Abstract
Bone marrow–derived mesenchymal stromal cells (MSCs) are promising for regenerative medicine applications, such as for renoprotection and repair in acute kidney injury (AKI). Erythropoietin (Epo) can also exert cytoprotective effects on various tissues including the kidney. We hypothesized that MSCs gene-enhanced to secrete Epo may produce a significant beneficial effect in AKI. Mouse Epo-secreting MSCs were generated, tested in vitro, and then implanted by intraperitoneal injection in allogeneic mice previously administered cisplatin to induce AKI. Epo-MSCs significantly improved survival of implanted mice as compared to controls (67% survival versus 33% with Vehicle only). Also, Epo-MSCs led to significantly better kidney function as shown by lower levels of blood urea nitrogen (72 ± 9.5 mg/dl versus 131 ± 9.20 mg/dl) and creatinine (74 ± 17 µmol/l versus 148±19.4µmol/l). Recipient mice also showed significantly decreased amylase and alanine aminotransferase blood concentrations. Kidney sections revealed significantly less apoptotic cells and more proliferating cells. Furthermore, PCR revealed the presence of implanted cells in recipient kidneys, with Epo-MSCs leading to significantly increased expression of Epo and of phosphorylated-Akt (Ser473) (P-Akt) in these kidneys. In conclusion, our study demonstrates that Epo gene-enhanced MSCs exert significant tissue protective effects in allogeneic mice with AKI, and supports the potential use of gene-enhanced cells as universal donors in acute injury.
Introduction
Bone marrow–derived mesenchymal stromal cells (MSCs), also often referred to as mesenchymal stem cells, have demonstrated potential for several therapeutic applications, such as for cardiovascular, bone and cartilage, and also kidney repair.1,2,3,4 MSCs have shown great promise in regenerative medicine due to the ability of these cells to differentiate into a wide variety of cell types which include renal cells, but more significantly due to the capacity of these cells to release beneficial factors.5,6,7,8,9,10,11 Studies have revealed that MSCs can exert renoreparative and renoprotective effects when delivered in mouse models of acute kidney injury (AKI).5,12,13 For instance, reports on the use of MSCs implanted intravenously in mice with cisplatin-induced AKI described lower impairment of kidney function.5,7,13 Besides intravenous delivery, intraperitoneal implantation of MSCs has also been found to reduce blood urea nitrogen levels and mortality in mice with cisplatin-induced AKI.5
Other than MSCs, erythropoietin (Epo) use has also demonstrated tissue protective and reparative abilities for various organs, such as heart, brain, and also kidneys.14,15,16 Studies have shown that Epo administration can exert renoprotective as well as prorecovery effects in rodents with AKI.17,18,19,20,21 For instance, reports revealed improved recuperation of kidney function following intraperitoneal injection of Epo in rats with cisplatin-induced AKI.17,21 An alternative to recombinant Epo is to instead use adult somatic cells, such as MSCs, that have been gene-enhanced to secrete Epo. Previous research, including ours, have established that MSCs can be efficiently gene-modified with Epo in vitro and then used in vivo as vehicles for Epo delivery.22,23,24,25,26 For example, we determined that Epo gene-enhanced MSCs lead to pharmacologically relevant Epo secretion when implanted in immunocompetent mice, and also when tested in mice with anemia from experimental chronic kidney disease.24,25,26
Besides being efficient cellular vehicles, MSCs have also been reported to home to sites of tissue damage, including injured kidneys.2,6,27,28 We therefore hypothesized that combining the renoreparative and renoprotective abilities of MSCs with those of Epo, through the use of MSCs gene-enhanced to produce Epo, may lead to a more significant therapeutic effect in AKI. These cells would allow continuous levels of Epo at the site of kidney injury and thus spare the systemic erythrocytic effects of frequent recombinant Epo injections. Furthermore, since for AKI, a rapid and transient effect would be sufficient to achieve a therapeutic outcome, we investigated Epo gene-enhanced MSCs that were allogeneic with regards to the recipient. We here report on our use of Epo-secreting MSCs delivered intraperitoneally in allogeneic mice with AKI. In brief, we observed favorable effects on kidney function and mouse survival, brought about by MSCs and particularly by Epo gene-enhanced MSCs. In addition, due to reported beneficial factors secreted by MSCs, i.e., the paracrine effects of these cells, we also assessed the action of their secretome in vitro on mouse kidney epithelial cells exposed to cisplatin, and noted prosurvival effects.
Results
Characterizations of MSCs and Epo-MSCs
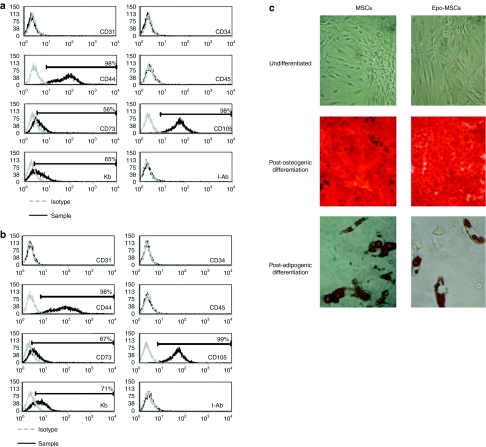
In order to determine if our preparations of MSCs and of Epo-MSCs express the cell surface antigens characteristic of MSCs, flow cytometry analysis was conducted. Results revealed that both MSCs and Epo-MSCs used in this study had the expected flow cytometry phenotype. As shown in Figure 1, these cell preparations were CD44+, CD73+, CD105+, major histocompatibility complex class I positive (MHCI+) (i.e., H-2Kb+), and CD31−, CD34−, CD45−, and MHCII− (i.e. I-Ab−), as expected.
Figure 1.
Characterization of mesenchymal stromal cells (MSCs) and Epo-MSCs. Flow cytometry analysis was conducted (a) on tissue-culture expanded primary MSCs and (b) Epo gene-enhanced MSCs to determine the expression of cell surface antigens CD31, CD34, CD44, CD45, CD73, CD105, H-2Kb (Kb) and I-Ab, as indicated in the Materials and Methods. The darker line represents the specific antibody, and the lighter line the isotype control. (c) Also, a portion of MSCs and Epo-MSCs were tested in vitro by exposure in culture to specific media inducing the osteogenic differentiation (center) or adipogenic differentiation (lower) of both cell populations as described in the Materials and Methods, and as revealed following Alizarin Red S (100X) or Oil Red O (400X) staining, respectively. These cells were photographed under bright-field microscopy with an Axiovert 25 microscope.
To further characterize our preparations of MSCs and verify that they maintained their progenitor cell properties, these cells were tested for their ability to differentiate into adipocytes and osteocytes in vitro when exposed to specific differentiation-inducing media. As shown in Figure 1c, both the MSCs and Epo-MSCs populations demonstrated their capacity for osteogenic differentiation as shown following Alizarin Red S staining, and for adipogenic differentiation as shown following Oil Red O staining.
In vitro effect of conditioned media from MSCs and Epo-MSCs on renal epithelial cells
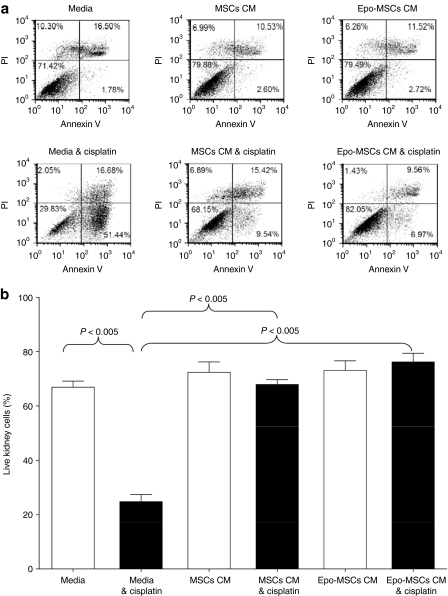
To reveal any beneficial effect of conditioned media (CM) from our preparations of MSCs and Epo-MSCs on mouse kidney cell survival in vitro, MM55.K mouse kidney epithelial cells were exposed to CM from MSCs and Epo-MSCs, in the presence and absence of cisplatin. As shown in Figure 2, the percentage of live kidney cells after 42 hours exposure to cisplatin (7.5 µg/ml) was 68 ± 1.8% with use of MSCs CM, and 76 ± 3.2% with Epo-MSCs CM, both values significantly higher (P < 0.005) than the 25 ± 2.7% survival observed with use of media alone. The kidney cells exposed to the CM from Epo-MSCs always showed greater survival and less apoptotic cells (representative example of flow cytometry results shown in Figure 2a), but it was not significantly different than the beneficial effect exerted by the MSCs CM, as shown by the results of three separate experiments (Figure 2b, black columns). Also, the survival of kidney cells exposed to MSCs CM or to Epo-MSCs CM was not significantly affected by cisplatin, whereas there was a significant drop in survival of kidney cells exposed to media alone in the presence of cisplatin versus in the absence of cisplatin, i.e., 25 ± 2.7% versus 67 ± 2.3% survival, respectively (P < 0.005) (Figure 2b, first two columns). In addition, we ascertained that there were no significant differences between the three conditions when the kidney cells were not exposed to cisplatin (Figure 2b, white columns).
Figure 2.
Effect of conditioned media (CM) from mesenchymal stromal cells (MSCs) and Epo-MSCs on kidney cell survival in vitro. MM55.K mouse kidney epithelial cells were exposed to CM from MSCs, CM from Epo-MSCs, or complete media, with or without cisplatin at 7.5 µg/ml for 42 hours as described in the Materials and Methods. (a) All kidney cells were then recovered, stained, and analyzed by flow cytometry for annexin V and propidium iodide (PI) expression. (b) Experiment was performed three separate times and average of live kidney cells (negative for both annexin V and PI) calculated. Mean ± SEM.
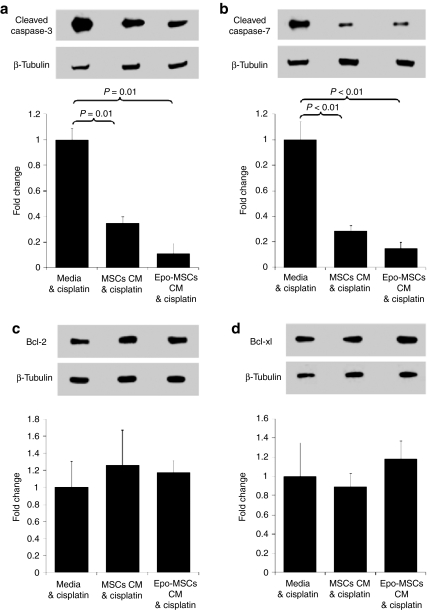
In order to further study this increased survival of cisplatin-treated MM55.K mouse renal cells when exposed to CM from MSCs or Epo-MSCs, as opposed to media only, western blot analyses were conducted on the renal cell lysates. As shown in Figure 3 by the representative images and by the averages of three separate experiments, the CM from MSCs and from Epo-MSCs led to significantly lower expression of proapoptotic Cleaved Caspase-3, i.e., 35 ± 4.7% and 11 ± 7.8%, respectively, of the level observed with use of media only (P = 0.01) (Figure 3a). Cleaved Caspase-3 expression with use of Epo-MSCs CM was considerably, but not significantly, lower than that seen with MSCs CM (P = 0.06) (Figure 3a). Similarly, MSCs and Epo-MSCs CM led to significantly reduced expression of proapoptotic Cleaved Caspase-7, i.e., 29 ± 4.5% and 15 ± 5.1%, respectively, of the level noted with media only (P < 0.01) (Figure 3b). Cleaved Caspase-7 expression level with Epo-MSCs CM use was notably, but not significantly, lower than that observed with MSCs CM (P = 0.26) (Figure 3b).
Figure 3.
Effect of mesenchymal stromal cells (MSCs) and Epo-MSCs conditioned media (CM) on protein expression in kidney cells. MM55.K mouse kidney epithelial cells were exposed, as described in Materials and Methods, to cisplatin at 7.5 µg/ml in the presence of CM from MSCs, CM from Epo-MSCs, or complete media for 42 hours. All kidney cells were then recovered, lysed, and used for Western blot analysis to assess expression of (a) Cleaved Caspase-3, (b) Cleaved Caspase-7, (c) Bcl-2, and (d) Bcl-xl, with β-tubulin levels used as internal control. The experiment was conducted three separate times and mean ± SEM values for fold change shown under representative blot for each protein.
Also, anti-apoptotic Bcl-2 expression levels with use of CM from MSCs and Epo-MSCs were 126 ± 40.8% and 117 ± 14.1%, respectively, of the level observed with media only, but the differences were not significant (P = 0.59 and P = 0.34) (Figure 3c). Furthermore, anti-apoptotic Bcl-xl expression levels with MSCs and Epo-MSCs CM use were 89 ± 14% and 118 ± 18.6%, respectively, of the level observed with media only, but not significantly different (P = 0.49 and P = 0.43) (Figure 3d).
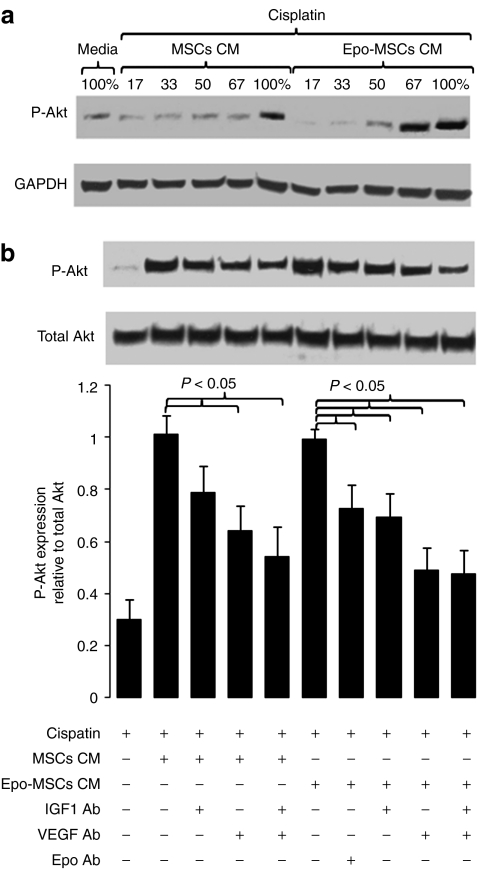
In addition, to help determine a contributing mechanism for the prosurvival effects of the CM from MSCs and Epo-MSCs, western blot analysis for the expression of phosphorylated-Akt (Ser473) (P-Akt) was conducted on these kidney cells. Results indicated that P-Akt expression was increased in cisplatin-treated kidney cells exposed to CM from MSCs and Epo-MSCs. More precisely, as can be seen in Figure 4a, the level of expression of P-Akt in these kidney cells rose with use of increasing concentrations of CM from MSCs, and particularly from Epo-MSCs. Furthermore, in a separate experiment, we observed that the increase in P-Akt expression seen with the use of MSCs CM was reduced by the addition of an insulin-like growth factor 1 (IGF-1) neutralizing antibody (Ab), but not significantly (P = 0.10), whereas it was significantly reduced by a vascular endothelial growth factor (VEGF) neutralizing Ab or by the combination of these two Abs (P < 0.05) (Figure 4b). With Epo-MSCs CM, we noted that the resulting increased P-Akt expression was significantly lowered by the IGF-1 neutralizing Ab, and more so by the VEGF neutralizing Ab or by these two Abs combined (P < 0.05) (Figure 4b). Furthermore, part of the rise in P-Akt expression seen with Epo-MSCs CM was significantly blocked by use of an Epo neutralizing Ab (P < 0.05) (Figure 4b).
Figure 4.
Effect of conditioned media (CM) from mesenchymal stromal cells (MSCs) and from Epo-MSCs on P-Akt expression in kidney cells. MM55.K mouse kidney epithelial cells were exposed to cisplatin, with/without MSCs CM or Epo-MSCs CM (a) at increasing concentrations or (b) at highest concentrations with/without a neutralizing anti-IGF-1 antibody (IGF1 Ab), and/or anti-VEGF antibody (VEGF Ab), or anti-Epo antibody (Epo Ab), as detailed in the Materials and Methods. Cells were collected 42 hours later and lysates used for western blot analysis of phosphorylated Akt (Ser473) (P-Akt) expression, as well as of loading controls GAPDH or total Akt expression. (b) The bar graph represents the mean ± SEM of seven independent experiments.
In vivo effect of MSCs and Epo-MSCs in allogeneic mice with cisplatin-induced AKI
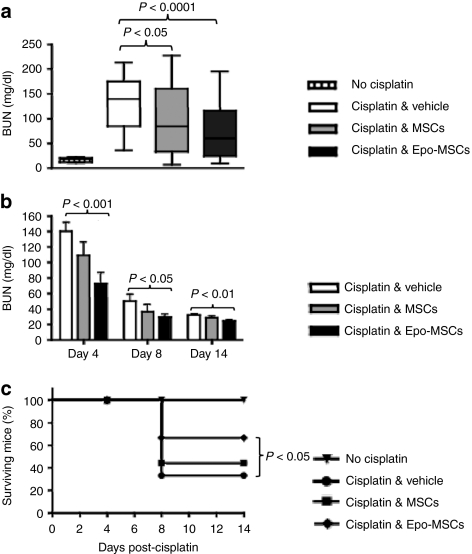
To determine the effect of our MSCs preparations in vivo in allogeneic recipients, C57Bl/6 mouse-derived MSCs and Epo-MSCs, at 5 × 106 cells/mouse, were delivered by intraperitoneal injection in MHC-mismatched Balb/c mice injected the previous day with cisplatin to induce AKI. As shown in Figure 5a, intraperitoneal delivery of MSCs and of Epo-MSCs led to blood urea nitrogen (BUN) levels of 97 ± 12 mg/dl and 72 ± 9.5 mg/dl, respectively, as compared to 131 ± 9.20 mg/dl noted with administration of Vehicle only, at day 4 following cisplatin treatment when peak BUN levels were determined to occur in these mice. The average BUN concentrations observed with MSCs and with Epo-MSCs were significantly lower than those seen with Vehicle only (P < 0.05 and P < 0.0001, respectively) (Figure 5a). BUN levels in surviving mice decreased with time, within 14 days, as seen in Figure 5b.
Figure 5.
Effect of mesenchymal stromal cells (MSCs) and Epo-MSCs on blood urea nitrogen (BUN) and survival of cisplatin-treated mice. (a) Mice given cisplatin were injected intraperitoneally with allogeneic MSCs (gray box, n = 30) or Epo-MSCs (black box, n = 30), or with Vehicle only (white box, n = 30), and BUN levels determined from blood collected at day 4 (median seen as horizontal bar within box in boxplot with whiskers from minimum to maximum), (b) and at three time points for mice kept long-term (mean ± SEM, n = 18 on day 4 for each of the three groups). (c) Survival of these mice over time was also assessed (n = 18). Normal mice not given cisplatin were used as controls.
Also, survival of cisplatin-treated mice was significantly increased by the administration of Epo-MSCs as compared to Vehicle only (P < 0.05) (Figure 5c). By day 14 postimplantation, 67% of cisplatin-treated mice that received Epo-MSCs were alive, as compared to 44% of those implanted with MSCs, and versus 33% of mice injected with Vehicle only (Figure 5c).
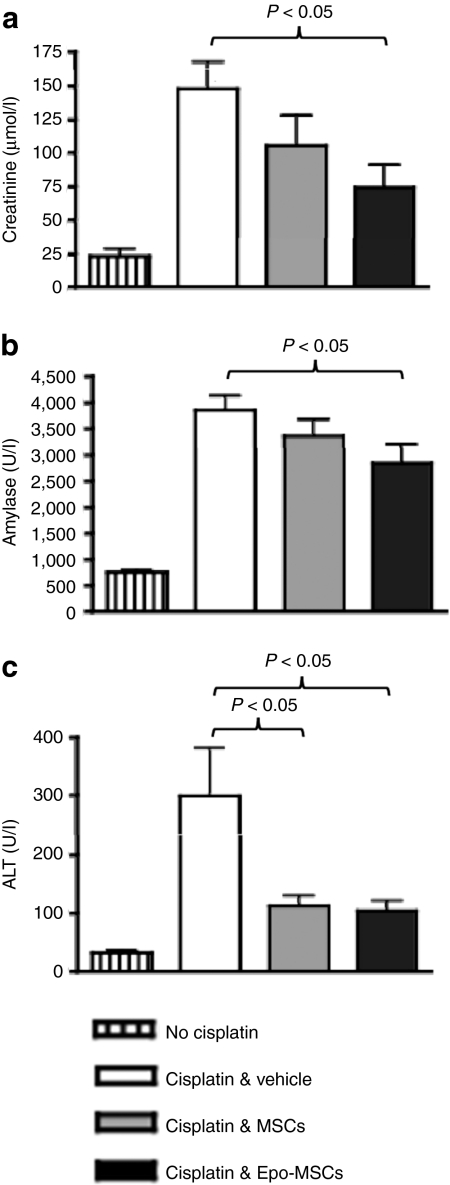
Further blood chemistry analysis conducted from higher blood volumes obtained from mice killed 4 days postimplantation showed that the creatinine level in cisplatin-treated mice implanted with Epo-MSCs was significantly lower than in those implanted with Vehicle only, i.e., 74 ± 17 µmol/l as compared to 148 ± 19.4 µmol/l, P < 0.05. MSCs also led to considerably decreased creatinine levels, but not significantly, i.e., 106 ± 22.4 µmol/l, P = 0.178. Moreover, blood amylase concentration in cisplatin-treated mice administered Epo-MSCs was significantly lower than the level with delivery of Vehicle only, i.e., 2,845 ± 354.6 U/l versus 3,852 ± 273.5 U/l, P < 0.05. Implantation of MSCs led to amylase value of 3,351 ± 325.2 U/l, and thus also to lowering of amylase as compared to the Vehicle group, but not significantly (Figure 6b). Furthermore, simultaneous assessment of blood alanine aminotransferase revealed significantly decreased levels in cisplatin-treated mice administered Epo-MSCs and also in those injected with MSCs, i.e., 104 ± 17.2 U/l and 112 ± 18.1 U/l respectively, as compared to alanine aminotransferase concentration in Vehicle only recipient mice, i.e., 299 ± 82.4 U/l (P < 0.05), (Figure 6c).
Figure 6.
Blood creatinine, amylase and alanine aminotransferase (ALT) levels in mice. Mice implanted by intraperitoneal injection with allogeneic mesenchymal stromal cells (MSCs) (gray columns) or Epo-MSCs (black columns), or with Vehicle only (white columns) 1 day following cisplatin administration, were killed 4 days later, and blood collected analyzed for (a) creatinine, (b) amylase and (c) ALT levels (n = 9–15, mean ± SEM). Normal mice not given cisplatin were utilized as controls (striped columns).
Immunohistochemical analysis of mouse kidneys
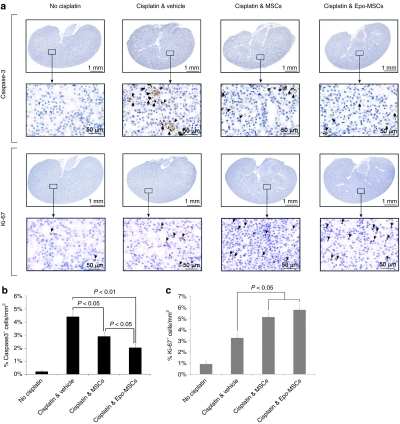
To assess whether intraperitoneal delivery of MSCs and Epo-MSCs in cisplatin-treated mice affects the amount of apoptotic cells and of proliferating cells in the kidneys, immunohistochemical detection of Caspase-3 and of Ki-67, respectively, was performed as shown in Figure 7. Results reveal that implantation of MSCs and Epo-MSCs significantly lowered the proportion of Caspase-3-expressing cells in the kidneys of cisplatin-treated mice, as 2.9 ± 0.3% and 2.0 ± 0.3%, respectively, were Caspase-3+ per mm2 tissue, as compared to 4.4 ± 0.4% seen with Vehicle only administration (P < 0.05 and P < 0.01, respectively) (Figure 7b). Furthermore, the decrease in Caspase-3+ cells achieved with use of Epo-MSCs was significantly greater than that observed with MSCs (P < 0.05) (Figure 7b).
Figure 7.
Immunohistochemical analysis of mouse kidneys. Mice implanted intraperitoneally with allogeneic mesenchymal stromal cells (MSCs), Epo-MSCs, or Vehicle only, 1 day following cisplatin administration, were killed 4 days later and kidneys removed, sectioned and stained for detection of Caspase-3 and Ki-67. (a) Caspase-3 (top panel) or Ki-67 (lower panel) expressing cells are indicated by black arrows in representative images. (b) The percentages of Caspase-3 positive cells and (c) Ki-67 positive cells per mm2 kidney area were also evaluated as described in the Materials and Methods (n = 4–6 mice/group, mean ± SEM). Normal mice not given cisplatin were used as controls.
In addition, MSCs and Epo-MSCs led to 5.1 ± 0.4% and 5.8 ± 0.6%, respectively, Ki-67+ cells per mm2 tissue in kidneys of recipient mice, in significant contrast to 3.3 ± 0.5% seen in Vehicle only-implanted mice (P < 0.05) (Figure 7c). Although more Ki-67+ cells were observed with Epo-MSCs than with MSCs, the difference was not significant.
PCR and western blot analysis of kidneys
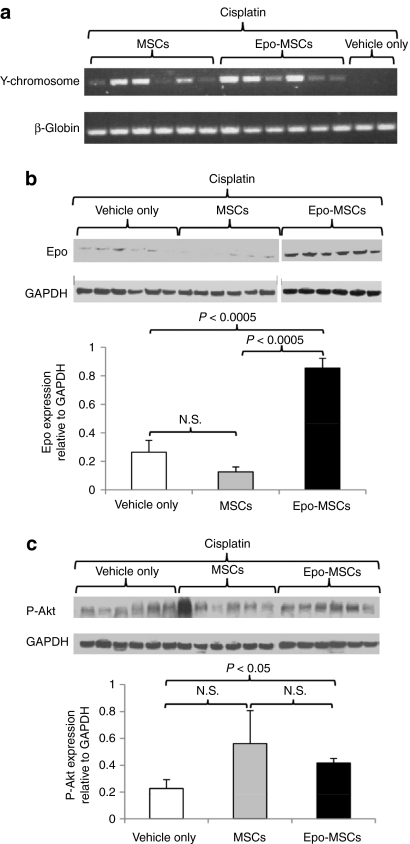
To establish if implanted male mouse-derived MSCs and Epo-MSCs engrafted in the kidneys of recipient female cisplatin-treated mice, PCR for detection of a mouse Y chromosome-specific fragment of 444 bp was conducted on kidney genomic DNA. As shown in Figure 8a, PCR amplification of the expected fragment was seen in tested DNA samples from kidneys of MSCs and Epo-MSCs–implanted mice and not in those from Vehicle only recipients.
Figure 8.
PCR and western blot analysis of kidneys. (a) Genomic DNA isolated from kidneys of cisplatin-treated mice implanted with mesenchymal stromal cells (MSCs), Epo-MSCs, or Vehicle only, were used to amplify a 444-base pair fragment of mouse Y chromosome and a 363-base pair fragment of internal control β-globin, and findings from individual mice shown. The same mouse kidneys were also used for isolation of total protein used for (b) western blot detection of Epo or (c) of phosphorylated-Akt (Ser473) (P-Akt), and (b,c) of GAPDH internal control. Results from individual mice are shown in upper portions of panels b and c, whereas lower portions reveal levels (means ± SEM (n = 6)) of Epo or P-Akt expression relative to GAPDH.
Furthermore, to determine the expression levels of Epo in the kidneys of these same mice, western blot analysis was performed on the protein isolated from kidneys of cisplatin-treated mice implanted with MSCs, Epo-MSCs, or Vehicle only, and the resulting blot shown in Figure 8b. Quantitative analysis of the intensity of the signal of Epo relative to that of the internal control GAPDH revealed that it was significantly greater in mice implanted with Epo-MSCs (i.e., 0.86 ± 0.07) as compared to MSCs (i.e., 0.13 ± 0.03, P < 0.0005), and to Vehicle only recipient mice (i.e., 0.26 ± 0.08, P < 0.0005) (Figure 8b). Moreover, similar evaluation of the levels of P-Akt in these same mouse kidneys revealed that the intensity of the signal of P-Akt relative to that of the GAPDH internal control was significantly higher in kidneys of mice implanted with Epo-MSCs, as compared to those implanted with Vehicle only (i.e., 0.42 ± 0.03 versus 0.23 ± 0.07, P < 0.05) (Figure 8c).
Discussion
In the present study, our main goal was to determine if Epo gene-enhanced MSCs can exert a renoprotective effect in allogeneic mice with cisplatin-induced AKI, and to analyze this effect not only in vivo but also in vitro. We first generated MSCs and Epo-secreting MSCs, and ascertained that they expressed the cell surface antigens characteristic of MSCs (Figure 1a,b). In addition, we demonstrated that they retained their mesenchymal progenitor cell properties as they were successfully induced to differentiate in vitro (Figure 1c). MSCs have shown increasing promise for tissue regeneration and repair.2,4,29 This is due in part to the ability of these cells to differentiate into several different cell types, such as osteocytes, adipocytes, chondrocytes, cardiomyocytes, astrocytes, and even into renal tubular cells, but mainly due to the capacity of these cells to release factors that exert beneficial outcomes.2,4,6,8,9,11,29 These latter paracrine effects of MSCs include anti-apoptotic, mitogenic, angiogenic and anti-inflammatory actions.5,6,7,10,11 Epo administration has also been reported to induce various cytoprotective cellular responses which include mitogenesis, angiogenesis, apoptosis inhibition, as well as vascular repair stimulation via endothelial progenitor cell mobilization from the bone marrow.14,16,18,19,20,30 Through our Epo gene-enhanced MSCs, we would thus investigate the potential of MSCs and Epo combined tissue reparative and protective abilities. We therefore decided to examine the effect of the secretomes on the survival of mouse kidney epithelial cells in vitro. We noted that the CM of MSCs and of Epo-MSCs protected kidney cells from death-induced by cisplatin (Figure 2). Both treatments led to significantly higher survival of kidney cells as compared to use of media only, and to less apoptosis. Survival was slightly, but not significantly, greater with CM from Epo-MSCs versus that from MSCs. Our observations with MSCs CM are consistent with those of another report where a prosurvival effect on murine proximal tubular cells exposed to cisplatin in vitro was similarly revealed with addition of murine MSCs CM.5 Therefore, MSCs CM possess a protective capability on renal cells.
Other than cell survival, we also examined apoptotic protein expression and noted that the CM from MSCs and from Epo-MSCs led to very significantly lower levels of active Caspase-3 and active Caspase-7 in cisplatin-treated kidney epithelial cells, as compared to media only (Figure 3). We observed that the reduction in the expression of these two proteins was more substantial with Epo-MSCs CM as compared to MSC CM, especially for active Caspase-3, but the differences were not significant (P = 0.06 for cleaved Caspase-3 and P = 0.26 for cleaved Caspase-7). Moreover, we showed that the CM of MSCs and of Epo-MSCs increased the expression level of phosphorylated serine/threonine protein kinase Akt1 (Ser473) (P-Akt) in mouse kidney epithelial cells exposed to cisplatin (Figure 4), with higher expression seen with use of increasing CM concentrations (Figure 4a). A previous study on AKI induced by cisplatin demonstrated the implication of the PI3K-Akt pathway in reducing apoptosis of renal tubular cells and preserving kidney function.31 We also demonstrated the involvement of IGF-1 and mainly of VEGF and of their combination in this effect, since the use of neutralizing anti-IGF-1 and anti-VEGF antibodies lowered the MSCs and Epo-MSCs CM-induced increase in P-Akt expression (Figure 4b). Furthermore, a neutralizing anti-Epo antibody reduced the rise in P-Akt expression caused by the Epo-MSCs CM, indicating the beneficial implication of the Epo secreted by the Epo-MSCs (Figure 4b). Our findings are consistent with previous reports using MSCs or Epo. In one study, murine MSCs were shown to decrease the amount of apoptotic cisplatin-treated proximal tubular cells in co-culture experiments, more specifically the percentage of Caspase-3 and Caspase-7 positive tubular cells.7 The role of IGF-1 was demonstrated in that study, since silencing IGF-1 in MSCs increased tubular cell apoptosis and also limited the protective effect of MSCs in vivo in mice with cisplatin-induced AKI.7 Other than IGF-1, the protective role of VEGF was revealed in a study where murine MSCs with small interfering RNA gene-silenced expression of VEGF showed decreased effectiveness in rats with ischemia/reflow-induced AKI.32 Besides MSCs, investigations have also linked Epo renoprotection with impeded activation of Caspase-3.33,34 For instance, recombinant Epo was observed to prevent Caspase-3 activation in serum-starved human proximal tubule epithelial cells.34
We thereafter tested our preparations of MSCs and Epo-MSCs in vivo, and more specifically by intraperitoneal implantation in allogeneic mice with cisplatin-induced AKI. In a previous study, we showed that MSCs will lead to a cellular immune response and their immune rejection in MHC-mismatched recipient mice.35 Therefore, allogeneic MSCs may not be suited for long-term use such as for chronic diseases. However, for acute diseases such as AKI when a rapid and short-lived effect may be sufficient for beneficial outcome, the use of these cells becomes even more promising since they may then be employed as universal donors. The results of our present investigation showed that Epo-MSCs implantation in allogeneic mice administered cisplatin the previous day led to significantly less impairment of kidney function, as compared to Vehicle only and as determined by significantly lower BUN levels (Figure 5a). Implantation of MSCs also considerably decreased BUN levels, but not as much as Epo-MSCs. We subsequently observed that in surviving mice in all groups, BUN returned to normal or near-normal values within 2 weeks, but that the recipients of the Epo-MSCs showed significantly lower levels than the Vehicle group (Figure 5b). In addition, we observed that blood creatinine levels were also reduced by MSCs and significantly so by Epo-MSCs (Figure 6a), confirming the significant protective effect of Epo-MSCs on kidney function. Furthermore, remarkable was the in vivo effect on survival that the implantation of Epo-MSCs caused in cisplatin-treated mice (Figure 5c). Two weeks following their intraperitoneal administration, these Epo-MSCs led to 67% survival, as compared to only 33% seen with the use of Vehicle only. MSCs that were not Epo gene-enhanced led to 44% survival of cisplatin-treated mice. Therefore, engineering the MSCs to secrete Epo augmented their beneficial action on survival. MSCs have been demonstrated to lead to renoprotective effects in AKI in mice.5,12,13 These studies were conducted in syngeneic recipients. In one investigation using allogeneic rat MSCs in a rat model of ischemia/reflow-induced AKI, these cells were also found to be effective at renoprotection although not as potently as the autologous MSCs.32 Although the effect of MHC-mismatched MSCs may not be as considerable, the use of these cells remains appealing, especially when gene-enhancing these such as with Epo, as we here showed, raised their beneficial effect. Transient presence of exogenous allogeneic cells also decreases any safety concerns raised with persistence of implanted cells longer than required.
The effectiveness of cisplatin, a widely used chemotherapeutic drug, is hindered by dose-limiting nephrotoxicity.36,37 Therefore, a chemoprotective approach, such as with our Epo gene-enhanced MSCs, would enhance the therapeutic potential of cisplatin and other chemotherapeutic agents with major dose-limiting toxicity to the kidneys. Other than nephrotoxicity, cisplatin also exerts further toxicities, such as neurotoxicity and ototoxicity. Its distribution in patients was reported to include different organs, mostly kidney, liver and testis, as well as pancreas, muscle, lung, and nerve tissue.38 Therefore, since cisplatin causes several toxicities and thus not only affects BUN and creatinine, we also looked at blood amylase as well as alanine aminotransferase levels. Epo-MSCs implantation significantly lowered the increase in amylase induced by cisplatin (Figure 6). MSCs also reduced blood amylase, but not significantly. MSCs and Epo-MSCs both significantly decreased the rise in blood alanine aminotransferase concentrations. These findings suggest that MSCs and mainly Epo-MSCs not only protect against cisplatin-induced injury to the kidneys but also to other sites affected by this chemotherapeutic agent, such as pancreas and liver. Reports have revealed the beneficial action of MSCs on pancreas and liver repair.39,40 The migration capability of MSCs in vivo to sites of injury therefore allows these cells to exert their reparative/protective actions where needed, an important added advantage of this cell therapy. Moreover, studies have shown that the prognosis of patients with AKI that also have related injury to other organs is further decreased,41,42,43,44 therefore indicating the added beneficial potential of our cell and gene therapy for toxicities/injuries that affect various organs.
To determine if the anti-apoptotic in vitro effect we observed on kidney cells also applies in vivo, we assessed the presence of Caspase-3-positive cells in kidneys of AKI mice implanted with our MSCs populations. Indeed, both the MSCs and Epo-MSCs significantly lowered the amount of Caspase-3-expressing cells in the kidneys of cisplatin-treated mice (Figure 7b). Although both were significant, Epo-MSCs led to a greater reduction, one which was even significantly lower than that obtained with MSCs. As indicated above, studies have revealed that both MSCs and recombinant Epo can exert anti-apoptotic actions. Our combining these two approaches through the use of Epo gene-enhanced MSCs appear here to enhance their anti-apoptotic effectiveness.
In addition, MSCs as well as recombinant Epo have also been reported to exert mitogenic effects. Therefore, we evaluated the amount of proliferating cells, i.e., Ki-67-positive, in the kidneys of our AKI mice. Both MSCs and Epo-MSCs administrations led to significantly increased numbers of proliferating cells in kidneys of cisplatin-treated mice, as compared to Vehicle only (Figure 7c). This effect with Epo-MSCs was higher than that achieved with MSCs, but not significantly. MSCs can secrete various factors such as VEGF, hepatocyte growth factor, and IGF-1, at levels which have various beneficial capabilities, such mitogenic, angiogenic, anti-inflammatory, and anti-apoptootic.5,6,7,10,11 Besides Epo, any potentially beneficial plasma soluble gene-product may also have the ability to improve the effectiveness of MSCs in AKI. In one study, kallikrein gene-modified MSCs brought about anti-apoptotic and anti-inflammatory effects following injection in a rat model of ischemia/reperfusion-induced AKI, leading to significant renoprotection.45
We chose to inject the cells rather than their CM because we aimed for a local effect and this would be feasible since MSCs have been found to migrate to sites of tissue injury through homing signals, and then ameliorate function and microenvironment principally through paracrine actions.2,6,27 In one such study, MSCs's CD44 expression was found to be implicated in their migration to injured kidneys in a mouse model of glycerol-induced AKI where expression of the CD44 ligand hyaluronic acid was elevated.28 We detected donor MSCs and Epo-MSCs in the kidneys of our implanted AKI mice (Figure 8a). In addition, we revealed that the kidneys of Epo-MSCs–implanted mice had significantly higher Epo expression levels than those of MSCs or Vehicle only-implanted mice (Figure 8b). This result may be attributed to Epo-MSCs engrafted in the kidneys, and/or to the effect of the Epo delivered by Epo-MSCs on Epo-secreting cells of the kidney. Moreover, the expression of P-Akt in the kidneys of Epo-MSCs–implanted mice was significantly higher than in those of MSCs or Vehicle only recipients (Figure 8c). Our findings indicate that Epo-MSCs delivered by intraperitoneal injection can home to damaged kidneys and reduce cisplatin-induced injury by delivering not only the beneficial growth factors secreted by all MSCs, such as VEGF, but also the secreted Epo from Epo-MSCs, leading to augmented expression of prosurvival P-Akt.
Other than in mice, an ongoing phase 1 clinical study is investigating the safety of human MSCs and their use for prevention and treatment in patients at increased risk of developing AKI subsequent to cardiac surgery.46 Therefore, our present investigation in mice revealing that gene-enhancing MSCs with Epo raises their therapeutic potential for kidney protection in AKI may lead to improved outcomes in future studies in patients.
Materials and Methods
Generation of mouse MSCs and Epo-MSCs. A male C57Bl/6 mouse (Harlan Laboratories, Indianapolis, IN) weighing ~20g was killed, femurs and tibias isolated, and whole bone marrow flushed out with complete media [i.e., α-MEM media (Invitrogen-GIBCO, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Wisent, St-Bruno, Quebec, Canada), 1% Penicillin/Streptomycin (Wisent) and 1% -glutamine (Wisent)], and placed in culture in a 37 °C incubator with 5% CO2. Five days later, the nonadherent hematopoietic cells were discarded and the adherent marrow MSCs cultured for ~9 passages in complete media to generate the population of MSCs used in this study. The generation of Epo gene–enhanced MSCs, i.e., Epo-MSCs, was performed by retroviral transduction of MSCs for 5 successive days. More specifically, for each round of transduction, 0.45 µm filtered retroviral supernatant from 293GP2 cells transfected with our previously published mouse Epo-containing retroviral construct25,26,35 and VSV-G expression vector according to the manufacturers' (Invitrogen/Life Technologies, Frederick, MD) protocol was used in presence of 6 µg/ml lipofectamine reagent (Invitrogen).
To determine the concentration of Epo secreted by Epo-MSCs, CM were collected from Epo-MSCs, and used in an enzyme-linked immunosorbent assay specific for mouse Epo (R&D Systems, Minneapolis, MN). The preparations of Epo-MSCs used in this study were thus noted to secrete ~1.4 µg Epo per one million cells per 24 hours. MSCs were also similarly assayed and no Epo secretion by these cells was detected.
Characterization of MSCs and Epo-MSCs. To verify the phenotype of our cell populations, flow cytometry was conducted on MSCs and Epo-MSCs at passage 9 for analysis of the expression of cell surface antigens CD31, CD34, CD44, CD45, CD73, CD105, MHCI, and MHCII. More specifically, MSCs were incubated with CD31-biotin/Streptavin-PE; CD34-biotin/Streptavin-PE; CD44-PE; CD45-PE; CD73-PE,; CD105-PE; H-2Kb-PE; I-Ab-PE, (BD Biosciences, San Diego, CA) for 1 hour in the dark at 4 °C and then washed with FACS Buffer (3% FBS in phosphate-buffered saline (PBS)). Cells that were incubated with the biotin-conjugated antibodies were stained with the secondary antibodies (streptavidin-PE, dilution 1:400) for 30 minutes. Isotypic control analyses were carried out in parallel. Cells were washed with FACS Buffer, fixed with 1% paraformaldehyde, and analyzed using a fluorescence-activated cell sorter (FACS) Calibur flow cytometer (BD Immunocytometry systems, San Jose, CA), with data analysis performed with FCSExpress (De Novo Software, Los Angeles, CA).
In addition, our MSCs and Epo-MSCs populations were tested in vitro for the maintenance of their mesenchymal progenitor cell properties, more specifically their capacity for osteogenic and adipogenic differentiation. In order to test for osteogenic differentiation of MSCs and Epo-MSCs, a portion of these cells when ~70–80% confluent were exposed to the following media: complete media supplemented with β-glycerol phosphate (10 mmol/l), ascorbic acid 2-phosphate (5 µg/ml), and dexamethasone (10−8 mol/l; Sigma-Aldrich Canada, Oakville, Ontario, Canada)47,48 for 4 weeks and then stained with Alizarin Red S (Sigma-Aldrich Canada; pH 4.1 using ammonium hydroxide).48 In order to test for adipogenic differentiation of MSCs and Epo-MSCs, a portion of these cells when ~70–80% confluent were exposed to the following media: complete media supplemented with insulin (10 µg/ml), dexamethasone (1 µmol/l), 3-isobutyl-methylxanthine (0.5 mmol/l), and indomethacin (46 µmol/l; Sigma-Aldrich Canada) for 7 days, and then stained with Oil Red O (Sigma-Aldrich Canada).47
Flow cytometry analysis of effects of MSCs and Epo-MSCs CM on kidney cell survival. The media was collected from MSCs and Epo-MSCs at ~80–90% confluency 96 hours after changing the media for 18 ml fresh complete media per 150 mm tissue-culture plate. Collected media were centrifuged to eliminate any floating cells and then supernatants, i.e., conditioned media (CM), placed at −80 °C until later utilized for in vitro assays. Mouse kidney epithelial cell line MM55.K (ATCC, Manassas, VA) was plated in 6-well tissue-culture dishes at 2 × 105 cells per well in Dulbecco's Modified Eagle's Medium (D-MEM) with NaHCO3 at 1.5 g/l (ATCC) supplemented with 10% heat-inactivated FBS and 1% Penicillin/Streptomycin (Wisent), and placed in a 37 °C incubator with 5% CO2. When kidney cell confluency was ~60% the following day, this media was removed and replaced with serum-free media in order to synchronize the cells, and after 4.5 hours replaced with MSCs CM, Epo-MSCs CM, or complete media i.e., α-MEM media (Invitrogen/Life Technologies) with 10% FBS and 1% Penicillin/Streptomycin (Wisent), with or without cisplatin (Mayne Pharma Canada, Montreal, Quebec, Canada) at 7.5 µg/ml. After 42 hours in the 37 °C incubator, all cells were recovered, adherent and floating, and analyzed by flow cytometry following annexin V and propidium iodide staining. More specifically for staining, cells were centrifuged, resuspended in 100 µl 1X binding buffer (BD Biosciences Pharmingen, Mississauga, ON) to which 5 µl annexin V FITC and 10 µl propidium iodide (BD Biosciences Pharmingen) were then added. Following 15-minute incubation in the dark at room temperature, cells were centrifuged, resuspended in 400 µl 1X binding buffer, and then within 1 hour analyzed with the FACSCalibur flow cytometer and FCSExpress.
Western blot analysis of CM effects on kidney cell Caspase-3, -7, Bcl-2 and Bcl-xl expression. MM55.K mouse kidney cells were plated in 10 cm tissue-culture dishes at 1 × 106 cells per plate and the next day serum-starved for 4.5 hours and exposed as described above, to MSCs CM, Epo-MSCs CM, or complete media, in presence of cisplatin at 7.5 µg/ml for 42 hours. All cells, floating and adherent, were then lysed using lysis buffer (20 mmol/l Tris (pH 7.5), 150 mmol/l NaCl, 1 mmol/l ethylene diamine tetraacetate (EDTA), 1 mmol/l ethylene glycol-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA), 1% Triton X-100, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l b-glycerophosphate, 1 mmol/l Na3VO4, 1 µg/ml leupeptin, 1 mmol/l phenylmethylsulfonyl fluoride), and used for western blot analysis with antibodies (all from Cell Signaling Technology, Beverly, MA) to determine expression of Cleaved Caspase-3, Cleaved Caspase-7, Bcl-2, Bcl-xl, all with β-tubulin levels assessed as internal control. Three different western blots for each condition were scanned (Scanner model) and transferred to Scion Image software (version 4.0.2). Blots were discriminated from surrounding background using the thresholding mode. In this mode, pixels equal to or greater than threshold level are displayed in black and all other pixels are displayed in white (background). Evaluation of blot size and intensity was performed using the Gelplot macro provided with the software.
Western blot analysis of CM and antibody effects on kidney cell P-Akt expression. Mouse MSCs and Epo-MSCs were seeded at 900 cells per cm2 and incubated for 5 days in complete culture medium containing αMEM, 10% FBS, 100 units/ml penicillin, 100 µg/ml streptomycin. The media were then harvested from the cells, cell debris were removed by centrifugation, and the supernatants (i.e., CM) were used as follows. First, the concentrations of Epo, IGF-1 and VEGF in the CM were determined by enzyme-linked immunosorbent assays specific for mouse Epo, IGF-1 and VEGF, respectively (R&D Systems). These were respectively 0, 0.68, and 0.58 ng/ml in MSCs CM, whereas 242, 0.02, and 0.4 ng/ml in Epo-MSCs CM. The MSCs CM was tested alone or in combination with neutralizing antibodies against IGF-1 (AF791) or VEGF (AF-493-NA) (R&D Systems), or against both IGF-1and VEGF. The Epo-MSCs CM was similarly tested alone or in combination with neutralizing antibodies against IGF-1, VEGF or Epo (MAB959) (R&D Systems), or against both IGF-1and VEGF. The amounts of the antibodies against Epo, IGF-1 and VEGF for neutralization were respectively 20-, 20- and 80-fold greater than the concentrations for half-maximal inhibition of the recombinant protein. For western blot analysis of the effect of these CM on P-Akt expression in MM55.K cells, these kidney cells when at 60% confluence were starved with serum-free α-MEM for 6 hours, and then incubated with either complete α-MEM-based culture medium or with CM (from MSCs or Epo-MSCs), without or with the neutralizing antibodies above for 42 hours, in presence of Cisplatin (7.5 µg/ml), 37 °C, 5% CO2. The adherent and floating cells were then harvested and lysed using a lysis buffer (same as described above). Protein concentrations were determined using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA). Whole cell lysates were separated by 4–20% Precise Protein Gels (Thermo Scientific, Rockford, IL) and transferred to a Nitrocellolose membrane (Bio-Rad). Subsequently, the membranes were incubated for 1 hour at room temperature in the solution of TBST (10 mmol/l Tris–HCl (pH 8.0), 150 mmol/l NaCl, and 0.05% Tween20) supplemented with 10% nonfat dry milk, and probed overnight at 4 °C with the appropriate primary antibody, i.e., against P-Akt (Ser473; 1:1000) (Cell Signaling Technology). The membrane was washed three times for 10 minutes each with Tris–buffered saline containing 0.1% Tween 20. The bound primary antibodies were visualized by incubating for 1 hour with a suitable secondary antibody conjugated with horseradish peroxidase (Bethyl Laboratories, Montgomery, TX). The membrane was washed and developed using Chemiluminescent HRP Substrate (Millipore, Billerica, MA). Blots were stripped with Re-Blot Plus Strong Solution (Millipore, Temecula, CA) and reprobed with antibodies against GAPDH (1:5000) or Total Akt (1:2000) (Cell Signaling Technology) as loading controls.
Implantation of MSCs and Epo-MSCs, and analysis of blood of recipient allogeneic AKI mice. As a chemically-induced mouse model of AKI, Balb/c mice, 8–10-week-old (Harlan Laboratories) were injected with cisplatin subcutaneously at 14.7 mg/kg following a 6-hour period of removal of food and water. One day following cisplatin delivery, allogeneic MSCs and Epo-MSCs were recovered by trypsinization, centrifuged, washed and then resuspended in RPMI media (Invitrogen) for intraperitoneal injection. The MSCs and Epo-MSCs were implanted intraperitoneally in the Balb/c mice with AKI at 5 × 106 cells per mouse, in 370 µl RPMI per mouse. As a control group, AKI mice were similarly implanted but with Vehicle only, i.e., RPMI, at 370 µl per mouse. We used Balb/c mice as recipients of the C57Bl/6 mouse derived MSCs and Epo-MSCs because these are MHC class I and class II mismatched and thus are allogeneic with regards to the implanted cells. Also, we used male donor C57Bl/6 mice in order to be able to detect the donor-derived cells in recipient female Balb/c mice through the presence of the Y chromosome.
Long-term experiments were conducted where blood collections were performed from the saphenous vein at various days after cisplatin delivery, and the concentration of urea nitrogen in plasma assessed by colorimetric method (Urea Nitrogen Reagent, Colorimetric Method; Teco Diagnostics, Anaheim, CA). Survival of these mice was also determined over time. The experiment was repeated, similar results obtained, and thus data combined.
Short-term experiments were also performed where mice on day 5 post-cisplatin were killed in order to collect higher blood volumes by cardiac puncture, and to harvest kidneys for several analyses as described below. Chemistry analysis to assess blood creatinine, amylase and alanine aminotransferase levels was conducted using the Abaxis VetScan VS2 chemistry analyzer (VetNovations, Barrie, ON).
Animals were handled under the guidelines promulgated by the Canadian Council on Animal Care.
Immunohistochemical staining and analysis of kidney sections. Sections were prepared and stained at the histology core facility of the Institute for Research in Immunology and Cancer (IRIC, Montreal, Quebec, Canada). Kidneys were removed, placed in formalin, embedded with paraffin, and sections were prepared of 3 µm thickness, and stained for Caspase-3 (apoptotic cells) and Ki-67 (proliferating cells). Immunohistochemical detection was conducted on a Discovery XT automatic immunostainer (Ventana Medical Systems, Tucson, AZ) performing deparaffinization and antigen retrieval with proprietary reagents (Cell Conditioning 1 from Ventana Medical Systems). The anti-Caspase-3 antibody (Biocare Medical; Concord, CA), diluted 1:100 with PBS, was incubated at room temperature for 2 hours. The anti-Ki-67 antibody (Biocare Medical), diluted 1:200 with PBS, was incubated at room temperature for 60 minutes. Subsequently, incubation with anti-rabbit biotin conjugated antibody (Jackson ImmunoResearch, West Grove, PA) was performed. Streptavidin-horseradish peroxidase, 3,3-diaminobenzidine detection kit and Avidin-Biotin Blocking kit were utilized as indicated in the manufacturer's instructions (Ventana Medical Systems). Sections were counterstained with hematoxylin, and a bluing reagent was applied for post-counterstaining.
Stained kidney sections were scanned using the NanoZoomer Virtual Microscopy System (Hamamatsu, Bridgewater, NJ). Images from three different regions per kidney section were processed using the Paint Shop Pro software (version 12.0) using a preprogrammed script, and then transferred to Scion Image software (version 4.0.2). Kidneys from 4 to 6 mice were processed per group, thus constituting 12–18 regions per group of mice. Caspase-3 or Ki-67 stained areas were discriminated from the background using the thresholding mode. In this mode, pixels equal to or greater than threshold level were displayed in black and all other pixels were displayed in white (background). Evaluation of the amount of stained cells was then conducted by enumerating the black spots.
Extraction of genomic DNA from kidney tissues and PCR analysis. Genomic DNA was isolated using AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Hilden, Germany) from mouse kidneys that had been harvested and kept at −80 °C until use. Since cells from male mice were implanted into female recipients, DNA samples (200 ng) were used to amplify a mouse Y chromosome fragment (444 bp) using the following primers: sense primer 5′ CTCCTGATGGACAAACTTTACG 3′ and antisense primer 5′ TGAGTGCTGATGGGTGACGG 3′. The PCR cycle conditions for Y-chromosome detection were the following: 95 °C for 15 minutes (1 cycle), 94 °C for 40 seconds, 59 °C for 1 minute, 72 °C for 1 minute (47 cycles), and 72 °C for 8 minutes. As the internal standard, a 363-bp β-globin product was amplified using the primers 5′ GAAGTTGGGTGCTTGGAGAC 3′ (sense) and 5′ GGAAGGTTGAGCAGAATAGC 3′ (antisense). The PCR cycle conditions for β-globin detection were: 95 °C for 15 minutes (1 cycle), 94 °C for 30 seconds, 57 °C for 1 minute, 72 °C for 1 minute (40 cycles), and 72 °C for 8 minutes. The HotstarTaq DNA Polymerase kit (Qiagen) was used to amplify the specific DNA fragments.
Protein extraction from kidney tissues and western blot analysis. Tissue protein was extracted from harvested mouse kidneys that had been placed at −80 °C until use. Briefly, 30 mg of kidney tissue was homogenized in 400 µl of ice-cold lysis buffer (same as above except also containing 1% sodium dodecyl sulfate). Protein concentrations were determined using Pierce BCA Protein Assay Kit (Thermo Scientific). The tissue lysates (90 µg of protein) were fractionated by 4–20% Precise Protein Gels (Thermo Scientific) and transferred to a Nitrocellolose membrane (Bio-Rad). The membrane was blocked for one hour at room temperature with Tris–buffered saline containing 10% nonfat dry milk and 0.05% Tween20, and then probed overnight at 4 °C with the primary antibody of P-Akt (Ser473; 1:1000). The bound antibodies were visualized with a suitable secondary antibody conjugated with horseradish peroxidase using Chemiluminescent HRP Substrate (Millipore). The blot was stripped with Re-Blot Plus Strong Solution (Millipore) and reprobed with an anti-Epo antibody (1:1000, R&D Systems) to determine Epo expression. The blot was similarly stripped and reprobed with an antibody against GAPDH (1:5000, Cell Signaling Technology) as a loading control.
Statistical analysis. The t-test was employed for comparing most results from the different test groups (other than survival results). For comparison of survival, the Logrank test was conducted. P ≤ 0.05 was considered significant.
Acknowledgments
This research was supported by a Special Grant from the Roche Foundation for Anemia Research (RoFAR) awarded to Nicoletta Eliopoulos. We thank Jacques Galipeau (Emory University) for valuable feedback. The authors declared no conflict of interest.
REFERENCES
- Brooke G, Cook M, Blair C, Han R, Heazlewood C, Jones B.et al. (2007Therapeutic applications of mesenchymal stromal cells Semin Cell Dev Biol 18846–858. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagrinati C, Ronconi E, Lazzeri E, Lasagni L., and, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14:277–285. doi: 10.1016/j.molmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Bi B, Schmitt R, Israilova M, Nishio H., and, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- Crisostomo PR, Markel TA, Wang Y., and, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143:577–581. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L.et al. (2007Insulin-like growth factor-1 sustains stem cell mediated renal repair J Am Soc Nephrol 182921–2928. [DOI] [PubMed] [Google Scholar]
- Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS., and, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD.et al. (1999Multilineage potential of adult human mesenchymal stem cells Science 284143–147. [DOI] [PubMed] [Google Scholar]
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C., and, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- Tögel F, Weiss K, Yang Y, Hu Z, Zhang P., and, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM., and, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041. [PubMed] [Google Scholar]
- Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M.et al. (2004Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure J Am Soc Nephrol 151794–1804. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F., and, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MJ. New insights into erythropoietin and epoetin alfa: mechanisms of action, target tissues, and clinical applications. Oncologist. 2003;8 Suppl 3:18–29. doi: 10.1634/theoncologist.8-suppl_3-18. [DOI] [PubMed] [Google Scholar]
- Westenfelder C. Unexpected renal actions of erythropoietin. Exp Nephrol. 2002;10:294–298. doi: 10.1159/000065304. [DOI] [PubMed] [Google Scholar]
- Bagnis C, Beaufils H, Jacquiaud C, Adabra Y, Jouanneau C, Le Nahour G.et al. (2001Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat Nephrol Dial Transplant 16932–938. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH., and, Fliser D. Erythropoietin and renoprotection. Curr Opin Nephrol Hypertens. 2009;18:15–20. doi: 10.1097/MNH.0b013e32831a9dde. [DOI] [PubMed] [Google Scholar]
- Fliser D., and, Haller H. Erythropoietin and treatment of non-anemic conditions–cardiovascular protection. Semin Hematol. 2007;44:212–217. doi: 10.1053/j.seminhematol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z., and, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006;69:1806–1813. doi: 10.1038/sj.ki.5000356. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Zhou XJ., and, Liao SY. Erythropoietin enhances recovery from cisplatin-induced acute renal failure. Am J Physiol. 1994;266 3 Pt 2:F360–F366. doi: 10.1152/ajprenal.1994.266.3.F360. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Patil S, Mackay A, Nelson M, Buyaner D, Hardy W.et al. (2001Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo Hum Gene Ther 121527–1541. [DOI] [PubMed] [Google Scholar]
- Daga A, Muraglia A, Quarto R, Cancedda R., and, Corte G. Enhanced engraftment of EPO-transduced human bone marrow stromal cells transplanted in a 3D matrix in non-conditioned NOD/SCID mice. Gene Ther. 2002;9:915–921. doi: 10.1038/sj.gt.3301727. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Al-Khaldi A, Crosato M, Lachapelle K., and, Galipeau J. A neovascularized organoid derived from retrovirally engineered bone marrow stroma leads to prolonged in vivo systemic delivery of erythropoietin in nonmyeloablated, immunocompetent mice. Gene Ther. 2003;10:478–489. doi: 10.1038/sj.gt.3301919. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Gagnon RF, Francois M., and, Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol. 2006;17:1576–1584. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Lejeune L, Martineau D., and, Galipeau J. Human-compatible collagen matrix for prolonged and reversible systemic delivery of erythropoietin in mice from gene-modified marrow stromal cells. Mol Ther. 2004;10:741–748. doi: 10.1016/j.ymthe.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chen CP, Lee YJ, Chiu ST, Shyu WC, Lee MY, Huang SP.et al. (2006The application of stem cells in the treatment of ischemic diseases Histol Histopathol 211209–1216. [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F.et al. (2007Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury Kidney Int 72430–441. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Hauser PV, Carvalhosa R., and, Camussi G. Contribution of stem cells to kidney repair. Curr Stem Cell Res Ther. 2009;4:2–8. doi: 10.2174/157488809787169129. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, de Groot K, Haller H., and, Fliser D. Erythropoietin: is it more than correcting anaemia. Nephrol Dial Transplant. 2004;19:20–22. doi: 10.1093/ndt/gfg455. [DOI] [PubMed] [Google Scholar]
- Kuwana H, Terada Y, Kobayashi T, Okado T, Penninger JM, Irie-Sasaki J.et al. (2008The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity Kidney Int 73430–445. [DOI] [PubMed] [Google Scholar]
- Tögel F, Cohen A, Zhang P, Yang Y, Hu Z., and, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane S, Ragolia L, Palaia T, Johnson B, Elzein H., and, Maesaka JK. Cytoprotection by darbepoetin/epoetin alfa in pig tubular and mouse mesangial cells. Kidney Int. 2004;65:452–458. doi: 10.1111/j.1523-1755.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M.et al. (2004Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion J Am Soc Nephrol 152115–2124. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Stagg J, Lejeune L, Pommey S., and, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- Arany I., and, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Pabla N., and, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Heydorn K, Rietz B, Kraruphansen A. Distribution of platinum in patients treated with cisplatin determined by radiochemical neutron activation analysis. J Trace Elem Exp Med. 1998;11:37–43. [Google Scholar]
- Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC.et al. (2008Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells Gastroenterology 1342111–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD.et al. (2006Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice Proc Natl Acad Sci USA 10317438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AC., and, Farrington K. Management of acute renal failure. Postgrad Med J. 2006;82:106–116. doi: 10.1136/pgmj.2005.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golab F, Kadkhodaee M, Zahmatkesh M, Hedayati M, Arab H, Schuster R.et al. (2009Ischemic and non-ischemic acute kidney injury cause hepatic damage Kidney Int 75783–792. [DOI] [PubMed] [Google Scholar]
- Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM.et al. (2007Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy Am J Physiol Renal Physiol 293F30–F40. [DOI] [PubMed] [Google Scholar]
- Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol. 2006;26:105–113. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Shen B, Chao L., and, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807–819. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch A, Doty J, Flores J, Swenson L, Toegel FE, Reiss GR.et al. (2008Initial report on a phase I clinical trial: Prevention and treatment of post-operative acute kidney injury with allogeneic mesenchymal stem cells in patients who require on-pump cardiac surgery Cell Ther Transplant 131–35. [Google Scholar]
- Meirelles Lda S., and, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Kopen G, Isaacson RL., and, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]