Abstract
Many genetic metabolic diseases manifest in infancy, therefore, it is important to develop effective treatments that could be implemented at this time. Adeno-associated virus serotype 8 (AAV8) gene transfer has been studied in neonatal mouse, cat, and dog models and shown some efficacy with a single hepatic injection at birth. AAV8-mediated liver gene transfer has also generated sustained therapeutic effects in feline and canine models of lysosomal storage disorders. In these models, delaying the age of vector treatment increased gene transfer stability. The growth rate of infant nonhuman primates is more similar to the growth trajectory of humans, thus infant monkeys provide an excellent model to study AAV gene transfer efficiency, stability, and safety. In this study, we report for the first time that AAV8-mediated hepatic gene transfer in infant monkeys is safe and efficient but less stable when compared to adolescent animals. Infant monkeys administered AAV8 intravenously at 1 week postnatal age achieved up to 98% transduction of hepatocytes within 7 days of injection; however, there was significant dilution of genomes and loss of transgene expression 35 days postadministration. Delaying the injection to 1 month postnatal age did not improve stability of transduction but decreased the antibody response to AAV8 capsid.
Introduction
Vectors based on adeno-associated virus serotype 8 (AAV8) have demonstrated superior liver transduction efficiency in many preclinical studies and are currently being tested in humans.1 Many studies have been conducted in adult animals from several different species, including mouse, rat, cat, dog, and nonhuman primates.2,3,4,5,6,7,8,9 Since many inherited metabolic diseases manifest at a young age, it is important to develop effective treatments and interventions early in life. For AAV-mediated liver gene therapy, the pediatric population presents both advantages and challenges. The presence of AAV neutralizing antibodies (NAb) has been shown to be low in pediatric patients when compared to adults.10 Young individuals are also more likely to be “naive” as they may have no prior natural AAV infections, therefore they do not have memory T cells to AAV capsid. In pediatric patients there is also less likelihood for tissue damage and/or accumulation of toxic metabolites in the liver, which can reduce the threshold for therapeutic efficiency. Moreover, if the potential for inflammation is reduced this may be of importance in evading transgene-specific immune responses.
One of the main challenges with pediatric gene transfer is the impact of liver growth on the stability of vector DNA persistence and transgene expression. This is because AAV vector DNA primarily exists in episomal vector genomes in transduced tissues.11As demonstrated in the partial hepatectomy adult mouse model or following AAV administration into neonatal mice,11,12,13,14 a rapid decline of transgene expression has been observed due to hepatocyte proliferation. In neonatal mice, stable transgene expression eventually persisted in 4–8% of hepatocytes.13 Nevertheless, successful long term rescue has been achieved by a single injection of AAV8 vectors at the neonatal stage in lethal mouse models of methylmalonic acidemia and propionic acidemia,15,16,17 or in the lethal infant model of glycogen storage disease type Ia mouse model.18 In these cases, expression of the normal gene in a small percentage of stably transduced cells account for the clinical effects. Interestingly, when vector is delivered at serially higher ages up to adulthood, transgene expression was shown to be progressively more durable, correlating with the decline in liver growth.13,18 Neonatal gene transfer by AAV8 vector in rat and cat models of mucopolysaccharidosis VI (MPS VI) has been shown to result in long term correction in the MPS VI cats bearing a missense mutation, however, in the null MPS VI rats, development of a neutralizing humoral immune response to the transgene product resulted in reduced levels of circulating enzyme levels after gene transfer and limited therapeutic efficacy.19 In the MPS VI cats, high vector doses were required to overcome the dramatic loss of transgene expression levels within the first month following vector administration.4,19 Delaying the vector injection from postnatal day 5 to postnatal day 50 significantly increased the stability of transgene expression levels and decreased the vector dose required to achieve therapeutic effects.4 In the canine model of glycogen storage disease type Ia, a single injection of AAV2/8 vector in 1-day-old dogs achieved transient correction. A second injection with an AAV2/1 vector 2 months after the initial AAV2/8 injection successfully restored therapeutic effects.20
AAV-mediated hepatic gene transfer has been well studied in juvenile and adult nonhuman primates.8,21,22,23,24 However, data on gene transfer in developing monkeys is limited.25,26 In this study, we report the efficiency, stability, and safety of AAV8-mediated hepatic gene transfer in infant rhesus monkeys. High levels of short-term expression in the liver were achieved. However, there was significant dilution of vector genomes and loss of transgene expression which may still provide therapeutic value in selected inherited metabolic diseases, such as methylmalonic acidemia and propionic acidemia15,16,17 or some lysosomal storage disorders.4,18
Results
Study design
To investigate the transduction efficiency of AAV8 in the liver of infant rhesus monkeys, 3 × 1012 genome copies (GC)/kg of AAV2/8.TBG.EGFP vector was administered intravenously to six 1-week-old male rhesus monkeys. Three of six were scheduled for tissue harvest 7 days after vector administration (d7-1w) and three of six were euthanized for tissue harvest 35 days after vector administration (d35-1w). The evaluation of enhanced green fluorescent protein (EGFP) expression was performed by analyzing EGFP-positive areas by direct fluorescence in liver sections, and EGFP concentration in total liver lysates. Vector genomes in liver and vector biodistribution were assessed in select tissues by quantitative-PCR (Q-PCR). Relative EGFP RNA levels in liver and spleen were measured by real-time reverse transcription-PCR. To evaluate if delaying vector administration to ~1 month postnatal age would enhance the persistence of gene transfer, three 1-month-old male rhesus monkeys were administered the same vector construct intravenously at the same dose with tissue harvests performed 45 days later (d45-1m). For all animals, body weights, complete blood counts, and clinical chemistry panels including liver enzymes were assessed regularly, and peripheral blood mononuclear cells (PBMCs) collected for evaluation of T cell responses to AAV8 capsid and EGFP.
Robust liver transduction by AAV8 in infant rhesus monkeys
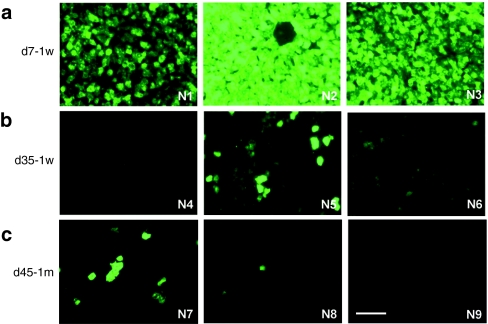
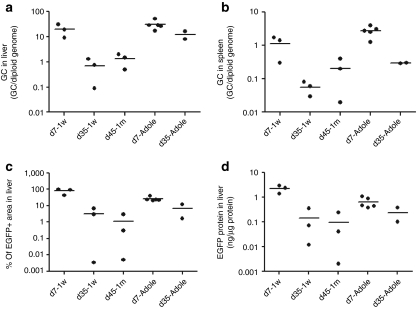
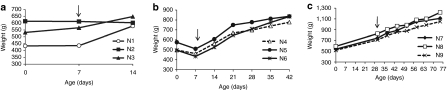
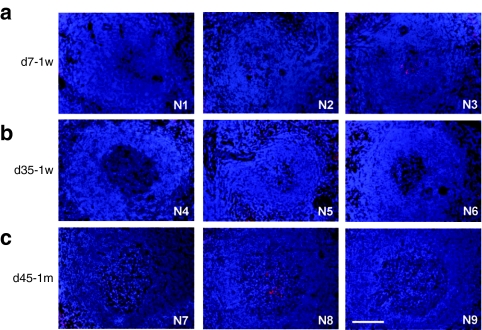
The livers collected 7 days after vector administration from the three 2-week-old monkeys showed extremely high levels of transduction (Figure 1a). EGFP-positive areas ranged from 44 to 98% and were found to be evenly distributed throughout the liver, with no peri-central transduction preference which has been seen in the mouse.3,13,27 Vector genome copies in the liver ranged from 8 to 32 copies per diploid genome (Table 1). Among the three animals in this early age group, the transduction efficiency appeared to be inversely correlated with growth rates as measured by body weight (Figure 3a and Table 1). Compared to the data generated from previous studies in five adolescent male rhesus monkeys that received the same vector at the same dose with tissues collected 7 days after vector administration,7,23 both groups showed similar vector genome copies in the liver (Figure 2a) (P = 0.27, two-tailed t test). However, infant monkeys showed significantly higher EGFP expression in the liver as measured by EGFP fluorescence and liver lysates (Figure 2c,d) (P < 0.01, two-tailed t test). These findings suggest that the liver-specific TBG promoter may be more active in the younger animals.
Figure 1.
EGFP expression in the livers of infant rhesus monkeys after intravenous administration of 3 × 1012 GC/kg of AAV2/8.TBG.EGFP. (a) Infant rhesus monkeys were administered vector at 1 week postnatal age and tissues collected 7 days post administration (d7-1w). (b) Infant rhesus monkeys were administered vector at 1 week postnatal age and tissues collected 35 days post administration (d35-1w). (c) Infant rhesus monkeys were administered vector at 1 month postnatal age and tissues collected 45 days post administration (d45-1m). Animal number is shown at the bottom right corner. Representative liver images of each animal are shown. Bar = 100 µm. AAV, adeno-associated virus; EGFP, enhanced green fluorescent protein.
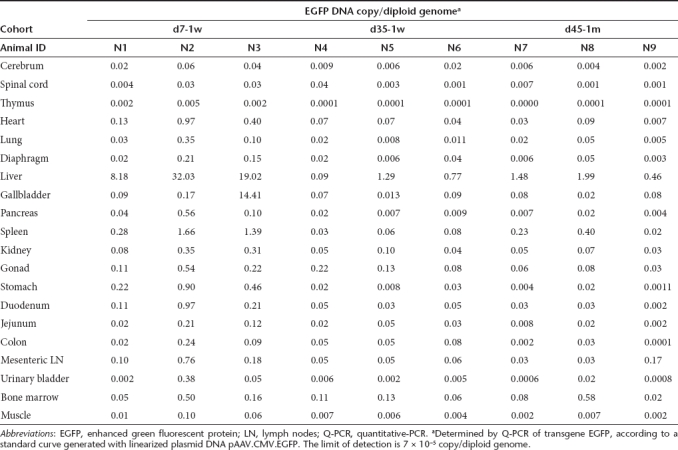
Table 1. Summary of gene transfer in infant rhesus monkeys after administration of AAV2/8.TBG.EGFP vector.
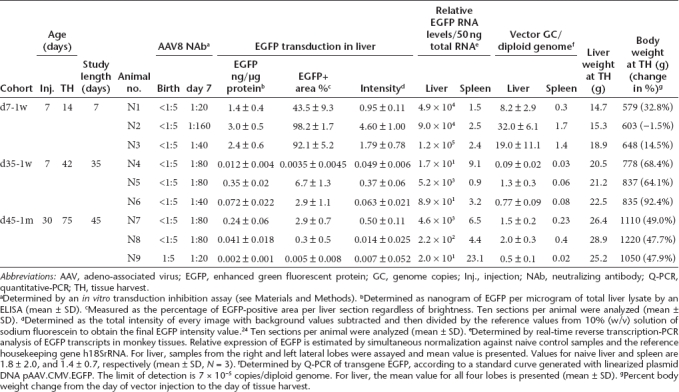
Figure 2.
Gene transfer and transduction efficiency in infant rhesus monkeys. Data from previously published adolescent rhesus monkeys (d7-Adole and d35-Adole) administered the same vector at the same dose7,23 are included for comparison. (a) Vector genome copies in liver. (b) Vector genome copies in spleen. (c) Quantitative morphometric analysis of the transduction efficiency based on percent transduction of liver area. (d) Quantification of EGFP in liver lysates by ELISA. Horizontal lines show the mean levels for each group. EGFP, enhanced green fluorescent protein; ELISA, enzyme-linked immunosorbent assay; GC, genome copies.
Dramatic reduction of gene transfer in infant monkeys 35 days after vector administration
In contrast to findings 7 days after vector administration, the three animals with tissue harvests 35 days after vector administration showed low levels of EGFP expression in the liver (Figure 1b). EGFP-positive cells appeared in clusters and ranged from <0.01% (#N4) to 2.9% (#N6) and 6.7% (#N5); liver vector genome copies ranged from 0.09 (#N4) to 0.8 (#N6) and 1.3 (#N5) copies per diploid genome (Table 1). Animal #N4 showed the lowest EGFP expression, and was the only animal with a transient elevation of serum transaminase when compared to prior values. At week 3 after vector administration, alanine aminotransferase and aspartate aminotransferase levels were increased threefold and 2.6-fold, respectively, when compared to the prior three time points. There were no T cell responses to AAV8 capsid or EGFP detected in the PBMCs isolated weekly after vector administration by IFN-γ ELISPOT (Supplementary Figure S1). Monkey #N6 showed greater weight gain (92%) when compared to monkey #N5 (64%) during the 35 days after vector administration and a twofold lower quantity of vector genomes and EGFP-positive areas in the liver (Figure 3b and Table 1). Compared to two adolescent rhesus monkeys from prior studies that were injected with the same vector at the same dose and followed for 35 days,23 vector genomes in the liver were significantly lower in the infant monkeys in this study (Figure 2a) suggesting dilution of vector genomes in these young animals. Interestingly, there were no significant differences in EGFP-positive areas and EGFP in the liver lysates when comparing the infant and adolescent monkeys (Figure 2c,d) suggesting that the liver-specific TBG promoter may be more active in younger animals.
Figure 3.
Body weight gain for infant rhesus monkeys from birth until tissue harvest. (a) d7-1 w cohort, (b) d35–1 w cohort, (c) d45-1m cohort. Arrow indicates the timepoint when AAV8 vector was administered. Body weights for all animals in this study were within the normative range for infant rhesus monkey based on historical data26 (A. Tarantal). AAV, adeno-associated virus.
Delaying vector administration to 1 month postnatal age did not improve the stability of gene transfer
To determine if the age of vector administration could improve the stability of gene transfer, three 1-month-old male rhesus monkeys were administered the same vector at the same dose and monitored for 45 days. As shown in Figure 1c, EGFP expression levels in the livers of this cohort of animals were no different than the d35-1w cohort, although the growth rates of the d45-1m cohort differed (Table 1). Variation in EGFP expression levels within this cohort was also observed although there was no clear relationship to growth rate (Figure 3c). Vector genome copies in the liver varied to a lesser degree from 0.5 to 2 copies per diploid genome (Table 1). Monkey #N9 showed the lowest liver transduction and was the only animal in the infant study that had low but detectable NAb to AAV8 at birth (titer = 1:5). Vector genomes in the liver were three to fourfold lower than the other two animals (#N7 and #N8) in this cohort (Table 1). Liver enzyme levels were within the normative range during the study and there were no detectable T cell responses to AAV8 capsid or EGFP in the PBMCs collected after vector administration (Supplementary Figure S1).
Detection of EGFP in spleen and the antibody response to EGFP
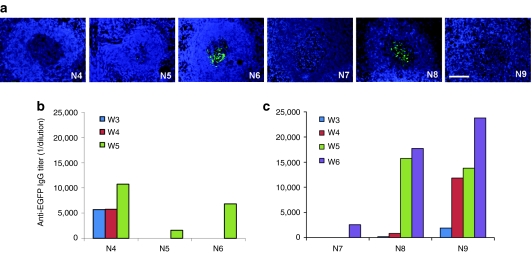
In our previously published study with adolescent rhesus monkeys, one of the animals (605103) showed high levels of EGFP in liver and also in the germinal centers of the spleen when harvested 35 days after vector administration.24 Therefore, we examined the spleens from all animals in the current study. Two animals, #N6 and #N8 (harvested at 35 and 45 days, respectively) showed high levels of EGFP in the splenic germinal centers (Figure 4a). Despite relatively high vector genome copies in the spleen, none of the d7-1w cohort animals had any significant levels of EGFP-specific RNA (Table 1), or detectable EGFP in the spleen (data not shown). The discordance of the absence of EGFP-specific RNA and the presence of EGFP in spleen suggests that EGFP is not expressed directly in the spleen as a result of intravenous vector administration, but may be transported from the liver to the spleen and trapped by cells such as follicular dendritic cells. The EGFP in the vector used in this study is not a secreted protein, but may be released from hepatocytes expressing EGFP by cytotoxic T-lymphocytes or during apoptosis. Since EGFP is a foreign protein to the monkey, it is possible that the animals could mount an immune response. T cell responses to EGFP were detected in our prior published study with adolescent rhesus monkeys,23 but was not observed in any of the infant monkeys in the current study (Supplementary Figure S1). Sera collected weekly from the animals were analyzed by enzyme-linked immunosorbent assay (ELISA) for the detection of EGFP-specific IgG. Except for the d7-1w animals, all animals in the d35-1w and d45-1m cohorts developed α-EGFP IgG (Figure 4b,c). Among them, #N4 and #N9 showed minimal to no EGFP expression in liver at tissue harvest, and developed α-EGFP antibodies as early as 3 weeks after vector administration with increasing titers over time. Interestingly, #N4 also showed a transient elevation over baseline of liver enzymes at week 3. Animal #N5 and #N7 both showed the highest EGFP expression in liver in their respective cohorts and had the lowest titers at the last time point assessed. Animal #N6 and #N8 showed EGFP in spleen, and developed α-EGFP antibodies at 5 weeks after vector administration. Overall, there appeared to be a relationship between the timing/antibody titers and the EGFP expression levels in liver and the presence of EGFP in spleen.
Figure 4.
Detection of EGFP in the germinal centers of spleen and development of anti-EGFP antibodies post-AAV8 gene transfer. (a) Detection of EGFP in the germinal centers of spleens at tissue harvest. (b) Anti-EGFP IgG titer in the serum of d35-1w cohort animals at 3, 4, and 5 weeks (W) after vector administration. (c) Anti-EGFP IgG titers in the serum of d45-1m cohort animals at 3, 4, 5, and 6 weeks (W) after vector administration. Bar = 100 µm. AAV, adeno-associated virus; EGFP, enhanced green fluorescent protein.
Antibodies to AAV capsid
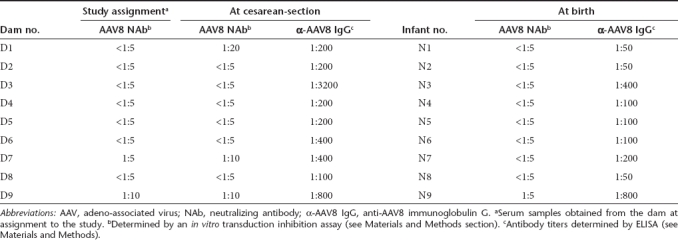
To avoid the complications associated with pre-existing AAV8 NAb which is prevalent in rhesus monkeys, all gravid animals were prescreened at assignment to the study (early gestation) for AAV8 NAb (<1:5 or 1:10). Serum samples were also collected from the dams at cesarean-section and from the newborns at birth and tested for AAV8 NAb and binding antibody (IgG) titers (Table 2). Among the nine dams, dam #1 (D1) seroconverted after assignment to the study as shown in the sample collected at cesarean-section; results were below the detection limit (<1:5) at study assignment and was 1:20 at delivery. Dam #7 (D7) had a slight increase of NAb titer from 1:5 during pregnancy to 1:10 at the time of cesarean-section delivery. With the exception of #N9 with a NAb of 1:5 at birth, all other eight newborn monkeys had no detectable AAV8 NAb at birth. As shown in Table 2, the binding antibody titers did not correlate well with the NAb titers. Maternal transfer of AAV8 binding antibodies was observed in some infant monkeys, albeit most infants had slightly lower titers than their dams.
Table 2. Pre-existing AAV8 antibody titers in the dams and newborns at birth.
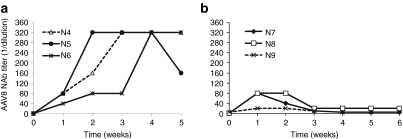
Infant monkey NAb responses to AAV8 capsid were monitored weekly after vector administration. All animals showed an increase in NAb titers 7 days after vector administration, ranging from 1:20 to 1:160 (Table 1). In the d35-1w cohort, NAb titers gradually rose to 1:320 2–3 weeks after vector injection and remained at that level in most cases (Figure 5a). In contrast, the magnitude and kinetics of NAb responses in the d45-1m cohort animals were very different. Here, low peak levels were reached at 1 week after vector administration then quickly declined to low levels of NAb at 1:5 (#N7 and #N9) or 1:20 (#N8) within 3 or 4 weeks after vector injection, respectively. These findings suggest that the age of the animals at the time of vector administration may influence the NAb response to AAV capsid. Additional studies with this age group are needed to confirm this interesting finding and to characterize the mechanism. A low NAb titer may allow re-administration of AAV vector at a later time period since hepatic gene transfer was efficient in animals with low NAb titers (≤1:10).23,24
Figure 5.
Development of AAV8 NAb after vector administration. AAV8 NAb titer in the serum of d35-1w cohort (a) and d45-1m cohort (b) animals after vector administration. AAV, adeno-associated virus; NAb, neutralizing antibody.
Vector biodistribution in infant monkeys following systemic delivery of AAV8 vector
For all nine animals evaluated at tissue harvest, a broad analysis of vector biodistribution in 20 different tissues was performed by Q-PCR (Table 3). Not surprisingly, liver was shown with the highest quantity of vector DNA, reflecting the liver tropism of AAV8. In the d7-1w cohort, spleen also showed relatively higher quantities of vector DNA when compared to other tissues although levels were 14-to 30-fold lower than the liver. When compared to the five adolescent monkeys from previously published studies,23,24 vector genome copies in the spleen of infant monkeys were twofold lower (Figure 2b). The d35-1w cohort had fivefold lower vector genome copies in spleen compared to adolescent rhesus monkeys harvested at day 35 (Figure 2b). In general, the d7-1w cohort showed higher vector DNA in many tissues when compared to the d35-1w and d45-1m cohorts, which may be associated with tissue proliferation. While vector DNA was detected in the gonads of animals injected at 1 week of age (average = 0.22 ± 0.17 per diploid genome, N = 6), prior studies have shown that this does not imply that vector sequences are present in the germ cells.28 Laser capture microdissection would be required to confirm transduction of germ cells, and in prior studies with rhesus monkeys there has been no evidence, to date, of gene transfer to the gonads in male offspring.
Table 3. Biodistribution of vector DNA in infant rhesus monkeys following intravenous vector administration.
Sequestration of AAV capsid in the germinal centers of spleen in infant monkeys
In previously published gene transfer studies in adolescent and adult monkeys, 58% of the animals (11 of 19 animals) had sequestration of AAV8 capsid in the splenic germinal centers.24 AAV antigen was colocalized with follicular dendritic cells in the spleen at both 7 days (10/16) and 35 days (1/3) after vector administration. Of the nine infant monkeys assessed in the current study, capsid antigen was detected in four animals: two in the d7-1w cohort (#N2 and #N3) and two in the d45-1m cohort (#N7 and #N8), whereas none of the d35-1w animals showed detectable capsid antigen in the spleen (Figure 6). The presence of AAV capsid appeared to be associated with the relatively high vector genome copies in spleen with the exception of #N1 (Table 1).
Figure 6.
Detection of AAV8 capsid protein in the germinal centers of infant rhesus monkey spleens. Spleens were collected at 7 (a), 35 (b), or 45 (c) days after vector administration. Immunostaining showed the presence of capsid protein (red) in germinal centers in some animals (#N2, #N3, #N7, #N8). Samples are counterstained with DAPI (shown in blue) to visualize nuclei. Bar = 100 µm. AAV, adeno-associated virus.
Discussion
We have previously reported the efficiency, stability, and safety of AAV8-mediated hepatic gene transfer in adolescent rhesus monkeys.23,24 AAV8 showed transduction of 24% and 40% of the liver lobes, respectively, 7 days after systemic vector delivery. Importantly, expression was detected in several animals after 35 days despite the development of transgene-specific T cells in the liver. The use of a liver-specific promoter greatly reduced T cell responses against the transgene product therefore improving the stability of transgene expression in the liver. In the current study, we report for the first time the transduction efficiency, stability, and safety in nine infant rhesus monkeys following a single intravenous injection of 3 × 1012 GC/kg of AAV2/8.TBG.EGFP, the same vector and dose used in the previously published adolescent monkey studies.
Based on the data generated in this study, the following conclusions are proposed:
AAV8 has very high transduction efficiency in infant monkey liver. Up to 98% of the liver lobes showed strong and evenly distributed EGFP expression when analyzed 7 days after vector administration. Although vector genome copies per cell were at similar levels when compared to those found in adolescent monkeys, overall gene expression levels in the infant monkeys were much higher, suggesting that either more vector DNA in the transduced infant liver were transcriptionally active or that the TBG promoter is more active in infant monkey liver (Figure 2).
Similar to what have been observed in neonatal mouse, rat, cat, and dog models,4,13,18,19,20 AAV gene transfer in infant monkey liver is unstable as growth may impact the stability of gene transfer and transduction. In the d7-1w cohort, there was a clear inverse correlation of body weight gain and gene transfer and transduction efficiency. During the first week of life newborn monkeys can lose weight (when compared to birth weight) which is typically followed by a relatively rapid weight gain. Monkey #N2 showed this typical trend and displayed the highest transduction efficiency, while #N1 showed an early growth trajectory (32.8%) and indicated the lowest vector genome copies and EGFP expression in liver. Significant dilution of vector genome and loss of transgene expression was noted in liver lobes analyzed 35 days after vector administration. Compared to #N2, #N5 showed a 25-fold reduction of vector genome/diploid genome and 15-fold reduction of EGFP-transduced area in the liver. In the previously published study in adolescent rhesus monkeys there were three and fourfold reductions of vector genomes and EGFP transduction in liver, respectively, between 7 days and 35 days (Figure 2).Unlike findings in mouse and cat models,4,13,18 delaying the age for vector administration to 1 month postnatal age in rhesus monkeys did not improve the outcome of transduction.
AAV8-mediated hepatic gene transfer in infant monkeys appears safe. All of the animals remained healthy with no adverse findings during the study period, and only one showed a transient (nonsignificant) elevation in liver enzymes (#N4) at 3 weeks after vector administration. No T cell responses were detected in the PBMCs isolated weekly from any of the animals after vector administration. In the previous published study with adolescent rhesus monkeys, two animals showed elevated liver enzymes that peaked at week 5 and T cell responses to EGFP in the spleen but not in PBMCs.23
Immune responses to transgene product were detected. While no T cell responses to capsid or EGFP were observed in PBMCs, T cell responses in the target tissue (liver) as observed in adolescent monkeys cannot be ruled out.23,29 One of the animals (#N4) had a transient elevation of liver enzymes which may represent a spurious finding or may indicate possible cytotoxic T-lymphocyte cell killing. This animal had the lowest number of vector genomes in the liver in this study. Four animals also developed antibodies to EGFP, a cytoplasmic protein. Animals that developed high titers of α-EGFP IgG as early as week 3 after vector administration, had the lowest EGFP expression in the liver within their respective cohort (#N4 and #N9). In contrast, animals with the lowest titer of α-EGFP IgG had the highest EGFP expression in the liver within their respective cohort (#N5 and #N7). Although the adolescent monkeys also developed α-EGFP IgG, there was no consistent correlation between EGFP expression levels and the kinetics or titers of α-EGFP IgG (L. Wang, unpublished results). In addition, EGFP was also detected in the germinal centers of the spleen in two animals (#N6 and #N8).These antigenic proteins could be potentially taken up by antigen presenting cells via cross-presentation to mount an immune response. Further studies would be required to confirm this hypothesis. Such potential transgene immune responses would be a concern in subjects with null mutations but are of less concern in those with point mutations.
There was a good correlation between vector genome copies, EGFP RNA levels, and EGFP expression in liver lobes among the six animals administered AAV8 at 1-week postnatal age. However, for the d45-1m cohort animals, vector genomes in the liver varied only fourfold, but EGFP RNA levels varied 20- to 250-fold, and EGFP expression varied dramatically from 3% of liver areas (#N7) to 0.3% (#N8) to none (#N9). Promoter shut-off is likely one of the main causes for a reduction in gene expression. Mechanisms that cause promoter shut-off will require further investigation.
In summary, AAV8-mediated hepatic gene transfer in infant monkeys has been shown to be safe and efficient but less stable than in adolescent animals. In some metabolic diseases, 3–7% transduction may be sufficient to provide therapeutic benefit. Additional improvements such as self-complementary AAV vector could be explored to further improve the transduction efficiency and overall therapeutic effects. Re-administration at a later time period may also be possible with selected age groups and could be explored to provide long term therapeutic effects.
Materials and Methods
Vector production. The AAV2/8.TBG.EGFP vector used in the current study was produced by a scaled production method based on PEI transfection and purified from supernatant as described.30 Vector genome titer (GC/ml) and other quality control assays were performed as previously described.23
Animals. All animal procedures conformed to the requirements of the Animal Welfare Act, and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. Normally cycling, adult female rhesus monkeys (Macaca mulatta; N = 9) with a history of prior pregnancy were bred and identified as pregnant, using established methods.31 All dams selected for the study were prescreened to ensure they were seronegative or had low titers for AAV8 antibodies, and had a male fetus.32 Activities related to animal care were performed as per California National Primate Research Center standard operating procedures. Fetuses were monitored sonographically during gestation to ensure normal growth and development31 and newborns were delivered by cesarean-section at term (160 ± 2 days gestation) according to established protocols.33 Newborns were placed in incubators postdelivery and nursery-reared. Infant health, food intake, and body weights were recorded daily or weekly (dependent on age) in the nursery according to established protocols. At 1 week (N = 6) or 1 month postnatal age (N = 3) infants were sedated with Telazol (5 mg/kg intramuscularly) in preparation for intravenous vector injection via a peripheral vessel of 3 × 1012 GC/kg (~ 1 ml injection volume). Blood samples were collected at birth (cord blood) then weekly from a peripheral vessel (~ 3 ml) to monitor complete blood counts and clinical chemistry panels, and to collect serum and PBMCs. PBMCs were cryopreserved using a controlled rate cryopreservation protocol.26 For infants administered vector at 1-week postnatal age (N = 6), three were sedated with ketamine (10 mg/kg intramuscularly) then euthanized by an overdose of pentobarbital at 7 days (#N1, #N2, #N3) or 35 days (#N4, #N5, #N6) postgene transfer. Infants administered vector intravenously at 1 month postnatal age (N = 3) were euthanized 45 days post gene transfer (#N7, #N8, #N9). Tissue harvests were performed according to established protocols.33 Blood samples were collected and the following specimens obtained at tissue harvest: thymus, spleen, all liver lobes (right lateral, left lateral, caudate, quadrate), gallbladder, pancreas, right and left adrenals, right and left kidneys, right and left gonads, urinary bladder, cerebrum, spinal cord, right and left diaphragm, right and left ventricles of the heart, all lung lobes (right and left cranial, middle, caudal, and accessory), lymph nodes (mesenteric), GI tract (stomach, duodenum, jejunum, colon), muscle (quadriceps), and bone marrow. Sections of tissue were placed in phosphate-buffered formalin or frozen in OCT embedding compound, and specimens were placed in cryotubes and quick frozen over liquid nitrogen for PCR.
Quantification of EGFP expression in liver. EGFP transduction in rhesus monkey liver was quantified using three different approaches: the percentage of area expressing EGFP,23 the intensity of EGFP,24 and the EGFP concentration in total liver lysate measured by ELISA.24
AAV8 antibody assays. Serum samples were collected from the dam at study assignment and at cesarean-section, and from newborns at birth then weekly post gene transfer as noted above. AAV8 NAb assays were performed on Huh7 cells as previously described.34 The limit of detection for the assay is 1:5 serum dilution. AAV8-specific total IgG titers were determined by an ELISA as described.22
Anti-EGFP antibodies. EGFP-specific IgG titers in monkey serum samples after vector administration were determined by ELISA. Ninety six-well ELISA plates (Corning, Oneonta, NY) were coated overnight at 4 °C with 20 ng/well of purified EGFP with 12-His tags (BioVision, Mountain View, CA) in 0.1 mol/l carbonate buffer (pH 9.6). Plates were washed and blocked with 5% Blotto (Bio-Rad, Hercules, CA) in phosphate-buffered saline for 1 hour at room temperature. Serum samples were diluted twofold serially in 5% Blotto with a starting dilution of 1:100 and incubated for 1 hour at room temperature. Eight wells with Blotto alone served as negative controls. The plates were then washed and incubated for 1 hour at room temperature with a horseradish peroxidase-conjugated goat anti-monkey antibody (1:2,000 dilution; Sigma, St Louis, MO). The plates were developed by tetramethylbenzidine substrate (Sigma) at room temperature for 5–10 minutes, stopped with 2N sulfuric acid, and read in a plate reader at 450 nm. Titers are reported using a three parameter curve fit to determine the theoretical serum dilution for optical density (O.D.) ≥ the cutoff value (mean O.D. ± 3× standard deviation for negative control wells (N = 8)).
Detection of AAV capsid and EGFP in tissues. Immunofluorescence was performed on frozen spleen sections to detect AAV8 capsid using rabbit serum raised against AAV8 as previously described.24 EGFP in spleen was detected on tissue samples that were fixed overnight in formalin, then frozen and sectioned but was equally visible in the fresh-frozen spleen samples used for capsid immunostaining.
Vector biodistribution analysis. Tissue DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Detection and quantification of vector genomes in extracted DNA were performed by real-time Q-PCR as described previously.35
Real-time reverse transcription-PCR. Total RNA was extracted from liver and spleen with TRIzol reagent (Invitrogen, Carlsbad, CA), treated with RNase-free DNase I (Roche, Indianapolis, IN), and purified with an RNeasy Plus mini kit (Qiagen). Total RNA was reverse transcribed with a high capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions. Fifty nanograms each of total RNA equivalent cDNA was used for TaqMan PCR reactions. Relative expression of EGFP was estimated by simultaneous normalization against mean Ct value of control samples from three naive rhesus monkeys and the reference housekeeping gene 18SrRNA. A combined absolute and relative quantification strategy as described previously29 was employed to assess the presence and level of EGFP transcripts.
Interferon-γ ELISPOT. Cryopreserved PBMCs were thawed and recovered overnight in RPMI/10% fetal bovine serum at the cell density of 106 cells/ml in a 37 °C incubator. Interferon-γ ELISPOT assays were performed using previously published protocols.36 PBMCs (2 × 105/well) were stimulated with 2 µg/ml of EGFP-peptide library (46-peptide pool containing 15-mers with a 10-amino acid overlap with the preceding peptide; Mimotopes, Clayton, Australia) or AAV8 capsid peptide library (three peptide pools-A, B, and C, spanning the entire VP1 region). Spots were counted with an AID ELISPOT reader system (AutoimmunDiagnostika, Strassberg, Germany). Peptide-specific cells were represented as spot forming units per 106 PBMCs.
SUPPLEMENTARY MATERIAL Figure S1. Absence of T cell response to EGFP or AAV8 capsid by IFN-γ ELISPOT in the PBMCs of infant rhesus monkeys post-AAV8 vector administration.
Acknowledgments
The authors thank Martin Lock and the Vector Core (Gene Therapy Program) for supplying vectors; Judith Franco, Qiuyue Qin, Surina Boyd, and Hongwei Yu (Gene Therapy Program); and the animal care staff at the California National Primate Research Center for technical assistance. This work was supported by NIH grants: P01-HD057247 (J.M.W.), P01-HL059407 (J.M.W.), P30-DK047757 (J.M.W.), the NHLBI Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases (HL085794) (A.F.T.), and the Primate Center base operating grant (RR00169)(A.F.T.). J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. L.W., P.B., J.L., R.C., and A.F.T. declared no competing financial interests.
Supplementary Material
Absence of T cell response to EGFP or AAV8 capsid by IFN-γ ELISPOT in the PBMCs of infant rhesus monkeys post-AAV8 vector administration.
REFERENCES
- Ponder KP. Hemophilia gene therapy: a Holy Grail found. Mol Ther. 2011;19:427–428. doi: 10.1038/mt.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T, McIntosh J, Work LM, Nathwani A., and, Baker AH. Performance of AAV8 vectors expressing human factor IX from a hepatic-selective promoter following intravenous injection into rats. Genet Vaccines Ther. 2008;6:9. doi: 10.1186/1479-0556-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW., and, Alexander IE. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotugno G, Annunziata P, Tessitore A, O'Malley T, Capalbo A, Faella A.et al. (2011Long-term amelioration of feline Mucopolysaccharidosis VI after AAV-mediated liver gene transfer Mol Ther 19461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan PE, Lothrop CD, Sun J, Hirsch ML, Kafri T, Kantor B.et al. (2010Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application Mol Ther 181907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM.et al. (2005Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy Blood 1053079–3086. [DOI] [PubMed] [Google Scholar]
- Bell P, Gao G, Haskins ME, Wang L, Sleeper M, Wang H.et al. (2011Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs Hum Gene TherIn press). [DOI] [PMC free article] [PubMed]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y.et al. (2007Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates Blood 1091414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN.et al. (2006Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver Blood 1072653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B.et al. (2006Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors Hum Gene Ther 17440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L., and, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J.et al. (2005Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart Nat Biotechnol 23321–328. [DOI] [PubMed] [Google Scholar]
- Cunningham SC, Dane AP, Spinoulas A, Logan GJ., and, Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Hu C, Busuttil RW., and, Lipshutz GS. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J Gene Med. 2010;12:766–778. doi: 10.1002/jgm.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RJ., and, Venditti CP. Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy. Mol Ther. 2010;18:11–16. doi: 10.1038/mt.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Carrasco N, Chandler RJ, Chandrasekaran S., and, Venditti CP. Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum Gene Ther. 2010;21:1147–1154. doi: 10.1089/hum.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RJ, Chandrasekaran S, Carrillo-Carrasco N, Senac JS, Hofherr SE, Barry MA.et al. (2011Adeno-associated virus serotype 8 gene transfer rescues a neonatal lethal murine model of propionic acidemia Hum Gene Ther 22477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu WH, Lee YM, Peng WT, Pan CJ, Mead PA, Mansfield BC.et al. (2010Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy Mol Ther 181076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Faella A, O'Malley T, Cotugno G, Doria M, Kunieda T.et al. (2008Biochemical, pathological, and skeletal improvement of mucopolysaccharidosis VI after gene transfer to liver but not to muscle Mol Ther 1630–37. [DOI] [PubMed] [Google Scholar]
- Weinstein DA, Correia CE, Conlon T, Specht A, Verstegen J, Onclin-Verstegen K.et al. (2010Adeno-associated virus-mediated correction of a canine model of glycogen storage disease type Ia Hum Gene Ther 21903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E.et al. (2010Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy Mol Ther 181983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH.et al. (2010The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques Mol Ther 18126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL.et al. (2011Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors impact of pre-existing immunity Hum Gene Therepub ahead of print) [DOI] [PMC free article] [PubMed]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE.et al. (2006Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo Circ Res 99e3–e9. [DOI] [PubMed] [Google Scholar]
- Tarantal AF., and, Lee CC. Long-term luciferase expression monitored by bioluminescence imaging after adeno-associated virus-mediated fetal gene delivery in rhesus monkeys (Macaca mulatta) Hum Gene Ther. 2010;21:143–148. doi: 10.1089/hum.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane AP, Cunningham SC, Graf NS., and, Alexander IE. Sexually dimorphic patterns of episomal rAAV genome persistence in the adult mouse liver and correlation with hepatocellular proliferation. Mol Ther. 2009;17:1548–1554. doi: 10.1038/mt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Jimenez DF, Kohn DB., and, Tarantal AF. Fetal gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta) Hum Gene Ther. 2005;16:417–425. doi: 10.1089/hum.2005.16.417. [DOI] [PubMed] [Google Scholar]
- Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L.et al. (2009Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses Hum Gene Ther 20930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z.et al. (2010Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale Hum Gene Ther 211259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantal AF.2005Ultrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fascicularis) macaques: Reproductive and Research Applications Wolfe-Coote S.ed). The Laboratory Primate Elsevier Academic Press: San Diego; 317–351. [Google Scholar]
- Jimenez DF., and, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol. 2003;32:315–319. doi: 10.1046/j.1600-0684.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, McDonald RJ, Jimenez DF, Lee CC, O'Shea CE, Leapley AC.et al. (2005Intrapulmonary and intramyocardial gene transfer in rhesus monkeys (Macaca mulatta): safety and efficiency of HIV-1-derived lentiviral vectors for fetal gene delivery Mol Ther 1287–98. [DOI] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J., and, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A.et al. (2006Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver Mol Ther 1434–44. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R.et al. (2006Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid Nat Med 12967–971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Absence of T cell response to EGFP or AAV8 capsid by IFN-γ ELISPOT in the PBMCs of infant rhesus monkeys post-AAV8 vector administration.