Abstract
For effective airway gene therapy of cystic fibrosis (CF), inhaled gene carriers must first penetrate the hyperviscoelastic sputum covering the epithelium. Whether clinically studied gene carriers can penetrate CF sputum remains unknown. Here, we measured the diffusion of a clinically tested nonviral gene carrier, composed of poly--lysine conjugated with a 10 kDa polyethylene glycol segment (CK30PEG10k). We found that CK30PEG10k/DNA nanoparticles were trapped in CF sputum. To improve gene carrier diffusion across sputum, we tested adjuvant regimens consisting of N-acetylcysteine (NAC), recombinant human DNase (rhDNase) or NAC together with rhDNase. While rhDNase alone did not enhance gene carrier diffusion, NAC and NAC + rhDNase increased average effective diffusivities by 6-fold and 13-fold, respectively, leading to markedly greater fractions of gene carriers that may penetrate sputum layers. We further tested the adjuvant effects of NAC in the airways of mice with Pseudomonas aeruginosa lipopolysaccharide (LPS)-induced mucus hypersecretion. Intranasal dosing of NAC prior to CK30PEG10k/DNA nanoparticles enhanced gene expression by up to ~12-fold compared to saline control, reaching levels observed in the lungs of mice without LPS challenge. Our findings suggest that a promising synthetic nanoparticle gene carrier may transfer genes substantially more effectively to lungs of CF patients if administered following adjuvant mucolytic therapy with NAC or NAC + rhDNase.
Introduction
Cystic fibrosis (CF) is a common genetic disorder where mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene causes abnormal ion transport across epithelial surfaces of the lung airways, as well as the gastrointestinal and genital tracts.1 Aberrant ion regulation leads to hyperviscoelastic mucus secretions that interfere with normal mucus functions, in particular mucus clearance mechanisms.2 In the CF lung, poor mucociliary clearance creates a permissible environment for chronic infection and inflammation, leading to purulent sputum accumulation and airway obstruction.2,3 Pulmonary complications are primarily responsible for CF-related morbidity and mortality.4
Gene therapy is the most direct therapeutic strategy to cure CF, and hope was first provided when the CFTR gene was discovered in 1989.5 Yet, there has been no cure to date, a challenge widely attributed to inefficient gene delivery to CF airway epithelia.6,7,8 A large number of gene delivery platforms, including viral and nonviral systems, have been developed and tested in the lungs of CF patients.6 Poor gene transfer has been attributed to numerous biological barriers, including limited cellular uptake across the apical membrane, unproductive intracellular trafficking, carrier toxicity, and immunogenicity of the carriers. A less elucidated bottleneck may be the adhesive and hyperviscoelastic sputum gel that may exclude gene carriers from reaching the epithelium, thereby limiting gene transfer to CF lungs.7,9 It remains unclear whether or not gene carriers previously and currently tested in clinical trials can penetrate CF sputum.
A majority of gene therapy trials have utilized viral gene carriers; nevertheless, viral gene carriers have a number of intrinsic shortcomings, including complicated manufacturing and immunologic inactivation upon repeated dosing.6,10 A particularly promising nonviral system is the highly compacted, rod-shaped DNA nanoparticle, pioneered by Copernicus Therapeutics.11 It is composed of a 30-mer poly--lysine polycation (for DNA compaction) conjugated via a single cysteine residue to a 10 kDa polyethylene glycol segment (for colloidal stability) (CK30PEG10k polymer).11 In a phase 1/2 study, Copernicus DNA nanoparticles were safely administered to nares of CF patients and offered partial correction in nasal potential difference (NPD).12 In this study, we sought to investigate whether CK30PEG10k/DNA nanoparticles (CK30PEG10k/DNA NPs hereafter), formulated to mimic the Copernicus DNA nanoparticles, are capable of penetrating dense sputum freshly expectorated from CF patients. We subsequently tested whether the common mucolytics, recombinant human DNase (rhDNase, Pulmozyme) and N-acetylcysteine (NAC, Mucomyst), improve the diffusion rate of CK30PEG10k/DNA NPs in CF sputum. Finally, we investigated whether mucolytic-improved sputum penetration ex vivo could be translated to improve gene transfer to the lungs in a mouse model with mucus hypersecretion.
Results
Physicochemical characterization of CK30PEG10k/ DNA nanoparticles
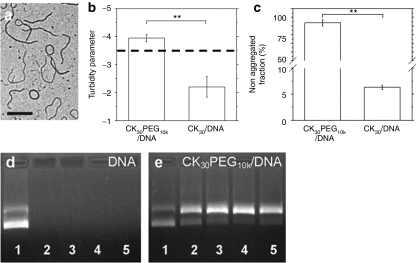
To confirm we did not significantly alter the properties of our gene carriers from the Copernicus DNA nanoparticles that are in human clinical trials, we performed a series of physicochemical characterizations of the CK30PEG10k/DNA NPs. CK30PEG10k/DNA NPs exhibited rod-shaped morphology, with average length and diameter of ~350 and ~10 nm, respectively (Figure 1a), and a roughly neutral ζ-potential (−1.5 ± 0.5 mV). CK30PEG10k/DNA NPs were colloidally stable in saline for at least 2 months at 4 °C (Figure 1b,c), and offered protection against DNase digestion for at least 2 hours, although there was increased fraction of nicked circular versus supercoiled DNA (Figure 1d,e). In summary, the size, morphology, surface charge, colloidal stability, and protection of compacted DNA were similar between CK30PEG10k/DNA NPs used here and Copernicus DNA nanoparticles.11
Figure 1.
Physicochemical characterization of CK30PEG10k/DNA NPs. (a) Transmission electron micrograph of CK30PEG10k/DNA NPs. Bar = 200 nm. (b) Turbidity parameter as indicator of particle stability (n = 3). A turbidity parameter greater than −3.5 (dashed line) indicates complexes that are stable and nonaggregated in solution, whereas below dashed line indicates aggregated complexes. Turbidity parameter of CK30PEG10k/DNA NPs is compared to that of uncoated counterpart (CK30/DNA NPs). (c) Sedimentation assay indicates nonaggregated fractions of gene carriers in saline after 2 months of storage at 4 °C (n = 3). (d,e) Stability of plasmid DNA against DNase digestion over 0, 0.5, 1, 1.5, and 2 hours (lanes 1–5). (d) Free DNA was degraded with 0.5 hour after DNase treatment. (e) Gene carriers protect compacted DNA against DNase treatment up to 2 hours. Upper and lower bands depict nicked circular and supercoiled conformations of DNA, respectively. The differences are statistically significant between the conditions indicated with asterisks (*) (**P < 0.01). Mean ± SEM.
Plasmid DNA association with CK30PEG10k polymers in CF sputum
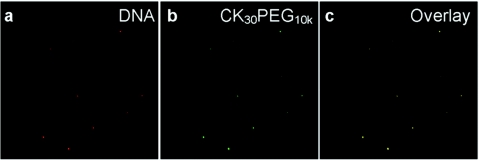
We covalently attached Alexa Fluor 488 (AF488) to the primary amines of poly--lysine at a low fluorophore:polymer ratio to enable direct microscopic observation of CK30PEG10k/DNA NPs in CF sputum. To confirm that CK30PEG10k/DNA NPs remain stable in CF sputum, we prepared gene carriers where the polymer and DNA were each labeled with a different color fluorophore. Both fluorophore signals were highly co-localized in CF sputum (Figure 2), suggesting that DNA remained associated with CK30PEG10k polymers in sputum, and that our diffusion studies were performed with intact CK30PEG10k/DNA NPs.
Figure 2.
Stability of CK30PEG10k/DNA NPs. (a) Cy3-labeled DNA (red) remained compacted with (b) AF488-labeled CK30PEG10k polymers (green) in (c) cystic fibrosis (CF) sputum as shown by their colocalization (yellow).
CK30PEG10k/DNA nanoparticles diffusion in human CF sputum
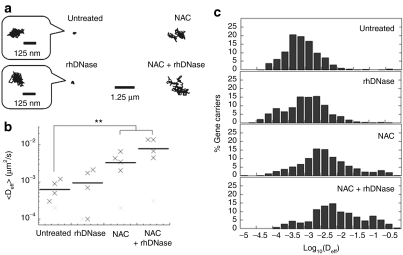
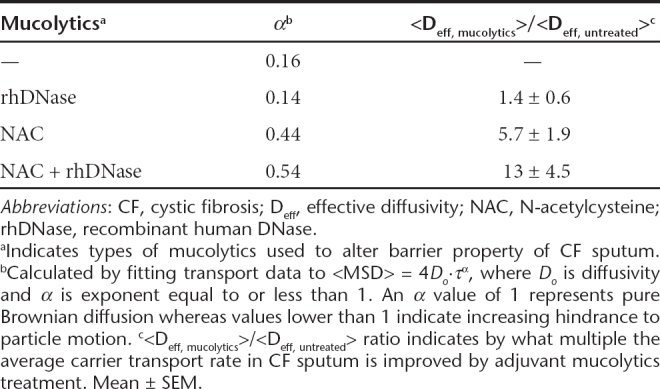
Using high resolution multiple particle tracking (MPT), we measured the translational motion of hundreds of individual, fluorescently labeled, CK30PEG10k/DNA NPs in purulent sputum freshly collected from CF patients. As evident by the highly constrained, non-Brownian time-lapse traces of individual particles (Figure 3a), diffusion of CK30PEG10k/DNA NPs was strongly hindered in CF sputum. The geometric averaged effective diffusivity (<Deff>) of CK30PEG10k/DNA NPs in five different pooled sputum samples was ~6.2 × 10−4 µm2/s at a time scale (τ) of 1 second (Figure 3b), comparable to the <Deff> previously reported for 200 nm latex particles that were immobilized by polyvalent adhesive interactions in CF sputum.13 For comparison, the theoretical effective diffusivity (Deff) for a spherical particle with the same hydrodynamic diameter as CK30PEG10k/DNA NPs is 8.7 µm2/second in water, suggesting that CK30PEG10k/DNA NPs are slowed by over 10,000-fold in CF sputum compared to in water. We observed virtually no fast moving outlier CK30PEG10k/DNA NPs (Figure 3c). The extent of hindrance to particle diffusion is also reflected by an extremely low α value (α = 0.16) (α is obtained by fitting transport data to <MSD> = 4Do·τα, where <MSD> is geometric averaged mean square displacement (MSD), Do is τ-independent diffusivity and α is exponent equal to or less than 1; α = 1 for pure Brownian diffusion; 0 < α < 1 for hindered diffusion with higher degree of obstruction for lower α values) (Table 1). Based on the measured Deff of hundreds of individual CK30PEG10k/DNA NPs, we used a previously described mathematical model13,14 to estimate that < < 1% of CK30PEG10k/DNA NPs are expected to traverse a 10 µm thick CF sputum layer within 2 hours (data not shown).
Figure 3.
Transport of CK30PEG10k/DNA NPs in untreated cystic fibrosis (CF) sputum or CF sputum pretreated with mucolytics (recombinant human DNase (rhDNase) and N-acetylcysteine (NAC), individually or in combination). (a) Representative trajectories of gene carriers in untreated and treated CF sputum during 20-second movies. The logarithmic effective diffusivities (Deff) of individual traces are within one standard deviation of the logarithmic ensemble-averaged Deff. (b) Ensemble-averaged geometric mean effective diffusivity (<Deff>) of gene carriers in different CF sputum samples at a time scale (τ) of 1 second. Differences in <Deff> of gene carriers in untreated sputum versus sputum treated with either NAC or NAC + rhDNase are statistically significant when one outlier sample (light gray cross mark), which showed no adjuvant effect regardless of mucolytic agent used, is excluded from the analysis (**P < 0.01). (c) Distribution of the logarithmic Deff of individual gene carriers at τ = 1 second. Data represent five independent experiments, with an average of n > 100 gene carriers per experiment.
Table 1. Transport of CK30PEG10k/DNA NPs in CF sputum.
Effect of mucolytics on CK30PEG10k/DNA nanoparticle diffusion in CF sputum
Since CK30PEG10k/DNA NPs were essentially immobile in CF sputum, we investigated whether common mucolytic agents could improve their diffusion through CF sputum. We first tested the effect of adding recombinant human DNase (rhDNase, Pulmozyme), a mucolytic agent commonly used by CF patients, to CF sputum prior to administration of CK30PEG10k/DNA NPs. Despite a marked decrease in bulk viscosity (by ~50% at a frequency of ω = 1 rad/second), rhDNase failed to improve the diffusion rates of CK30PEG10k/DNA NPs in CF sputum, as reflected by the highly constrained sample trajectories (Figure 3a). The <Deff> of CK30PEG10k/DNA NPs in rhDNase-treated sputum was ~9.2 × 10−4 µm2/second (τ = 1), which is similar to that of the NPs in untreated sputum, and there was a large overlap in the distribution of individual Deff values (Figure 3b,c). The α value of CK30PEG10k/DNA NPs in sputum treated with rhDNase was ~0.14, further indicating that their diffusion remained strongly hindered (Table 1).
We next assessed the effectiveness of NAC as an adjuvant; similar to rhDNase, NAC also greatly reduced bulk viscoelasticity of CF sputum (by ~60% at ω = 1 rad/second). In contrast to rhDNase, CK30PEG10k/DNA NPs in NAC-treated sputum displayed trajectories that spanned substantially greater distances than that in untreated sputum (Figure 3a). The <Deff> of CK30PEG10k/DNA NPs in NAC treated sputum samples was approximately sixfold greater than that in untreated sputum (Figure 3b and Table 1). The difference was statistically significant when one outlier sputum sample, which exhibited no adjuvant effect regardless of the type of mucolytics utilized, was excluded from the analysis (P < 0.01). Roughly 20% of CK30PEG10k/DNA NPs in NAC-treated sputum exhibited Deff larger than 0.01 µm2/second, compared to ~2% in untreated sputum (Figure 3c). NAC also improved the average α value from 0.16 to 0.44 (Table 1), suggesting that the degree to which CK30PEG10k/DNA NPs were hindered by CF sputum was substantially reduced.
We next sought to test if the simultaneous use of both mucolytics may substantially enhance gene carrier transport. The <Deff> of CK30PEG10k/DNA NPs in sputum treated with rhDNase + NAC was ~13-fold (τ = 1 second) greater than that in untreated sputum (Figure 3b). Over 35% of CK30PEG10k/DNA NPs displayed Deff larger than 0.01 µm2/second in sputum treated with both mucolytics (Figure 3c). While the difference in <Deff> between the combined treatment versus NAC alone was not statistically significant, the combined treatment increased <Deff> in three out of five sputum samples compared to NAC alone, and exhibited comparable <Deff> in the other two samples. The average α value for CK30PEG10k/DNA NPs in sputum treated with NAC + rhDNase was 0.54 (Table 1).
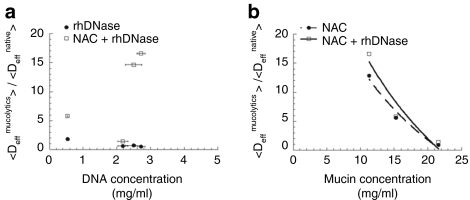
Effect of mucin and DNA concentrations on the adjuvant effect of mucolytics
The extent of the improvement in CK30PEG10k/DNA NP diffusion due to addition of mucolytic agents varied substantially across different pooled sputum samples. We thus investigated whether the effectiveness of the mucolytics was dependent on the concentration of the two primary sputum constituents, DNA and mucin. We found the concentrations of DNA and mucin in CF sputum samples varied between 0.53–2.7 and 11–22 mg/ml, respectively, in agreement with previous reports (Figure 4).7 The diffusion of CK30PEG10k/DNA NPs in all CF sputum samples treated with rhDNase was not substantially altered regardless of the DNA concentration (Figure 4a). The adjuvant effect of treatment with rhDNase + NAC was also independent of DNA concentration. In contrast, mucin concentration appeared to be inversely correlated with the <Deff> of CK30PEG10k/DNA NPs in CF sputum treated with NAC (r2 ~0.97). While NAC improved <Deff> by as high as 13-fold in the sputum sample with the lowest mucin concentration (11 mg/ml), NAC had virtually no effect on CK30PEG10k/DNA NP diffusion in the sputum sample with the highest mucin concentration (~22 mg/ml) (Figure 4b). Mucin concentration was similarly inversely correlated with the <Deff> of CK30PEG10k/DNA NPs in sputum samples treated with rhDNase + NAC (r2 ~0.92). There was no correlation between mucin concentration and the adjuvant effect of rhDNase (data not shown).
Figure 4.
Effect of mucin and DNA concentrations on the adjuvant effect of mucolytics. Ratios of ensemble-averaged effective diffusivity (<Deff>) of CK30PEG10k/DNA NPs in sputum pretreated with mucolytics to <Deff>of the same carriers in untreated cystic fibrosis (CF) sputum, as a function of (a) DNA concentration in CF sputum or (b) mucin concentration in CF sputum. The trendlines are logarithmic fit of individual data. Mean ± SEM.
Gene transfer to airways of lipopolysaccharide (LPS)-challenged mice
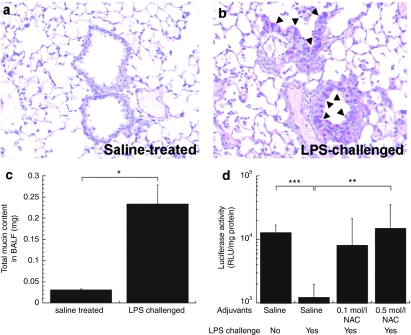
In CF lungs, the airways are covered with hyperviscoelastic sputum due to chronic infection and inflammation. Therefore, we challenged C57 mice with Pseudomonas aeruginosa LPS by inhalation to cause increased mucin secretion and inflammation. We confirmed airway inflammation and subsequent mucus cell metaplasia in LPS-challenged mice by histological examination. In agreement with previous studies,15 hematoxylin and eosin-stained lung tissue sections from LPS-challenged mice revealed massive immune cell infiltration in the airways and lung parenchyma (Supplementary Figure S1a,b). We detected periodic acid Schiff-positive mucus cells in both upper and lower airways of LPS-challenged mice by day 4, whereas no periodic acid Schiff-positive cells were observed in control (saline-treated) mice (Figure 5a,b). We also found that the total mucin content in bronchioalveolar lavage fluid from LPS-challenged mice was approximately eightfold higher than that in saline-treated mice (P < 0.05) (Figure 5c). This suggests the LPS dosing regimen used successfully established mucus cell metaplasia and mucus hypersecretion throughout the mouse airways.
Figure 5.
In vivo gene transfer to airways of C57 mice intranasally challenged with Pseudomonas aeruginosa lipopolysaccharide (LPS). Periodic acid Schiff (PAS) staining of paraffin sections of lung tissues from mice treated/challenged twice either with (a) isotonic saline or (b) 2 mg/ml LPS in the interval of 2 days. On day 4, PAS-positive mucus cells (magenta color; indicated with triangles) were detected in the airways of LPS-challenged mice whereas no PAS-positive cells were detected in the airways of saline-treated mice. (c) Total mucin contents in bronchioalveolar lavage fluid (BALF) collected from mice treated/challenged with isotonic saline or LPS. (d) In vivo gene transfer to airways of mice treated/challenged twice either with saline or LPS. On day 4, mice were treated with adjuvants, either saline (n = 7) or NAC solution (n = 10) with varying concentrations (0.1 and 0.5 mol/l) 30 minutes prior to the administration of CK30PEG10k/DNA NPs carrying pd1GL3-RL (luciferase). Subsequently, CK30PEG10k/DNA NPs (DNA dose of 50 µg/mouse) were intranasally instilled to each mouse and the luciferase activity was measured 24 hours after the administration. The differences are statistically significant between the conditions indicated with asterisks (*) (*P < 0.05, **P < 0.01, ***P < 0.001). Mean ± SEM.
LPS-induced mucus hypersecretion reduced the average luciferase expression induced by inhaled CK30PEG10k/DNA NPs by approximately tenfold compared to native, saline-treated mice at 24 hours postadministration (P < 0.001) (Figure 5d). Pretreating mucus-hypersecreting mice with 5 and 25 µmol/l NAC per mouse 30 minutes prior to administration of CK30PEG10k/DNA NPs increased airway gene expression on average by ~7- and ~12-fold, respectively (Figure 5d). Notably, the improvement mediated by 25 µmol/l NAC in LPS-challenged mice was statistically significant (P < 0.01), and the average luciferase activity was restored to levels comparable to that of LPS-null, saline-treated mice. We also immunohistochemically confirmed transgene expression in airway epithelial cells of LPS-challenged mice that were dosed intranasally with 25 µmol/l NAC followed by CK30PEG10k/DNA NPs (Supplementary Figure S2).
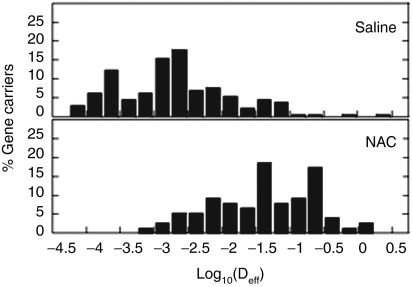
To confirm if the enhanced gene transfer in mice receiving NAC adjuvant may be attributed to improved gene carrier diffusion across sputum, we used MPT to quantify the motion of CK30PEG10k/DNA NPs added to the trachea of NAC- or saline-treated LPS-challenged mice (Figure 6). Substantially more fast moving CK30PEG10k/DNA NPs were observed in tracheas of NAC-treated mice than in saline-treated mice. The α values were 0.12 and 0.43, respectively, for NPs in saline- and NAC-treated mice, suggesting that NAC is capable of substantially enhancing the diffusion of CK30PEG10k/DNA NPs in the tracheas of LPS-challenged mice.
Figure 6.
Transport of CK30PEG10k/DNA NPs in the tracheas of lipopolysaccharide (LPS)-challenged mice (n = 2). On day 4, mice were treated with saline or 0.5 mol/l N-acetylcysteine (NAC) as adjuvants, tracheas were taken out, and then the gene carriers were administered on the lumen of tracheas. Distribution of the logarithmic effective diffusivity (Deff) of individual gene carriers at τ = 1 second.
Discussion
An early study using a CF airway cell line suggested that only ~5% of airway epithelial cells need to express functional CFTR proteins to restore chloride ion imbalance in the CF lung.16 However, it is now suggested that a greater fraction of cells must have functional CFTR to maintain proper ion homeostasis.17 Thus, for effective gene therapy in the CF airways, it is essential that a large fraction of gene carriers avoid immobilization in sputum so they may reach the underlying epithelium. Here, we provide evidence that one of the most promising nonviral gene delivery systems for the lungs,6,18 CK30PEG10k/DNA NP, is immobilized in fresh, purulent sputum obtained from CF patients. Trapped gene carriers will reach the airway epithelium of CF patients at very low efficiency at best, presumably leading to poor gene transfer in the airways analogous to what was observed in mice with LPS-induced mucus hypersecretion. Our findings confirm that, in addition to cellular and immunological barriers, the hyperviscoelastic sputum likely represents a critical bottleneck to efficient gene delivery in the airways. Previous failures to achieve therapeutic end points in CF patients may be caused by the inability of gene carriers to rapidly penetrate CF sputum.
We previously reported that the average 3D mesh spacing of CF sputum is ~140 ± 50 nm, with individual spacings spanning the range of 60–300 nm.13 Thus, the extensive hindrance of carrier movement in CF sputum may be attributed in part to steric obstruction imposed by sputum mesh structures, since the average length of the rod-shaped CK30PEG10k/DNA NPs (~350 nm) is substantially larger than the average mesh spacing. We recently found that NAC treatment markedly increased the average mesh spacing in CF sputum (to 230 ± 50 nm), with spacings spanning 50–1300 nm.19 Thus, the improved diffusion of CK30PEG10k/DNA NPs in human CF sputum and tracheal mucus of LPS-challenged mice may be at least partially due to reduced steric obstruction from the sputum mesh. Nevertheless, despite enhanced NP penetration upon NAC treatment, the average diffusion rate of CK30PEG10k/DNA NPs remained ~40-fold lower than that of substantially larger, spherical muco-inert nanoparticles (diameter ~500 nm) in NAC-treated CF sputum.19 This suggests that the diffusion of CK30PEG10k/DNA NPs in CF sputum is also strongly hindered by adhesive interactions with sputum constituents.
CK30PEG10k/DNA NPs are coated with neutrally-charged, hydrophilic PEG polymers, and thus exhibit a roughly neutral surface charge that should, in theory, reduce adhesion based on electrostatic interactions. However, the PEG coating may not be sufficiently dense to completely shield the cationic poly--lysine core from adhesive interactions between particles and anionic sputum constituents.20 Inadequate PEG coverage on CK30PEG10k/DNA NPs is reflected by the increased fraction of nicked circular versus supercoiled DNA upon DNase digestion, since the PEG coating did not completely block access of DNase to cargo DNA. We note that synthetic particles that rapidly penetrate human cervicovaginal mucus and CF sputum were densely coated with muco-inert PEG polymers at estimated densities of ~1 PEG molecules per nm2.20 We estimate CK30PEG10k/DNA NPs to be covered with ~25–50-fold lower surface PEG density (~1–2 PEG per 50 nm2; calculated from the NP surface area and number of PEG molecules per NP). Based on our previous observation that ~40% reduction in PEG coverage of latex nanoparticles led to a 700-fold decrease in the average NP transport rate in cervicovaginal mucus,20 the relatively lower PEG coverage of CK30PEG10k/DNA NPs is unlikely to adequately prevent mucoadhesion. A dense PEG coating may be even more critical to achieve rapid penetration of gene carriers through CF sputum due to the elevated adhesivity of CF sputum compared to that of normal mucus.21 The use of 10 kDa PEG in CK30PEG10k/DNA NPs may also increase mucoadhesion; we have previously found that nanoparticles densely coated with 10 kDa PEG were trapped in human cervicovaginal mucus, whereas particles densely coated with 2–5 kDa PEG readily penetrated the same mucus secretions.20
Effects of NAC or its derivatives on diffusion of a variety of gene carriers have been indirectly studied in vitro and in vivo by monitoring gene expression in mucus/sputum covered tissues or cells.22,23,24 Nacystelyn, a lysine salt of NAC, has been shown to improve gene transfer to nares and airways of inbred mouse strains via viral and nonviral gene carriers.23,24 However, mice typically produce considerably less mucus in their airways than humans25 and, hence, native mice may not be an optimal animal model to assess adjuvant effects of mucolytics on airway gene transfer. To our knowledge, this work provides the first demonstration that NAC can improve gene transfer in the airways of a mucus-hypersecreting mouse model. Mice with CFTR knockout or mutation also may not be ideal animal models for studying the effect of mucolytics on airway gene transfer, since few genetically engineered CF mouse models spontaneously develop human-like CF lung disease.26,27 Alternatively, P. aeruginosa,26,27 the most prevalent pathogen affecting airways of CF patients, and its components (such as LPS),15,28 have been shown to mediate clinical airway pathophysiology of lung inflammation and subsequent mucus cell metaplasia/hypersecretion in normal and CF mice. Thus, an increasing number of airway gene transfer studies have been performed with the P. aeruginosa infection mouse model.29,30,31 We show that NAC is capable of significantly increasing CK30PEG10k/DNA NP-mediated gene transfer to the lungs of mice with LPS-induced mucus hypersecretion. The enhanced gene transfer may be attributed in part to the NAC-mediated increase in the mobility of CK30PEG10k/DNA NP in the mucus layers of LPS-challenged mice, as suggested here by observations of CK30PEG10k/DNA NP mobility in human CF sputum and in LPS-challenged mouse tracheal mucus. It is also possible that the intrinsic anti-inflammatory capacity of NAC32 led to improved gene expression in the inflamed mouse airways by reducing neutrophil/macrophage infiltration and/or inhibiting mucus hypersecretion. Despite being speculative, Kushwah and coworkers also noted in their study that pretreatment with nacystelyn may improve airway gene transfer by combination of its antioxidant, mucolytic and anti-inflammatory effects.24
Airway gene transfer observed in LPS-challenged mice is not likely due to uptake of CK30PEG10k/DNA NP by infiltrating immune cells. We found that the airway gene transfer in LPS-challenged mice, where immune cell infiltration is elevated, was significantly decreased compared to unchallenged mice. Furthermore, if transfection of immune cells were significant, NAC would likely decrease gene transfer to LPS-challenged mice, since NAC is known to diminish immune cell accumulation mediated by LPS challenge.33 We also immunohistochemically confirmed that CK30PEG10k/DNA NP-mediated gene transfer specifically to airway epithelial cells of LPS-challenged mice pretreated with NAC. This is in good agreement with a previous observation that CK30PEG10k/DNA NP primarily transfect airway epithelial cells of the same strain of mice used in this study, albeit without LPS challenge.11
Mucins may influence transport of gene carriers more than DNA in CF sputum, since NAC improved the transport of CK30PEG10k/DNA NPs, whereas rhDNase alone exhibited minimal effect. Furthermore, the adjuvant effect of NAC appears to be critically dependent on mucin concentration of the sputum sample. It has been reported that mucin concentration in sputum increased in CF patients in progressive stages, and decreases in the airways of stabilized patients.34 Thus, the effectiveness of NAC treatment on improving carrier transport may depend on disease state. The variability in NAC-induced improvement in gene carrier transport is unlikely caused by poor distribution of NAC within CF sputum since NAC is a small, hydrophilic molecule, and thus able to diffuse freely in aqueous pores of CF sputum. An alternative possibility is that the dose of NAC used in this study (20 mmol/l) does not sufficiently reduce disulfide crosslinks in sputum with high mucin content to mediate improved CK30PEG10k/DNA NP diffusion. We previously demonstrated that 20 mmol/l NAC mediates rapid penetration of 200 nm PEG-coated latex particles in CF sputum,19 whereas Ferrari and coworkers showed that much higher concentration (300–400 mmol/l) is required to improve liposome- and polymer-based gene delivery to ex vivo model of sheep tracheal epithelium.23 Assuming that more NAC is required to promote efficient carrier diffusion in CF sputum with high mucin concentration, higher NAC doses could be considered to fully realize its adjuvant effect.
It remains unclear why NAC, but not rhDNase, improves the carrier diffusion in CF sputum given their similar effects on reducing sputum bulk viscosity. However, this finding is in good agreement with previous observations that rhDNase exhibited only minimal to no effect on the diffusion of latex nanoparticles35 and lipid-based gene carriers36 in CF sputum. Dawson and coworkers suggested that rhDNase may induce a higher microviscosity (viscosity in the watery channels) in CF sputum due to the release of soluble DNA fragments; fast and slow moving outlier particles were eliminated upon treatment of sputum with rhDNase.35 More recently, we showed that NAC markedly improved the penetration rates of muco-inert nanoparticles in CF sputum.19 Cleaved mucin fibers may remain associated with the sputum mesh via entanglements and/or adhesive interactions, perhaps giving rise to enlarged pores without releasing soluble mucins that can increase the microviscosity in CF sputum.
Although mucolytics markedly improve the diffusion of CK30PEG10k/DNA NPs in CF sputum, large fractions of CK30PEG10k/DNA NPs still remained hindered by CF sputum, leading to a concern about the clinical relevance of this approach. The slow carrier diffusion may be attributed to adhesive interactions between gene carriers and sputum constituents. To further improve the penetration of gene carriers across sputum, it is likely essential to further reduce the muco-affinity of gene carriers. One such approach is to engineer a dense coating of low MW PEG on the nanoparticle surface.9,20 We have recently shown that well-coated latex nanoparticles were able to penetrate NAC-treated sputum at rates approaching their theoretical speeds in water.19 A denser PEG coating may also improve protection of compacted DNA upon DNase exposure.
In summary, we found that synthetic highly compacted polymeric gene carriers are immobilized by CF sputum. This suggests that overcoming the sputum barrier should be considered an essential design criterion in developing a gene delivery platform capable of achieving clinical end points for CF gene therapy. We also showed that a common mucolytic agent, NAC, enhances penetration of this promising gene carrier through sputum, and improves airway gene transfer in mice, suggesting mucolytic adjuvants may substantially improve the effectiveness of clinically studied gene carriers.
Materials and Methods
CF sputum collection and rheological characterization. Sputum spontaneously expectorated from male and female CF patients of age 23–44, was collected at the Johns Hopkins Adult Cystic Fibrosis Program. The procedures conformed to the Declaration of Helsinki and the sputum collection was performed under informed consent on a protocol approved by the Johns Hopkins Medicine Institutional Review Board. Two to four samples were acquired from the weekly CF outpatient clinic, placed on ice upon collection and during transport, pooled together to minimize patient-to-patient variation, and studied the same day. We used sputum samples from CF patients who had not been treated with mucolytics at least for 12 hours prior to the sputum collection. The total number of individual samples used for the present studies was 12. Samples for rheological characterization were stored at −20 °C until cone-and-plate rheometry (Rheometrics HADV-III; Brookfield, Middleboro, MA) was performed, as previously described.13 Briefly, the rheometer was set to dynamic mode and specimens were subjected to 1% strain at a broad spectrum of shear rates to measure the elastic and viscous moduli of CF sputum.
CK30PEG10k/DNA nanoparticle formulation. CK30PEG10k polymer was prepared by reacting equal molar ratios of a 30-mer poly--lysine peptide with methoxy-PEG-maleimide (10 kDa; Rapp Polymere, Tübingen, Germany), as described elsewhere.11 CK30PEG10k polymers were fluorescently labeled by conjugating the amine reactive probe, Alexa Fluor 488 sulfodichlorophenol (5-SDP) ester (AF 488; Invitrogen, Carlsbad, CA), to the epsilon amines of lysine (reaction stoichiometry was one AF 488 fluorophore per CK30PEG10k polymer chain) following manufacturers protocol. Unreacted AF 488 was removed by fractionating the reaction mixture on a Sephadex G25 column (GE Healthcare, Piscataway, NJ) pre-equilibrated with 50 mmol/l ammonium acetate. Fractions containing CK30PEG10k polymers, as measured by UV absorbance, were pooled and lyophilized.
The plasmids, pd1GL3-RL (luciferase; 7.9 kbp; a kind gift from Professor Alexander Klibanov of M.I.T, Cambridge, MA) and pUMVC-nt-β-gal (nuclear targeted β-galactosidase; 7.5 kbp; Aldevron, Fargo, ND), were propagated in Escherichia coli DH5α. Plasmid DNA was collected and purified using Qiagen Endofree Giga Prep kits (Qiagen, Valencia, CA) per manufacturer's protocol.
Highly compacted CK30PEG10k/DNA NPs were formed upon the drop wise addition of 9 volumes of DNA at a concentration of 0.222 mg/ml to a vortexing solution of 1 volume AF488-labeled CK30PEG10 polymers at N/P = 2 (the number of free amines on CK30PEG10k polymers were determined by the trinitrobenzesulphonic acid assay). CK30PEG10k/DNA NPs were allowed to stand at room temperature for 30 minutes before removing aggregates by syringe filtration (0.2 µm). To remove free polymers, CK30PEG10k/DNA NPs were diluted with 0.9% NaCl and reconcentrated to 0.2 mg/ml using a Vivaspin 6 centrifugal concentrator (100,000 MWCO; Vivaproducts, Littleton, MA) two times. For in vivo experiments, CK30PEG10k/DNA NPs were concentrated to 1 mg/ml. DNA concentration of CK30PEG10k/DNA NPs were determined via absorbance at 260 nm, measured with a NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE) after correcting for the contribution to the absorbance by AF 488.
Physicochemical characterization of CK30PEG10k/DNA nanoparticles. To determine morphology and size, CK30PEG10k/DNA NPs were imaged using transmission electron microscopy (TEM). ζ-Potential, a measure of particle surface charge, was determined by laser Doppler anemometry using a Nanosizer ZS90 (Malvern Instruments, Southborough, MA).
The ability of CK30PEG10k/DNA NPs to protect DNA against DNase digestion was assayed as follows. Ten microliters containing 0.5 µg of CK30PEG10k/DNA NPs or DNA alone was incubated with 1 µl of recombinant DNase I (0.5 U; RNase free; Roche Diagnostics, Mannheim, Germany) at 37 °C for varying durations. After DNase treatment, DNase was inactivated by addition of 5 µl of 0.5 mol/l EDTA followed by 15 minutes incubation at room temperature. To release DNA for gel electrophoresis, 1 µl of 0.25% Trypsin (Sigma, St Loius, MO) was incubated with CK30PEG10k/DNA at 37 °C for 10 minutes. Gel electrophoresis was carried out on a 1% agarose gel containing 50 µg/ml ethidium bromide.
To determine whether gene carriers remain stable in presence of CF sputum, dual labeled CK30PEG10k/DNA NPs were formulated by labeling DNA with Cy3 (Mirus Bio, Madison, WI) followed by compaction with AF488-labeled CK30PEG10k polymers. Dual labeled CK30PEG10k/DNA NPs were incubated in CF sputum for 30 minutes and the colocalization of polymer and DNA was monitored via an inverted epifluorescence microscope (Marianas, Zeiss, Thornwood, NY) equipped with dual Cascade II 512 EMCCD cameras (Photometrics, Tucson, AZ).
Treating CF sputum with mucolytic agents. RhDNase (Pulmozyme; 1 mg/ml; Genentech, San Francisco, CA) was diluted to 350 µg/ml, and 1 mol/l solution of NAC (Sigma) was prepared and adjusted to pH ~7, since its mucolytic activity is optimal with a pH of 7.0–9.0. To study effects of individual mucolytics on the transport of gene carriers, CF sputum was treated with either rhDNase (final concentration of 7 µg/ml) or NAC (final concentration of 20 mmol/l), for 30 minutes at 37 °C prior to the addition of CK30PEG10k/DNA NPs (2% dilution of CF sputum). For the combined treatment of rhDNase and NAC, both mucolytics were simultaneously added to CF sputum at the aforementioned final concentrations. Changes in the bulk rheological properties (elastic and viscous moduli) upon mucolytic treatments were confirmed at 37 °C with a sensitive strain-controlled cone-and-plate rheometer.
MPT in CF sputum. CK30PEG10k/DNA NPs were added to ~30 µl of CF sputum (3% dilution), with or without mucolytic (rhDNase and NAC) pretreatment, transferred to custom-made 30 µl microwells, and equilibrated for 30 minutes at 37 °C prior to microscopy. The dynamics of CK30PEG10k/DNA NPs were quantified using MPT.37 Briefly, 20-second movies at 67 ms temporal resolution were acquired via dual Cascade II 512 EMCCD cameras on an inverted epifluorescence microscope (3-I Marianas, Zeiss) with ×100/1.4 NA objective. Movies were analyzed with Metamorph software (Universal Imaging, Glendale, WI) to extract x, y positional data over time. Time-averaged mean square displacement and effective diffusivity (Deff) for each CK30PEG10k/DNA NP were calculated as a function of time scale (τ).37 CF sputum was assumed to be locally isotropic but not necessarily homogeneous; thus, 2D diffusivity is equal to 3D diffusivity.37 Bulk transport properties were calculated by geometric ensemble-averaging of individual transport rates. The tracking resolution was 10 nm, determined by tracking displacement of particles immobilized with a strong adhesive.38 Fractions of gene carriers expected to penetrate a 10 µm CF sputum layer was calculated as previously reported.13,14,19
Measurement of mucin and DNA concentration. DNA concentration was determined by a fluorometric assay based on the reaction of diaminobenzoic acid (DABA) 2HCl (Sigma) and DNA, as previously described.39 The sputum was pretreated with rhDNase (10 µg/ml sputum), diluted fivefold and vortexed. Three hundred microliters of this suspension was reacted with 300 µl of 20% w/v DABA solution and incubated at 60 °C for 1 hour. Ten milliliters of 1.73 mol/l HCl was added to stop the reaction and the florescence was measured at excitation and emission wavelengths of 390 and 530 nm, respectively. Mucin concentration was determined based on the reaction of 2-cyanoacetamide with O-glycosylated glycoproteins, where the sputum is pretreated with rhDNase (10 µg/ml sputum), diluted 20-fold, vortexed and incubated in a 60 °C water bath for 1 hour.40 One hundred microliters of this suspension was reacted with 120 µl of an alkaline solution of 2-cyanoacetamide (200 µl of 0.6 mol/l 2-cyanoacetamide; Sigma) at 100 °C for 30 minutes. After incubation, 1 ml of 0.6 mol/l borate buffer at pH 8.0 was added and the fluorescence was measured at an excitation and an emission wavelength of 336 and 383 nm, respectively.
In vivo airway gene transfer. All procedures performed with mice were approved and strictly regulated by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. C57 mice (female, 6–8 weeks) were anesthetized in a perfusion chamber with 1–1.5% isoflurane in 1–1.5 l/minute oxygen flow, and then intranasally challenged twice with 50 µl of 2 mg/ml P. aeruginosa LPS (Sigma) on day 0 and 2 (48-hour interval). Control mice were treated with saline. On day 4, lung tissues were excised from the chest, inflated with and immersed overnight in formalin. Subsequently, lung tissues were paraffin-sectioned and stained for hematoxylin and eosin and periodic acid Schiff11 to confirm whether LPS induced airway inflammation and mucus cell metaplasia. To test whether the LPS challenge mediated mucus hypersecretion, total mucin content in mouse airway was determined. Briefly, bronchioalveolar lavage was performed ex vivo three times with 1 ml 1X phosphate-buffered saline each time, and the bronchioalveolar lavage fluid was pooled for each mouse. Supernatant of bronchioalveolar lavage fluid was collected by centrifugation at 500g for 8 minutes, lyophilized overnight, and resuspended in 250 µl of distilled water. The total mucin content in each sample was measured as described above.
Four days after the initial saline treatment or LPS challenge, C57 mice were anesthetized and intranasally treated with 50 µl of either isotonic saline (control) or NAC (0.1 and 0.5 mol/l), 30 minutes prior to the administration of gene carriers. All mice were anesthetized again and intranasally instilled with CK30PEG10k/DNA NPs carrying pd1GL3-RL at a dose of 50 µg DNA per mouse. To examine airway gene expression, we measured the luciferase activity on lung tissue homogenates. Briefly, mice were euthanized 24 hours after the administration of CK30PEG10k/DNA NPs, perfused with 3 ml phosphate-buffered saline via the heart to remove blood, and then lung tissues were harvested and ground with a homogenizer (Power Gen 125; Fisher Scientific, Pittsburg, PA). The homogenates were subjected to three freeze-and-thaw cycles to assure complete cell lysis, and supernatants were obtained by centrifugation. Luciferase activity in the supernatant was measured using a standard luciferase assay kit (Promega, Madison, WI) and a 20/20n luminometer (Turner Biosystems, Sunnyvale, CA). The relative light unit measured was normalized by total protein content measured by bicinchoninic acid assay.
To examine the airway distribution of transgene expression, LPS-challenged mice were intranasally dosed with either saline only (as an untreated control) or 0.5 mol/l NAC and CK30PEG10k/DNA NPs carrying pUMVC-nt-β-gal (100 µg DNA per mouse) with a 30-minute interval. Lungs were harvested 24 hours after the administration, inflated with a 50:50 mixture of optimal cutting temperature compound and 1X phosphate-buffered saline, embedded in 100% optimal cutting temperature compound, frozen in liquid N2, and cryosectioned at −22 °C. For immunohistochemical staining, tissue sections were first fixed with acetone for 10 minutes at 4 °C and permeabilized with 0.2% Triton X-100. Slides were blocked in goat serum for 1 hour at room temperature and incubated with rabbit anti-β-galactosidase IgG (Invitrogen) overnight at 4 °C. Slides were then incubated with Alexa Fluor 555 goat anti-rabbit IgG (Invitrogen) for 2 hours at room temperature. Subsequently, tissue sections were stained with ProLong Gold antifade reagent with DAPI (Molecular Probes, Eugene, OR) and images were captured with a fluorescence microscope.
Diffusion of CK30PEG10k/DNA nanoparticles in mouse tracheal mucus. LPS-challenged mice on day 4 were anesthetized and intranasally treated either with isotonic saline or 0.5 mol/l NAC. Twenty minutes later, each mouse was euthanized and a small piece of trachea (~5 mm) was dissected. The trachea was then cut open longitudinally and lay flat on a glass slide (mucosal side up). A low volume (0.1 µl) of labeled CK30PEG10k/DNA NPs was applied to the luminal surface of the section and the trachea was promptly sealed with a coverslip using a thin layer of super glue around the periphery of the coverslip to minimize dehydration. To measure the transport rates of CK30PEG10k/DNA NPs, MPT was performed as described above.
Statistical analysis. Statistically significant differences between two groups were analyzed with a two-tailed Student's t test, assuming unequal variances. Multiple comparisons were performed by analysis of variances of logarithmically transformed data using analysis of variance followed by post hoc test. P values less than 0.05 were considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Lung inflammation induced by Pseudomonas aeruginosa lipopolysaccharide (LPS). Figure S2. Immunohistochemical staining of transgene expression in lungs of LPS-challenged mice.
Acknowledgments
Funding was provided by the National Institutes of Health (NIH 1R01 EB003558, P01 HL51811, and CTSA UL1 RR 025005) and Cystic Fibrosis Foundation (CFF HANES08G0 and R025-CR07). We thank Meghan Ramsay and Sharon Watts at the Johns Hopkins Adult Cystic Fibrosis Center for CF sputum collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Lung inflammation induced by Pseudomonas aeruginosa lipopolysaccharide (LPS).
Immunohistochemical staining of transgene expression in lungs of LPS-challenged mice.
REFERENCES
- Aitken ML., and, Fiel SB. Cystic fibrosis. Dis Mon. 1993;39:1–52. doi: 10.1016/0011-5029(93)90028-2. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- Davis PB, Drumm M., and, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- Flume PA. Pulmonary complications of cystic fibrosis. Respir Care. 2009;54:618–627. doi: 10.4187/aarc0443. [DOI] [PubMed] [Google Scholar]
- Higgins CF. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- Griesenbach U., and, Alton EW, UK Cystic Fibrosis Gene Therapy Consortium Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv Drug Deliv Rev. 2009;61:128–139. doi: 10.1016/j.addr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Sanders N, Rudolph C, Braeckmans K, De Smedt SC., and, Demeester J. Extracellular barriers in respiratory gene therapy. Adv Drug Deliv Rev. 2009;61:115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziady AG, Davis PB., and, Konstan MW. Non-viral gene transfer therapy for cystic fibrosis. Expert Opin Biol Ther. 2003;3:449–458. doi: 10.1517/14712598.3.3.449. [DOI] [PubMed] [Google Scholar]
- Lai SK, Wang YY., and, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., and, Flotte TR. Gene therapy for cystic fibrosis. Clin Rev Allergy Immunol. 2008;35:164–178. doi: 10.1007/s12016-008-8080-3. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Gedeon CR, Miller T, Quan W, Payne JM, Hyatt SL.et al. (2003Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo Mol Ther 8936–947. [DOI] [PubMed] [Google Scholar]
- Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ.et al. (2004Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution Hum Gene Ther 151255–1269. [DOI] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP.et al. (2009The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles Biomaterials 302591–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M.et al. (2009Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier Proc Natl Acad Sci USA 10619268–19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K, Seki M., and, Cheng PW. Lipopolysaccharide Induces Mucus Cell Metaplasia in Mouse Lung. Am J Respir Cell Mol Biol. 2001;24:66–73. doi: 10.1165/ajrcmb.24.1.4122. [DOI] [PubMed] [Google Scholar]
- Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R., and, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH.et al. (2009CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium PLoS Biol 7e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PB., and, Cooper MJ. Vectors for airway gene delivery. AAPS J. 2007;9:E11–E17. doi: 10.1208/aapsj0901002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP., and, Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine (Lond) 2011;6:365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Lai SK, Suk JS, Pace A, Cone R., and, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed Engl. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA., and, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- Stern M, Caplen NJ, Browning JE, Griesenbach U, Sorgi F, Huang L.et al. (1998The effect of mucolytic agents on gene transfer across a CF sputum barrier in vitro Gene Ther 591–98. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Kitson C, Farley R, Steel R, Marriott C, Parkins DA.et al. (2001Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium Gene Ther 81380–1386. [DOI] [PubMed] [Google Scholar]
- Kushwah R, Oliver JR, Cao H., and, Hu J. Nacystelyn enhances adenoviral vector-mediated gene delivery to mouse airways. Gene Ther. 2007;14:1243–1248. doi: 10.1038/sj.gt.3302968. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Song Y., and, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol, Cell Physiol. 2003;284:C2–15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- Kukavica-Ibrulj I., and, Levesque RC. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab Anim. 2008;42:389–412. doi: 10.1258/la.2007.06014e. [DOI] [PubMed] [Google Scholar]
- Stotland PK, Radzioch D., and, Stevenson MM. Mouse models of chronic lung infection with Pseudomonas aeruginosa: models for the study of cystic fibrosis. Pediatr Pulmonol. 2000;30:413–424. doi: 10.1002/1099-0496(200011)30:5<413::aid-ppul8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Szarka RJ, Wang N, Gordon L, Nation PN., and, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997;202:49–57. doi: 10.1016/s0022-1759(96)00236-0. [DOI] [PubMed] [Google Scholar]
- Rejman J, De Fino I, Paroni M, Bragonzi A, Demeester J, De Smedt S.et al. (2010Impact of chronic pulmonary infection with Pseudomonas aeruginosa on transfection mediated by viral and nonviral vectors Hum Gene Ther 21351–356. [DOI] [PubMed] [Google Scholar]
- Sirninger J, Muller C, Braag S, Tang Q, Yue H, Detrisac C.et al. (2004Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector Hum Gene Ther 15832–841. [DOI] [PubMed] [Google Scholar]
- Van Heeckeren AM, Scaria A, Schluchter MD, Ferkol TW, Wadsworth S., and, Davis PB. Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L717–L726. doi: 10.1152/ajplung.00227.2003. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Anti-inflammatory drugs and experimental bronchitis. Eur J Respir Dis Suppl. 1986;146:245–257. [PubMed] [Google Scholar]
- Moon C, Lee YJ, Park HJ, Chong YH., and, Kang JL. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am J Respir Crit Care Med. 2010;181:374–387. doi: 10.1164/rccm.200907-1061OC. [DOI] [PubMed] [Google Scholar]
- Henke MO, John G, Germann M, Lindemann H., and, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175:816–821. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- Dawson M, Wirtz D., and, Hanes J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J Biol Chem. 2003;278:50393–50401. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- Sanders NN, Van Rompaey E, De Smedt SC., and, Demeester J. On the transport of lipoplexes through cystic fibrosis sputum. Pharm Res. 2002;19:451–456. doi: 10.1023/a:1015139527747. [DOI] [PubMed] [Google Scholar]
- Suh J, Dawson M., and, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev. 2005;57:63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Apgar J, Tseng Y, Fedorov E, Herwig MB, Almo SC., and, Wirtz D. Multiple-particle tracking measurements of heterogeneities in solutions of actin filaments and actin bundles. Biophys J. 2000;79:1095–1106. doi: 10.1016/S0006-3495(00)76363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F., and, Demeester J. Cystic fibrosis sputum: a barrier to the transport of nanospheres. Am J Respir Crit Care Med. 2000;162:1905–1911. doi: 10.1164/ajrccm.162.5.9909009. [DOI] [PubMed] [Google Scholar]
- Crowther RS., and, Wetmore RF. Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem. 1987;163:170–174. doi: 10.1016/0003-2697(87)90108-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lung inflammation induced by Pseudomonas aeruginosa lipopolysaccharide (LPS).
Immunohistochemical staining of transgene expression in lungs of LPS-challenged mice.