Abstract
Glycogen storage disease type Ia (GSD-Ia) is caused by the deficiency of glucose-6-phosphatase (G6Pase). Long-term complications of GSD-Ia include life-threatening hypoglycemia and proteinuria progressing to renal failure. A double-stranded (ds) adeno-associated virus serotype 2 (AAV2) vector encoding human G6Pase was pseudotyped with four serotypes, AAV2, AAV7, AAV8, and AAV9, and we evaluated efficacy in 12-day-old G6pase (−/−) mice. Hypoglycemia during fasting (plasma glucose <100 mg/dl) was prevented for >6 months by the dsAAV2/7, dsAAV2/8, and dsAAV2/9 vectors. Prolonged fasting for 8 hours revealed normalization of blood glucose following dsAAV2/9 vector administration at the higher dose. The glycogen content of kidney was reduced by >65% with both the dsAAV2/7 and dsAAV2/9 vectors, and renal glycogen content was stably reduced between 7 and 12 months of age for the dsAAV2/9 vector-treated mice. Every vector-treated group had significantly reduced glycogen content in the liver, in comparison with untreated G6pase (−/−) mice. G6Pase was expressed in many renal epithelial cells of with the dsAAV2/9 vector for up to 12 months. Albuminuria and renal fibrosis were reduced by the dsAAV2/9 vector. Hepatorenal correction in G6pase (−/−) mice demonstrates the potential of AAV vectors for the correction of inherited diseases of metabolism.
Introduction
Glycogen storage disease type Ia (GSD-Ia) was first described as “hepatonephromegalia glycogenica” by von Gierke in 1929.1 The deficiency of glucose-6-phosphatase (G6Pase), primarily in liver and kidney, underlies GSD-Ia; (MIM +232200), an autosomal recessive disorder of metabolism associated with life-threatening hypoglycemia during fasting.2 Dietary therapy is focused upon preventing hypoglycemia by frequent ingestion of uncooked cornstarch or by frequent meals complemented by continuous nocturnal tube feeding. The acute complications of GSD-Ia have responded favorably to dietary therapy that prevents hypoglycemia, including renal tubular dysfunction and growth failure, even though dietary therapy has significant limitations.2 Long-term complications of GSD-Ia frequently fail to respond to dietary therapy, including delayed growth relative to genetic potential, proteinuria occasionally progressing to renal failure, osteopenia, formation of hepatic adenomas and occasionally hepatocellular carcinoma, and rarely pancreatitis or pulmonary hypertension.2 Dietary therapy requires constant vigilance to avoid acute metabolic decompensation accompanied by hypoglycemia and lactic acidosis.2 Hypoglycemia occurs daily, even during monitored compliance with cornstarch supplementation.3
Renal involvement in GSD-Ia typically presents as a late complication.2 Up to 70% of patients develop proteinuria, and perhaps 30% develop end-stage renal disease requiring dialysis.4,5 The majority of patients with type I glycogen storage disease have proteinuria by the third decade of life. In some patients, renal function has deteriorated and progressed to failure, requiring dialysis and transplantation. It has been suggested that the correction of biochemical abnormalities will result in reversal and/or prevention of renal disease, because improved metabolic control through dietary control has resolved proteinuria in some cases.2
Liver transplantation has been performed successfully in >20 patients; however, any beneficial effects upon renal involvement following liver transplantation remain to be proven.2 Hypoglycemia resolved immediately following liver transplantation, and accelerated growth was observed in several younger patients.6,7 The progression to renal failure has been reported following liver transplantation, potentially related to cyclosporine toxicity;6 however, the risk for progression to renal failure following liver transplantation has not been delineated. Hence, a combined liver and kidney transplantation for advanced GSD-Ia has been advocated by some.8 The critical factor in the decision to pursue transplantation has recently been limited to life-threatening complications, including enlarging hepatic adenomas with a high risk for hepatocellular carcinoma.2
GSD-Ia is caused by deficiency of the catalytic subunit of G6Pase-α, an integral membrane protein localized to the endoplasmic reticulum. GSD-Ia is inherited in an autosomal recessive manner, and incidence is roughly 1 in 100,000 births with a carrier frequency of about 1 in 150.2 A great number of disease causing mutations in the G6Pase catalytic subunit (G6PC) gene have been described in GSD-Ia.2 Definitive therapy for GSD-Ia will presumably require expressing G6Pase above a therapeutic threshold in at least the most severely affected organs. The level of replacement needed to correct hypoglycemia and related biochemical abnormalities has been estimated at 10% of normal G6Pase activity in the liver.9 Therefore, the correction of G6Pase deficiency within a fraction of cells might have an efficacious effect due to the cell autonomous nature of this disease. No cross-correction of nontransduced cells will be generated from gene replacement therapy in GSD-Ia, due to the membrane localization and absence of receptor-mediated uptake of G6Pase-α.
The development of new therapy for GSD-Ia, such as gene therapy, might prevent long-term complications that arise due to recurrent hypoglycemia and related biochemical abnormalities. Efficacy from liver-targeted gene therapy in GSD-Ia might be expected, given the experience with human patients following liver transplantation. Early attempts at gene therapy in GSD-Ia did not fully correct G6Pase deficiency in the liver of G6Pase knockout mice. Homozygous G6pase (−/−) mice suffer >85% mortality by 3 weeks of age, in absence of gene therapy to correct G6Pase deficiency.10,11 An adeno-associated virus serotype 2 (AAV2) vector encoding murine G6Pase failed to prolong survival of neonatal G6pase (−/−) mice; however, coadministration with an adenovirus vector encoding murine G6Pase prolonged survival in a parallel experiment. Newer AAV serotypes have enhanced tropism for the liver and kidney, and enhanced the efficacy of gene therapy for GSD-Ia in preclinical experiments. A comparison of a double-stranded (ds) AAV vector encoding human G6Pase and pseudotyped as AAV1 (AAV2/1) or AAV8 (AAV2/8) revealed that the AAV2/8 vector achieved higher efficacy in 2-week-old infant G6pase (−/−) mice, when a single administration of fewer vector particles (1 × 1013 vector particles (vp)/kg) prevented both mortality and hypoglycemia during fasting.12 The AAV2/1 pseudotyped vector failed to reduce renal glycogen content in infant G6pase (−/−) mice.12 Ghosh et al. compared the efficacy of AAV2/1 and AAV2/8 vectors encoding murine G6Pase in neonatal G6pase (−/−) mice, and found that the AAV2/1 vector featured increased efficacy in association with partial correction of the kidney manifestations.13 The vector evaluated by Ghosh et al. contained a viral enhancer as part of the CBA promoter, and subsequently it was associated with cytotoxic T-cell responses and rapid clearance in the liver, when administered to young G6pase (−/−) mice.14 It seems likely that AAV vector-mediated gene therapy could achieve long-lasting efficacy in GSD-Ia, given the sustained efficacy for 8 years with an AAV2 vector in hemophilia B dogs.15
AAV vectors with packaging size <2.4 kbp, termed self-complementary (sc)AAV or dsAAV, have markedly increased transduction efficiency in vivo.16,17,18 This increased transduction results from bypassing the need for the conversion of single-stranded to ds AAV vector genomes, a rate-limiting step for transduction.19,20,21 The scAAV vectors have a deletion within the 5′ terminal repeat that vastly increases the packaging of ds vector genomes.
A dsAAV2/8 vector containing a human G6Pase mini-gene demonstrated high efficacy in GSD-Ia.12 A minimal human G6Pase promoter and the human G6Pase cDNA were contained within the packaging size of 2.1 kb for the vector, AAV-G6Pase. The minimal human G6Pase promoter, starting at position −298, contains a cyclic adenosine monophosphate response element and insulin response sequences sufficient to mediate glucose, dexamethasone, and insulin responsiveness.22,23,24 The resulting transgene was small enough to be packaged within a ds AAV vector genome, although this vector did not contain the terminal repeat mutation converting it to a self-complementary AAV vector that would drive uniform ds vector genome packaging. Only 3% of the AAV vector genomes were ds as determined by denaturing gel electrophoresis. Importantly, efficient transgene expression was observed in the liver.12 Therefore, high-level transgene expression was attributable to the annealing of single-stranded (+ and −) AAV vector genomes contained within the same AAV particle, which bypasses the need for second-strand DNA synthesis associated with traditional, single-stranded AAV vectors.25
The renal involvement in GSD-Ia represents a severe long-term complication that should be the focus of efforts to develop new therapy for this rare, orphan disease, including gene therapy. In an effort to better characterize the correction of renal involvement in GSD-Ia, we have pseudotyped dsAAV-G6Pase vector with three additional serotypes, AAV2, AAV2/7, and AAV2/9, and evaluated efficacy in G6pase (−/−) mice, in comparison with the dsAAV2/8 vector previously characterized.12 AAV2 was included despite the previous report of low efficacy for that serotype, due to the clinical experience with AAV2 and the improved performance of small genome AAV2 vectors that can be packaged as dsAAV.16,25 The dsAAV2/1 vector was not studied further, because it failed to reduce glycogen storage in the kidney.12 The newer serotypes demonstrated high efficacy by stabilizing blood glucose for 2–8 hours fasting at up to 12 months age. Renal function was partially restored with the AAV2/9 vector, reflecting the higher tropism of newer AAV serotypes and of dsAAV vectors for the kidney.
Results
Reversal of hypoglycemia and associated abnormalities following AAV vector administration
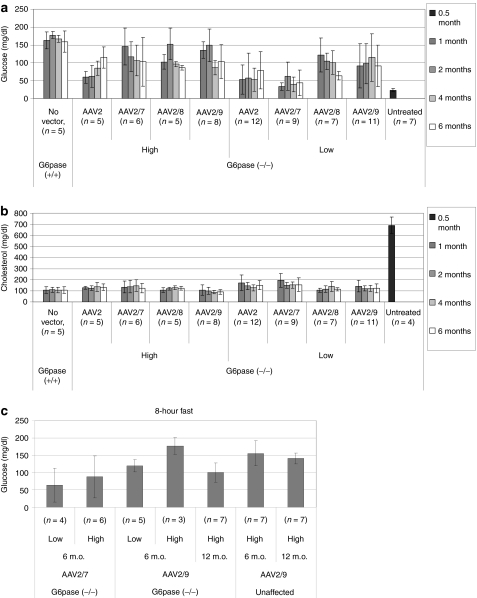
The dsAAV2/8 vector encoding human G6Pase prevented hypoglycemia during fasting, which represents the primary metabolic abnormality in GSD-Ia;12 however, the vector dose exceeded the highest dose administered in a clinical trial of AAV vector-mediated gene therapy (1 × 1013 vp/kg).26 Therefore, a reduced dose was administered in G6pase (−/−) mice (2 × 1012 vp/kg) at 12 days of age for four pseudotypes of the AAV-G6Pase vector, and hypoglycemia during fasting (plasma glucose <100 mg/dl) was prevented for >6 months by the AAV2/8 and AAV2/9 vectors (Figure 1a). The dsAAV2/1 vector was not included in the current study, due to its reduced transduction of kidneys, in comparison with the dsAAV2/8 vector.12 The AAV2/7 vector prevented hypoglycemia only at the higher dose (1 × 1013 vp/kg). The higher dose of the AAV2 vector prevented hypoglycemia at 4 and 6 months, consistent with a more gradual onset of transgene expression. Further evidence for biochemical correction was demonstrated by the normalization of blood cholesterol following the administration of the AAV2/8 and AAV2/9 vectors at either dose (Figure 1b).
Figure 1.
Prevention of hypoglycemia during fasting in glucose-6-phosphatase (G6pase) (−/−) mice following adeno-associated virus (AAV) vector administration. Glucose and cholesterol were determined in plasma following a timed fast. High vector dose (1 × 1013 vp/kg, except AAV2 5 × 1012 vp/kg) or low vector dose (2 × 1012 vp/kg) was administered at 12 days of age as indicated. Mean ± SD are shown. (a) Plasma glucose following a 2-hour fast. (b) Plasma cholesterol following a 2-hour fast. (c) Plasma glucose following an 8-hour fast for G6pase (−/−) and (+/+) mice following administration of the high dose of AAV vector particles. Untreated wild-type mice were not retained for 12 months, but blood glucose following fasting was equivalent between wild-type mice and unaffected, vector-treated G6pase (±) mice at 6 months of age (159 ± 29 mg/dl and 155 ± 36 mg/dl, respectively). Thus, the latter group was chosen as the normal control group at 12 months of age. m.o., months old.
Prolonged fasting for 8 hours at 6 months of age revealed very significant correction of hypoglycemia following AAV2/9 vector administration at the higher dose (Figure 1c), whereas the AAV2/7 vector did not prevent hypoglycemia (glucose <100 mg/dl). Mice treated with AAV2 and AAV2/8 vectors were not fasted for 8 hours, because blood glucose was deemed too low following a 2-hour fast at 6 months of age (<100 mg/dl; Figure 1a). Prolonged fasting at 12 months of age did induce mild hypoglycemia following AAV2/9 vector administration, in comparison with unaffected littermates treated with the same vector dose (Figure 1c).
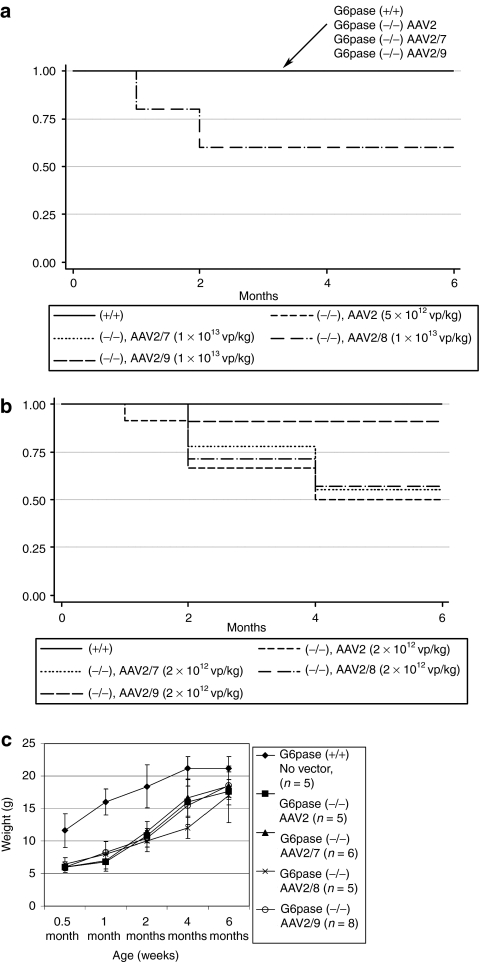
All untreated G6pase (−/−) mice died prior to weaning.27,28 AAV2, AAV2/7, and AAV2/9 vectors sustained uniform survival to 6 months at the higher dose, whereas two of five AAV2/8 treated mice died (Figure 2a). No AAV2/9 treated G6pase (−/−) mice died among a group retained for 12 months (data not shown). The lower dose of each vector failed to prevent mortality, although AAV2/9 treated G6pase (−/−) mice survived at higher frequency (90%) than did those treated with other pseudotypes (Figure 2b). Increased weight gain was demonstrated following vector administration. G6pase (−/−) mice were initially much smaller than G6pase (+/+) mice at 2 weeks of age, and later grew to almost an equivalent size following vector treatment (Figure 2c).
Figure 2.
Prolonged survival in glucose-6-phosphatase (G6Pase) (−/−) mice following adeno-associated virus (AAV) vector administration. Affected mice did not survive until 1 month of age (data not shown), unless the AAV vector was administered at 12 days of age. Kaplan–Meier survival analysis following (a) high vector dose (1 × 1013 vp/kg, except AAV2 = 5 × 1012 vp/kg), or (b) low vector dose (2 × 1012 vp/kg) was administered as indicated. (c) Body weight for G6pase (−/−) and G6pase (+/+) mice at the indicated age. Mean ± SD are shown.
Correction of biochemical abnormalities in kidney and liver
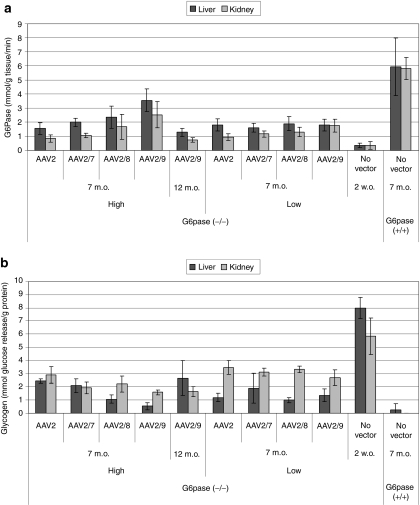
G6Pase activity was analyzed in tissues of G6pase (−/−) mice to evaluate expression of G6Pase encoded by the AAV vector genome at 7 months and 12 months of age (Figure 3). G6Pase activity reached 43% of normal activity following AAV2/9 vector administration at 7 months of age (Figure 3a). G6Pase activity was significantly increased in the kidney for vector-treated groups of mice at 7 months of age, with the exception of the low dose AAV2-treated group, in comparison with untreated G6pase (−/−) mice (Wilcoxon rank–sum test). G6Pase activity in the liver was significantly increased for vector-injected mice at 7 months of age, in comparison with the liver of untreated G6pase (−/−) mice, and up to 48% of normal activity was demonstrated for the higher dose of the AAV2/9 vector (Figure 3a). Liver G6Pase activity was significantly increased by the AAV2/9 vector at the higher dose at 7 months of age, in comparison with AAV2 and AAV2/7 vector-treated groups (P ≤ 0.03 for each comparison). However, G6Pase activity decreased between 7 and 12 months of age in both the liver and kidney for G6pase (−/−) mice treated with the higher dose of AAV2/9 vector.
Figure 3.
Correction of glucose-6-phosphatase (G6Pase) deficiency in the kidney and liver of G6Pase (−/−) mice following adeno-associated virus (AAV) vector administration. Analysis of G6pase (−/−) mice (n = 4, except for AAV2, Low, n = 3) and (+/+) mice (n = 5) following administration of the indicated number of vector particles at 12 days of age. Tissues collected at the indicated ages. High vector dose (1 × 1013 vp/kg, except AAV2 5 × 1012 vp/kg) or low vector dose (2 × 1012 vp/kg) was administered as indicated. Mean ± SD are shown. (a) G6Pase activity quantitated as the degradation of glucose-6-phosphate. Mean ± SD are shown. (b) Glycogen content quantified as the release of glucose by amyloglucosidase digestion. m.o., months old; w.o., weeks old.
Glycogen content was analyzed to evaluate the biochemical activity of G6Pase expressed with each AAV vector at 7 months of age. In the kidney the higher dose of the AAV2/9 vector significantly reduced glycogen content of G6pase (−/−) mice in comparison with each of the other vector-treated groups (Figure 3b; P ≤ 0.03 for each comparison), with the exception of the high dose AAV2/7 and AAV2/8 groups. Glycogen did not increase between 7 and 12 months of age in the kidney of the AAV2/9 vector-treated group, and it was reduced by 93% in comparison with untreated affected mice. The AAV2 vector-treated groups had significantly higher glycogen content in the kidney, in comparison with the other three vectors. Every vector-treated group had significantly reduced glycogen content in the liver, in comparison with the liver of untreated G6pase (−/−) mice (Figure 3b; P ≤ 0.03 for each comparison). In the liver the higher dose of the AAV2/9 vector significantly reduced glycogen content of G6pase (−/−) mice in comparison with each of the other vector-treated groups (Figure 3b), with the exception of the high dose AAV2/7 and AAV2/8 groups (P ≤ 0.03 for each comparison). However, glycogen content increased slightly between 7 and 12 months in the liver for G6pase (−/−) mice treated with the AAV2/9 vector.
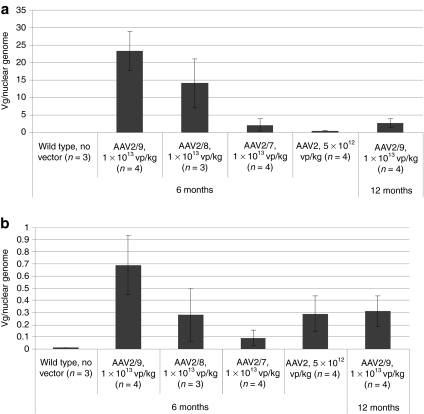
Realtime PCR quantification of vector genome (vg) copy number revealed that the AAV2/7 vector copy number was lower in the liver, in comparison with AAV2/9 (2.1 ± 1.7 versus 23 ± 6 vg/nuclear genome) or AAV2/8 (14 ± 7 vg/nuclear genome); however, AAV2/7 vector copy number was higher than the AAV2/2 copy number (0.5 ± 0.1 vg/nuclear genome) in the liver (Figure 4a). Similarly, the AAV2/9 vector transduced kidney more efficiently than other pseudotypes (Figure 4b). The AAV2/9 vector-injected mice retained a higher vector copy number in kidney at 7 months of age than did AAV2/8 vector-injected mice (0.7 ± 0.2 versus 0.3 ± 0.2 vg/nuclear genome); however, by 12 months of age the residual AAV2/9 vector DNA had fallen by >50% (to 0.3 ± 0.1 vg/nuclear genome). The AAV2/7 vector copy number was lower than AAV2/9 or AAV2/8 vector copy number in kidney at 7 months of age (0.1 ± 0.1 vg/nuclear genome), despite the significant degree of biochemical correction achieved with the AAV2/7 vector (Figure 3). Surprisingly, the AAV2 vector copy number was higher in the kidney than the AAV2/7 copy number (Figure 4b).
Figure 4.
Vector genome quantification in the kidney and liver of glucose-6-phosphatase (G6Pase) (−/−) mice following adeno-associated virus (AAV) vector administration. Tissues collected at the indicated ages. High vector dose (1 × 1013 vp/kg, except AAV2 5 × 1012 vp/kg) or low vector dose (2 × 1012 vp/kg) was administered as indicated. Mean ± SD are shown. (a) Liver. (b) Kidney.
Microscopic examination of the liver revealed markedly vacuolated hepatocytes in untreated 2-week-old G6pase (−/−) mice (Figure 5a). Vacuolated hepatocytes were largely absent at 7 and 12 months of age in G6pase (−/−) mice following administration of the higher dose of the AAV2/9 vector; however, residual, moderately vacuolated hepatocytes were present in the liver of G6pase (−/−) mice that received the lower vector dose (Figure 5a). Microscopic examination of the kidney revealed diffuse glycogen accumulation in markedly vacuolated renal tubular cells, and tubular atrophy and tubular basement membrane thickening in untreated 2-week-old G6pase (−/−) mice (Figure 5b). Although focal segmental glomerulosclerosis occurs frequently in the kidneys of humans with GSD-Ia,2 it was absent from the kidneys of G6pase (−/−) mice evaluated at 0.5, 7, and 12 months of age (Figure 5b). Seven-month-old G6pase (−/−) mice treated with a lower dose of the AAV2/9 vector exhibited vacuolation of renal tubules and increased Bowman's space (surrounding the glomerular tufts, arrows) in an multifocal pattern, in contrast to diminished vacuolation of the tubular epithelial cells present in 7-month-old G6pase (−/−) mice treated with the higher dose of the AAV2/9 vector, as well as large clusters of nonvacuolated tubules (Figure 5b). Glycogen deposition was clearly decreased to a greater extent by the higher dose of the AAV2/9 vector than for the lower dose (Figure 5b). However, many nonvacuolated tubules persisted at 12 months following administration of the higher dose of the AAV2/9 vector.
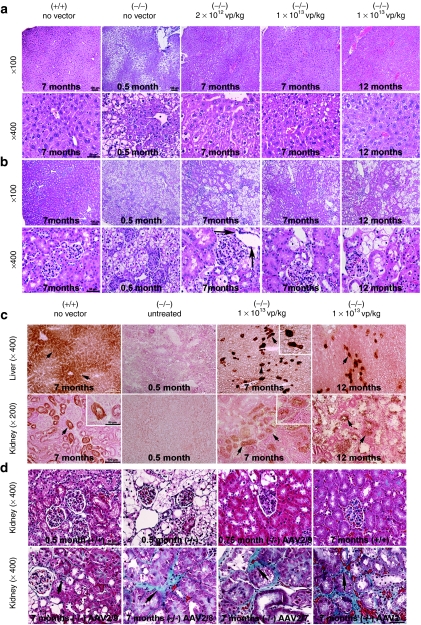
Figure 5.
Reversal of vacuolation in the liver and kidney of glucose-6-phosphatase (G6pase) (−/−) mice following AAV2/9 vector administration. Representative images are shown (three samples were evaluated for each image shown). Magnification of original image indicated. Tissues were collected at the indicated ages following the administration of the indicated number of adeno-associated virus (AAV) vector particles at 12 days of age. (a) Hematoxylin and eosin staining of liver. (b) Hematoxylin and eosin staining of kidney. Bowman's capsule indicated (arrows). (c) Histochemical staining of G6Pase in liver and kidney at the indicated age administration of the indicated number of AAV2/9 vector particles. Renal tubules indicated (arrows and high magnification inserts). (d) Masson's trichrome staining of kidney. Age, vector, and vector dosage indicated.
G6Pase expression was demonstrated in the liver and kidney of G6pase (−/−) mice that received the higher vector dose by histochemical staining (Figure 5c). In the liver widespread G6Pase expression was detected at 7 months of age, accompanied by focal areas of very intense G6Pase expression. At 12 months of age, the G6Pase expression was limited to very darkly stained foci of a few hepatocytes, suggesting that very long-term expression was limited to a few transduced hepatocytes. In the kidney, moderately intense G6Pase expression was detected in tubular foci of renal epithelial cells at 7 months of age (Figure 5c). At 12 months of age, fewer renal epithelial cells expressed G6Pase (Figure 5c), although many foci of nonvacuolated epithelial cells were present (Figure 5b). Masson's trichrome staining revealed increased fibrosis in the kidney of mice treated with all vectors except for the AAV2/9 vector at 7 months of age (Figure 5d). Dramatic improvement in the vacuolation of renal tubules was observed by 3 weeks of age, following AAV2/9 vector administration, and the degree of correction in the kidney was greater for the AAV2/9 vector than for other vectors at 7 months of age (Figure 5d).
Preservation of renal function by G6Pase expression in the kidney
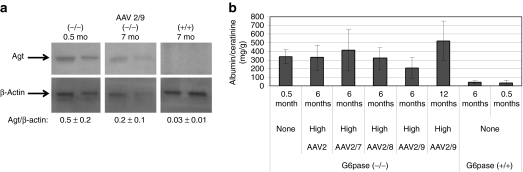
Western blot detection of angiotensinogen revealed increased expression in the kidney of 2-week-old G6pase (−/−) mice,7 and this analysis was performed for untreated and vector-treated G6pase (−/−) mice. The normalized signal for angiotensinogen was decreased in the kidney of G6pase (−/−) mice by the high dose of the AAV2/9 vector, in comparison with untreated 2-week-old G6pase (−/−) mice; however, angiotensinogen was further decreased in the kidney of age-matched G6pase (+/+) mice, reflecting incomplete renal correction following vector administration (Figure 6a). Renal involvement in GSD-Ia progresses from microalbuminuria to proteinuria, and therefore urinary albumin:creatinine ratio was quantified for vector-treated G6pase (−/−) mice. Albumin:creatinine ratio was statistically less following administration of the AAV2/9 vector, in comparison with the AAV2/7 vector (P = 0.05; Figure 6b). There was a trend toward reduced albumin:creatinine ratio 6 months following administration of the AAV2/9 vector, in comparison with untreated G6pase (−/−) mice, suggesting that preservation of renal function was possible with the AAV2/9 vector (P = 0.08; Figure 6b). However, the albumin:creatinine ratio was significantly elevated by 12 months of age for the AAV2/9 vector, in comparison with 6 months of age (P < 0.001). Thus, a trend toward reversal of activation of renin–angiotensin and proteinuria was demonstrated for >6 months following treatment with the AAV2/9 vector.
Figure 6.
Improvement in renal function following adeno-associated virus (AAV) vector administration. (a) Western blot analysis of angiotensinogen (Agt) in G6pase (−/−) and (+/+) mouse kidneys. Each lane represents an individual mouse. (b) Analysis of G6pase (−/−) mouse urine following administration of the indicated number of vector particles at 12 days of age (1 × 1013 vp/kg, except AAV2 5 × 1012 vp/kg); urine sampled at the indicated ages. Number of mice per group as follows: AAV2 (n = 5), AAV2/7 (n = 5), AAV2/8 (n = 3), AAV2/9 at 6 months (n = 9), AAV2/9 at 12 months (n = 4), G6pase (+/+) at 6 months (n = 7), G6pase (+/+) at 0.5 months (n = 3), and G6pase (−/−) at 0.5 months (n = 4). Mean ± SD are shown.
Discussion
The availability of an accurate mouse model has facilitated the preclinical testing of gene therapy vectors for GSD-Ia, including adenovirus, lentiviral, and AAV vectors, and prior work demonstrated partial biochemical correction of the kidney in this model.10,12,13,14,27,28,29 The correction of G6Pase deficiency, glycogen storage, histologic abnormalities, and albuminuria has demonstrated the potential for AAV vectors to correct inherited diseases of the kidney. This study demonstrated that the dsAAV2/9 vector was superior to other serotypes with regard to transduction of the kidney, consistent with reports of AAV2/9 vectors in other models,30,31 although the advantage for the dsAAV2/9 vector in comparison with the dsAAV2/8 vector was slight. The dsAAV2/8 vector achieved a degree of biochemical correction equivalent to that of the AAV2/9 vector in both the kidney and liver, despite the presence of lower vg copy number and increased renal fibrosis observed following dsAAV2/8 vector administration.
One of the primary abnormalities in GSD-Ia, glycogen storage in the kidney, was evaluated by demonstrating reduced glycogen content following AAV vector administration in comparison with untreated G6pase (−/−) mice. The degree of glycogen clearance in the kidney and the duration of correction compared favorably with previous studies, and the impact of gene therapy upon renal function has not been reported.12,13,14,27,28 Studies in normal mice have identified AAV9 as an advantageous serotype for the transduction of multiple tissues. While early published reports focused upon the efficiency of transduction of the heart and skeletal muscle with AAV2/9, recently it has become evident that AAV2/7 and AAV2/9 vectors transduced liver and kidney with the highest efficiency in a comparison of multiple AAV serotypes.30 Currently, the AAV2/9 vector featured much greater biochemical correction of the kidney than the AAV2 vector following intravenous injection, and the AAV2/9 vector prevailed when each vector was administered at the higher dose. All vectors evaluated did prolong survival, which surpassed previous results for an AAV2 vector in GSD-Ia. This new benchmark for an AAV2 vector in GSD-Ia demonstrated the advantage of the small genome AAV vector, capable of being packaged as double-stranded AAV.12,27,32 AAV2 vectors previously transduced renal tubules only following direct injection of the kidney, not intravenous injection, indicating that the main obstacle to renal gene therapy with AAV was delivery to the kidney.33,34,35 Intriguingly, an AAV2 vector transduced renal tubule cells more effectively than AAV2/1, AAV2/3, AAV2/4, or AAV2/5 vectors following direction injection of the kidney.36 Clearly, an AAV2/1 vector can transduce the kidney in mice with GSD-Ia and achieve a degree of biochemical correction,13 although the dsAAV2/1 vector was less effective than the equivalent dsAAV2/8 vector in our earlier study.12 However, the potential for AAV2/7 and AAV2/9 vectors to transduce kidney cells has been demonstrated following intravenous injections of vectors containing reporter genes in normal adult mice,30,37 and we currently confirmed that dsAAV2, dsAAV2/7, dsAAV2/8, and dsAAV2/9 vectors were partially efficacious in the kidney of mice with GSD-Ia (in order of ascending efficacy).
Renal involvement in GSD-Ia has been attributed to glomerular damage causing hyperfiltration, which gradually progresses from microalbuminuria to proteinuria.38,39,40 The majority of patients with GSD-Ia developed microalbuminuria during childhood in one series of 36 patients with GSD-Ia;39 moreover, 29% of children had microalbuminuria and 4% had proteinuria, while 67% of adults with GSD-Ia had microalbuminuria and 42% had proteinuria in a series of 39 patients with GSD-Ia.40 During childhood microalbuminuria was associated with poor metabolic control.40 Mundy and Lee hypothesized that the pathogenesis of renal involvement in GSD-Ia appears similar to that of diabetes mellitus, because glomerular hyperfiltration is associated with increased renal angiotensinogen expression.41 Indeed, angiotensin upregulation and fibrosis was demonstrated in the kidney of G6pase (−/−) mice, validating the renal involvement in the mouse model for GSD-Ia.7
Prevention of hypoglycemia during fasting represents a critical endpoint for gene therapy in GSD-Ia. Intercurrent hypoglycemia and related metabolic abnormalities have been linked to progression of renal involvement in GSD-Ia.42,43 The newly established benchmark of maintaining normal blood glucose (>100 mg/dl) for 8 hours of fasting would be clinically relevant in humans with GSD-Ia, who currently require absolute adherence to continuous cornstarch supplementation to prevent life-threatening hypoglycemia. The duration of normoglycemia for 8 hours following AAV2/9 vector administration surpassed the established limit of 6 hours normoglycemia achieved with an AAV2/8 vector in G6pase (−/−) mice.14
None of the vectors administered corrected liver G6Pase deficiency to the degree observed following high-dose treatment with the dsAAV2/8 pseudotyped vector, when levels reached those of wild-type mice.12 However, the equivalent number of dsAAV2/9 vector particles reduced liver glycogen content to a greater degree than the dsAAV2/8 vector did in that earlier study (93% vs 80%), and the currently studied dsAAV2/8 vector stock performed similarly to the previously published vector by reducing glycogen content in the liver by 86%. The latter data suggested a widespread expression of G6Pase with the new dsAAV2/9 vector, at least equivalent to the level achieved with the dsAAV2/8 vector, and microscopic examination of liver and kidney sections revealed a widespread reduction in glycogen vacuolation at 6 and 12 months.
The enhanced transduction of hepatocytes with scAAV vectors has been demonstrated with several different AAV serotypes. The earliest data demonstrated a tenfold increase in transduction of mouse hepatocytes with an scAAV2 vector, in comparison to an ssAAV vector, from <5% to up to 50%.16 Optimization of gene therapy for hemophilia B revealed enhanced transduction of hepatocytes with scAAV vectors pseudotyped as AAV8.44,45,46 These studies demonstrated efficacious transgene expression from a low number of vector particles.
The current study demonstrated efficacy with a dsAAV vector in both male and female mice with GSD-Ia, and an equivalent number of each sex survived to the end of the observation period (data not shown). This is noteworthy, because reduced transgene expression in adult female mice has been linked to the androgen-dependence of AAV vector transduction. The androgen-dependence of second-strand DNA synthesis for AAV vectors was previously demonstrated as the cause for reduced transduction in female mice,47 indicating that a dsAAV vector might at least partially overcome this hurdle. Furthermore, transgene expression decreased more rapidly in young female mice following AAV vector administration than their male counterparts, which has clear implications for AAV vector-mediated therapy in G6pase (−/−) mice treated prior to weaning.48
An scAAV2/9 vector delivered hepatocyte growth factor efficaciously in a mouse model for renal fibrosis in COL4A3-deficient mice.31 That study also identified AAV2/9 as the most efficient pseudotype for transduction of renal tubular epithelium, in agreement with the current data. Similarly to this study, Schievenbusch et al. included a renal-active regulatory cassette for transgene expression. The endocrine effect of liver-expressed hepatocyte growth factor represents an inherent advantage for gene therapy in murine renal fibrosis, whereas the need for intrarenal transgene expression to replace membrane bound G6Pase in GSD-Ia seems a greater challenge. Nonetheless, we found a nearly equivalent biochemical correction of the kidney and liver with dsAAV2/9-G6Pase in G6pase (−/−) mice, in contrast to the transduction of kidney being reduced by approximately 100-fold in COL4A3-deficient mice.31 The gradual accumulation of intramembranous G6Pase might explain how a high degree of biochemical correction was achieved in the kidney of G6pase (−/−) mice with dsAAV2/9-G6Pase. Whether an scAAV2/9 has a disadvantage with regard to transduction of the kidney, versus a dsAAV2/9 vector that contained the intact terminal repeat sequence, remains to be determined. Overall, either an scAAV2/9 or dsAAV2/9 vector seems to have significant advantages with regard to the treatment of renal diseases in murine models.
The current study revealed that the dsAAV2/9-G6Pase vector can express G6Pase for up to a year in the kidney and liver with high efficacy in mice with GSD-Ia, and the dsAAV2, dsAAV2/7, and dsAAV2/8 vectors were efficacious to a lesser extent with regard to hepatorenal correction. Thus, further preclinical development of gene therapy with dsAAV vectors should be pursued in GSD-Ia, given a favorable safety profile and the potential for preventing severe, life-threatening complications including recurrent hypoglycemia and renal failure.
Materials and Methods
Preparation of AAV vectors encoding human G6Pase. The AAV vector plasmid, pAAV-G6PaseHA, contained a transgene comprised of the human G6Pase minimal promoter to drive a human G6Pase cDNA followed by a human growth hormone polyadenylation signal.12 The AAV vectors were pseudotyped with AAV capsid proteins as described (packaging plasmids courtesy of Dr Wilson, University of Pennsylvania, Philadelphia, PA), and the helper plasmid was pAdHelper (Stratagene, La Jolla, CA).12
AAV vector administration to G6Pase (−/−) mice. Carrier G6Pase (±) mice were housed in the Duke Vivarium and bred to produce homozygous, affected G6Pase (−/−) offspring. Affected genotype was confirmed by PCR analysis of tail DNA with primers within and flanking the neo gene insertion in the G6Pase gene as described.28 G6Pase (−/−) mice were injected with vectors via the retroorbital sinus at 12 ± 1 days of age without regard to sex, and both males and females were included in all groups. Injection was performed following isoflurane anesthesia with a 28 gauge insulin syringe (10 µl/g volume), and hemostasis was achieved by brief manual pressure. Daily injection of 0.1–0.2 ml 10% dextrose subcutaneously was initiated at 3 days of age and continued for 2–3 weeks. Mice were fasted periodically for 2 hours beginning at 10:00 , or for 8 hours beginning at 8:00 . All mouse procedures were done in accordance with Duke University Institutional Animal Care and Use Committee-approved guidelines.
Evaluation of biochemical correction. Enzyme analysis was performed as described.12 Briefly, tissues were flash-frozen and stored at −70 °C. Glycogen content was measured by complete digestion of polysaccharide using amyloglucosidase (Sigma Chemical, St Louis, MO). The structure of the polysaccharide was inferred by using phosphorylase free of the debranching enzyme to measure the yield of glucose-1-phosphate. Specific G6Pase activity was measured by using glucose-6-phosphate as substrate after subtraction of nonspecific phosphatase activity as estimated by β-glycerophosphate. Glucose and cholesterol were analyzed with a Kodak Biolyzer (Eastman Kodak, Rochester, NY) according to the manufacturer's recommendations. Western blot analyses were performed with antibodies against angiotensin (N-10) (Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (Abcam, Cambridge, MA). Detection of epitope-specific immunostaining was accomplished by chemiluminescence reaction using ECL western blotting substrate (Promega, Madison, WI) and Kodak BioMax MR film (VWR Scientific, Radnor, PA). Standard densitometric analysis of angiotensinogen and β-actin bands was conducted using the UN-SCAN-IT 6.1 gel analysis software program (Silk Scientific, Orem, UT). Individual band intensities were quantified and data were expressed as relative optical density with associated range of variance.
Quantification of vector DNA. Realtime quantitative reverse transcription-PCR was performed using SYBR green in a LightCycler 480II (Roche, Basel, Switzerland) following the manufacturer's instructions. Gene-specific primers for human G6Pase cDNA (sense 5′-AGTGCCCACACAGTGCGACGT-3′, and antisense 5′-CCTCGTAGCGCCTGTTAGCTG-3′), and for mouse β-actin (sense 5′-AGAGGGAAATCGTGCGTGAC-3′ and antisense 5′-CAATAGTGATGACCTGGCCGT-3′) were used for each reaction. Plasmid DNA corresponding to 0.01 copy to 100 copies of human GAA gene (in 500 ng genomic DNA) was used in a standard curve. To determine the vg copy number, the Cp values of samples were compared to the standard curve.
G6Pase histochemical detection. G6Pase was detected qualitatively in frozen sections (6 µm) mouse liver by an optimized cerium-diaminobenzidine method as described.49 Liver sections were incubated in medium consisting of 5 mmol/l cerium chloride (Sigma), 10 mmol/l glucose-6-phosphate (Sigma), and 50 mmol/l tris-maleate buffer (pH 6.5) for 5 minutes at room temperature. The first incubation was stopped in 50 mmol/l tris-maleate buffer (pH 8.0). G6Pase was visualized by a subsequent incubation in 10 mmol/l glucose-6-phosphate, 10 mmol/l calcium chloride, 5 mmol/l sodium azide and 50 mmol/l tris-maleate buffer (pH 6.5) for 5 minutes at room temperature, rinsed with distilled water, counter-stained with nuclear fast red, and mounted with Shur/Mount medium (Triangle Biomedical Sciences, Durham, NC).
Quantification of urine albumin excretion. Urine collections were obtained on mice at 6 months and 12 months, with free access to water and food. After collection, urine was immediately centrifuged for 3 minutes (13,000g) to remove contamination, and the supernatant was stored at −80 °C before analysis. Albuminuria was determined by ELISA (Albuwell; Exocell, Philadelphia, PA), and creatinine was measured using the alkaline picrate method (The Creatinine Companion; Exocell).
Statistical analysis of survival and other endpoints. Reported P values are two-tailed and were derived from nonparametric methods. Pairwise comparisons used the Wilcoxon rank–sum test. Survival analysis included production of Kaplan–Meier curves and P values presented were from log rank test.
Acknowledgments
We wish to acknowledge inspiration and support from Dr Emory and Mrs Mary Chapman and their son Christopher, and from Dr and Mrs John Kelly. D.D.K., X.Y., S.L., and A.B. were supported by the Children's Fund for GSD Research. We deeply appreciate the dedication shown by Duke Vivarium staff, when caring for mice with GSD-Ia. We wish to acknowledge excellent technical support from Alison Homstad, Alison Byrd, Michael Maranzano, Hiruni Amarasekara, and Colby Bird.
REFERENCES
- Von Gierke E. Hepato-nephro-megalia glycogenica (glykogenspeicher-krankheit der leber und nieren) Beitr Pathol Anat. 1929;82:497. [Google Scholar]
- Chen YT.2001Glycogen storage diseases Scriver CR, Beaudet AL, Sly WS., and, Valle D.eds). The Metabolic and Molecular Bases of Inherited Disease8th edn. McGraw-Hill: New York; 1521–1551. [Google Scholar]
- Wolfsdorf JI., and, Crigler JF., Jr Cornstarch regimens for nocturnal treatment of young adults with type I glycogen storage disease. Am J Clin Nutr. 1997;65:1507–1511. doi: 10.1093/ajcn/65.5.1507. [DOI] [PubMed] [Google Scholar]
- Chen YT, Coleman RA, Scheinman JI, Kolbeck PC., and, Sidbury JB. Renal disease in type I glycogen storage disease. N Engl J Med. 1988;318:7–11. doi: 10.1056/NEJM198801073180102. [DOI] [PubMed] [Google Scholar]
- Talente GM, Coleman RA, Alter C, Baker L, Brown BI, Cannon RA.et al. (1994Glycogen storage disease in adults Ann Intern Med 120218–226. [DOI] [PubMed] [Google Scholar]
- Matern D, Starzl TE, Arnaout W, Barnard J, Bynon JS, Dhawan A.et al. (1999Liver transplantation for glycogen storage disease types I, III, and IV Eur J Pediatr 158 Suppl 2S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu WH, Pan CJ, Ruef RA, Peng WT, Starost MF, Mansfield BC.et al. (2008Angiotensin mediates renal fibrosis in the nephropathy of glycogen storage disease type Ia Kidney Int 73716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belingheri M, Ghio L, Sala A, Menni F, Trespidi L, Ferraresso M.et al. (2007Combined liver-kidney transplantation in glycogen storage disease Ia: a case beyond the guidelines Liver Transpl 13762–764. [DOI] [PubMed] [Google Scholar]
- Keller KM, Schütz M, Podskarbi T, Bindl L, Lentze MJ., and, Shin YS. A new mutation of the glucose-6-phosphatase gene in a 4-year-old girl with oligosymptomatic glycogen storage disease type 1a. J Pediatr. 1998;132:360–361. doi: 10.1016/s0022-3476(98)70463-9. [DOI] [PubMed] [Google Scholar]
- Lei KJ, Chen H, Pan CJ, Ward JM, Mosinger B, Jr, Lee EJ.et al. (1996Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse Nat Genet 13203–209. [DOI] [PubMed] [Google Scholar]
- Zingone A, Hiraiwa H, Pan CJ, Lin B, Chen H, Ward JM.et al. (2000Correction of glycogen storage disease type 1a in a mouse model by gene therapy J Biol Chem 275828–832. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Pinto C, Sun B, Li S, Kozink DM, Benjamin DK., Jret al. (2008AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia Mol Ther 16665–672. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Allamarvdasht M, Pan CJ, Sun MS, Mansfield BC, Byrne BJ.et al. (2006Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer Gene Ther 13321–329. [DOI] [PubMed] [Google Scholar]
- Yiu WH, Lee YM, Peng WT, Pan CJ, Mead PA, Mansfield BC.et al. (2010Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy Mol Ther 181076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J.et al. (2009Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy Blood 113797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P., and, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Monahan PE., and, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X.et al. (2003Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain Mol Ther 8911–917. [DOI] [PubMed] [Google Scholar]
- Alexander IE, Russell DW., and, Miller AD. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J Virol. 1994;68:8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Alexander IE., and, Miller AD. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FK, Samulski T, Shenk T., and, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Morris DW., and, Chou JY. The role of HNF1alpha, HNF3gamma, and cyclic AMP in glucose-6-phosphatase gene activation. Biochemistry. 1997;36:14096–14106. doi: 10.1021/bi9703249. [DOI] [PubMed] [Google Scholar]
- Schmoll D, Wasner C, Hinds CJ, Allan BB, Walther R., and, Burchell A. Identification of a cAMP response element within the glucose- 6-phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem J. 1999;338 (Pt 2):457–463. [PMC free article] [PubMed] [Google Scholar]
- Vander Kooi BT, Streeper RS, Svitek CA, Oeser JK, Powell DR., and, O'Brien RM. The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J Biol Chem. 2003;278:11782–11793. doi: 10.1074/jbc.M212570200. [DOI] [PubMed] [Google Scholar]
- McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Sun MS, Pan CJ, Shieh JJ, Ghosh A, Chen LY, Mansfield BC.et al. (2002Sustained hepatic and renal glucose-6-phosphatase expression corrects glycogen storage disease type Ia in mice Hum Mol Genet 112155–2164. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Sun BD, Damodaran TV, Brown T, Millington DS, Benjamin DK., Jret al. (2006Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia Gene Ther 131281–1289. [DOI] [PubMed] [Google Scholar]
- Grinshpun A, Condiotti R, Waddington SN, Peer M, Zeig E, Peretz S.et al. (2010Neonatal gene therapy of glycogen storage disease type Ia using a feline immunodeficiency virus-based vector Mol Ther 181592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G., and, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Schievenbusch S, Strack I, Scheffler M, Nischt R, Coutelle O, Hösel M.et al. (2010Combined paracrine and endocrine AAV9 mediated expression of hepatocyte growth factor for the treatment of renal fibrosis Mol Ther 181302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RM, Jackson M, Peterson D, Bird A, Brown T, Benjamin DK., Jret al. (2002Delivery of glucose-6-phosphatase in a canine model for glycogen storage disease, type Ia, with adeno-associated virus (AAV) vectors Gene Ther 91015–1022. [DOI] [PubMed] [Google Scholar]
- Ito K, Chen J, Khodadadian JJ, Vaughan ED, Jr, Lipkowitz M, Poppas DP.et al. (2008Adeno-associated viral vector transduction of green fluorescent protein in kidney: effect of unilateral ureteric obstruction BJU Int 101376–381. [DOI] [PubMed] [Google Scholar]
- Chen S, Agarwal A, Glushakova OY, Jorgensen MS, Salgar SK, Poirier A.et al. (2003Gene delivery in renal tubular epithelial cells using recombinant adeno-associated viral vectors J Am Soc Nephrol 14947–958. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Yamashita T, Miyazaki T, Taira H., and, Suzuki A. Gene delivery to renal tubular epithelial cells using adeno-associated virus vector in domestic cats. Res Vet Sci. 2009;87:408–412. doi: 10.1016/j.rvsc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Takeda S, Takahashi M, Mizukami H, Kobayashi E, Takeuchi K, Hakamata Y.et al. (2004Successful gene transfer using adeno-associated virus vectors into the kidney: comparison among adeno-associated virus serotype 1-5 vectors in vitro and in vivo Nephron Exp Nephrol 96e119–e126. [DOI] [PubMed] [Google Scholar]
- Bostick B, Ghosh A, Yue Y, Long C., and, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- Baker L, Dahlem S, Goldfarb S, Kern EF, Stanley CA, Egler J.et al. (1989Hyperfiltration and renal disease in glycogen storage disease, type I Kidney Int 351345–1350. [DOI] [PubMed] [Google Scholar]
- Ozen H, Ciliv G, Koçak N, Saltik IN, Yüce A., and, Gürakan F. Short-term effect of captopril on microalbuminuria in children with glycogen storage disease type Ia. J Inherit Metab Dis. 2000;23:459–463. doi: 10.1023/a:1005608113270. [DOI] [PubMed] [Google Scholar]
- Martens DH, Rake JP, Navis G, Fidler V, van Dael CM., and, Smit GP. Renal function in glycogen storage disease type I, natural course, and renopreservative effects of ACE inhibition. Clin J Am Soc Nephrol. 2009;4:1741–1746. doi: 10.2215/CJN.00050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy HR., and, Lee PJ. Glycogenosis type I and diabetes mellitus: a common mechanism for renal dysfunction. Med Hypotheses. 2002;59:110–114. doi: 10.1016/s0306-9877(02)00199-8. [DOI] [PubMed] [Google Scholar]
- Wolfsdorf JI, Laffel LM., and, Crigler JF., Jr Metabolic control and renal dysfunction in type I glycogen storage disease. J Inherit Metab Dis. 1997;20:559–568. doi: 10.1023/a:1005346824368. [DOI] [PubMed] [Google Scholar]
- Wolfsdorf JI., and, Crigler JF., Jr Effect of continuous glucose therapy begun in infancy on the long-term clinical course of patients with type I glycogen storage disease. J Pediatr Gastroenterol Nutr. 1999;29:136–143. doi: 10.1097/00005176-199908000-00008. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN.et al. (2006Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver Blood 1072653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y.et al. (2007Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates Blood 1091414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T.et al. (2008Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose Mol Ther 16280–289. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Zhou J, Spence Y., and, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- Dane AP, Cunningham SC, Graf NS., and, Alexander IE. Sexually dimorphic patterns of episomal rAAV genome persistence in the adult mouse liver and correlation with hepatocellular proliferation. Mol Ther. 2009;17:1548–1554. doi: 10.1038/mt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonges GN, Van Noorden CJ., and, Gossrau R. Quantitative histochemical analysis of glucose-6-phosphatase activity in rat liver using an optimized cerium-diaminobenzidine method. J Histochem Cytochem. 1990;38:1413–1419. doi: 10.1177/38.10.2169492. [DOI] [PubMed] [Google Scholar]