal,34 neonatal30 and adult mice38 it should be noted that hepatocellular carcinomas were observed 18 months after neonatal gene transfer.37 Thus long-term monitoring of injected offspring will be necessary to address the likelihood of tumorigenesis.
At equivalent doses, splenic transduction with AAV5 was significantly higher than hepatic VCN, and was two log-fold higher than splenic transduction with AAV8. This observation partly concurs with the differential vector distribution of AAV5 and AAV8 in adult NHP recipients of gene transfer,33 and may be due to a predilection of AAV5 for reticuloendothelial tissue due to capsid protein-cell receptor interactions, which may help explain the apparent greater immunogenicity of AAV5. The degree of maternal tissue transduction as a result of transplacental passage was several log-fold lower compared to their injected offspring. Despite the presence of hFIX mRNA in maternal livers (Figure 5c) and the absence of anti-hFIX and neutralizing antibodies (NAb), mothers did not express detectable plasma hFIX, an observation possibly related to low vector copies in maternal liver (median maternal VCN of 0.1 per hepatocyte versus median VCN of 78 per hepatocyte in infants, at birth, Figure 5b). In our experience with postnatal gene transfer in NHPs, circulating AAV8 was cleared more rapidly than AAV5, with consequently less immune activation.33 This observed transplacental passage of intrauterine-delivered AAV mirrors the vertical transmission from mother to fetus of wild-type AAV in human pregnancies.39 Receptor-mediated endocytosis or inflammatory damage in the syncytiotrophoblast may play a part in vertical transmission of viruses across the placenta, although exact mechanisms are unclear. Size of biological agents is not the only determining factor, as other cellular and paracellular mechanisms may also be involved.40,41 Serious concern remains over possible adverse consequences for the mother. Although there has been no correlation established between wild-type AAV and infectious pathology in humans despite detection of viral DNA in multiple tissues,42 AAV2 has been detected in spontaneous first trimester abortuses, with the effects of AAV in pregnancy being less well-understood.39,43 In contrast to previous efforts to delineate maternal germline transmission through laser-dissection of block tissues44 which may still include cells not of oocyte origin, we have isolated pure oocytes through ovarian hyperstimulation and reassuringly demonstrate the absence of maternal germline transmission. Germline transduction was not assessed in injected infants as, when the atresia of oocytes and high turn-over of sperm in reproductively-capable animals is considered, this parameter only becomes relevant in adolescent NHP around 4–6 years of age. Our finding of the absence of immunotoxicity, tumor formation, or maternal germline transduction is therefore cautiously optimistic for translational application.
In conclusion our study demonstrated the efficacy of IUGT with scAAV5 and scAAV8 vectors after intravenous delivery in late gestation. While our proof-of-principle model of Hemophilia B is unlikely to be a first-line candidate for intrauterine treatment, this IUGT-NHP model holds more value in its potential application to perinatal genetic diseases that require a high level of liver transduction and transgene expression to succeed. We believe that our data will be informative in predicting clinical IUGT outcomes in which late-gestation intervention may still hold potential benefit, where diagnosis is made in advanced pregnancy, or in conditions associated with permanent early-onset neurological defects or with a high perinatal mortality such as congenital Factor VII and X deficiency, Protein C deficiency, and ornithine transcarbamylase deficiency.45
Materials and Methods
Macaques and intrauterine gene transfer. Animal procedures were approved by the Institutional Animal Care and Use Committee at the Singapore Health Services and conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited facility. Female M. fascicularis of reproductive age (4–8 years) weighing 3.5–5kg were screened for pre-existing immunity to AAV serotypes 5 and 8. Seronegative females were time-mated and monitored by transabdominal ultrasound for pregnancy (4C3V curvilinear probe at 7 MHz; GE LP5, GE, Fairfield, CT). Viable pregnancies were dated and serial fetal biometry (biparietal diameter, femur length) performed to assess growth using standard fetal growth charts.46 At 0.9G (135–141 days) IUGT was performed by inserting a 22G siliconized hypodermic needle (ECHOtip; Cook Medical) percutaneously into the fetal intrahepatic vein under continuous transabdominal ultrasound and general anesthesia (GA). After aspirating fetal blood (200 µl) for baseline analyses, 4 × 1012 vgs of AAV2/8-LP1-FIXco (n = 5) or AAV2/5-LP1-FIXco (n = 7) were administered as a slow bolus in 4 ml saline under continuous ultrasound. Fetal heart-rate was monitored up to 5 minutes postprocedure. Fetal viability was confirmed in all injected fetuses up to 72 hours postprocedure with a hand-held Doppler machine. Maternal blood samples were collected 5 minutes post-IUGT and 24-hourly for 72 hours postinjection. Mothers were given dexamethasone (2.4 mg intramuscular over 24 hours) in case of preterm delivery. Animals were screened monthly with complete blood counts and liver function tests.
Delivery and monitoring of transplanted offspring. With the exception of five infants born spontaneously, all fetuses were delivered by classical Caesarean section between 143–147 gestational days performed via midline incisions under GA. Biopsies were obtained from maternal liver, ovary, myometrium, omentum, skin, subcutaneous fat, and skeletal muscle. Newborns were hand-reared until 6 months of age and housed separately from their mothers. Plasma was collected monthly from mother and offspring for hFIX and antibody assays. Liver biopsies were obtained under GA at 6-monthly intervals from offspring and dams. The superior and inferior surfaces of the right, median, and left hepatic lobes were carefully inspected and palpated for superficial and parenchymal growths during laparotomy or subsequent necropsy. Animals that expired during the course of the study were subjected to full necropsies at which all organs were bisected and examined for tumors.
scAAV-hFIX vector production and purification. The scAAV vector includes a liver-specific regulatory element (LP1) consisting of core domains from the human apolipoprotein hepatic control region and the human α-1-antitrypsin gene promoter, followed by a codon-optimized human FIX (hFIX) cDNA.23 Vectors were made by adenovirus-free transient transfection using chimeric AAV2 Rep-5Cap and AAV2 Rep-8Cap packaging plasmids (pLT-RCO3 and pAAV8-2) to generate scAAV5- and scAAV8-pseudotypes, and purified and characterized as previously described.33 Vector genome titers were determined by real-time quantitative PCR (qPCR) using linearized plasmids as standards.
Detection of hFIX in macaque plasma. Human-specific FIX antigen levels were determined by enzyme linked immunosorbent assay as described previously.47 Briefly, 96-well plates (NUNC, Rochester, NY) were coated with polyclonal rhesus antihuman Factor IX antibody and were in turn serum-blocked, incubated with samples or standards (diluted 1:50), polyclonal goat anti-hFIX IgG (1:2,000), and urea hydrogen peroxide and OPD peroxidase substrate (SIGMA FAST P9187; Sigma, St Louis, MI), with the color reaction quenched after 5 minutes with 3 mol/l HCl. Maximum UV absorption was measured at 490 nm.
Detection of Anti-AAV5 and anti-AAV8 antibodies. 96-well plates (NUNC) were coated overnight with 5 × 108 AAV viral particles and processed as previously described.47 Absorbance was read at 490 nm, normalized to baseline (negative control, plasma from an uninjected macaque fetus) and expressed as a ratio of the positive control (plasma from a seropositive macaque).
Detection of NAb to AAV. The presence of NAb was determined by inhibition of 293T cell transduction with rAAV-CMV-eGFP. Briefly, 50 µl plasma (1:100) was incubated with 5 × 1010 rAAV5 or rAAV8 particles before further incubation with 1 × 105 293T cells as previously described.47 Transduction was evaluated by flow cytometry. The presence of NAb was determined by a reduction in 293T transduction of 50%.
Detection of maternal viraemia. Cell-free DNA was extracted from maternal plasma as previously described.33 qPCR was performed using 1× SYBR Green Master Mix (Applied Biosystems/Life Technologies, Carlsbad, CA) and 100 nmol/l each of forward primer 5′-GGGCAAGTATGGCATCTACA-3′ and reverse primer 5′-AAAGCATCGAGTCAGGTCAG-3′ that amplified an 84bp region of the transgene encompassing the 3′ end of the codon-optimized hFIX transgene and the 5′ region of the SV40 late poly A sequence. Cycling conditions were 95 °C for 5 minutes, 55 cycles of 95 °C for 10 seconds and 60 °C for 30 seconds. Viral load was expressed as copies per µl maternal plasma.
Quantitative PCR for vgs. Genomic DNA was extracted from snap-frozen tissue with phenol:chloroform:isoamylalcohol 25:24:1 (Sigma-Aldrich, St Louis, MO) after overnight digestion at 55 °C in lysis buffer (10 mmol/l Tris, 50 mmol/l NaCl, 5 mmol/l EDTA, 1 mg/ml Proteinase K, 0.5% Sodium Dodecyl Sulfate) and ethanol precipitation. 15 ng of genomic DNA was subjected to qPCR as above. To check equivalent loading, a 52 bp region of the macaque β-actin gene was amplified using forward primer 5′-TCCTGTGGCATCCACGAAA-3′ and reverse primer 5′-CCACGTCACACTTCATGATGG-3′. Vector copies were expressed per diploid genome (6.6 pg of DNA, VCNs per cell) and the calculated limit of detection was 1 vg per 455 diploid genomes.
Reverse transcription PCR. Transgene expression was determined by RT-PCR. Total RNA was extracted from snap-frozen macaque tissues using TRIzol (Invitrogen/Life Technologies) and subjected to DNA digestion before 1 µg was reverse-transcribed using the Superscript II First Strand cDNA synthesis kit (Invitrogen/Life Technologies) in a total volume of 19 µl. Two microlitres of cDNA was used to amplify a 617 bp region of the hFIX transgene using forward primer 5′-TTTCCTGATGTGGACTATGT-3′ and reverse primer 5′-TCATGGAAGCCAGCACAGAACATG-3′. Integrity of cDNA was determined by amplifying a 604 bp region of the macaque β-actin gene using forward primer 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and reverse primer 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ in a 50 µl reaction. The cycling conditions were: 95 °C for 5 minutes, 40 cycles of 95 °C for 30 seconds, 57 °C for 30 seconds (hFIX) or 60 °C for 30 seconds (β-actin) and 72 °C for 60 seconds, with final extension of 72 °C for 7 minutes, and the product resolved on 2% agarose gel.
Oocyte harvest and analysis of germline transmission. Maternal ovarian hyperstimulation was performed to evaluate gamete transduction. From day 2 of menstruation, dams received 30 IU twice-daily of recombinant follicular stimulating hormone (Gonal-F, Merck; Puregon, Organon/MSD) subcutaneously. From day 6, 250 µg daily of gonadotrophin releasing hormone antagonist (Ganirelix acetate, Orgalutran, MSD) was administered subcutaneously to prevent premature ovulation. Medications were continued until day 12 when both ovaries were harvested. Ovarian follicles were aspirated using a 22G needle and oocytes dissociated from oocyte-cumulus complexes via incubation in hyaluronidase (10 IU/ml) using a flame-pulled fine-bore glass pipette. Oocytes from each animal were pooled, washed five times in phosphate-buffered saline, heated at 65 oC for 10 minutes in 1 µl lysis buffer (0.2 mol/l potassium hydroxide in MiliQ water), and neutralized in 1 µl of 0.2 mol/l tricine (C6H13NO5). Of the final cell-free DNA 1 µl was used to determine VCN. Sensitivity of this qPCR was 1 vg in 455 diploid genomes.
Fluorescent in-situ hybridization. AAV was visualized in frozen liver biopsy specimens that were thawed, fixed and hybridized with digoxigenin dUTP labeled LP1-hFIXco or a randomly selected macaque BAC clone (CH250-279C2) probe. Specific hybridization signals were detected using FITC conjugated antidigoxigenin antibodies; the slides were then counterstained in DAPI and analyzed under epi-fluorescence microscopy.
LAM-PCR integration site analysis. LAM-PCR was used to isolate AAV genome junctions after IUGT. Unknown AAV flanking sequences were amplified from 75–250 ng of genomic DNA extracted from animals 8-001, 8-002, and 8-003 as described previously48 with inclusion of the following primers specific for the AAV vector sequence: 5′-GTCGAGTGGATGTTGGAGGTG-3′ for linear PCR, 5′-AGCTCCAAGGTCAGCAGGCA-3′ for the first exponential PCR and 5′-GTGTTTGCTGTTTGCTGCTTGC-3′ for the second exponential PCR. For next generation pyrosequencing (GS FLX 454 pyrosequencing, Roche, Basel, Switzerland), an additional PCR amplification reaction with bar-coded fusion-primers (5′-GCCTCCCTCGCGCCATCAG(N)6GCCCATTTTAGGGGTCACGA-3′ specific for the rAAV vector and 5′-GCCTTGCCAGCCCGCTCAGAGTGGCACAGCAGTTAGG-3′ specific for the linker cassette) was performed. LAM-PCR sequences obtained by 454 pyrosequencing were analyzed by automated bioinformatical data mining, including alignment using UCSC BLAT analyzing tools and identification of integrating features as previously described.49 Furthermore, we estimated the presence of integration sites versus concatemers by measuring the retrieval frequency of individual LAM-PCR amplicons. The retrieval frequency of all integration sites was calculated from the total number of 454 reads whose 5′end matched the rAAV vector and whose 3′end continued in a different nucleotide-configuration. LAM-PCR amplicons resembling concatemeric rearrangements of the vector were defined when the sequences following the ITR breakpoint matched the AAV vector. For identified AAV integration sites the sequences following the ITR breakpoint were mapped to the rhesus genome.
Statistics. Nonparametric data were shown as median and range, and analyzed using the Mann–Whitney U Test. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Fluorescent in situ hybridization of transduced hepatocyte. Table S1. Long-term trends in hFIX expression and hepatic VCN in injected infants monitored for 2 months or more. Table S2. Molecular characterization of integration events in the liver of animals 8-001, 8-002 and 8-003 different time points after IUGT.
Acknowledgments
We acknowledge assistance from Mark Chong, Lay-Geok Tan, Sam Chong (National University of Singapore), and Darvi Michell, Vivienne Liang and Chin-Yong Kiong (Singhealth Experimental Medicine Centre). This study was funded by the BioMedical Research Council (BMRC 09/1/21/19/5 and 06/1/21/19/450) and National Medical Research Council (CSA/012/2009) Singapore, and the European Union (CLINIGENE). M.C. and J.C. received salary support from the Clinician Scientist Award, NMRC (CSA/007/2009 and CSA/012/2009). We thank Merck for supplying Gonal-F and MSD for Puregon and Ganirelix. The authors declare no competing financial interests.
Supplementary Material
Fluorescent in situ hybridization of transduced hepatocyte.
Long-term trends in hFIX expression and hepatic VCN in injected infants monitored for 2 months or more.
Molecular characterization of integration events in the liver of animals 8-001, 8-002 and 8-003 different time points after IUGT.
REFERENCES
- Santore MT, Roybal JL., and, Flake AW. Prenatal stem cell transplantation and gene therapy. Clin Perinatol. 2009;36:451–471, xi. doi: 10.1016/j.clp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF.et al. (1999Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors Nat Med 564–70. [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jèze G, Marodon G, Salomon BL, Catala M., and, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- Porada CD, Park PJ, Almeida-Porada G, Liu W, Ozturk F, Glimp HA.et al. (2005Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer Mol Ther 11284–293. [DOI] [PubMed] [Google Scholar]
- Brenner WE, Edelman DA., and, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126:555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- Seppen J, van der Rijt R, Looije N, van Til NP, Lamers WH., and, Oude Elferink RP. Long-term correction of bilirubin UDPglucuronyltransferase deficiency in rats by in utero lentiviral gene transfer. Mol Ther. 2003;8:593–599. doi: 10.1016/s1525-0016(03)00234-x. [DOI] [PubMed] [Google Scholar]
- Dejneka NS, Surace EM., and, Bennett J. Gene therapy for Leber congenital amaurosis. Adv Exp Med Biol. 2003;533:415–422. doi: 10.1007/978-1-4615-0067-4_53. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Nivsarkar MS, Mistry AR, Buckley SM, Kemball-Cook G, Mosley KL.et al. (2004Permanent phenotypic correction of hemophilia B in immunocompetent mice by prenatal gene therapy Blood 1042714–2721. [DOI] [PubMed] [Google Scholar]
- Weatherall DJ. Academy of Medical Sciences: London; 2006. The Use of Non-Human Primates in Research. [Google Scholar]
- Waddington SN, Buckley SM, David AL, Peebles DM, Rodeck CH., and, Coutelle C. Fetal gene transfer. Curr Opin Mol Ther. 2007;9:432–438. [PubMed] [Google Scholar]
- Tarantal AF, Lee CI, Ekert JE, McDonald R, Kohn DB, Plopper CG.et al. (2001Lentiviral vector gene transfer into fetal rhesus monkeys (Macaca mulatta): lung-targeting approaches Mol Ther 4614–621. [DOI] [PubMed] [Google Scholar]
- Lai L, Davison BB, Veazey RS, Fisher KJ., and, Baskin GB. A preliminary evaluation of recombinant adeno-associated virus biodistribution in rhesus monkeys after intrahepatic inoculation in utero. Hum Gene Ther. 2002;13:2027–2039. doi: 10.1089/10430340260395884. [DOI] [PubMed] [Google Scholar]
- Jimenez DF, Lee CI, O'Shea CE, Kohn DB., and, Tarantal AF. HIV-1-derived lentiviral vectors and fetal route of administration on transgene biodistribution and expression in rhesus monkeys. Gene Ther. 2005;12:821–830. doi: 10.1038/sj.gt.3302464. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, McDonald RJ, Jimenez DF, Lee CC, O'Shea CE, Leapley AC.et al. (2005Intrapulmonary and intramyocardial gene transfer in rhesus monkeys (Macaca mulatta): safety and efficiency of HIV-1-derived lentiviral vectors for fetal gene delivery Mol Ther 1287–98. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Lee CC, Jimenez DF., and, Cherry SR. Fetal gene transfer using lentiviral vectors: in vivo detection of gene expression by microPET and optical imaging in fetal and infant monkeys. Hum Gene Ther. 2006;17:1254–1261. doi: 10.1089/hum.2006.17.1254. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM., and, Tuddenham EG. Prospects for gene therapy of haemophilia. Haemophilia. 2004;10:309–318. doi: 10.1111/j.1365-2516.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y.et al. (2005Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models Mol Ther 11875–888. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y.et al. (2004Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1 Blood 10385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A.et al. (2004In utero gene therapy rescues vision in a murine model of congenital blindness Mol Ther 9182–188. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Mackenzie TC, Peranteau W, Edmonson S, Campagnoli C, Liu YL.et al. (2007Persistent expression of hF.IX After tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice Mol Ther 151677–1685. [DOI] [PubMed] [Google Scholar]
- Koppanati BM, Li J, Xiao X., and, Clemens PR. Systemic delivery of AAV8 in utero results in gene expression in diaphragm and limb muscle: treatment implications for muscle disorders. Gene Ther. 2009;16:1130–1137. doi: 10.1038/gt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN.et al. (2006Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver Blood 1072653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Chérel Y, Chenuaud P.et al. (2008Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle J Virol 827875–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz GS, Titre D, Brindle M, Bisconte AR, Contag CH., and, Gaensler KM. Comparison of gene expression after intraperitoneal delivery of AAV2 or AAV5 in utero. Mol Ther. 2003;8:90–98. doi: 10.1016/s1525-0016(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Tarantal AF., and, Lee CC. Long-term luciferase expression monitored by bioluminescence imaging after adeno-associated virus-mediated fetal gene delivery in rhesus monkeys (Macaca mulatta) Hum Gene Ther. 2010;21:143–148. doi: 10.1089/hum.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker L., and, Marshall-Clarke S. Functional responses of human neonatal B lymphocytes to antigen receptor cross-linking and CpG DNA. Clin Exp Immunol. 2003;134:409–419. doi: 10.1111/j.1365-2249.2003.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M.et al. (2004Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns Blood 1031030–1032. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Kennea NL, Buckley SM, Gregory LG, Themis M., and, Coutelle C. Fetal and neonatal gene therapy: benefits and pitfalls. Gene Ther. 2004;11 Suppl 1:S92–S97. doi: 10.1038/sj.gt.3302375. [DOI] [PubMed] [Google Scholar]
- Cunningham SC, Dane AP, Spinoulas A, Logan GJ., and, Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L., and, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Young SM., Jr, and, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y.et al. (2007Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates Blood 1091414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D.et al. (2011Long-term Safety and Efficacy Following Systemic Administration of a Self-complementary AAV Vector Encoding Human FIX Pseudotyped With Serotype 5 and 8 Capsid Proteins Mol Ther 19876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz BR., and, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Piao C, Kotchey NM, Wu X., and, Nakai H. Frequency and spectrum of genomic integration of recombinant adeno-associated virus serotype 8 vector in neonatal mouse liver. J Virol. 2008;82:9513–9524. doi: 10.1128/JVI.01001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW.et al. (2007AAV vector integration sites in mouse hepatocellular carcinoma Science 317477. [DOI] [PubMed] [Google Scholar]
- Bell P, Wang L, Lebherz C, Flieder DB, Bove MS, Wu D.et al. (2005No evidence for tumorigenesis of AAV vectors in a large-scale study in mice Mol Ther 12299–306. [DOI] [PubMed] [Google Scholar]
- Burguete T, Rabreau M, Fontanges-Darriet M, Roset E, Hager HD, Köppel A.et al. (1999Evidence for infection of the human embryo with adeno-associated virus in pregnancy Hum Reprod 142396–2401. [DOI] [PubMed] [Google Scholar]
- Bianchi DW. Robert E. Gross Lecture. Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. J Pediatr Surg. 2007;42:12–18. doi: 10.1016/j.jpedsurg.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Burton GJ., and, Watson AL. The Structure of the Human Placenta: Implications for Initiating and Defending Against Virus Infections. Rev Med Virol. 1997;7:219–228. doi: 10.1002/(sici)1099-1654(199712)7:4<219::aid-rmv205>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Schlehofer JR., and, Dupressoir T. Infectiology and pathology of human adeno-associated viruses. Contrib Microbiol. 2000;4:59–67. doi: 10.1159/000060326. [DOI] [PubMed] [Google Scholar]
- Tobiasch E, Rabreau M, Geletneky K, Laruë-Charlus S, Severin F, Becker N.et al. (1994Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion J Med Virol 44215–222. [DOI] [PubMed] [Google Scholar]
- Lee CC, Jimenez DF, Kohn DB., and, Tarantal AF. Fetal gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta) Hum Gene Ther. 2005;16:417–425. doi: 10.1089/hum.2005.16.417. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Kramer MG, Hernandez-Alcoceba R, Buckley SM, Themis M, Coutelle C.et al. (2005In utero gene therapy: current challenges and perspectives Mol Ther 11661–676. [DOI] [PubMed] [Google Scholar]
- Tarantal AF., and, Hendrickx AG. Prenatal growth in the cynomolgus and rhesus macaque (macaca fasicularis and macaca mulatta): a comparison by ultrasonography. Am J Primatol. 1988;15:309–323. doi: 10.1002/ajp.1350150405. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A.et al. (2002Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques Blood 1001662–1669. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schwarzwaelder K, Bartholomae C, Zaoui K, Ball C, Pilz I.et al. (2007High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR) Nat Methods 41051–1057. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I.et al. (2009Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy Science 326818–823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescent in situ hybridization of transduced hepatocyte.
Long-term trends in hFIX expression and hepatic VCN in injected infants monitored for 2 months or more.
Molecular characterization of integration events in the liver of animals 8-001, 8-002 and 8-003 different time points after IUGT.