Abstract
Hepatic adeno-associated virus serotype 2 (AAV2)-mediated gene transfer failed to achieve sustained transgene product expression in human subjects. We formulated the hypothesis that rejection of AAV-transduced hepatocytes is caused by AAV capsid-specific CD8+ T cells that become reactivated upon gene transfer. Although this hypothesis was compatible with clinical data, which showed a rise in circulating AAV capsid-specific T cells following injection of AAV vectors, it did not explain that AAV vectors achieved long-term transgene expression in rhesus macaques, which are naturally infected with AAV serotypes closely related to those of humans. To address this apparent contradiction, we tested human and rhesus macaque samples for AAV capsid-specific T cells by intracellular cytokine staining combined with staining for T-cell subset and differentiation markers. This highly sensitive method, which could provide a tool to monitor adverse T-cell responses in gene transfer trials, showed that AAV capsid-specific CD8+ and CD4+ T cells can be detected in blood of naturally infected humans and rhesus macaques. They are present at higher frequencies in rhesus macaques. Furthermore, T cells from humans and rhesus macaques exhibit striking differences in their differentiation status and in their functions, which may explain the disparate duration of AAV-mediated gene transfer in these two species.
Introduction
In a phase 1 clinical trial,1 human subjects with severe hemophilia B were infused with recombinant adeno-associated virus (AAV) vectors derived from the human serotype 2 (AAV2) expressing factor IX (AAV2-F.IX) for intrahepatic expression. At the highest dose of vector, one of the patients developed therapeutic levels of F.IX by week 2, but by 4 weeks after vector infusion, levels of F.IX started to decrease and within a few weeks returned to pregene therapy levels. At the time when F.IX levels started to decrease, the patient showed an asymptomatic increase in transaminases, which eventually resolved after F.IX had decreased to baseline.1 The trial was continued with a reduced dose of vector. The next patient did not develop detectable levels of F.IX upon gene transfer. Nevertheless, he also presented with transaminitis after gene transfer with a time course relative to therapy identical to that seen in the other patient. In the 2nd patient, T-cell responses to the capsid antigen of the AAV2 vector and the transgene product were assessed by ELISpot assays for interferon-γ (IFN-γ) before and after gene transfer.2 He had no detectable AAV2 capsid-specific T cells in blood before gene transfer. Such a response developed after gene transfer and then eventually subsided. Responses to F.IX could not be detected before or after gene transfer. Overall these findings were compatible with the hypothesis that humans due to natural infections carry pre-existing T cells to antigens of AAV capsid, which are reactivated upon AAV-mediated gene transfer and eliminate the transduced cells.2 The clinical results were in contrast to preclinical data generated in a number of species including mice,3,4,5 dogs,3,6,7 and nonhuman primates (NHPs)8,9,10 where the AAV2 vector had resulted in sustained gene transfer and in correction of disease in animal models of hemophilia B. It was postulated that rejection was not encountered in preclinical models with species that do not become infected with primate AAVs such as mice or canines and thus have no immunological memory to antigens of AAV capsid. Nevertheless, attempts to mimic the clinical findings in rodents exposed to antigens of AAV capsid prior to AAV gene transfer have failed thus far.11,12 Furthermore, the hypothesis of immune-mediated destruction of AAV-transduced hepatocytes in individuals with immunological memory to AAV could not explain why sustained AAV-mediated gene transfer could be achieved in rhesus macaques, which are naturally infected with AAVs that show sufficient homology with strains that infect humans to allow for cross-recognition by T cells.2 As was shown previously, AAVs appear to persist more commonly and at higher copy numbers in NHPs such as rhesus macaques than in humans.13,14 These studies also showed evidence of cross-species AAV transmission with an identical clade being isolated from humans and rhesus macaques.14
Here, we tested human and rhesus macaque samples for AAV capsid-specific T cells. Specifically we tested for T cells to capsid antigens of AAV serotypes that have been isolated from either species, i.e., AAV215 and AAV8,13 respectively, although it should be pointed out that these two viruses share 83% homology at the nucleotide level and therefore T-cell epitopes.16 Our results show that both AAV capsid-specific CD8+ and CD4+ T cells can readily be detected in blood of both species but are present at higher frequencies in rhesus macaques. Our results also identify differences in AAV capsid-specific T-cell differentiation status and functions between humans and NHPs, which may contribute to the disparate duration of AAV-mediated gene transfer in these two species.
Results
Magnitude and differentiation status of human AAV capsid-specific T-cell responses
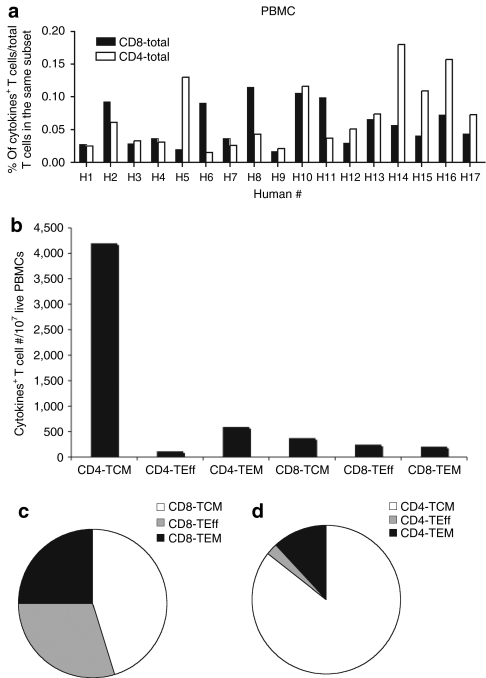
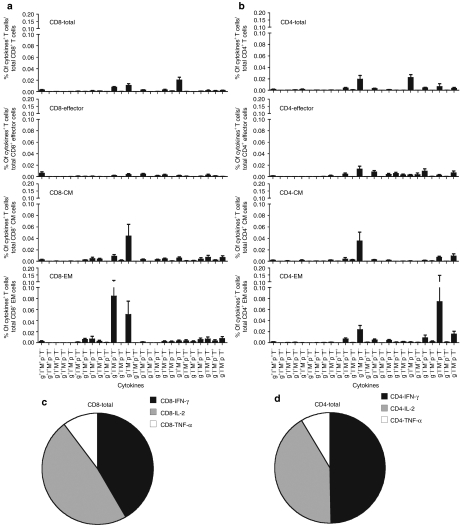
Frequencies of AAV capsid-specific T cells isolated from peripheral blood mononuclear cells (PBMCs) of 17 healthy adults from the Philadelphia area were determined by multicolor staining with antibodies to differentiation markers and cell functions including IFN-γ, interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), perforin and macrophage inflammatory protein-1α (MIP-1α) followed by flow cytometric analyses (Figure 1). Of the human subjects 8 out of 17 had detectable AAV capsid-specific CD8+ T cells with frequencies of above 0.05% in blood. The sum of frequencies of CD8+ T cells producing any of the possible combinations of factors was calculated (Figure 2a). To determine subset distribution the sum of frequencies was then normalized to numbers of cells present within 107 live PBMCs (Figure 2b). Using numbers of cells as a read out showed that approximately half of the AAV capsid-specific CD8+ T cells belonged to the central memory T cells (TCM) subset while the more activated effector T cells (TEff) and effector memory T cells (TEM) cells represented about a quarter of AAV capsid-specific CD8+ T cells each (Figure 2c).
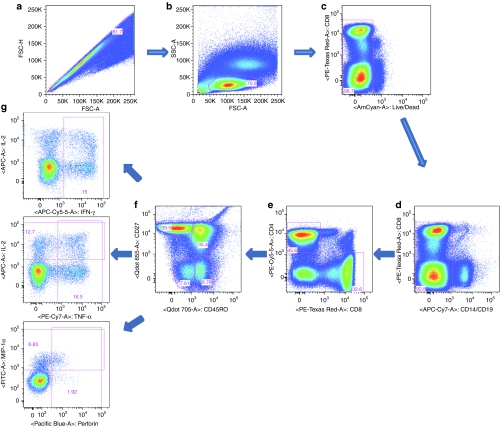
Figure 1.
Gating for the human cells. Cells were first gated onto (a) single cells and then on (b) lymphocytes and (c) AmCyan negative live cells. (d) APC-Cy7 was used as a dump gate for CD14 and CD19. (e) Cells were then gated onto CD8+ or CD4+ cells, which were then gated further according to (f) expression of CD45RO and CD27. (g) CD45ROhiCD27hi cells (central memory cells, TCM), CD45RO hiCD27low cells (effector memory cells, TEM) and CD45RO lowCD27low cells (effector cells, TEff) were then gated onto interferon-γ (IFN-γ), or interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α), perforin and macrophage inflammatory protein-1α (MIP-1α) as shown.
Figure 2.
Frequencies and cell numbers of adeno-associated virus serotype 2 (AAV2) capsid-specific CD8+ or CD4+ peripheral blood mononuclear cells (PBMCs) in humans. Lymphocytes isolated from 17 human blood samples were tested by intracellular cytokine staining for the production of interferon-γ, interleukin-2, tumor necrosis factor-α, perforin, and macrophage inflammatory protein-1α upon stimulation with the AAV2 capsid peptide pool. (a) The sum of the responses in total CD8+ and CD4+ T cells in different people are shown. (b) The numbers of AAV2 capsid-specific cells of each subset per 107 live PBMCs are shown. The average relative distributions of the (c) AAV2 capsid-specific different CD8+ and (d) CD4+ T-cell subsets were calculated. Background responses (responses without peptides) were subtracted prior to plotting of responses. TCM, central memory cells; TEff, effector cells; TEM, effector memory cells.
AAV capsid-specific CD4+ T cells could be detected in 9 of the 17 samples (Figure 2a). Cell frequencies were normalized and numbers of specific cells with 107 live PBMCs were determined (Figure 2b). The AAV capsid-specific CD4+ T-cell response in humans was markedly dominated by TCM cells, which contributed over 80% of AAV capsid-specific CD4+ T cells; the rest mainly belonged to the TEM subset. Numbers of highly activated CD4+ TEff cells were negligible (Figure 2d).
Magnitude and differentiation status of NHP AAV capsid-specific T-cell responses
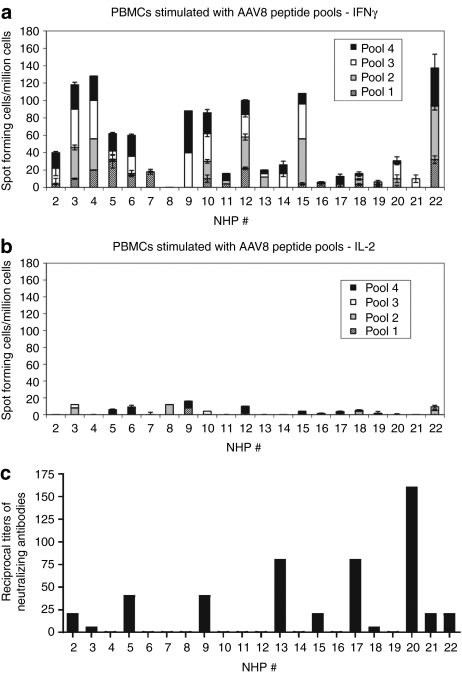
T-cell responses to AAV8 capsid were first tested from PBMCs of 21 young rhesus macaques using ELISpot assays for both IFN-γ (Figure 3a) and IL-2 (Figure 3b), a method that had been used to assess T-cell responses to AAV gene transfer in humans.2 In addition, plasma from the same set of NHPs were analyzed for neutralizing antibodies to AAV8 (Figure 3c). Eleven animals had neutralizing antibodies to AAV8 and nine animals had circulating T cells that produced IFN-γ upon in vitro stimulation with AAV8 peptide pools with a cut-off for positive responses of 55 spots/106 PBMCs. None of the samples were positive for T cells producing IL-2 to the AAV8 peptide pools. IFN-γ responses were low and there was no correlation between magnitude of T-cell responses and neutralizing antibody titers.
Figure 3.
ELISPOTs for interferon-γ (IFN-γ) and interleukin-2 (IL-2) responses and neutralizing antibodies to adeno-associated virus serotype 8 (AAV8) in nonhuman primates (NHPs). Freshly isolated peripheral blood mononuclear cells (PBMCs) from naive rhesus macaques were test by an ELISpot assay for IFN-γ and IL-2 secretion upon stimulation with four different peptide pools containing overlapping peptides of the AAV8 capsid. The graphs showed responses of 21 animals. Responses to medium (negative control) were subtracted from responses to each pool. (a,b) Our cut-off for positive responses was 55 spots/106 PBMCs. Error bar showed the SD of two different wells. (c) Plasmas from these rhesus macaques were also collected and titers of neutralizing antibodies to AAV8 were tested.
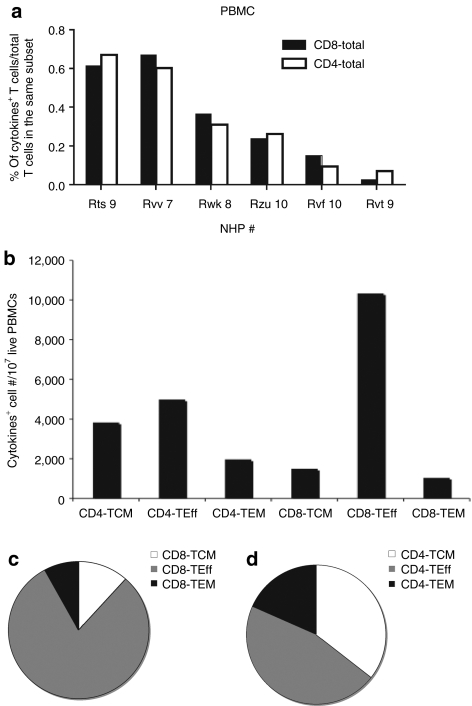
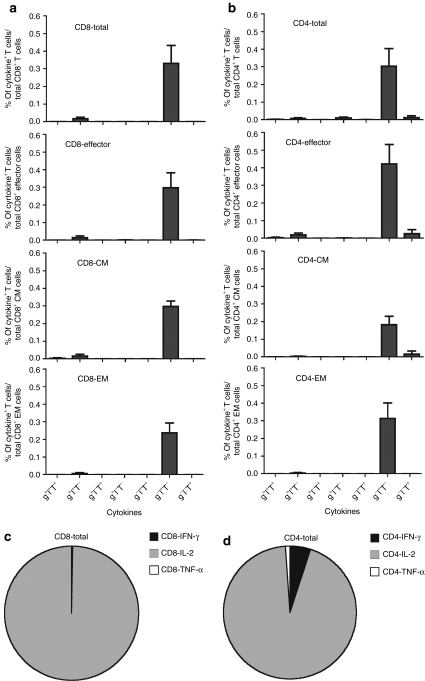
We tested PBMCs from six additional rhesus macaques for T-cell responses to AAV8 capsid by intracellular cytokine staining (ICS) and multicolor flow cytometry for production of IL-2, IFN-γ and TNF-α. AAV8 capsid-specific CD8+ T cells could be detected in five out of six samples (Figure 4a). The AAV capsid-specific CD8+ T-cell response was strongly dominated by TEff cells, which contributed more than 80% of the responding cells. TCM and TEM CD8+ cells could be detected at approximately equal numbers (Figure 4b,c). AAV capsid-specific CD4+ T cells could be detected in all samples (Figure 4a); numbers of specific CD4+ TCM and TEff cells were similar while those of TEM cells were slightly lower (Figure 4b,d).
Figure 4.
Frequencies and cell numbers of adeno-associated virus serotype 8 (AAV8) capsid-specific CD8+ or CD4+ peripheral blood mononuclear cells (PBMCs) in nonhuman primates (NHPs). PBMCs from six naive rhesus macaques were tested by intracellular cytokine staining for production of interferon-γ, interleukin-2 and tumor necrosis factor-α upon stimulation with the AAV8 capsid peptide pool. (a) The sum of the responses in total CD8+ and CD4+ T cells in different NHP samples are shown. (b) The numbers of AAV8 capsid-specific cells of each subset per 107 live PBMCs are shown. The average relative distributions of the (c) AAV8 capsid-specific different CD8+ and (d) CD4+ T-cell subsets were calculated. Background responses (responses without peptides) were subtracted before plotting of responses. TCM, central memory cells; TEff, effector cells; TEM, effector memory cells.
Functionality of human T cells
Assessing five different functions for each T-cell subset allows in theory for the detection of 31 populations in each of the six T-cell subsets that produce either only one factor or different combinations of two, three, four or all five factors. Perforin is commonly present in human T cells that are not stimulated by antigen in vitro and has to be produced in combination with another factor to allow for identification of an antigen-responsive population. T cells that upon stimulation with AAV peptides exhibited production of perforin only were thus not taken into account in the analyses (hence 31 and not 32 populations). Results shown in the figures only include functions exhibited by any of the AAV-specific T-cell subsets under interrogation.
In blood AAV capsid-specific CD8+ TEff were rare and the largest population that could be detected produced multiple factors, i.e., IFN-γ (g), IL-2 (l), and perforin (p). Those producing IFN-γ only dominated CD8+ TCM cells, although smaller populations producing a variety of factors or combinations of factors could also be detected. The response profile was similar for AAV capsid-specific CD8+ TEM cells for which a second dominant peak of cells producing IFN-γ together with perforin could be detected (Figure 5a). Upon normalization for CD8+ T cells producing IFN-γ, IL-2 or TNF-α, numbers and percentages of cells producing IFN-γ or IL-2 were similar while only ~10% produced TNF-α (Figure 5c).
Figure 5.
Functional properties of CD8+ or CD4+ T cells from human blood. The same 17 human peripheral blood mononuclear cells (PBMCs) shown in Figure 2 were tested for secretion of interferon-γ (IFN-γ) (g), interleukin-2 (IL-2) (l), tumor necrosis factor-α (TNF-α) (T), perforin (p) and macrophage inflammatory protein-1α (MIP-1α) (M) by intracellular cytokine staining of CD8+ or CD4+ T cells that had been stimulated with the adeno-associated virus serotype 2 (AAV2) peptide pool. (a,b)The bars show the frequencies of different subsets of cells that stained for the different function either alone or in combinations. Frequencies <0.005% over all cells of the subset and frequencies of T cells producing perforin only are not shown. The error bar shows the standard error of mean of 17 human PBMCs. (c,d)The average relative distribution of IFN-γ, IL-2 or TNF-α secreting AAV2 capsid-specific CD8+ and CD4+ T cells are shown. Background responses (responses without peptides) were subtracted.
CD4+ TEff in blood produced a wide variety of combinations of factors. TCM cells mainly produced IFN-γ. Most CD4+ TEM cells were positive for single functions, i.e., IFN-γ, MIP-1α (M) or TNF-α (T) (Figure 5b). Upon normalization to live PBMCs about half of the CD4+ T cells produced IFN-γ alone or with other function, 40% produced IL-2 and the rest produced TNF-α (Figure 5d).
Functionality of T cells from NHPs
AAV capsid-specific T cells from NHPs were analyzed for only three functions, i.e., IFN-γ (g), IL-2 (l), and TNF-α (T) (Figure 6a,b), because suitable antibodies were not available for perforin and MIP-1α. Cells producing only IL-2 dominated responses of all CD8+ and CD4+ T-cell subsets (Figure 6c,d), which contrasted to the responses of human PBMCs. A summary of the three functions, i.e., IFN-γ, IL-2 and TNF-α for different CD8+ T-cell subsets from human and NHP samples is shown in Supplementary Table S1.
Figure 6.
Functional properties of CD8+ or CD4+ T cells from nonhuman primates (NHPs). The same NHP peripheral blood mononuclear cells (PBMCs) shown in Figure 4 were tested for secretion of interferon-γ (IFN-γ) (g), interleukin-2 (IL-2) (l) and tumor necrosis factor-α (TNF-α) (T) by intracellular cytokine staining of CD8+ or CD4+ T cells that had been stimulated with adeno-associated virus serotype 8 (AAV8) peptide pool. (a,b) The bars showed the frequencies of different subsets of cells that secreted one, two or three cytokines alone or in combinations. The error bar showed the standard error of mean of six NHPs. (c,d) The average relative distribution of IFN-γ, IL-2 or TNF-α secreting AAV8 capsid-specific CD8+ and CD4+ T cells are shown. Background responses (responses without peptides) were subtracted.
Phenotypes of human and NHP AAV capsid-specific T cells
CD8+ and CD4+ T cells from human and NHPs that secreted IFN-γ and IL-2, the two most common functions observed in human and NHP AAV capsid-specific T cells, were analyzed for expression of PD1, an immunoinhibitory marker that is highly expressed on recently activated as well as on so-called exhausted T cells. In addition, cells were analyzed for intracellular expression of Ki-67, a molecule that is associated with cell proliferation (Figure 7a). Noncytokine secreting cells of the same subset as well as naive cells (CD27hiCD45ROlow) were analyzed in parallel. Levels of expression of these markers on AAV capsid-specific CD4+ or CD8+ T cells were comparable to those on non-AAV-specific T cells from the same subsets in both humans and NHPs and clearly distinct from those of naive T cells, with the exception that PD1 was downregulated on AAV capsid-specific CD8+ TEM and TEff cells from NHPs, while Ki67 was upregulated in the AAV capsid-specific CD8+ TEff and CD4+ TEM cells. Nevertheless, differences were subtle (Figure 7b–e). T cells were in addition stained for antibodies to CD160, another immunoinhibitory molecule and CD57, a marker of terminal T-cell differentiation. Antibodies that react with these molecules from NHPs are not available and this analysis was therefore restricted to human samples. AAV capsid-specific CD8+ T cells of the TCM and TEM subsets expressed neither CD160 nor CD57, while ~59% of AAV capsid-specific CD8+ TEff cells and ~69% of other CD8+ TEff cells expressed CD57. CD4+ TEff cells expressed elevated levels of CD57 with frequencies of 50% in AAV capsid-specific T cells and 65% in cells with other specificities. CD160 expression was only increased on a fraction of CD8+ TEff cells (35% for AAV capsid-specific CD8+ TEff cells versus 46% of non-AAV capsid-specific CD8+ TEff cells, data not shown). Overall phenotypic analyses did not reveal phenotypic differences between AAV capsid-specific T cells compared to T cells directed to other antigens in humans and NHPs.
Figure 7.
Phenotypes of adeno-associated virus (AAV) capsid-specific different CD8+ and CD4+ T-cell subsets. The average expression levels of PD1, Ki67 were determined on interferon-γ (IFN-γ) and/or interleukin-2 (IL-2) secreting (TCM+,TEM+,TEff+) and IFN-γ and IL-2 double negative (TCM−,TEM−,TEff−) CD8+ and CD4+ T-cell subsets in both nonhuman primate (NHP) and human peripheral blood mononuclear cells (PBMCs). (a) The expression levels in naive cell population are shown for comparison. (b–e) The geometric mean fluorescence intensity (GMFI) of PD1, Ki67 expression in different cell subsets are shown. The error bars show the SD of three to four different samples which had sufficient numbers of cells for the analysis in each group.
Discussion
Experiments described in this manuscript were designed to address two issues. First, we wanted to develop a method that is suitable to monitor human CD4+ and CD8+ T-cell responses to AAV capsid antigens to aid AAV gene transfer trials. Previous trials tested for T cells using mainly ELISpot assays. Such assays only detected AAV capsid-specific T-cell responses in blood of 2 out of 46 or in spleens of 1 out of 28 human subjects prior to gene transfer although most had antibodies to AAV indicating prior infections. Following gene transfer AAV capsid-specific T cells could transiently be detected in blood of AAV vector recipients enrolled into different trials.2,17,18
Till recently, ELISpot assays were viewed as being more sensitive than ICS to detect memory T cells present at low frequencies. Nevertheless, advances in flow cytometry that now allow to detect up to 18 different fluorochromes on individual cells, exclusion of dead cells, testing for subsets by staining for differentiation markers, and use of antibodies for multiple functions have reduced the background of ICS and increased its sensitivity to that of or above ELISpot assays.19,20 Results obtained by ICS are clearly more informative than those gathered by ELISpots as the former but not the latter distinguishes between responses by CD4+ or CD8+ T cells and allows for identification of different T-cell subsets. Furthermore ICS can monitor T-cell samples simultaneously for production of multiple cytokines, chemokines or other factors that are induced upon antigenic stimulation while ELISpot assays only measure one function. Although it is technically feasible to use ELISpot assays for an array of functions, the number of cells required for multiple parallel testing would be prohibitive. Last but not least, as we reported previously,21 ELISpot assays may not be optimal to test for terminally differentiated T cells as those may die during the 18–24-hour incubation period of the assay. Our data show that multicolor ICS followed by flow cytometric analysis may be better suited than ELISpot assays to monitor immune responses in AAV gene transfer trials.
The ICS assay we used here for human samples measures five functions produced by different T-cell subsets. Samples from NHPs were only tested for three functions, as antibodies for the other two functions, i.e., MIP-1α and perforin, were not available. Frequencies of AAV-specific T cells in NHPs are thus presumably underestimated compared to those of humans. Notwithstanding, NHPs, which do not reject AAV upon gene transfer, have on average 4–5 times higher frequencies and numbers of AAV capsid-specific T cells circulating in blood compared to humans, which appear to eliminate AAV-transduced cells. In addition to differences in frequencies, there were also marked differences in subset distribution of AAV capsid-specific CD4+ and CD8+ T cells. The human response was dominated by CD4+ T cells, which mainly belonged to the TCM subset while in NHPs TEff CD4+ T cells were more common followed by TCM cells. Approximately 50% of AAV capsid-specific CD8+ T cells in humans belonged to the TCM subset, while in NHPs more than 80% were activated CD8+ TEff cells. Differences in memory cell distribution may reflect differences in the AAVs lifecycle in the two species. As was shown previously, AAVs appear to persist more commonly and at higher copy numbers in NHPs such as rhesus macaques than in humans.13,14 These studies also showed evidence of cross-species AAV transmission with an identical clade being isolated from humans and rhesus macaques.14 AAV most commonly infects together with adenoviruses. There is mounting evidence that infections with adenoviruses, which provide helper functions to AAVs that as dependoviruses cannot replicate by themselves, are more pervasive in NHPs than in humans.22 Most healthy NHPs shed adenoviruses while this is only observed during or shortly after acute infections of humans.23,24 Under the assumption that in simians helper functions to AAVs are provided by adenoviruses and that AAVs co-persist with adenoviruses in the same cells, this may indicate that reactivation of endogenous AAVs might also be more common in NHPs. This in turn could affect not only frequencies of circulating AAV-specific T cells but in addition their functionality, as continued exposure to high levels of antigen, as was shown upon chronic infections of mice with certain strains of lymphocytic choriomeningitis virus25 or of humans with human immunodeficiency26 or hepatitis virus,27 can lead to functional impairment of CD8+ T cells, which due to exhaustion or terminal differentiation could lose the ability to expand upon antigenic stimulation or to eliminate antigen presenting target cells.
AAV capsid-specific T-cell responses in humans exhibited a number of functions while those of NHPs were functionally more restricted. Comparing functions of AAV capsid-specific T cells between the two species showed that human CD8+ and CD4+ T cells produced mainly IFN-γ or IL-2 alone or in combination with other factors such as MIP-1α or perforin in contrast to those from NHPs, which produced nearly exclusively IL-2. This was unexpected as in general IL-2 is more commonly produced upon reactivation of resting TCM cells while IFN-γ is produced by more activated TEff or TEM cells. In addition results from the ELISpot analyses for PBMCs from NHPs scored positive for T cells producing IFN-γ in response to antigen and negative for those producing IL-2 while which is in contrast to results from ICS again stressing that these two methods may not necessarily give concordant results as T cells, especially highly activated T cells, may die rapidly upon in vitro culture.21
AAV capsid-specific T cells from human and NHPs were further tested for some phenotypic markers that are typically used to assess T-cell functionality. Again the analysis was more comprehensive for human samples due to availability of antibodies to more markers. Levels of expression of PD-1 and Ki-67, which are used as indicators for T-cell exhaustion or for the T cells' proliferative potential, were very similar on AAV capsid-specific T cells and non-AAV capsid-specific antigen-experienced T cells in either species. In the same token, CD57, a marker for terminal T-cell differentiation, and CD160, which is also a marker of exhaustion, were similar on both populations in humans. Neither of these stains thus supported the notion that AAV capsid-specific T cells were impaired or phenotypically distinct from other antigen-experienced T cells.
Overall, differences in sustained gene transfer by AAV vectors in humans and NHP, correlate well with the observed differences in T cells' differentiation status and functionality. TCM cells, which are relatively more common in humans, proliferate copiously upon re-encounter of their antigens, unlike the more activated TEM or TEff cells. One would thus expect that upon AAV-mediated gene transfer, expansion of AAV capsid-specific TCM cells would in the end result in higher numbers of effector cells in humans than the more modest expansion of activated AAV capsid-specific T cells in NHPs. In addition, AAV capsid-specific T cells in humans produced IFN-γ, a cytokine that upregulates major histocompatibility complex (MHC) class I expression. MHC class I molecules are crucial for CD8+ T-cell recognition and previous studies showed that in absence of IFN-γ hepatic transfer of highly immunogenic adenovirus vectors results in sustained gene transfer.28 AAV capsid-specific CD8+ T cells from NHPs failed to produce IFN-γ when tested by ICS. We assume that this may allow for the escape of AAV-transduced cells in monkeys while in humans production of IFN-γ by AAV capsid-specific CD8+ T cells may lead to upregulation of MHC class I molecules on hepatocytes and their recognition and destruction by AAV capsid-specific CD8+ T cells. One could view this as a circular argument, as T cells require recognition of their target antigen such as peptides derived from the AAV capsid in association with MHC molecules to produce cytokines including IFN-γ. Nevertheless, the initial T-cell stimulation could be provided by cells other than hepatocytes present in the liver resulting in IFN-γ production, which would then trigger upregulation of molecules of the antigen presentation pathway in hepatocytes.
While our first goal, to develop a more sensitive method for monitoring of AAV-mediated human gene therapy trials was easily met by multicolor flow cytometry. Our second goal, to elucidate why AAV gene transfer readily achieves sustained transgene expression in mice and monkeys but only transient expression in humans touches the very complex and not yet fully understood pathways that govern CD8+ T-cell differentiation. Monkeys and humans develop AAV capsid-specific CD8+ T cells upon natural infections while such T cells can readily be induced in mice by their immunization with an appropriate viral vector.11 In mice, AAV capsid-specific memory CD8+ T cells can recognize their cognate antigen and mount a proliferate response29 but they fail to reduce liver-specific expression of the AAV vector's transgene. We speculate that this reflects that in mice AAV-transduced hepatocytes fail to express sufficient densities of MHC class I–AAV capsid epitope complexes to allow for their lysis through CD8+ T cells. We assume that this pathway ultimately contributes to sustained transgene product expression upon hepatic AAV transfer into NHPs. The high pervasiveness of Ad viruses in this species may allow for concomitant synthesis of AAV proteins. Constant production of AAV capsid antigens would result in recurrent T-cell activation and initiate a differentiation program characterized by reduced functionality, i.e., a loss of proliferative potential and reduced production of cytokine and lytic enzymes. In contrast, in humans, many of the AAV capsid-specific CD8+ T cells differentiate into memory cells, which provide indirect evidence that production of AAV antigens might be of more transient duration. Upon re-encounter of their antigen such memory cells can proliferate vigorously and become fully competent effector cells able to exhibit a full spectrum of functions including secretion of IFN-γ, which increases molecules' need for optimal antigen presentation, perforin and granzyme, which are able to lyse cells expressing the CD8+ T cells' antigen.
While hepatic gene transfer of single-stranded AAV2 vector for hF.IX did not result in sustained transgene expression, more recent studies with a self-complementary AAV8 vector yielded more promising results.30 Whether this reflects differences in T-cell responses, vector preparations or differential processing of capsids from serologically distinct AAVs within hepatocytes remains to be investigated.
Materials and Methods
Human samples. Human bloods were drawn from 18- to 55-year-old, normal healthy humans from the Philadelphia area.
NHPs samples. Samples from 2- to 3-year-old, healthy and simian immunodeficiency virus-uninfected Indian origin Macaca mulatta that had not yet been enrolled into any experiments were used.
Isolation and preservation of lymphocytes. PBMCs were isolated as described.31 They were tested immediately after isolation or frozen in 90% fetal bovine serum and 10% dimethyl sulfoxide (Sigma, St Louis, MO) at −80 °C.
ELISpot assays. The ELISpot assays for IFN-γ and IL-2 were conducted as described.21 Experimental wells were incubated with pools of AAV capsid peptides each at a final concentration of 1 µg/ml, negative control wells were incubated with medium and positive control wells were incubated with phorbol 12-myristate 13-acetate (PMA) (final concentration 0.05 µg/ml) and ionomycin (final concentration 1 µg/ml). Based on previous publications,32,33 samples were scored positive if they had at least 55 spots per 106 cells upon antigenic stimulation, if numbers of spots/well in presence of the peptide pools were >3 times higher than number of spots/well that were cultured without peptides and if numbers of spots in experimental wells were at least 3 SD above those in control wells.
ICS
Human samples: The function of AAV capsid-specific CD8+ T cells was assessed by ICS after stimulation with a AAV2 capsid peptide pool. All peptides were used at a final concentration of 2.5 µg of each peptide per ml. Frozen cells were thawed and incubated in RPMI 1640 medium supplemented with 10% fetal bovine serum overnight at 37 °C in 10% CO2. Then cells were washed with Hank's Balanced Salt Solution (HBSS) supplemented with 2 units/ml DNase I, resuspended with RPMI 1640 medium and stimulated for 6 hours in the presence of anti-CD28 (clone CD28.2), anti-CD49d (clone 9F10), and Brefeldin A.31 Cells were stained with LIVE/DEAD Fixable Aqua dead cell staining kit-AmCyan (Invitrogen, Carlsbad, CA), anti-CD14-APC-Cy7 (clone TüK4; Invitrogen), anti-CD19-APC-Cy7 (clone SJ25-C1; Invitrogen), anti-CD8-PE-Texas Red (clone SFCI21Thy2D3; Beckman Coulter, Fullerton, CA), anti-CD4-PE Cy5.5 (clone S3.5; Invitrogen), anti-CD27-QD 655 (clone CLB-27/1; Invitrogen), and anti-CD45RO-QD 705 (UPenn, Philadelphia, PA) for 30 minutes at room temperature (RT) in the dark. After fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) for 20 minutes at RT, cells were then stained with anti-CD3-PerCP-Cy5.5 (clone SP34-2), anti-IFN-γ-APC-Cy5.5 (clone B27), anti-IL-2-APC (clone MQ1-17H12), anti-TNF-α-PE-Cy7 (clone MAb11), anti-Perforin-Pacific Blue (UPenn) and anti-MIP1α-FITC (clone 93342; R&D Systems, Minneapolis, MN) for 45 minutes at RT in dark. Cells were washed twice, fixed with BD Stabilizing Fixative (BD Biosciences), and then analyzed by FACS using LSRII (BD Biosciences) and DiVa software. Post-acquisition analyses were performed with FlowJo (TreeStar, Ashland, OR). Single color controls used CompBeads Anti-Mouse Ig, k (BD Biosciences). Unless otherwise noted, antibodies were purchased from BD (BD Biosciences). Samples were only scored positive if at least 0.05% of a given subpopulation stained for a cytokine or factor; this percentage for most samples required more than 10 positive events on the flow cytometry blots.
For the phenotypic analysis of AAV2 capsid-specific CD8+ and CD4+ T cells, cells were first stained with LIVE/DEAD Fixable Aqua dead cell staining kit-AmCyan, anti-CD14-APC-Cy7, anti-CD19-APC-Cy7, anti-CD8-PE-Texas Red, anti-CD4-PE Cy5.5, anti-CD27-QD 655, anti-CD45RO-QD 705, and anti-PD1-PE-Cy7 (clone EH12.2H7; BioLegend, San Diego, CA) for 30 minutes at RT in the dark. After fixation and permeabilization with Cytofix/Cytoperm for 20 minutes at RT, cells were then stained with anti-CD3-PerCP-Cy5.5, anti-IFN-γ-PE (clone B27), anti-IL-2-FITC (clone MQ1-17H12), anti-Ki67-APC-Cy5.5 (clone B56) for 45 minutes at RT in dark. The last three antibodies were purchased from BD (BD Biosciences).
Cells were gated as follows (Figure 1): Single cells (Figure 1a) were first gated onto lymphocytes (Figure 1b), and then gated onto the live cells (Figure 1c) (AmCyan as dead cell staining). APC-Cy7 used as a dump gate for CD14+ and CD19+ cells (Figure 1d). Live cells were then gated onto CD8+ cells or CD4+ cells (Figure 1e), which were then gated onto CD45RO and CD27 (Figure 1f). CD45RO hiCD27hi cells (TCM), CD45RO hiCD27low cells (TEM) and CD45ROlowCD27low cells (TEff). Subsets were then gated onto IFN-γ, or IL-2 and TNF-α, Perforin and MIP-1α (Figure 1g) which shows results for CD8+ T cells stimulated with polyclonal activators.
NHP samples: The function of AAV capsid-specific CD8+ T cells in NHPs was tested by ICS after stimulation with AAV8 capsid peptide pool using the similar methods as described above. Frozen cells were thawed and washed, then immediately used for the ICS. Cells were first stained with LIVE/DEAD Fixable Violet Dead Cell stain Kit-Pacific Blue (Invitrogen), anti-CD14-Pacific Blue (clone M5E2), anti-CD16-Pacific Blue (clone 3G8), anti-CD20-Pacific Blue (clone 2H7, AbD serotec, Oxford, UK), anti-CD8-APC-H7 (clone SK1), anti-CD4-Alexa700 (clone OKT4; eBioscience, San Diego, CA), anti-CD95-PE-Cy5 (clone DX2), and anti-CD28-PE-Texas Red (clone CD28.2; Beckman Coulter) for 30 minutes at 4 °C. Additionally, cells were stained with anti-CCR7-PE (clone 150503; R&D Systems). After fixation and permeabilization, cells were stained with anti-IFN-γ-APC (clone B27), anti-IL-2-FITC (clone MQ1-17H12), anti-TNF-α-PE-Cy7 (clone MAb11) and anti-CD3-PerCP-Cy5.5 for 30 minutes at 4 °C. Unless otherwise noted, antibodies were purchased from BD (BD Biosciences).
For the analysis of phenotypes of AAV8 capsid-specific CD8+ and CD4+ T cells, cells were first stained with LIVE/DEAD Fixable Violet Dead Cell stain Kit-Pacific Blue, anti-CD14-Pacific Blue, anti-CD16-Pacific Blue, anti-CD20-Pacific Blue, anti-CD8-APC-H7, anti-CD4-QD605 (clone OKT4; eBioscience), anti-CD95-PE-Cy5, and anti-CD28-PE-Texas Red, anti-PD1-PE-Cy7 for 30 minutes at 4 °C. Additionally, cells were stained with anti-CCR7-PE. After fixation and permeabilization, cells were stained with anti-IFN-γ-APC, anti-IL-2-FITC, anti-Ki67-APC-Cy5.5 and anti-CD3-PerCP-Cy5.5 for 30 minutes at 4 °C in dark.
Cells were gated as follows: single cells were first gated onto CD3 (PerCP-Cy5.5 and Pacific Blue used as a dump gate for CD14+, CD16+ and CD20+cells and dead cells). Live CD3+ cells were then gated onto CD8+ cells (or CD4+ cells), which were then gated onto CD95 and CD28. CD95hiCD28hi cells (effector cells) were gated onto IL-2 and IFN-γ or IL-2 and TNF-α. CD95intCD28low cells (memory cells) were gated onto CCR7. CCR7hi (TCM) and CCR7low (TEM) cells were then gated onto the different fluorochromes identifying antibodies to cytokines.
For both human and nonhuman primate samples cells incubated in medium without peptides were used as negative controls and cells incubated in PMA and ionomycin (final concentration 5 µg/ml) were used as positive controls.
Titration of AAV neutralizing antibodies. The AAV8 neutralizing antibody titers in plasma of NHP were tested as described before11 on 293 HEK cells infected with AAV8 vector expressing green fluorescent protein.
Statistics. Significance was determined by one-tailed Student's t-tests comparing results obtained from cytokines positive individual populations to those obtained from cytokine negative individual populations. Significance was set at P ≤ 0.05.
SUPPLEMENTARY MATERIAL Table S1. Percent of cytokine+ T cells/ total CD8+ T cells in the same subset (average ± SEM).
Acknowledgments
This work is supported by a grant from the National Institutes of Health (P01HL078810) and by institutional grants to the Wistar Institute including an NCI Cancer Core Grant (CA10815). K.A.H. is supported by the Howard Hughes Medical Institute. We would like to acknowledge Debbie Davis in Wistar Phlebotomy department for collecting blood samples for healthy human volunteers for our study.
Supplementary Material
Percent of cytokine+ T cells/ total CD8+ T cells in the same subset (average ± SEM).
REFERENCES
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y.et al. (2004Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1 Blood 10385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ.et al. (1997Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus Proc Natl Acad Sci USA 945804–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D.et al. (1997Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors Nat Genet 16270–276. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW.et al. (2005Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model Blood 1053458–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ, Tazelaar J, Xiao X, Nichols TC, Bellinger DA.et al. (1998Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia Gene Ther 540–49. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y.et al. (2005Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models Mol Ther 11875–888. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y.et al. (2007Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates Blood 1091414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toromanoff A, Chérel Y, Guilbaud M, Penaud-Budloo M, Snyder RO, Haskins ME.et al. (2008Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle Mol Ther 161291–1299. [DOI] [PubMed] [Google Scholar]
- Li H, Murphy SL, Giles-Davis W, Edmonson S, Xiang Z, Li Y.et al. (2007Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes Mol Ther 15792–800. [DOI] [PubMed] [Google Scholar]
- Wang L, Figueredo J, Calcedo R, Lin J., and, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ.et al. (2003Adeno-associated viruses undergo substantial evolution in primates during natural infections Proc Natl Acad Sci USA 1006081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X.et al. (2004Clades of adeno-associated viruses are widely disseminated in human tissues J Virol 786381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Srivastava A, Berns KI., and, Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983;33:135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Mingozzi F, Hui DJ, Chen H, Colosi P, Ertl HC.et al. (2005Identification of mouse AAV capsid-specific CD8+ T cell epitopes Mol Ther 121023–1033. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT.et al. (2009Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy Proc Natl Acad Sci USA 10616363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA.et al. (2009AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells Blood 1142077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara RR. Methods for quantitating antigen-specific T cell responses using functional assays in rhesus macaques. Methods Mol Biol. 2009;485:417–424. doi: 10.1007/978-1-59745-170-3_28. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E.et al. (2006Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry Nat Med 12972–977. [DOI] [PubMed] [Google Scholar]
- Reyes-Sandoval A, Fitzgerald JC, Grant R, Roy S, Xiang ZQ, Li Y.et al. (2004Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes J Virol 787392–7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Vandenberghe LH, Kryazhimskiy S, Grant R, Calcedo R, Yuan X.et al. (2009Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates PLoS Pathog 5e1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JP, Brandt CD, Wassermann FE, Hall CE, Spigland I, Kogon A.et al. (1969The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness Am J Epidemiol 8925–50. [DOI] [PubMed] [Google Scholar]
- Fox JP, Hall CE., and, Cooney MK. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977;105:362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R., and, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S.et al. (2006PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression Nature 443350–354. [DOI] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M.et al. (2007Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression J Virol 812545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xiang Z, Ertl HC., and, Wilson JM. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tuyishime S, Wu TL, Giles-Davis W, Zhou D, Xiao W.et al. (2011Adeno-associated virus vectors serotype 2 induce prolonged proliferation of capsid-specific CD8+ T cells in mice Mol Ther 19536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rosales C, McIntosh J, Riddell A, Rustagi P.et al. (2010Early clinical trial results following administration of a low dose of a novel self complementary adeno-associated viral vector encoding human factor ix in two subjects with severe haemophilia B Blood 116114 [Google Scholar]
- Tatsis N, Lasaro MO, Lin SW, Haut LH, Xiang ZQ, Zhou D.et al. (2009Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens J Immunol 1826587–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW.et al. (2007Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors J Virol 816594–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Merck V520-016 Study Group et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent of cytokine+ T cells/ total CD8+ T cells in the same subset (average ± SEM).