Abstract
Muscular dystrophies, and other diseases of muscle, arise from recessive and dominant gene mutations. Gene replacement strategies may be beneficial for the former, while gene silencing approaches may provide treatment for the latter. In the last two decades, muscle-directed gene therapies were primarily focused on treating recessive disorders. This disparity at least partly arose because feasible mechanisms to silence dominant disease genes lagged behind gene replacement strategies. With the discovery of RNA interference (RNAi) and its subsequent development as a promising new gene silencing tool, the landscape has changed. In this study, our objective was to demonstrate proof-of-principle for RNAi therapy of a dominant myopathy in vivo. We tested the potential of adeno-associated viral (AAV)-delivered therapeutic microRNAs, targeting the human Facioscapulohumeral muscular dystrophy (FSHD) region gene 1 (FRG1), to correct myopathic features in mice expressing toxic levels of human FRG1 (FRG1−high mice). We found that FRG1 gene silencing improved muscle mass, strength, and histopathological abnormalities associated with muscular dystrophy in FRG1−high mice, thereby demonstrating therapeutic promise for treatment of dominantly inherited myopathies using RNAi. This approach potentially applies to as many as 29 different gene mutations responsible for myopathies inherited as dominant disorders.
Introduction
The concept of muscle gene therapy arose soon after dystrophin mutations were identified as the underlying cause of Duchenne muscular dystrophy (DMD) in 1987.1 Because DMD was a recessive disorder caused by the lack of normal dystrophin in muscle, several research groups began developing dystrophin gene replacement strategies as potential treatments for DMD.2 For many years, this was the sole focus of the nascent muscle gene therapy field, but as mutations in other myopathy-related genes were subsequently identified, the field expanded beyond DMD to include other muscle disorders.3 These disease gene identification studies, combined with important advancements in adeno-associated viral (AAV) vector development and delivery over the last two decades, contributed to several successful preclinical gene therapy trials in animal models of various myopathies.2,4,5,6,7,8,9,10,11,12,13 Importantly, one recently translated study showed the first promising Phase I clinical trial data for gene therapy of limb girdle muscular dystrophy in humans.14 Thus, steady progress in a relatively short period of time supports that gene therapy may someday be an effective method for treating inherited disorders of muscle.
Nevertheless, most of the current progress in the field has been primarily directed toward developing therapies for recessive disorders, while approaches to treat dominant myopathies were largely unexplored by comparison.2,4,5,6,7,8,9,10,11,13 This disparity in research focus is significant, as two of the three most common muscular dystrophies are dominant (facioscapulohumeral muscular dystrophy, FSHD; myotonic dystrophy, DM), and more than half of all currently known myopathy-related disease genes are linked to dominant disorders.15 One reason the muscle gene therapy field principally focused on recessive myopathies relates to the technical feasibility of the strategies necessary to treat each class of disorders. Specifically, recessive disorders require gene replacement, while dominant diseases would potentially benefit from disease gene silencing.15 Historically, feasible molecular tools existed to accomplish the former, but not the latter. This disparity could change however, with the recent emergence of RNA interference (RNAi) as a promising therapeutic approach to silence dominant disease genes.16 The initial work in this area mostly focused on treating neurodegenerative disease,16 but we hypothesized that RNAi could also be an effective mechanism to silence genes associated with dominant myopathies, which has not been previously illustrated.15 The goal of this study was to demonstrate proof-of-principle that RNAi-based gene therapy could correct muscle abnormalities in a mouse model of dominant myopathy. To do this, we used the FRG1−high transgenic mouse line, which develops myopathy caused by muscle-specific over-expression of the human FSHD region gene 1 (FRG1).17 As the gene name suggests, FRG1−high mice were initially developed as a putative model of autosomal dominant FSHD, but the pathogenic mechanisms underlying this disorder are not altogether resolved; indeed, recent data support a model in which DUX4 over expression is a primary pathogenic insult underlying FSHD.17,18,19,20,21,22,23 Thus, both FRG1 and DUX4 may be candidate targets for RNAi therapy, but there are no published animal models stably expressing the latter. We therefore focused on FRG1 in this study, and tested the potential of AAV-delivered, FRG1-targeted microRNAs to correct myopathy in FRG1−high mice. Our results demonstrate the therapeutic promise of RNAi therapy for FSHD candidate genes specifically, and dominant myopathies in general.
Results
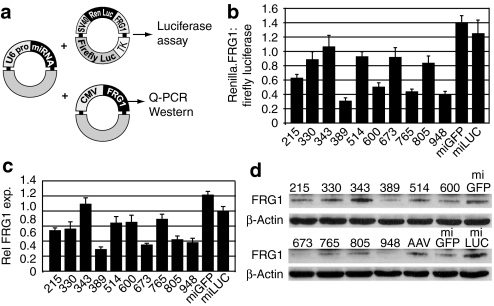
Myopathy in FRG1−high mice results from muscle-specific over-expression of the human FRG1 coding region.17 We therefore hypothesized that FRG1 knockdown using RNAi would improve myopathic phenotypes. To do this, we designed ten different U6 promoter-driven artificial microRNAs targeting sequences in the human FRG1 coding region (Supplementary Figure S1). We then identified our lead FRG1-targeted microRNA (miFRG1) using in vitro screening assays. First, we cotransfected U6.miFRG1 constructs with a plasmid expressing a Renilla luciferase-FRG1 fusion transcript and a separate firefly luciferase transfection control (Figure 1a). We then measured miFRG1-mediated gene silencing indirectly by determining the ratio of Renilla to firefly luciferase activity from transfected cell lysates, 2 days later (Figure 1b). To confirm these gene silencing data against a normal FRG1 open reading frame, we cotransfected individual U6.miFRG1 plasmids with a CMV.FRG1 expression vector into HEK293 cells and measured FRG1 transcript and protein levels by real-time PCR and western blot, respectively (Figure 1c–d). We identified two different miFRG1 sequences that consistently silenced FRG1 using all three assays, and we chose one of these (miFRG1.948; heretofore referred to as miFRG1) for in vivo studies because it catalyzed slightly better silencing at the protein level (Figure 1d).
Figure 1.
In vitro screen to identify lead miFRG1 sequences. (a) Plasmids used for testing FRG1 gene silencing in HEK293 cells. (Left) Each FRG1-targeted miRNA (miFRG1) was cloned downstream of the mouse U6 promoter (U6 pro). U6.miFRG1 expression plasmids were cotransfected with FRG1 target plasmids expressing Renilla luciferase (RenLuc)-FRG1 fusion transcripts (right, top) or the human FRG1 open reading frame (right, bottom). In the Luciferase-FRG1 expression plasmid, human FRG1 was placed downstream of the Renilla luciferase stop codon, thereby serving as a 3′ UTR. This plasmid also contained a separate Firefly luciferase reporter, which was useful as a transfection control. SV40, SV40 promoter; TK, herpes simplex virus (HSV) thymidine kinase promoter; CMV, cytomegalovirus promoter. (b) Luciferase assay screen. FRG1 gene silencing was initially determined by measuring the ratio of Renilla:Firefly luciferase activity from cotransfected cell lysates. Numbers on X-axis indicate miFRG1 sequences; numbers correspond to position on the FRG1 cDNA. MiGFP and miLUC control miRNAs do not target FRG1. (c) Relative FRG1 mRNA and (d) protein expression in HEK293 cells cotransfected with CMV.FRG1 and indicated miFRG1 expression plasmids. (c) FRG1 levels were determined by Taqman assay and normalized to human β-actin expression. Data represent SEM from two independent experiments performed in triplicate. Western blot in (d) shows representative data from three independent experiments. The U6.miFRG1.948 sequence consistently knocked down human FRG1 levels in all three assays. It was subsequently used in all in vivo experiments. eGFP, enhanced green fluorescent protein; MiGFP, microRNAs targeting eGFP.
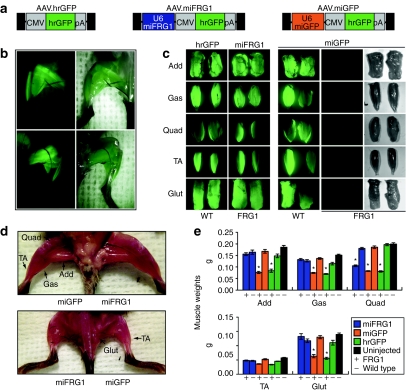
We next cloned U6.miFRG1 or an U6.miGFP control microRNA into our AAV.CMV.hrGFP proviral vector.24 The miGFP control microRNA, which targets sequences in the enhanced green fluorescent protein (eGFP) gene, does not direct knockdown of FRG1 or our hrGFP reporter. We then made AAV6 viral vectors expressing hrGFP alone (AAV.hrGFP), or hrGFP with miFRG1 or microRNAs targeting eGFP (miGFP) (AAV.miFRG1 or AAV.miGFP, respectively; Figure 2a), and injected 5 × 1010 DNAse resistant particles of each vector into the lower limbs of newborn FRG1−high or wild-type male littermates. For all wild-type mouse injections, and FRG1−high mice injected with AAV.miFRG1 vectors, this delivery approach produced robust and widespread hrGFP expression in most major muscles of the lower limbs, including the adductors (add), gastrocnemius (gas), tibialis anterior (TA), and gluteus maximus (glut), up to 14 weeks post injection (Figure 2b–c). The quadriceps muscle was inconsistently hrGFP positive, and showed the least amount of transduction of all major lower limb muscles (Figure 2c). In contrast, we did not observe abundant hrGFP expression in AAV.hrGFP or AAV.miGFP-injected FRG1−high mice 14 weeks after injection, although it was present at 3 weeks (Supplementary Figure S3).
Figure 2.
FRG1 gene silencing improves muscle mass in FRG1−high mice. (a) Adeno-associated viral (AAV) vectors used for in vivo studies. All contain a CMV promoter-driven humanized Renilla GFP (hrGFP) cassette with an SV40 polyA (pA) signal. The AAV.miFRG1 and AAV.miGFP vectors also contain upstream U6.miRNA expression sequences. The miGFP sequences target eGFP, and do not impact levels of hrGFP, which is a different gene. Flanking black rectangles indicate AAV inverted terminal repeats (ITRs). (b) GFP epifluorescence shows near saturation of lower limb musculature in adult mice injected intramuscularly as newborns with 5 × 1010 DNAse resistant particles (DRP) of AAV6. (c) In all groups except FRG1-high mice injected with the AAV.miGFP virus, this delivery method produced high transduction in adductors (add), gastrocnemius (gas), tibialis anterior (TA), and gluteus (glut) muscles, shown by hrGFP epifluorescence in isolated muscles. Transduction was lower and less consistent in the quadriceps (quad). In contrast, 14 weeks after AAV.miGFP injections, hrGFP epifluorescence was not evident in lower limbs of FRG1−high mice. (d,e) AAV.miFRG1 significantly improved muscle mass in FRG1−high mice compared to uninjected or control-injected muscles. In (d) vectors were unilaterally injected as indicated. Dorsal (bottom) and ventral (top) views from the same representative animal are shown. In (e) data represent the mean weights ± s.e.m. of male FRG1−high (+) or wild-type (–) muscles injected with indicated vectors (n = 14 muscles per group). *Indicates significant difference from wild-type counterpart (ANOVA with Bonferroni post-test, P < 0.05). CMV, cytomegalovirus promoter; GFP, green fluorescent protein; miGFP, microRNAs targeting eGFP.
We next examined muscle size in AAV.miFRG1- and control-treated mice, since muscle size deficits are the most obvious gross abnormality in FRG1−high animals. We found that AAV.miFRG1-treated lower limb muscles were visually larger than AAV.miGFP-injected controls, and isolated add, gas, and glut muscles from the former weighed significantly more than those from animals that received AAV.miGFP or AAV.hrGFP vectors (approximately twofold average increase; Figure 2d–e). Moreover, AAV.miFRG1-injected FRG1−high add, gas, and glut muscles were indistinguishable in size from wild-type controls (Figure 2d–e). We observed similar trends in TA muscle sizes of all treated and control groups, but none of these changes were statistically significant. In contrast, AAV.miFRG1 treatment did not restore FRG1−high quad muscles to wild-type sizes. This insignificant ∼1.3-fold mean quadriceps size correction was likely due to low average transduction, since quad weights trended higher in individual FRG1−high mice with the most AAV.miFRG1-derived hrGFP expression (Figure 2d–e and Supplementary Figure S2).
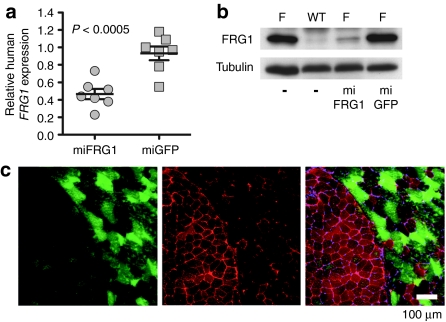
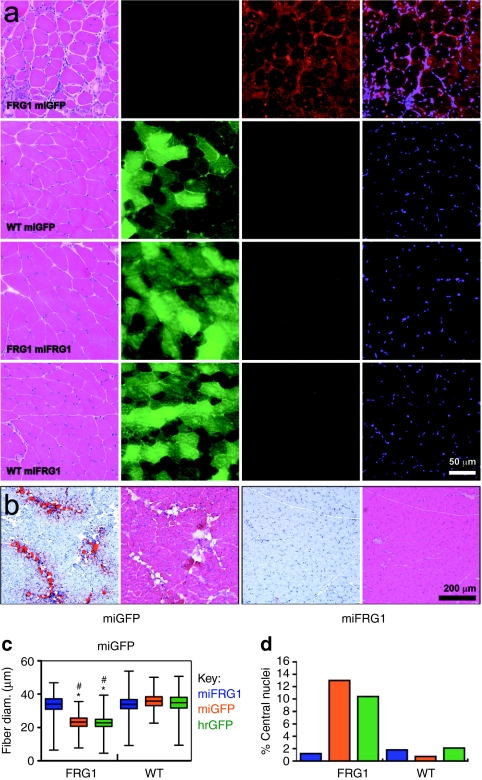
We next correlated these gross muscle improvements with FRG1 mRNA and protein knockdown using real-time PCR, western blot, and immunofluorescence staining. We measured a statistically significant ∼55% reduction of over expressed human FRG1 mRNA in FRG1−high muscles treated with AAV.miFRG1 compared to AAV.miGFP controls (Figure 3a). AAV.miFRG1 also reduced endogenous mouse FRG1 mRNA by ∼50%, despite having one mismatch with this transcript (Supplementary Figure S1). Expectedly, total FRG1 mRNA knockdown corresponded to a marked decrease in FRG1 protein levels by western blot (Figure 3b), but since RNAi rarely produces complete knockdown of abundant targets like FRG1 in FRG1−high mice, we were surprised to note that human FRG1 was nearly or completely undetectable in moderately- to highly-transduced myofibers (Figure 3c). This knockdown in AAV.miFRG1-transduced FRG1−high muscles was associated with wild-type histology. Specifically, all wild-type groups and transduced muscles from AAV.miFRG1-treated FRG1−high mice lacked the fibrosis, fat deposition, and myofiber degeneration and regeneration (indicated by increases in myofibers with smaller diameters and/or centrally-located nuclei) seen in untreated or control-treated FRG1−high animals (Figure 4).
Figure 3.
In vivo knockdown of FRG1 in FRG1−high mice. (a) Taqman assay showed that adeno-associated viral (AAV).miFRG1 vectors reduced FRG1 mRNA expression by a statistically significant average of 55% in FRG1−high muscles, compared with AAV.miGFP-injected controls (n = 7; ANOVA). FRG1 expression was determined by Taqman assay (human FRG1 primer/probe) normalized to mouse GAPDH expression. (b) Representative western blot confirmed FRG1 protein knockdown in vivo. F, FRG1−high muscles; (−), uninjected. Tubulin served as a loading control. (c) Immunofluorescence stain of AAV.miFRG1-injected FRG1−high muscle cryosections. Green shows transduced myofibers (humanized Renilla GFP (hrGFP) epifluorescence) and red stain shows FRG1 expression by immunostaining with DMA-AP-1 FRG1 primary antibody and Alexa-594 labeled secondary antibody. Importantly, FRG1 immunofluorescence inversely correlated with AAV.miFRG1 transduction, thereby demonstrating the efficacy of our FRG1 knockdown strategy. GFP, green fluorescent protein; miGFP, microRNAs targeting eGFP.
Figure 4.
FRG1 gene silencing improved myopathic histology in FRG1−high mice. FRG1−high mice show several histological indicators of myopathy, including fibrosis, fat deposition, myofiber size variability, and myofibers with centrally located nuclei. (a) Control adeno-associated viral (AAV).miGFP vectors had no impact on any of these histolopathological phenotypes. In contrast, AAV.miFRG1-transduced FRG1−high muscles were indistinguishable from all wild-type groups. Muscle cryosections were stained with (left to right): hematoxylin and eosin (H&E), humanized Renilla GFP (hrGFP) (epifluorescence), FRG1 antibodies followed by red-labeled secondaries, and DAPI. (b) Oil Red O stain (with corresponding H&E-stained serial sections) shows fat-infiltrated lesions in AAV.miGFP- but not AAV.miFRG1-transduced FRG1−high muscles. (c,d) FRG1 gene silencing normalized myofiber size defects and the number of centrally nucleated myofibers in FRG1−high mice. In c, *indicates significant differences between the comparable wild-type group, P < 0.05. #Indicates significant differences from FRG1−high mice injected with miFRG1, P < 0.05. Statistics were determined using one way ANOVA with Kruskal–Wallis post-test. GFP, green fluorescent protein; miGFP, microRNAs targeting eGFP.
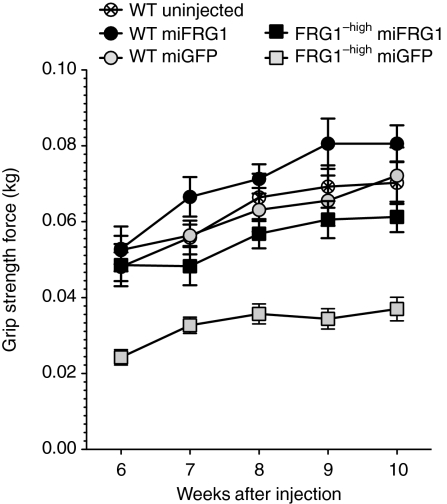
Finally, we determined whether FRG1 knockdown in FRG1−high mice improved overall hind limb muscle function. To do this, we measured grip strength weekly in AAV.miGFP-, AAV.miFRG1-, or AAV.hrGFP-injected FRG1−high and wild-type male mice for 5 weeks, and compared these values to uninjected wild-type littermates. We found that AAV.miFRG1-treated FRG1−high animals were significantly stronger (∼1.7-fold average increase) than age-matched AAV.miGFP- or AAV.hrGFP-injected controls (Figure 5 and Supplementary Figure S4). In contrast, hind limb grip strength from AAV.miFRG1-treated FRG1−high and wild-type mice, and all injected wild-type animals, were not significantly different from uninjected wild-type controls (Figure 5 and Supplementary Figure S4). We therefore concluded that RNAi-mediated knockdown of FRG1 improved myopathy in individual myofibers, isolated muscles, and whole limbs from FRG1−high mice.
Figure 5.
FRG1 gene silencing improved strength in FRG1−high mice. Hind limb grip strength assay showed that adeno-associated viral (AAV).miFRG1-treated FRG1−high animals were significantly stronger than counterparts injected with AAV.miGFP controls. In contrast, the former group and all injected wild-type controls were not significantly different from uninjected wild-type animals at any time point between 6–10 weeks post injection. Data represent means ± SEM using eight male mice per group (ANOVA with Bonferroni post-test). GFP, green fluorescent protein; miGFP, microRNAs targeting eGFP.
Discussion
Gene therapy refers to a therapeutic approach for disease that uses nucleic acids instead of drugs (http://www.asgct.org/about_gene_therapy/defined.php). For many years, this definition was almost exclusively synonymous with gene replacement, the strategy typically used to treat recessive diseases. As RNAi and microRNA-based expression systems emerged in recent years, the gene therapy field evolved to include gene silencing as another possible therapeutic approach. Thus, the number of diseases potentially treatable with nucleic acid therapies expanded. We viewed this expansion as an opportunity to begin developing gene therapies for dominant myopathies, which historically has been an under-represented area of research.
The “dominant myopathies” classification refers to a diverse group of clinically distinct, currently incurable, and potentially devastating muscle disorders caused by mutations in at least 29 different genes.15 As a group, dominant myopathies are relatively abundant, possibly affecting as many as 1 in 2,400 to 1 in 3,200 individuals.15,25 We hypothesized that a common RNAi-based therapeutic strategy, with modifications depending on etiology of each disorder, could potentially benefit a large population of patients affected by dominantly inherited muscle disease. We therefore set out to demonstrate proof-of-principle for this strategy in vivo. Accomplishing this required a disease animal model that developed obvious myopathic phenotypes arising from expression of a gene linked to a dominant human myopathy. We used the FRG1−high mouse model in this study, which was initially developed to test the hypothesis that FRG1 over expression was a primary pathogenic insult underlying FSHD.17,20 Although the progressive myopathy produced in these mice strongly supported this hypothesis, there have been some conflicting data arguing against the involvement of FRG1 in FSHD, or at least minimizing its role as a primary pathogenic insult.17,18,19,20,21,22,23,26,27,28,29,30,31,32,33,34,35,36 Thus, it is fair to say that FRG1 is a controversial FSHD candidate gene.37 Nevertheless, for this study, we were unconcerned with this ongoing debate, because our primary goal was to demonstrate proof-of-principle for RNAi therapy of dominant myopathies in general, and the FRG1−high line was useful as an outstanding model of dominant muscle disease.17,20 We reasoned that its involvement in FSHD, or lack thereof, was irrelevant to the goal of this study. We therefore developed a gene therapy strategy to knockdown pathological levels of human FRG1 in FRG1−high mouse muscles. Here, we reported that AAV6-delivered artificial microRNAs reduced toxic FRG1 levels and improved histological and functional muscle abnormalities associated with FRG1 over expression in mice. Our work therefore supports the therapeutic potential of RNAi therapy for dominant myopathies in general. In addition, it could be applied to FSHD, if additional evidence supporting FRG1 involvement in the disease emerges; alternatively, our strategy could be modified to target other FSHD candidate genes, such as DUX4.18,19,21,22,23,38
Finally, we note that over expression of an otherwise normal gene, such as in FSHD and FRG1−high mice, is a unique pathogenesis mechanism for dominant muscle diseases. Indeed, most other dominant myopathies arise from point mutations in one allele of a disease gene, while the other allele remains normal. In some cases, the remaining normal alleles encode essential proteins, and sufficient levels of the wild-type allele may be required to maintain some level of normal muscle function. Thus, any therapeutic benefits of reducing the dominant mutant allele could be counterbalanced if a similar reduction of the remaining wild-type allele causes haploinsufficiency-related myopathy. For example, dominant negative caveolin-3 (CAV3) mutations that result in 97% loss of normal CAV3, cause severe limb-girdle muscular dystrophy type 1C, but mutations resulting in 16% or 50% normal CAV3 levels produce only mild hyperCKemia without muscle weakness, or normal phenotypes, respectively.39,40,41 Thus, in addition to dominant mutations, CAV3 loss of function below a certain threshold also contributes to myopathic phenotypes. This was not a concern in our proof-of-principle study here, because dominant myopathy in FRG1−high mice was caused by increased dosage of an otherwise normal gene; we therefore only needed to reduce FRG1 to sufficiently nontoxic levels. In contrast, for most other dominant myopathies, such as the CAV3 example above, disease allele-specific RNAi strategies may be required. Importantly, several studies support the feasibility of engineering inhibitory RNA sequences that can distinguish between two alleles differing by a single nucleotide.42 Each allele-discriminating miRNA must be uniquely designed and empirically validated, since mismatches do not necessarily prevent gene silencing (Supplementary Figure S1 and ref. 42). Such strategies often require designing additional mismatches in the miRNA with the goal of destabilizing interactions with the normal allele of a disease gene.42 Thus, our RNAi strategy can be modified for disease allele-specificity, when applicable. Our work therefore supports that RNAi-based gene therapy is a promising candidate strategy for treating dominant myopathies, regardless of the causal genetic mutation. Future studies demonstrating the practicability of allele-specific silencing of dominant myopathy genes will further strengthen this conclusion.
Materials and Methods
Cloning of FRG1-targeted microRNAs. Mouse U6 promoter-driven artificial microRNAs targeting human FRG1 (called miFRG1s) were cloned using common molecular techniques as previously described.43 All microRNAs were based on human mir-30 sequences and structure, but the mature mir-30 portions were replaced by sequences derived from the FRG1 coding region. Ten different miFRG1s were generated; nomenclature indicates the first position of the miRNA binding site relative to +1 of the FRG1 coding region. Control U6-driven miGFP and microRNAs targeting firefly luciferase (miLuc) were previously described.43,44
Luciferase assay. The luciferase reporter plasmid (Figure 1a) was modified from Psicheck2 (Promega, Madison, WI). Human FRG1 cDNA was cloned downstream of the Renilla luciferase stop codon, thereby functioning as a 3′ UTR. A separate TK promoter driven firefly luciferase cassette served as a transfection control. HEK293 cells were cotransfected in triplicate wells (Lipofectamine-2000; Invitrogen, Carlsbad, California) with the luciferase. FRG1 reporter and individual U6.microRNA expression plasmids in a 1:5 molar ratio. FRG1 gene silencing was determined by measuring Renilla and firefly luciferase activity (Dual Luciferase Reporter Assay System, Promega) 48 hours post-transfection, following manufacturer's instructions. Triplicate data were averaged, and individual experiments performed 3 times; results were reported as the mean ratio of renilla to firefly activity ± SEM.
Real-time PCR and western blot. For in vitro work, U6.miFRG1 or control microRNA plasmids were cotransfected with a CMV.FRG1 expression vector into HEK293 cells (5:1 molar ratio). Forty-eight hours later, RNA or protein was extracted (Trizol from Fisher, Waltham, MA and M-PER from Pierce, Rockford, IL respectively). For in vivo work, RNA or protein was extracted from muscles injected 11–14 weeks prior, using previously described methods.23 RNA was quantified by Nanodrop, DNase-treated (DNA-Free, Ambion, TX), and reverse transcribed using random hexamers (Applied Biosystems cDNA Archive Kit; Applied Biosystems, Foster City, CA). Subsequent cDNA samples were then used as template for Taqman Assay using predesigned FRG1 and human β-actin or mouse GAPDH control primer/probe sets (Applied Biosystems). Two independent experiments were performed, with each sample run in triplicate. All in vitro data were normalized to miLuc-expressing samples. For westerns, protein was quantified by Lowry assay (BioRad, Hercules, CA), 50 µg samples were separated on 15% SDS-PAGE, transferred to PVDF membrane, and incubated with the following antibodies: commercial primary mouse monoclonal antibodies to FRG1 (1:8,000, Abnova, Taipei City, Taiwan); custom polyclonal FRG1 antibodies kindly provided by Dr Peter Jones (DMA-AP-1, 1:500)45; mouse monoclonal β-actin antibodies (1:60,000; Sigma, St Louis, MO); or rabbit polyclonal α-tubulin antibodies (1:5,000; Abcam, Cambridge, MA) overnight at 4 °C. Following washes, blots were then probed with HRP-coupled goat anti-mouse or goat anti-rabbit secondary antibodies (1:100,000; Jackson ImmunoResearch, West Grove, PA) for 1 hour at room temperature and then developed using Immobilon Western HRP substrate (Millipore, Billerica, MA).
AAV vector delivery to mouse muscle. U6.miGFP and U6.miFRG1.948 were cloned into our AAV.CMV.hrGFP proviral plasmid upstream of CMV.hrGFP. AAV6 particles were generated and titrated as previously described by the Viral Vector Core Facility at The Research Institute at Nationwide Children's Hospital.23 FRG1−high colonies were maintained by breeding hemizygous FRG1−high mice to C57BL/6 animals. Male FRG1−high and negative littermates were identified by PCR genotyping of genomic DNA from newborn mice (P1 or P2) using primers detecting the HSA.FRG1 transgene (5′-CCAGGGTAAAAAGACCATTGTCG-3′ and 5′-TCGTGCTCAAGGGAACCAAG-3′) and the mouse Y chromosome (SRY gene; 5′-GTGTCACAGAGGAGTGGCATTTTAC-3′ and 5′-TTGCTGCTGGTGGTGGTTATGG-3′). Following genotyping, male P1 or P2 mice were injected in the lower limbs with 5 × 1010 DNAse resistant particles per leg with indicated vectors. All mouse procedures were performed following guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at the Research Institute at Nationwide Children's Hospital.
Imaging and histology. In vivo AAV transduction was determined by hrGFP epifluorescence using a fluorescent dissecting microscope (MZ16FA, Leica, Wetzlar, Germany) at ×4.63 magnification. Dissected muscles were placed in O.C.T. Compound (Tissue-Tek, Torrance, CA), frozen in liquid nitrogen-cooled isopentane, cut onto slides as 10 µm cryosections, and stained with hematoxylin and eosin (H&E; following standard protocols), Oil Red O/Harris hematoxylin, or DMA-AP-1 FRG1 polyclonal antibodies. Oil Red O stains were performed using a filtered 60% stock solution in dH2O (stock, 2.5 g Oil red O powder in 500 ml isopropanol). Cryosections were post fixed in 10% formalin for 10 minutes, washed in tap water, and stained in Oil red O working solution for 10 minutes. Slides were then washed in tap water, counter stained in Harris hemotoxylin for 1 minute, rinsed, blued in ammonia water, and washed in tap water. H&E and Oil Red O sections were covered with crystal-mount (Electron Microscopy Sciences, Hatfield, PA), and mounted with Permount (Fisher Scientific). For FRG1 immunohistochemistry, cryosections were post fixed in 4% paraformaldehyde, washed, blocked in 5% milk/phosphate-buffered saline-tween (PBST), incubated overnight at 4 °C with DMA-AP-1 FRG1 primary antibody (1:200 in 1% BSA, 20% goat serum, and phosphate-buffered saline), and then with AlexaFluor-594 conjugated goat anti-rabbit secondary antibodies (1:500; 1 hour at RT; Molecular Probes, Carlsbad, CA). Slides were covered in Vectashield plus DAPI. All images were taken from mouse tissue harvested from 11-14 week old male mice, except in Supplementary Figure S2 (3 week old mice). Muscle cross-sectional fiber diameters and percentage of myofibers with centrally-located nuclei were determined as previously described from five different animals per group (five fields per leg).23
Grip strength. Hindlimb grip strength was measured weekly between 6–10 weeks of age as previously described (n = 8 male animals per group).23 Data represent means ± SEM.
SUPPLEMENTARY MATERIAL Figure S1 miFRG1 sequences. Figure S2 HrGFP transduction in isolated muscles from male mice used in this study. Figure S3 AAV.miGFP transduction in 3 week-old FRG1-high mice intramuscularly injected with 5 × 1010 DRP AAV.miGFP at post natal day 1 (P1). Figure S4 Additional controls for the grip strength assay (Figure 5).
Acknowledgments
This work was funded by a Research Development Grant from the Muscular Dystrophy Association (to SQH), an NIH KL2 Clinical and Translational Scholar Award (KL2 RR025754 to SQH) and startup funds from the Research Institute at Nationwide Children's Hospital (to SQH).
Supplementary Material
miFRG1 sequences.
HrGFP transduction in isolated muscles from male mice used in this study.
AAV.miGFP transduction in 3 week-old FRG1-high mice intramuscularly injected with 5 × 1010 DRP AAV.miGFP at post natal day 1 (P1).
Additional controls for the grip strength assay (Figure 5).
REFERENCES
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C., and, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Scott JM, Li S, Harper SQ, Welikson R, Bourque D, DelloRusso C.et al. (2002Viral vectors for gene transfer of micro-, mini-, or full-length dystrophin Neuromuscul Disord 12 Suppl 1S23–S29. [DOI] [PubMed] [Google Scholar]
- Lim LE., and, Campbell KP. The sarcoglycan complex in limb-girdle muscular dystrophy. Curr Opin Neurol. 1998;11:443–452. doi: 10.1097/00019052-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Li J, Dressman D, Tsao YP, Sakamoto A, Hoffman EP., and, Xiao X. rAAV vector-mediated sarcogylcan gene transfer in a hamster model for limb girdle muscular dystrophy. Gene Ther. 1999;6:74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- Cordier L, Hack AA, Scott MO, Barton-Davis ER, Gao G, Wilson JM.et al. (2000Rescue of skeletal muscles of gamma-sarcoglycan-deficient mice with adeno-associated virus-mediated gene transfer Mol Ther 1119–129. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF.et al. (2002Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy Nat Med 8253–261. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L.et al. (2004Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping Science 3061796–1799. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG.et al. (2004Systemic delivery of genes to striated muscles using adeno-associated viral vectors Nat Med 10828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah C, Pacak CA, Cresawn KO, Deruisseau LR, Germain S, Lewis MA.et al. (2007Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors Mol Ther 15501–507. [DOI] [PubMed] [Google Scholar]
- Pacak CA, Walter GA, Gaidosh G, Bryant N, Lewis MA, Germain S.et al. (2007Long-term skeletal muscle protection after gene transfer in a mouse model of LGMD-2D Mol Ther 151775–1781. [DOI] [PubMed] [Google Scholar]
- Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN.et al. (2008A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs Mol Ther 161944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR.et al. (2009Follistatin gene delivery enhances muscle growth and strength in nonhuman primates Sci Transl Med 16ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Montgomery CL, Mendell JR., and, Chicoine LG. AAV-mediated gene therapy to the isolated limb in rhesus macaques. Methods Mol Biol. 2011;709:287–298. doi: 10.1007/978-1-61737-982-6_19. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S.et al. (2010Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D Ann Neurol 68629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace LM, Garwick SE., and, Harper SQ.2010RNAi therapy for dominant muscular dystrophies and other myopathies Duan D.ed). Muscle Gene Therapy Springer, New York; 99–115. [Google Scholar]
- Boudreau RL., and, Davidson BL. RNAi therapeutics for CNS disorders. Brain Res. 2010;1338:112–121. doi: 10.1016/j.brainres.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Gabellini D, D'Antona G, Moggio M, Prelle A, Zecca C, Adami R.et al. (2006Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1 Nature 439973–977. [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Gang EJ, Galindo CL, Liu M, Simsek T.et al. (2008An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies EMBO J 272766–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit M, Ansseau E, Tassin A, Winokur S, Shi R, Qian H.et al. (2007DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1 Proc Natl Acad Sci USA 10418157–18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellini D, Green MR., and, Tupler R. Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell. 2002;110:339–348. doi: 10.1016/s0092-8674(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Kowaljow V, Marcowycz A, Ansseau E, Conde CB, Sauvage S, Mattéotti C.et al. (2007The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein Neuromuscul Disord 17611–623. [DOI] [PubMed] [Google Scholar]
- Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camaño P, Dauwerse JG.et al. (2010A unifying genetic model for facioscapulohumeral muscular dystrophy Science 3291650–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace LM, Garwick SE, Mei W, Belayew A, Coppee F, Ladner KJ.et al. (2011DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo Ann Neurol 69540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q.et al. (2005RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model Proc Natl Acad Sci USA 1025820–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSERM and French Ministry of Health 2008Prevalence of Rare Diseases: Bibliographic Data. In Orphanet Report : < http://orpha.net/orphacom/cahiers/docs/GB /Prevalence_of_rare_diseases_by_alphabetical_list.pdf >
- Arashiro P, Eisenberg I, Kho AT, Cerqueira AM, Canovas M, Silva HC.et al. (2009Transcriptional regulation differs in affected facioscapulohumeral muscular dystrophy patients compared to asymptomatic related carriers Proc Natl Acad Sci USA 1066220–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodega B, Ramirez GD, Grasser F, Cheli S, Brunelli S, Mora M.et al. (2009Remodeling of the chromatin structure of the facioscapulohumeral muscular dystrophy (FSHD) locus and upregulation of FSHD-related gene 1 (FRG1) expression during human myogenic differentiation BMC Biol 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celegato B, Capitanio D, Pescatori M, Romualdi C, Pacchioni B, Cagnin S.et al. (2006Parallel protein and transcript profiles of FSHD patient muscles correlate to the D4Z4 arrangement and reveal a common impairment of slow to fast fibre differentiation and a general deregulation of MyoD-dependent genes Proteomics 65303–5321. [DOI] [PubMed] [Google Scholar]
- Hanel ML, Wuebbles RD., and, Jones PL. Muscular dystrophy candidate gene FRG1 is critical for muscle development. Dev Dyn. 2009;238:1502–1512. doi: 10.1002/dvdy.21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Jones TI, Tang VW, Brieher WM., and, Jones PL. Facioscapulohumeral muscular dystrophy region gene-1 (FRG-1) is an actin-bundling protein associated with muscle-attachment sites. J Cell Sci. 2010;123 Pt 7:1116–1123. doi: 10.1242/jcs.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne RJ, Welle S, Venance SL, Thornton CA., and, Tawil R. Expression profile of FSHD supports a link between retinal vasculopathy and muscular dystrophy. Neurology. 2007;68:569–577. doi: 10.1212/01.wnl.0000251269.31442.d9. [DOI] [PubMed] [Google Scholar]
- Reed PW, Corse AM, Porter NC, Flanigan KM., and, Bloch RJ. Abnormal expression of mu-crystallin in facioscapulohumeral muscular dystrophy. Exp Neurol. 2007;205:583–586. doi: 10.1016/j.expneurol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Lemmers RJ, Grewal PK, van Geel M, Romberg S, Dauwerse HG.et al. (1996Identification of the first gene (FRG1) from the FSHD region on human chromosome 4q35 Hum Mol Genet 5581–590. [DOI] [PubMed] [Google Scholar]
- Winokur ST, Barrett K, Martin JH, Forrester JR, Simon M, Tawil R.et al. (2003Facioscapulohumeral muscular dystrophy (FSHD) myoblasts demonstrate increased susceptibility to oxidative stress Neuromuscul Disord 13322–333. [DOI] [PubMed] [Google Scholar]
- Winokur ST, Chen YW, Masny PS, Martin JH, Ehmsen JT, Tapscott SJ.et al. (2003Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation Hum Mol Genet 122895–2907. [DOI] [PubMed] [Google Scholar]
- Wuebbles RD, Hanel ML., and, Jones PL. FSHD region gene 1 (FRG1) is crucial for angiogenesis linking FRG1 to facioscapulohumeral muscular dystrophy-associated vasculopathy. Dis Model Mech. 2009;2:267–274. doi: 10.1242/dmm.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Maarel SM, Frants RR., and, Padberg GW. Facioscapulohumeral muscular dystrophy. Biochim Biophys Acta. 2007;1772:186–194. doi: 10.1016/j.bbadis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Snider L, Asawachaicharn A, Tyler AE, Geng LN, Petek LM, Maves L.et al. (2009RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy Hum Mol Genet 182414–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L, Carbone I, Capanni C, Sabatelli P, Tortorelli S, Sotgia F.et al. (2002Familial isolated hyperCKaemia associated with a new mutation in the caveolin-3 (CAV-3) gene J Neurol Neurosurg Psychiatr 7365–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M.et al. (1998Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy Nat Genet 18365–368. [DOI] [PubMed] [Google Scholar]
- Traverso M, Gazzerro E, Assereto S, Sotgia F, Biancheri R, Stringara S.et al. (2008Caveolin-3 T78M and T78K missense mutations lead to different phenotypes in vivo and in vitro Lab Invest 88275–283. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lebron E., and, Paulson HL. Allele-specific RNA interference for neurological disease. Gene Ther. 2006;13:576–581. doi: 10.1038/sj.gt.3302702. [DOI] [PubMed] [Google Scholar]
- McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I.et al. (2008Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi Proc Natl Acad Sci USA 1055868–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, Beck CR, Fineberg SK, Stein C, Ochoa D.et al. (2006Optimization of feline immunodeficiency virus vectors for RNA interference J Virol 809371–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel ML, Sun CY, Jones TI, Long SW, Zanotti S, Milner D.et al. (2011Facioscapulohumeral muscular dystrophy (FSHD) region gene 1 (FRG1) is a dynamic nuclear and sarcomeric protein Differentiation 81107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miFRG1 sequences.
HrGFP transduction in isolated muscles from male mice used in this study.
AAV.miGFP transduction in 3 week-old FRG1-high mice intramuscularly injected with 5 × 1010 DRP AAV.miGFP at post natal day 1 (P1).
Additional controls for the grip strength assay (Figure 5).