Abstract
We performed a cost-effectiveness analysis exploring the cost and benefits of LDLT using outcomes data from the Adult to Adult Living Donor Liver Transplantation Cohort Study (A2ALL). A multistage Markov decision analysis model was developed with treatment strategies including medical management only (strategy 1), waiting list with possible deceased donor liver transplant (strategy 2), and waiting list with possible LDLT or DDLT (strategy 3) over ten years. Decompensated cirrhosis with medical management offered 2.0 quality adjusted life years (QALY) survival while costing an average of $65,068, waiting list with possible DDLT offered 4.4 QALY survival and a mean cost $151,613, and waiting list with possible DDLT or LDLT offered 4.9 QALY survival and a mean cost $208,149. Strategy 2 had an incremental cost effectiveness ratio (ICER) of $35,976 over strategy 1 while strategy 3 produced an ICER of $106,788 over strategy 2. On average, strategy 3 cost $47,693 more per QALY than strategy 1. Both DDLT and LDLT are cost-effective compared to medical management of cirrhosis over our ten year study period. The addition of LDLT to a standard waiting list DDLT program is effective at improving recipient survival and preventing waiting list deaths but at a greater cost.
Keywords: living donor, liver transplantation, cost-effectiveness, economic evaluation, liver cirrhosis
Introduction
Living Donor Liver Transplantation (LDLT) is an alternative to traditional deceased donated transplants but there is little reliable outcomes data for adult-to-adult LDLT on which to base clinical decisions, patient counseling, or health policy. Of primary concern, the exposure to potential donor morbidity and mortality has not been evaluated systematically and case series reported in the literature vary in claims of donor morbidity in the immediate perioperative period from minimal (1) to 18% (2, 3). Although analyses of costs (4-9), outcomes (1, 2, 10-12), and quality of life (13-22) in relation to LDLT, have been published, few have evaluated the true cost-effectiveness of LDLT using a formal medical decision analysis (23-26). Previously published studies were also hindered by a lack of accurate data in regard to donor outcomes and incomplete accounting of donor morbidity.
In the year 2000, the U.S. National Institutes of Health organized a multicenter prospective cohort study of adult-to-adult living donor liver transplantation performed at several large transplant centers in the U.S. over a five year period (27, 28). This cohort study has been given the acronym A2ALL. When completed in 2009, the study will report all significant surgical and clinical outcomes for adult-to-adult LDLT candidates, recipients, and donors at nine major transplant centers in the U.S. This data assessment by the A2ALL consortium is the largest, most current, systematic report of the LDLT experience in the U.S. and includes outcomes from 819 transplant candidates, 1011 potential living donors, and 392 successful living donors. The aim of the current study is to evaluate the cost-effectiveness of adult-to-adult LDLT compared to DDLT using the most comprehensive and current data on variables such as donor morbidity and mortality, complication events, and quality-of-life estimates derived from A2ALL, the United Network for Organ Sharing (UNOS), and the latest published literature.
Methods
Decision Analysis Model
Cost-effectiveness analysis using Markov models has been described elsewhere (29). The model developed for this simulation considers six health states that can occur for patients with end-stage liver disease any time over a ten year time horizon including the pre-transplantation, perioperative, and post-transplant time periods. Figure 1 graphically displays the health states and transitions represented in the model. The model provides a conceptual framework for organizing the relationship of events, costs, and the utility of different outcomes for patients with end-stage liver disease.
Figure 1.
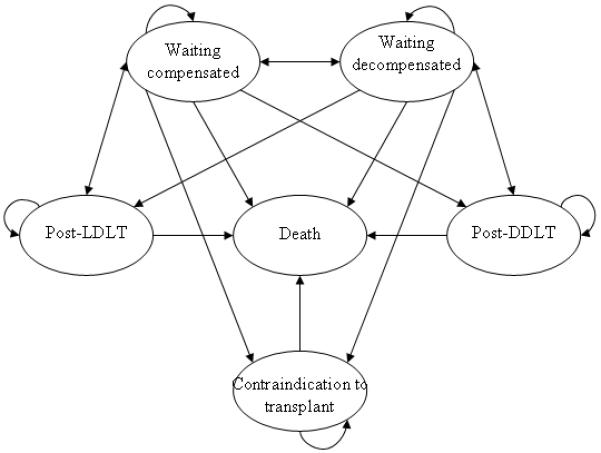
The basic health states of the Markov model.
Three separate treatment strategies are simulated in the model: 1) supportive care / medical management only for decompensated liver disease, 2) standard MELD-based wait-listing for DDLT, 3) DDLT wait-listing in addition to an evaluation of prospective donors for LDLT. A Monte Carlo simulation of the Markov decision model was used to estimate the distribution of events that would occur for 1,000 subjects (cohort members) over ten years. All event probabilities in the model were calculated using a one-month cycle length, which was selected as the most clinically pertinent time increment to simulate chronic liver disease and transplantation events. Half-cycle corrections were included (except for the first and last cycle) to account for mid-cycle cost and utility accumulation (29). In the model, members cycle through one of six basic health states as shown in Figure 1. By definition, every candidate entering into the model is referred for transplantation with a potential living donor available for assessment. Patients can remain “compensated” on the waiting list or can have various complications of cirrhosis including esophageal variceal bleeding, hepatocellular carcinoma, ascites flare, etc. When these acute events “resolve” they are returned to the theoretical “stable” waiting list after appropriate costs and utility tolls are assigned for the events. A similar construct of a stable health state, interrupted by complications is used in the post-transplant section of the model. To account for inflation, all costs are represented in adjusted year 2002 U.S. dollars. All utilities and costs are discounted by 3% yearly in order to account for the decreased present value of future costs and benefits (30). Data for Healthcare©, version 3.5 (TreeAge Software, Williamstown, MA) was used for modeling and SAS©, version 9.1 (Cary, NC) was used for statistical analysis and dataset manipulation. The University of Virginia Institutional Review Board for Human Research approved this study.
Event Probabilities
All major events in the pre-transplant, peri-transplant, and post-transplant treatment of cirrhosis were modeled. Table 1 lists the baseline estimates for event probabilities used in the model, the range of values used for sensitivity analysis, and the sources of the data. LDLT events, especially those related to donor complications were derived from the A2ALL dataset when available. A complete description of the A2ALL cohort patient population is published elsewhere (31). DDLT event probabilities were drawn from data supplied by the United Network for Organ Sharing (UNOS) transplant registry (32). The UNOS supplied database, the Standard Transplant Analysis and Research (STAR) dataset, was queried to calculate actual event rates and distributions that occurred during all adult liver transplants between January 1, 1999 and November 16, 2003. A complete population description of the STAR dataset is outlined at www.unos.org or www.ustransplant.org. Base-case probabilities were derived from exact calculations when available from the UNOS dataset or from A2ALL data. When exact calculations were not available, data were abstracted from the literature. In a few cases, mainly the complications of cirrhosis in the pre-transplant phase, enough data were available to calculate weighted averages for probabilities. When none of these choices were available, a point prevalence or percentage was used based on the published literature. Sensitivity margins attempted to encompass the span of the available literature on the event.
Table 1.
Event probabilities in the model.
| Base Case | Range Used in Sensitivity Analysis |
Sources | |
|---|---|---|---|
| Pre-transplantation | |||
|
| |||
| Yearly percent chance of developing symptomatic ascites on waitlist | 20.3% | 10-40% | (32, 72, 73) |
| Percent chance of receiving TIPS for ascites or bleeding | 20.5% | 5-40% | (32, 72, 74) |
| Percent chance of death related to each TIPS procedure | 3.8% | 1-8% | (72, 74) |
| Yearly percent chance of contracting SBP in patients with symptomatic ascites |
4.3% | 2-8% | (72, 75-78) |
| Percent chance of death related to each episode of SBP | 20.0% | 10-40% | (72, 75, 77-79) |
| Yearly percent chance of having encephalopathy requiring admission | 23.5% | 11-46% | (32, 80) |
| Percent chance of death from each episode of encephalopathy | 11.5% | 5-25% | (81, 82) |
| Yearly percent chance of developing HCC on waitlist | 10.3% | 5-20% | (32, 83) |
| Yearly percent chance of in patients with HCC of progression resulting in delisting (equivalent to 5.4% per 90-days on the waiting list) |
21.7% | 10-40% | (32, 84) |
| Yearly percent chance of variceal hemorrhage | 22.4% | 11-44% | (32, 85-87) |
| Percent chance of death from each episode of variceal hemorrhage | 14.2% | 7-28% | (86-88) |
| Yearly percent chance of remaining stable on waitlist, without complications | 18.7% | 9-36% | (89) |
| Yearly percent chance of death once delisted for a contraindication to transplant |
32.4% | 16-64% | (90-93) |
|
| |||
| Post DDLT | |||
|
| |||
| Percent chance of DDLT recipient death within 30 days of transplant | 4.1% | 2-9% | (32, 94, 95) |
| Percent chance of graft failure due to disease recurrence | 2.0% | 1-10% | (32) |
| Percent chance of successful retransplantation in patient with graft failure secondary to recurrent disease |
24.9% | 12-50% | (32) |
| Yearly percent chance of admission for non-biliary sepsis post DDLT | 9.9% | 4-20% | (96-98) |
| Percent chance of death from each sepsis event after DDLT | 14.1% | 7-28% | (96-98) |
| Percent chance of needing non-transplant, no-biliary reoperation more than 30 days after DDLT |
22.5% | 11-45% | (99) |
| Percent chance of death after each non-transplant reoperation after DDLT | 10.5% | 1-20% | (99) |
| Percent chance of a biliary complication after DDLT | 21% | 10-40% | (100-104) |
| Probability of death from biliary complications after DDLT | 4.7% | 2-9% | (100, 101, 104, 105) |
| Percent chance of receiving retransplantation in a recipient with biliary complications after DDLT |
4.8% | 2-9% | (102, 106, 107) |
| Percent chance of requiring non-transplant reoperation in a recipient with biliary complications after DDLT |
8.0% | 4-16% | (100, 102, 104, 108) |
| Percent chance of acute rejection severe enough for hospitalization after DDLT |
35.9% | 15-60% | (11, 109, 110) |
| Percent chance of death from an episode of acute rejection after DDLT | 0.2% | 0.1-0.5% | (110) |
| Percent chance of requiring retransplantation because of severe acute rejection after DDLT |
1.3% | 0.5-3% | (110) |
|
| |||
| Post LDLT | |||
|
| |||
| Donor | |||
|
| |||
| Probability of donor death after LDLT procedure | 0.28% | 0.01-1% | (51-55) |
| Probability of donor having major complications (Clavien grade 3 or 4) after LDLT procedure |
1.5% | 0.5-4% | A2ALL,(52-54, 56, 57, 111-116) |
| Probability of donor having minor or major complications (Clavien grade 2 or greater) after LDLT procedure |
13% | 6-25% | (52-54, 56, 57, 111- 116) |
| Recipient | |||
| Percent chance of LDLT recipient death within 30 days of transplant | 4.6% | 2-9% | A2ALL, (31, 32, 117) |
| Percent chance of graft failure due disease recurrence | 3.2% | 1.5-7% | A2ALL, (31, 118-122) |
| Percent chance of successful retransplantation in patient with graft failure secondary to recurrent disease |
21.4% | 12-50% | A2ALL, (32) |
| Yearly percent chance of recipient admission for non-biliary sepsis post LDLT | 9.9% | 4-20% | A2ALL, (31, 96-98) |
| Percent chance of recipient death from each sepsis event after LDLT | 14.5% | 7-28% | A2ALL, (31, 96-98) |
| Percent chance of recipient requiring non-transplant, non-biliary reoperation after LDLT |
22.5% | 11-45% | A2ALL, (31, 51, 112) |
| Percent chance of recipient death after each non-transplant reoperation after LDLT |
10.5% | 1-20% | (99) |
| Percent chance of a recipient biliary complication after LDLT | 37% | 15-60% | A2ALL, (31, 51, 104, 108, 117, 123, 124) |
| Percent chance of recipient death from biliary complications after LDLT | 9.7% | 4-19% | A2ALL, (31, 100, 101, 104, 108, 117, 123, 124) |
| Percent chance of receiving retransplantation in a recipient with biliary complications after LDLT |
4.9% | 2-10% | (100, 101, 104, 117, 123, 124) |
| Percent chance of requiring non-transplant reoperation in a recipient with biliary complications after LDLT |
54.9% | 20-90% | A2ALL,(104, 117, 123, 124) |
| Chance of having acute rejection severe enough for hospitalization after LDLT | 33.9% | 15-60% | A2ALL, (11, 109, 110, 122, 125) |
| Percent chance of death from an episode of acute rejection after LDLT | 0.2% | 0.1-0.5% | A2ALL, (11, 110, 122, 125) |
| Percent chance of requiring retransplantation because of rejection after LDLT | 1.3% | 0.5-3% | A2ALL, (11, 110, 122, 125) |
Financial Costs
All costs represented in the model are based on the medical center cost point of view. All direct and indirect outpatient and inpatient costs accrued over the ten year study period are accounted for in the model. Abstract costs such as lost wages and emotional costs are not measured in this model. Accurate, easily generalized liver transplantation cost data were not available from the literature, A2ALL dataset, or from the STAR dataset. Cost data for this analysis were obtained from mean values derived from liver transplant patient hospitalizations and physician administrative data abstracted from the University of Virginia Health System Clinical Data Depository (CDR) (33). The CDR is a secure comprehensive clinical database that captures all inpatient and outpatient clinical contacts in the UVA Health System. The CDR uses micro-costing algorithms to capture extensive cost data in an actual utilization (non-DRG) framework. Financial transactions are recorded in the CDR as both third-party charges and actual costs and are calculated using real-time discharge utilization algorithms. The development, accuracy, and validity of the UVA CDR have been published elsewhere (34-37). Cost data from the CDR have been used successfully in other decision analysis models and publications and costs calculated using the CDR have been shown to be comparable to adjusted national costs (38). Table 2 shows the estimated cost data components for the model.
Table 2. Cost data used in model.
All costs are reported in year 2002 adjusted U.S. dollars. See text for derivation of costs.
| Monthly Costs |
Monthly Cost Range* |
|
|---|---|---|
| Baseline Health State Costs | ||
|
| ||
| Baseline average monthly outpatient costs for patient with compensated cirrhosis | 63 | 31-126 |
| Baseline average monthly costs for subjects with permanent contraindication to transplant | 777 | 389-1554 |
| Baseline average monthly costs for recipients post transplantation | 772 | 386-1544 |
|
| ||
| Cost Tolls for Specific Events | ||
|
| ||
| Average cost of TIPS procedure. Includes revisions, complications, hospitalizations, imaging, and outpatient follow-up |
18,192 | 9,096-36,384 |
| Average cost of an episode of SBP. Includes treatment, hospitalization, complications, and imaging |
10,248 | 5,124-20,496 |
| Average cost of ascites and peripheral edema requiring admission to the hospital | 6,197 | 3,098-12,394 |
| Average cost of encephalopathy admission. | 4,297 | 2,148-8,594 |
| Average monthly cost of HCC. Includes imaging, procedures, and follow-up | 3,755 | 1,877-7,510 |
| Average cost of variceal bleeding. Includes hospitalization, procedures, and follow-up | 11,964 | 5,892-23,928 |
| One-time cost of DDLT procedure. Includes deceased donor expenses and organ acquisition costs from OPO, hospitalization, and pharmacy (126). |
103,806 | 51,903-207,612 |
| One-time cost of LDLT procedure. Includes workup costs for 1.23 potential donors (49, 50), donor procedure without complications, hospitalization, and pharmacy (126). Donor costs are normalized to DDLT OPO charges to avoid double-charging living donors. The actual estimated costs for one time live donor procedure without complications is $129,144 (see text). |
103,806 | 51,903-207,612 |
| One-time cost for donor having major complications. Includes hospitalization, procedures, pharmacy, and follow-up. |
16,892 | 8,446-33,784 |
| One-time cost for donor death. Estimated at 75% of the cost of a major complication. | 12,669 | 6,335-25,338 |
| One-time cost for recurrent disease causing graft failure. This is only applied to subjects not eligible for re-transplantation. Based on costs incurred for care when transplant is contraindicated. |
4,662 | 2,331-9,324 |
| Average cost for post-transplant subjects with non-biliary infectious complications. Includes hospitalization, imaging, pharmacy, and follow-up. |
6,952 | 3,476-13,904 |
| Average cost for post transplant recipients that require non-transplant re-operation. Based on the cost of laparotomy. |
16,892 | 8,446-33,784 |
| Average cost for post-transplant subjects with clinically significant biliary complications. Includes cost associated with chronic biliary strictures, and 2 ERCP’s. (104) |
7,292 | 3,646-14,584 |
| One-time cost of post-transplant recipients that require non-transplant reoperation for biliary complication. Does not include ERCP costs. Based on the cost of laparotomy. |
18,607 | 9,303-37,214 |
| Average cost of post-transplant treatment of acute rejection. Includes hospitalization, procedures, pharmacy, and follow-up. |
6,798 | 3,339-13,596 |
Ranges are derived as 50% and 200% of calculated costs.
Health Related Utility Measures
Health outcomes research and health decision analysis depend on analyzing not only the length of time spent in a health state but the quality of life, or utility, associated with that state. Quantification of this level of sickness and the prorating of years of life spent in illness (compared to perfect health) enables a decision analysis to best quantify survival and standardize quality of life in order to more accurately compare medical interventions (39). Several studies have reported health state utilities associated with chronic liver disease and liver transplantation derived by standardized and validated methods (40-47). Table 3 lists the utility values for the health states in the model and specific event related utility tolls, or penalties, for adverse events in the model.
Table 3.
Utility data used in the model.
| Base Case Utility | Utility Range | Sources | |
|---|---|---|---|
| Baseline Health State Utilities | |||
|
| |||
| Utility of compensated cirrhosis (Childs B) | 0.71 | 0.44-0.98 | (40-47) |
| Utility of decompensated cirrhosis (Childs C) | 0.56 | 0.30-0.67 | (40-47) |
| Utility of recipient post liver transplantation | 0.80 | 0.63-0.87 | (41, 43, 45, 47, 127) |
| Utility penalty accrued every month after transplant when donor dies secondary to donation complication. Donors are assumed to be in perfect health before donation. |
Recipient utility – 1.0 | 0.75-1.0 | Expert opinion |
| Utility penalty accrued every month after transplant when donor has major complications secondary to donation complication (128). Donors are assumed to be in perfect health before donation. |
Recipient utility – 0.3 | 0.25-1.0 | Expert opinion |
|
| |||
| Utility Tolls for Specific Events |
Percent Toll From
Baseline |
||
|
| |||
| Monthly utility penalty for refractory ascites | −25% | 0-80% | (44) |
| One-time utility penalty from TIPS | −25% | 0-80% | (42) |
| One-time utility penalty for SBP. Based on utility for refractory ascites. |
−25% | 0-80% | (44) |
| Monthly utility penalty for HCC | −10% | 0-50% | (41, 43, 44) |
| Monthly utility penalty for encephalopathy | −25% | 0-80% | (42, 44) |
| Monthly utility penalty for variceal bleeding | −25% | 0-80% | (42, 44) |
| One-time utility penalty for recent major surgery | −20% | 0-80% | (58) |
| Monthly utility penalty for major complication of transplantation | −25% | 0-80% | (41, 43, 45, 47, 127) |
| One-time utility penalty for undergoing DDLT | −50% | 25-75% | (41, 43, 45, 47, 127) |
| One-time utility penalty for undergoing LDLT. Includes combined donor and recipient penalties |
−75% | 25-90% | (41, 43, 45, 47, 127) and expert opinion |
Donor Complications and Costs
Previously published models of LDLT have not adequately accounted for donor morbidity, mortality, or costs (25, 26, 48). Evidence from the literature indicates that prospective donors are frequently disqualified from the donation process following discovery of pre-existing medical conditions, tissue incompatibilities, or by their eventual unwillingness to participate (49, 50). Donor death and serious morbidity significantly affect the overall utility and costs of the LDLT process. The probability of these events is uncertain, but is becoming clearer as the published literature on donor complications expands (51-55).
Living donor costs are not well accounted for in the current reimbursement system in the U.S. and there is little published data on this topic. We performed an informal multicenter survey regarding the costs and charges assigned to the living donor in LDLT. It was generally agreed upon that typical costs paid to the organ procurement organization (OPO) for DDLT should not be attributed to the living donor. A detailed analysis at two of the A2ALL centers showed that the costs attributed to the living donor approximates the costs paid to the OPO for DDLT [unpublished data]. Many of the centers, for accounting purposes, assigned a cost to the LDLT recipient that was equal to the OPO charges for DDLT. Therefore, we chose to equalize the costs for the hospitalizations of the living donor to the OPO DDLT costs in the model. This resulted in only a small difference for the actual DDLT and LDLT hospitalizations. Repeated trial runs of the model using both the equalized costs and the individual estimated costs for both procedures yielded nearly the exact same results (see Table 2). Thus, the authors chose to use the most common accounting practice in the final model. A wide variation was used in the sensitivity analysis to test the impact of this decision.
Our model assumes that all living donors enter the simulation in a state of perfect health. Based on the A2ALL experience, for each recipient of an LDLT, 1.23 prospective donors are evaluated. This accounts for the extra cost of evaluating donors that are eventually deemed ineligible (49, 50). Variables were introduced into the model in order to account for differential rates of major complications between LDLT and DDLT and the various costs associated with these complications. The major complication rates for donors were extracted from the A2ALL dataset and include all donor complications considered grade 3 or above according to the Clavien grading system (3, 56, 57). Donor deaths incur a penalty of 1.0 utility point per month for the remainder of the simulation. Donor severe complications incur a penalty of 0.30 utility points per month for the remainder of the simulation. This is based upon the documented health utility after complications from major surgery (58). Donor utility penalties continue to accrue for the remainder of the simulation in order to account for the loss of life for the donor who has died or of the quality of life for donors who suffer a major complication.
Sensitivity Analysis
The cost-effectiveness analysis results were assessed for sensitivity to each of the individual estimated probabilities, costs, and utilities in the model. The range of minimum and maximum values considered for each estimated component in the model are shown in the tables of the model probabilities, costs, and utilities.
Results
Model Validation and Unadjusted Recipient Survival
Table 4 lists results from the Monte Carlo simulation of the Markov decision analysis model iterating 1,000 theoretical subjects with decompensated cirrhosis. Ten-year post-transplant survival rates for DDLT recipients (542 of 687, 79.0%%) and LDLT recipients (510 of 715, 71.3%) were comparable to reported survival rates in the literature. The 10-year survival rate for subjects with no access to transplantation (3.6%) was comparable to that reported in the literature for end-stage liver disease. The mean waiting time for all candidates was 149 days, which is comparable to the current waiting times for DDLT in the U.S. The LDLT simulation yielded 2 donor deaths (0.2%) and 17 major complications in donors (2.4%). The maximum survival was attained in the LDLT-exposed treatment branch. In this branch there were 715 LDLT and 233 DDLT procedures with 112 subjects (11.2%) dying on the waitlist and 285 subjects (28.5%) dying after transplantation. This treatment branch terminated at 10 years with 24 subjects still alive and awaiting transplantation.
Table 4. Model validation and survival data after ten year, 1000 subject trial.
Results from a 120-month Monte Carlo simulation of 1,000 theoretical subjects with base case values for all variables.
| Variable | No transplant available |
Listed for DDLT |
Listed for DDLT with LDLT available |
|---|---|---|---|
| Mean cost per patient, 2002 USD | 65,068 | 151,613 | 208,149 |
| Mean unadjusted lifespan (years) | 3.1 | 6.4 | 7.3 |
| Mean quality adjusted lifespan (QALY) | 1.9 | 4.4 | 4.9 |
| Number receiving DDLT (primary or retransplant) | - | 687 | 233 |
| Number receiving LDLT (primary) | - | - | 715 |
| Number dead after 1 year | 175 | 158 | 136 |
| Number dead after 5 years | 630 | 335 | 256 |
| Number dead after 10 years | 964 | 454 | 397 |
| Number receiving primary transplant at one year | - | 309 | 520 |
| Number receiving primary transplant at five years | - | 516 | 662 |
| Number dead pre-transplant or on waitlist* (%) | 964 | 309 (30.9) | 112 (11.2) |
| Mean cost to prevent one pre-transplant death, 2002 USD | - | 122,516 | 199,942 |
| Number dead after transplant (%) | - | 145 (14.5) | 285 (28.5) |
| Living donor deaths (%) | - | - | 2 (0.2) |
| Living donor serious complications, excluding deaths (%) | - | - | 17 (2.4) |
This represents a 10 year cumulative mortality for the entire cohort. The yearly mortality rate on the waiting list is roughly one-tenth of this value.
The major contributor to the increased five-year survival rate in the LDLT exposed treatment arm when compared to the DDLT-only treatment arm is the decreased number of subjects dying on the waiting list. The DDLT-only treatment arm had a 30.9% mortality rate on the waiting list while the LDLT treatment arm had 11.2% waitlist mortality over the ten years of the simulation. This yielded a relative risk of waitlist mortality of 2.75 in the DDLT-only group compared to the LDLT exposed group with a relative risk reduction for waitlist death of 63.8%. This is equivalent to a number needed to treat (NNT) of 5 to prevent one waitlist death, i.e., for every 5 patients listed at a transplant program with access to LDLT, one waitlist death was prevented compared to programs with only DDLT access. This finding is in agreement with previously published recipient survival improvements afforded by LDLT (59, 60).
Costs, Utility, and Cost-effectiveness Analysis
Cost-effectiveness analysis results for the baseline case are summarized in Table 5 for each treatment strategy. Per person costs for the DDLT-only cohort were $87,000 more than non-transplant care. The LDLT approach was $64,000 more expensive than DDLT-only. The increased cost of the LDLT strategy was due to fewer waitlist deaths and thus more ongoing post-transplant expenses in the survivors, and donor procedures and complications. See Figure 2 for cost details.
Table 5. Results of the cost-effectiveness analysis.
Expected costs are those expected for a subject entering the treatment strategy arm, including all outcomes and complications in year 2002 USD. Effectiveness (survival) is expressed in quality-adjusted life years.
| Treatment Strategy |
Expected Cost (x$1000) |
Marginal Cost (x$1000) |
Survival (QALY) |
Marginal Survival (QALY) |
C/E Ratio | Incremental C/E Ratio (ICER) |
|---|---|---|---|---|---|---|
| No Transplant | 63 | - | 2.0 | - | $32,969 | - |
| Listed for DDLT | 150 | 87 | 4.4 | 2.4 | $34,648 | $35,976 |
|
Listed for DDLT
with LDLT available |
214 | 64 | 4.9 | 0.5 | $43,487 | $106,788 |
Figure 2. Mean costs per patient for each treatment strategy by phase of simulation.
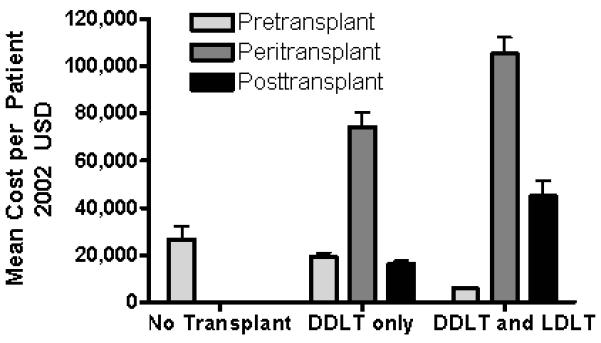
Costs are reported in 2002 USD with standard deviation. The addition of LDLT to a transplant program significantly decreases pretransplant costs but increases post-transplant and peritransplant costs.
Effectiveness is reported as quality-adjusted life years. The “no transplant” strategy offered a quality-adjusted expected survival of 2.0 QALY’s, and DDLT-only offered 4.4 QALY’s. The combined LDLT strategy resulted in 4.9 QALY expected survival, which was 0.5 QALY more than the DDLT-only strategy. Cost-effectiveness ratios are reported in dollars per QALY. The LDLT strategy yielded the highest cost per QALY. Both transplant approaches were more effective and of reasonable cost compared to pure supportive care / medical management of cirrhosis. The DDLT-only strategy cost an average of $35,976 per QALY over medical management while the availability of LDLT strategy cost $47,693 per QALY. LDLT quality adjusted survival was hindered by donor morbidity and, to a lesser extent, donor death. The incremental cost (ICER) of moving from the DDLT-only strategy to the LDLT strategy was approximately $106,788 per QALY.
Sensitivity Analysis
One-way sensitivity analyses were performed on all variables in the model. The basic relationships between DDLT and LDLT and cost-effectiveness were not affected by any clinically relevant range of values for any single variable in the model. Repeated analyses using extreme estimates for each of these variables did not change the preferred treatment strategy with respect to cost-effectiveness (see Figure 3). In general, improvement in pre-transplant variables decreased the cost-effectiveness of both forms of transplantation in proportional amounts. Conversely, improvements in post-transplantation variables moderately improved the cost-effectiveness of both forms of transplantation. There were no circumstances in the sensitivity analysis that enabled LDLT to be less costly than DDLT and only with extreme assumptions could their costs approach equality (see Figure 4). Similarly, because of donor morbidity and mortality, aggregate adjusted quality of life was always only marginally better for LDLT than DDLT, despite the decrease in deaths on the waitlist. The improvement in quality of life and decrease in costs associated with less waitlist death in the LDLT strategy was offset by the increased exposure of recipients to post-transplant complications and costs. Because of this, there were no realistic interventions in the model that could bring the ICER of the LDLT strategy below $50,000/QALY. This remained true when DDLT waiting times were varied through extreme ranges. When average waiting times for DDLT approached three years, the DDLT and LDLT strategies yielded very similar ICER values. Conversely, when DDLT waiting times were less than two months, the LDLT strategy was both less effective and more costly than the LDLT approach.
Figure 3. Sensitivity of the model to cost variables.
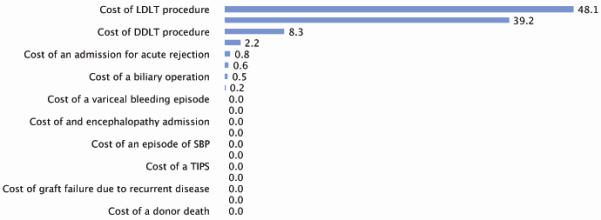
Number shown is percent of cost-dependent model variability attributable to the listed cost as determined by a tornado diagram sensitivity analysis. Note that within the ranges of the sensitivity analysis, none of the cost variability was able to change the fundamental cost-effectiveness of the treatment strategies. See text for details. All values listed as 0.0 were not statistically significant contributors to overall model variability.
Figure 4. Two-way sensitivity analysis on costs of individual transplant procedures.
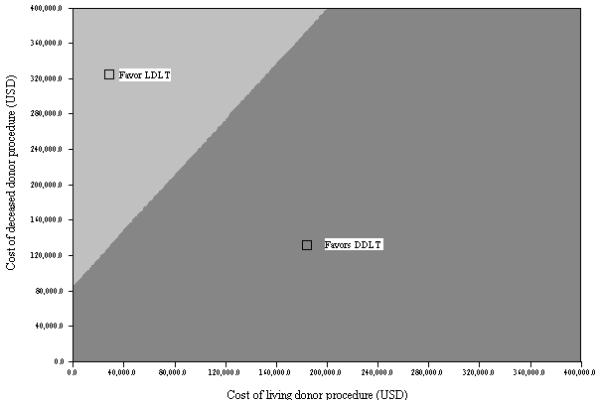
Only unrealistic differences in the cost of the individual procedures would swing the cost-effectiveness superiority to LDLT. See text for details.
The probability of donor death and complications after LDLT were significant influences on the cost-effectiveness of LDLT in the extreme cases. Because of the improved overall recipient quality of life after transplantation and diminished waitlist death rates, donor death only had a marginal influence on aggregate quality of life and adjusted survival. Only when donor death rates exceeded an unrealistic 24% was the aggregate adjusted survival after LDLT less than that of DDLT. Donor morbidity had little effect on overall adjusted survival and quality of life although costs were significant. Because of a steep learning curve in performing the LDLT procedure (31), data regarding complications and outcomes were separately analyzed using a cutoff of 20 LDLT procedures or less per center. The assumption was that less experienced centers would have more complications and therefore the cost-effectiveness of the procedure would improve after the learning curve was overcome. Despite significant improvement in some complications, cost-effectiveness in experienced centers was only minimally improved because the fundamental costs involved with more exposure to the post-transplant health state were not significantly affected. In the present model, if more than 40% of waitlisted subjects have HCC, then the ICER of LDLT approaches the commonly accepted $50,000/QALY but at the cost of a 17% increase in waitlist death (282 versus 331), mainly in the non-HCC subjects. More comprehensive simulations focused specifically on HCC have been published elsewhere (48).
The cost effectiveness measurements of all treatment strategies in the model were highly sensitive to the time horizon (i.e. the total observation time for each subject entering the model) chosen for the analysis. Because the majority of expense and morbidity occurs early in the course of liver transplantation, the major cost and quality of life benefit of liver transplantation is in the long-term survival advantage offered by either form of liver transplantation. In the present model, at one-year observation time (from presentation for evaluation with decompensated cirrhosis), neither form of liver transplantation is cost effective (ICER of DDLT > $2,252,000/QALY while LDLT was more costly and had lower survival). With a two year time horizon this trend continued (ICER of DDLT > $513,000/QALY and LDLT still inferior). Only with a total observation period of nine years or greater does the DDLT strategy become cost effective with an ICER less than $50,000/QALY while the LDLT strategy remains greater than $135,000/QALY. Because the mean survival of liver transplant recipients is greater than 10 years in the U.S., a time horizon of ten years was chosen in this model. This idea of accepting early increases in costs and morbidity for a long term benefit are critical in policy making decisions about liver transplantation. Conversely, earlier transplantation would only be expected to be a cost-effective treatment method in candidates at a high likelihood of death in the short term. Other researchers have estimated a threshold of survival benefit of liver transplantation to be above a MELD score of 17 (61).
Discussion
This simulation measures the cost-effectiveness of LDLT combined with the existing standard of care DDLT strategy for the treatment of end-stage liver disease using a Markov decision analysis model to simulate the major events that occur before, during, and after both living and deceased donor liver transplantation. Unlike many previous models, extensive consideration was given to costs related to workup of potential donors that are eventually deemed ineligible for donation, the real impact of donor mortality and morbidity, and the effect on quality adjusted survival and quality of life related to donor complications. The course of chronic liver disease and liver transplantation simulated by the model closely approximates to the course of events reported in the literature.
We found that liver transplantation is an expensive but effective treatment for end-stage liver disease and cirrhosis. The ICER for the standard-of-care DDLT-only approach was more than $35,000 when measured over a ten year time frame. While modestly more effective, mostly due to less time spent waiting for transplantation, addition of LDLT to the DDLT approach was an expensive but effective alternative. The ICER of the LDLT strategy was approximately $106,000. Interpretation of an ICER based on a simulation is a subjective matter and is influenced by societal willingness to pay and by the validity of the model and its assumptions (62). Previously reported ICER for routinely performed medical interventions in the U.S. include $86,362 for screening for colorectal cancer in people over age 65 (63), between $8,000 and $900,000 (depending on age and type of drug used) for treatment of hypertension (64, 65), and $112,000 for screening for HCC in cirrhosis patients with ultrasound and alpha fetoprotein (66), and more than $708,000 for intravenous proton pump inhibitor therapy peptic ulcers (67). In contrast, the traditional willingness to pay benchmark in the U.S. is based on the cost of chronic ambulatory hemodialysis (68-70). While an ICER of less than $50,000 has been traditionally accepted as a cost-effective addition to the medical system in the U.S., some authors have argued that based on different economic calculations and assumptions, a cost-effective medical intervention could range from as low as $24,000 to as high as $428,000 per QALY (71). In fact, if cost-effectiveness values associated with hemodialysis derived from studies in the late 1980’s are adjusted for year 2004 USD, an ICER of $75,000 may be a more proper benchmark for modern cost-effectiveness analyses.
All cost-effectiveness studies based on modeling have some inherent weaknesses. Ultimately, the quality of the model output and its resulting analysis is dependent on the quality of the model, its approximation of reality, and its probabilities, utilities, and costs used for the calculations. In the design of the current model, we have taken extreme care in designing a model that is flexible yet adequately represents most of the major events in chronic liver disease and liver transplantation. While quite complex, the model is an approximation of reality and cannot truly represent all the possible outcomes in this complicated disease process. However, we have based the probabilities and health state utilities on the best available and most pertinent data. We agree that much of the reported literature may be biased in one way or another but we have attempted to represent average reported values and used wide ranges in the sensitivity analysis when the data was insufficient or weak. Finally, when no published data was available, expert opinion and unpublished data was used but these occurrences were few and the following sensitivity analyses were conducted over a broad range.
Cost data was center specific and this inherent weakness was unavoidable in this simulation. Using strong micro-costing algorithms and averaging several years’ adjusted costs minimized this inherent weakness. The wide range of all costs (50-200%) used in the sensitivity analysis also helped guard against inaccurate cost data. In the analysis, the costs yielded from this model are consistent with other published cost data in the literature. There have been two published analyses from U.S. universities assessing costs in the setting of LDLT and DDLT (7, 8). While these studies did not publish specific costs but related comparative costs on the basis of “Cost Units”, the costs related to LDLT and DDLT in those reports were comparable to those used in this model. Similarly, European studies have published abridged cost data (25) and after accounting for currency conversion rates and inflation, the costs in this model compare similarly.
We chose a ten year time horizon because data for transplantation in the modern era are available for approximately the last ten years. It was also felt that extrapolating beyond ten years with event rates was very speculative and potentially inaccurate. This time frame also yields a reasonable time frame to judge the benefit of transplant beyond the immediate post-surgical complications and waiting list morbidities. Also the A2ALL study period was roughly ten years as initially funded. In theory, a longer time frame of observation could change our eventual conclusion concerning cost-effectiveness although no solid data is available at the present time to base this speculation and there is no clear advantage to LDLT or DDLT in this regard. Changing the overall time horizon in the model has two opposite effects: 1. Shortening the length of time that candidates are exposed to waiting list mortality, and 2. Shortening the extension of life in the post-transplant phase that successfully transplanted candidates are allowed to experience. These opposite effects cause a waiting list mortality decrease at the same time as a decrease in post-transplant quality of life benefit. The overall effect on the model is not strong unless very short time horizons are used. Thus, the ten year time frame was chosen to simulate reality as much as possible while still using data that are dependable. Another unexplored factor in this paper is regional variation in donor utilization and organ allocation. This model assumes a national distribution of organs and the fundamental data in this paper is based on national averages. Regional variation is a potential source of widely variable practice in the U.S. but investigation of regional changes in cost-effectiveness and LDLT practice is beyond the scope of this manuscript. It was the goal of this analysis to combine the representative data from a national sample of transplant programs in order to give a broad view of the practice of LDLT in the U.S.
In summary, this simulation presents an extensive cost-effectiveness model simulating chronic liver disease and cirrhosis with treatment options including the standard of care DDLT-only and the addition of LDLT to the treatment paradigm. Considering living donor costs, morbidity, mortality, and quality of life, this is the first model to accurately account for the true consequences to the donor in the LDLT treatment strategy. When using traditionally defined standards of cost-effectiveness, DDLT-only proved to be a cost-effective treatment for cirrhosis with an ICER of approximately $50,000 per QALY. However, LDLT in combination with DDLT, proved to be modestly more effective but much more expensive than the DDLT-only strategy per QALY saved. This simulation, along with the decision analysis model, should be a useful tool for policymakers and transplant centers in allocating resources and guiding further investigation into the field of cirrhosis and liver transplantation.
Acknowledgements
This study was supported by National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (listed below). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS). The following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
Columbia University Health Sciences, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-PI: Robert S. Brown, Jr., MD, MPH; Study Coordinators: Rudina Odeh-Ramadan, PharmD; Scott Heese, BA
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-PI: Andreas Blei, MD; Study Coordinator: Patrice Al-Saden, RN, CTCC
University of Pennsylvania Health System, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD; Co-PI: Kim M. Olthoff, MD; Study Coordinators: Mary Kaminski, PA-C; Mary Shaw, RN, BBA
University of Colorado Health Sciences Center, Denver, CO (DK62536): PI: James F. Trotter, MD; Co-PI: Igal Kam, MD; Study Coordinators: Carlos Garcia, BS
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Ronald W. Busuttil, MD, PhD; Co-PI: Sammy Saab, MD; Study Coordinator: Janet Mooney, RN, BSN
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-PI: Norah A. Terrault, MD; Study Coordinator: Dulce MacLeod, RN
University of Michigan Medical Center, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD; DCC Staff: Anna S.F. Lok, MD; Akinlolu O. Ojo, MD, PhD; Brenda W. Gillespie, PhD; Margaret Hill-Callahan, BS, LSW; Terese Howell, BS; Lan Tong, MS; Tempie H. Shearon, MS; Karen A. Wisniewski, MPH; Monique Lowe, BS
University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD; Study Coordinator: Carrie A. Nielsen, MA
University of Virginia (DK62484): PI: Carl L. Berg, MD; Co-PI: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN
Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-PI: Mitchell L. Shiffman, MD; Study Coordinators: Ede Fenick, RN; April Ashworth, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD; Leonard B. Seeff, MD; Patricia R. Robuck, PhD; Jay H. Hoofnagle, MD
Abbreviations
- LDLT
living donor liver transplant
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- DDLT
deceased donor liver transplant
- QALY
quality adjusted life years
- ICER
incremental cost effectiveness ratio
- NIH
National Institutes of Health
- UNOS
United Network for Organ Sharing
- MELD
model for end-stage liver disease
- STAR
Standard Transplant Analysis and Research
- CDR
University of Virginia Health System Clinical Data Repository
- OPO
organ procurement organization
- HCC
hepatocellular carcinoma
- HRSA
Health Resources and Services Administration
- ASTS
American Society of Transplant Surgeons
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
This is publication number 10 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
Supplemental data used to generate A2ALL baseline estimates for the simulation modeling were supplied to the A2ALL Data Coordinating Center at the University of Michigan by Arbor Research Collaborative for Health (Arbor Research). Arbor Research is the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Portions of this data were presented in brief abstract form at the American Society of Transplantation Annual Meeting - American Transplantation Congress 2007, San Francisco, CA; May 2007.
References
- 1.Hashikura Y, Kawasaki S, Terada M, Ikegami T, Nakazawa Y, Urata K, et al. Long-term results of living-related donor liver graft transplantation: a single-center analysis of 110 transplants. Transplantation. 2001;72(1):95–99. doi: 10.1097/00007890-200107150-00019. [DOI] [PubMed] [Google Scholar]
- 2.Fujita S, Kim ID, Uryuhara K, Asonuma K, Egawa H, Kiuchi T, et al. Hepatic grafts from live donors: donor morbidity for 470 cases of live donation. Transplant International. 2000;13(5):333–339. doi: 10.1007/s001470050710. [DOI] [PubMed] [Google Scholar]
- 3.Ghobrial R, Freise C, Trotter J, Tong L, Ojo A, Fair J, et al. Donor morbidity and mortality of adult living donors for liver transplantation (abstract) Am J Transpl. 2006;6(S2):115. [Google Scholar]
- 4.Azoulay D, Linhares MM, Huguet E, Delvart V, Castaing D, Adam R, et al. Decision for retransplantation of the liver: an experience- and cost-based analysis. Annals of Surgery. 2002;236(6):713–721. doi: 10.1097/01.SLA.0000036264.66247.65. discussion 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonsel GJ, Klompmaker IJ, Essink-Bot ML, Habbema JD, Slooff MJ. Cost-effectiveness analysis of the Dutch liver transplantation programme. Transplantation Proceedings. 1990;22(4):1481–1484. [PubMed] [Google Scholar]
- 6.Freeman R, Tsunoda S, Supran S, Warshaw A, Smith J, Fairchild R, et al. Direct costs for one year of liver transplant care are directly associated with disease severity at transplant. Transplantation Proceedings. 2001;33(1-2):1436–1437. doi: 10.1016/s0041-1345(00)02543-4. [DOI] [PubMed] [Google Scholar]
- 7.Kam I. Cadaveric versus living donor liver transplantation--analysis of costs. Transplantation Proceedings. 2003;35(3):971. doi: 10.1016/s0041-1345(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 8.Trotter JF, Mackenzie S, Wachs M, Bak T, Steinberg T, Polsky P, et al. Comprehensive cost comparison of adult-adult right hepatic lobe living-donor liver transplantation with cadaveric transplantation. Transplantation. 2003;75(4):473–476. doi: 10.1097/01.TP.0000047310.04069.ED. [DOI] [PubMed] [Google Scholar]
- 9.Filipponi F, Pisati R, Ferrara R, Mosca F. Cost and outcome evaluation of liver transplantation at Cisanello Hospital: (2) results. Transplantation Proceedings. 2003;35(3):1041–1044. doi: 10.1016/s0041-1345(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 10.Buell JF, Cronin DC, Blahnik L, Lo A, Trimbach C, Layman R, et al. The impact of donor factors on the outcomes following liver transplantation. Transplantation Proceedings. 2002;34(5):1495–1496. doi: 10.1016/s0041-1345(02)02944-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003;75(3 Suppl):S6–11. doi: 10.1097/01.TP.0000046533.93621.C7. [DOI] [PubMed] [Google Scholar]
- 12.Seaman DS. Adult living donor liver transplantation: current status. Journal of Clinical Gastroenterology. 2001;33(2):97–106. doi: 10.1097/00004836-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Belle SH, Porayko MK, Hoofnagle JH, Lake JR, Zetterman RK. Changes in quality of life after liver transplantation among adults. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Liver Transplantation Database (LTD) Liver Transplantation & Surgery. 1997;3(2):93–104. doi: 10.1002/lt.500030201. [DOI] [PubMed] [Google Scholar]
- 14.De Bona M, Ponton P, Ermani M, Iemmolo RM, Feltrin A, Boccagni P, et al. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. Journal of Hepatology. 2000;33(4):609–615. doi: 10.1034/j.1600-0641.2000.033004609.x. [DOI] [PubMed] [Google Scholar]
- 15.Gross CR, Malinchoc M, Kim WR, Evans RW, Wiesner RH, Petz JL, et al. Quality of life before and after liver transplantation for cholestatic liver disease. Hepatology. 1999;29(2):356–364. doi: 10.1002/hep.510290229. [DOI] [PubMed] [Google Scholar]
- 16.Karam V, Castaing D, Danet C, Delvart V, Gasquet I, Adam R, et al. Longitudinal prospective evaluation of quality of life in adult patients before and one year after liver transplantation. Liver Transplantation. 2003;9(7):703–711. doi: 10.1053/jlts.2003.50148. [DOI] [PubMed] [Google Scholar]
- 17.Karam VH, Gasquet I, Delvart V, Hiesse C, Dorent R, Danet C, et al. Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation. 2003;76(12):1699–1704. doi: 10.1097/01.TP.0000092955.28529.1E. [DOI] [PubMed] [Google Scholar]
- 18.Moore KA, Mc LJR, Burrows GD. Quality of life and cognitive function of liver transplant patients: a prospective study. Liver Transplantation. 2000;6(5):633–642. doi: 10.1053/jlts.2000.9743. [DOI] [PubMed] [Google Scholar]
- 19.Moore D, Feurer I, Speroff T, Shaffer D, Nylander W, Kizilisik T, et al. Survival and quality of life after organ transplantation in veterans and nonveterans. American Journal of Surgery. 2003;186(5):476–480. doi: 10.1016/j.amjsurg.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto T, Yamaoka Y, Tanaka K, Ozawa K. Quality of life among donors of liver transplants to relatives. New England Journal of Medicine. 1993;329(5):363–364. doi: 10.1056/NEJM199307293290518. [DOI] [PubMed] [Google Scholar]
- 21.Pinson CW, Feurer ID, Payne JL, Wise PE, Shockley S, Speroff T. Health-related quality of life after different types of solid organ transplantation. Annals of Surgery. 2000;232(4):597–607. doi: 10.1097/00000658-200010000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M, et al. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transplantation. 2002;8(3):263–270. doi: 10.1053/jlts.2002.31345. [DOI] [PubMed] [Google Scholar]
- 23.Saab S, Ly D, Han SB, Lin RK, Rojter SE, Ghobrial RM, et al. Is it cost-effective to treat recurrent hepatitis C infection in orthotopic liver transplantation patients? Liver Transplantation. 2002;8(5):449–457. doi: 10.1053/jlts.2002.32717. [DOI] [PubMed] [Google Scholar]
- 24.Cheng SJ, Pratt DS, Freeman RB, Jr., Kaplan MM, Wong JB. Living-donor versus cadaveric liver transplantation for non-resectable small hepatocellular carcinoma and compensated cirrhosis: a decision analysis. Transplantation. 2001;72(5):861–868. doi: 10.1097/00007890-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 25.Sagmeister M, Mullhaupt B, Kadry Z, Kullak-Ublick GA, Clavien PA, Renner EL. Cost-effectiveness of cadaveric and living-donor liver transplantation. Transplantation. 2002;73(4):616–622. doi: 10.1097/00007890-200202270-00025. [DOI] [PubMed] [Google Scholar]
- 26.Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33(5):1073–1079. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health-National Institutes of Diabetes D, and Kidney Diseases [Accessed January 2006]; http://www.nih-a2all.org/
- 28.Adult-to-adult living donor liver transplantation cohort study (A2ALL) Hepatology. 2003;38(4):792. doi: 10.1002/hep.510380402. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Medical Decision Making. 1993;13(4):322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA. 1989;262(20):2879–2886. doi: 10.1001/jama.262.20.2879. [DOI] [PubMed] [Google Scholar]
- 31.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242(3):314–323. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 323-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organ Procurement and Transplantation Network (OPTN) Standard Transplant Analysis and Research Database. Nov, 2003. Available from:
- 33.University of Virginia Clinical Data Repository. 2004 http://www.med.virginia.edu/cdr/ Available from:
- 34.Pates RD, Scully KW, Einbinder JS, Merkel RL, Stukenborg GJ, Spraggins TA, et al. Adding value to clinical data by linkage to a public death registry. Medinfo. 2001;10(Pt 2):1384–1388. [PubMed] [Google Scholar]
- 35.Einbinder JS, Scully KW, Pates RD, Schubart JR, Reynolds RE. Case study: a data warehouse for an academic medical center. Journal of Healthcare Information Management. 2001;15(2):165–175. [PubMed] [Google Scholar]
- 36.Scully KW, Pates RD, Desper GS, Connors AF, Harrell FE, Jr., Pieper KS, et al. Development of an enterprise-wide clinical data repository: merging multiple legacy databases. Proceedings/AMIA Annual Fall Symposium. 1997:32–36. [PMC free article] [PubMed] [Google Scholar]
- 37.Northup PG, Berg CL. Cost minimization in endoscopy center scheduling: a case-controlled study. J Clin Gastroenterol. 2005;39(4):268–272. doi: 10.1097/01.mcg.0000155138.81056.d7. [DOI] [PubMed] [Google Scholar]
- 38.Arseneau KO, Cohn SM, Cominelli F, Connors AF., Jr. Cost-utility of initial medical management for Crohn’s disease perianal fistulae. Gastroenterology. 2001;120(7):1640–1656. doi: 10.1053/gast.2001.24884. [see comment] [DOI] [PubMed] [Google Scholar]
- 39.Froberg DG, Kane RL. Methodology for measuring health-state preferences--II: Scaling methods. Journal of Clinical Epidemiology. 1989;42(5):459–471. doi: 10.1016/0895-4356(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 40.Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterology. 2001;96(2):579–583. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 41.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98(3):630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 42.Rubenstein JH, Eisen GM, Inadomi JM. A cost-utility analysis of secondary prophylaxis for variceal hemorrhage. Am J Gastroenterol. 2004;99(7):1274–1288. doi: 10.1111/j.1572-0241.2004.04153.x. [DOI] [PubMed] [Google Scholar]
- 43.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the Cost-Effectiveness of a Single Course of Interferon-alpha2b in Patients with Histologically Mild Chronic Hepatitis C. Ann Intern Med. 1997;127(10):855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 44.Stein K, Rosenberg W, Wong J. Cost effectiveness of combination therapy for hepatitis C: a decision analytic model. Gut. 2002;50(2):253–258. doi: 10.1136/gut.50.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim WR, Poterucha JJ, Hermans JE, Therneau TM, Dickson ER, Evans RW, et al. Cost-Effectiveness of 6 and 12 Months of Interferon-alpha Therapy for Chronic Hepatitis C. Ann Intern Med. 1997;127(10):866–874. doi: 10.7326/0003-4819-127-10-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 46.Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment Evaluation of Chronic Hepatitis C: Risks, Benefits, and Costs. JAMA. 1998;280(24):2088–2093. doi: 10.1001/jama.280.24.2088. [DOI] [PubMed] [Google Scholar]
- 47.Bryce CL, Angus DC, Switala J, Roberts MS, Tsevat J. Health status versus utilities of patients with end-stage liver disease. Qual Life Res. 2004;13(4):773–782. doi: 10.1023/B:QURE.0000021685.83961.88. [DOI] [PubMed] [Google Scholar]
- 48.Patel D, Terrault NA, Yao FY, Bass NM, Ladabaum U. Cost-effectiveness of hepatocellular carcinoma surveillance in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2005;3(1):75–84. doi: 10.1016/s1542-3565(04)00443-4. [DOI] [PubMed] [Google Scholar]
- 49.Valentin-Gamazo C, Malago M, Karliova M, Lutz JT, Frilling A, Nadalin S, et al. Experience after the evaluation of 700 potential donors for living donor liver transplantation in a single center. Liver Transpl. 2004;10(9):1087–1096. doi: 10.1002/lt.20223. [DOI] [PubMed] [Google Scholar]
- 50.Trotter JF, Wachs M, Trouillot T, Steinberg T, Bak T, Everson GT, et al. Evaluation of 100 patients for living donor liver transplantation. Liver Transplantation. 2000;6(3):290–295. doi: 10.1002/lt.500060323. [DOI] [PubMed] [Google Scholar]
- 51.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-Adult Transplantation of the Right Hepatic Lobe from a Living Donor. N Engl J Med. 2002;346(14):1074–1082. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 52.Marcos A. Right-lobe living donor liver transplantation. Liver Transpl. 2000;6(6 Suppl 2):S59–63. doi: 10.1053/jlts.2000.19011. [DOI] [PubMed] [Google Scholar]
- 53.Broelsch CE, Malago M, Testa G, Gamazo C Valentin. Living donor liver transplantation in adults: outcome in Europe. Liver Transpl. 2000;6(6 Suppl 2):S64–65. doi: 10.1053/jlts.2000.19015. [DOI] [PubMed] [Google Scholar]
- 54.Todo S, Furukawa H, Jin MB, Shimamura T. Living donor liver transplantation in adults: outcome in Japan. Liver Transpl. 2000;6(6 Suppl 2):S66–72. doi: 10.1053/jlts.2000.19009. [DOI] [PubMed] [Google Scholar]
- 55.Renz JF, Roberts JP. Long-term complications of living donor liver transplantation. Liver Transpl. 2000;6(6 Suppl 2):S73–76. doi: 10.1053/jlts.2000.18686. [DOI] [PubMed] [Google Scholar]
- 56.Morbidity after live donor hepatectomy based on clavien scoring. Transplantation. 2006;82(1 Suppl 2):116. [Google Scholar]
- 57.Tamura S, Sugawara Y, Kaneko J, Yamashiki N, Kishi Y, Matsui Y, et al. Systematic grading of surgical complications in live liver donors according to Clavien’s system. Transpl Int. 2006;19(12):982–987. doi: 10.1111/j.1432-2277.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 58.Gazelle GS, Hunink MG, Kuntz KM, McMahon PM, Halpern EF, Beinfeld M, et al. Cost-effectiveness of hepatic metastasectomy in patients with metastatic colorectal carcinoma: a state-transition Monte Carlo decision analysis. Ann Surg. 2003;237(4):544–555. doi: 10.1097/01.SLA.0000059989.55280.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berg CL. Living donor liver transplantation reduces the risk of death of transplant candidates [Presentation]. American Association for the Study of Liver Diseases, 55th Annual Meeting; Boston, MA. 2004 October 31.2004. [Google Scholar]
- 60.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr., Abecassis MM, Trotter JF, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133(6):1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 62.Bambha K, Kim WR. Cost-effectiveness analysis and incremental cost-effectiveness ratios: uses and pitfalls. 2004. pp. 519–526. [DOI] [PubMed]
- 63.Wagner JL, Herdman RC, Wadhwa S. Cost effectiveness of colorectal cancer screening in the elderly. Nov, 1991. pp. 807–817. 1915. [DOI] [PubMed]
- 64.Johannesson M. The cost-effectiveness of the switch towards more expensive antihypertensive drugs. Health Policy. 1994;28(1):1–13. doi: 10.1016/0168-8510(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 65.Johannesson M. The impact of age on the cost-effectiveness of hypertension treatment: an analysis of randomized drug trials. Med Decis Making. 1994;14(3):236–244. doi: 10.1177/0272989X9401400305. [DOI] [PubMed] [Google Scholar]
- 66.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48(2):251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiegel BM, Dulai GS, Lim BS, Mann N, Kanwal F, Gralnek IM. The cost-effectiveness and budget impact of intravenous versus oral proton pump inhibitors in peptic ulcer hemorrhage. Clin Gastroenterol Hepatol. 2006;4(8):988–997. doi: 10.1016/j.cgh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Hornberger JC. The hemodialysis prescription and cost effectiveness. Renal Physicians Association Working Committee on Clinical Guidelines. J Am Soc Nephrol. 1993;4(4):1021–1027. doi: 10.1681/ASN.V441021. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Carmona A, Fontan M Perez, Bouza P, Falcon T Garcia, Valdes F. The economic cost of dialysis: a comparison between peritoneal dialysis and in-center hemodialysis in a Spanish unit. Adv Perit Dial. 1996;12:93–96. [PubMed] [Google Scholar]
- 70.Tousignant P, Guttmann RD, Hollomby DJ. Transplantation and home hemodialysis: their cost-effectiveness. J Chronic Dis. 1985;38(7):589–601. doi: 10.1016/0021-9681(85)90048-7. [DOI] [PubMed] [Google Scholar]
- 71.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 72.Gines P, Cardenas A, Arroyo V, Rodes J. Management of Cirrhosis and Ascites. N Engl J Med. 2004;350(16):1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 73.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38(1):258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 74.Russo MW, Sood A, Jacobson IM, Brown RS., Jr. Transjugular intrahepatic portosystemic shunt for refractory ascites: an analysis of the literature on efficacy, morbidity, and mortality. American Journal of Gastroenterology. 2003;98(11):2521–2527. doi: 10.1111/j.1572-0241.2003.08664.x. [DOI] [PubMed] [Google Scholar]
- 75.Rimola A, Garcia-Tsao G, Navasa M, Piddock LJV, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. Journal of Hepatology. 2000;32(1):142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Tsao G. Spontaneous bacterial peritonitis. Gastroenterology Clinics Of North America. 1992;21(1):257–275. [PubMed] [Google Scholar]
- 77.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. Journal Of Hepatology. 1993;18(3):353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 78.Pinzello G, Simonetti R, Camma C, Dino O, Milazzo G, Pagliaro L, et al. Spontaneous bacterial peritonitis: An update. Gastroenterology International. 1993;6(1):54–60. [Google Scholar]
- 79.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of Intravenous Albumin on Renal Impairment and Mortality in Patients with Cirrhosis and Spontaneous Bacterial Peritonitis. N Engl J Med. 1999;341(6):403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 80.Jalan R, Hayes PC. Hepatic encephalopathy and ascites. The Lancet. 1997;350(9087):1309–1315. doi: 10.1016/S0140-6736(97)07503-X. [DOI] [PubMed] [Google Scholar]
- 81.Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. Journal of Hepatology. 1999;30(5):890–895. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 82.Forrest EH, Stanley AJ, Redhead DN, McGilchrist AJ, Hayes PC. Clinical response after transjugular intrahepatic portosystemic stent shunt insertion for refractory ascites in cirrhosis. Alimentary Pharmacology & Therapeutics. 1996;10(5):801–806. doi: 10.1046/j.1365-2036.1996.60202000.x. [DOI] [PubMed] [Google Scholar]
- 83.Figueras J, Jaurrieta E, Valls C, Benasco C, Rafecas A, Xiol X, et al. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: a comparative study. Hepatology. 1997;25(6):1485–1489. doi: 10.1002/hep.510250629. [DOI] [PubMed] [Google Scholar]
- 84.Merion R. SRTR analysis after waitlisting for HCC patients under MELD-based allocation. OPTN/UNOS Symposium on Liver Allocation Policy for HCC; Miami, Florida. January 26, 2003. [Google Scholar]
- 85.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22(1):332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 86.Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39(3):746–753. doi: 10.1002/hep.20126. [DOI] [PubMed] [Google Scholar]
- 87.Okano H, Shiraki K, Inoue H, Kawakita T, Deguchi M, Sugimoto K, et al. Long-term follow-up of patients with liver cirrhosis after endoscopic ethanol injection sclerotherapy for esophageal varices. Hepato-Gastroenterology. 2003;50(53):1556–1559. [PubMed] [Google Scholar]
- 88.Sorbi D, Gostout CJ, Peura D, Johnson D, Lanza F, Foutch PG, et al. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. American Journal of Gastroenterology. 2003;98(11):2424–2434. doi: 10.1111/j.1572-0241.2003.t01-1-07705.x. [DOI] [PubMed] [Google Scholar]
- 89.Gines P, Quintero E, Arroyo V, Teres J, Bruguera M, Rimola A, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7(1):122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 90.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [see comment] [DOI] [PubMed] [Google Scholar]
- 91.Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepato-Gastroenterology. 2002;49(43):7–11. [PubMed] [Google Scholar]
- 92.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50(1):123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 94.Chui AKK, Shi LW, Rao ARN, Anasuya A, Hagl C, Pillay P, et al. Primary graft dysfunction after liver transplantation. Transplantation Proceedings. 2000;32(7):2219–2220. doi: 10.1016/s0041-1345(00)01642-0. [DOI] [PubMed] [Google Scholar]
- 95.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55(4):807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 96.Singh N, Gayowski T, Wagener MM, Marino IR. Bloodstream infections in liver transplant recipients receiving tacrolimus. Clinical Transplantation. 1997;11(4):275–281. [PubMed] [Google Scholar]
- 97.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transplantation. 2000;6(1):54–61. doi: 10.1002/lt.500060112. [DOI] [PubMed] [Google Scholar]
- 98.Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. American Journal of Infection Control. 1992;20(5):239–247. doi: 10.1016/s0196-6553(05)80197-x. [DOI] [PubMed] [Google Scholar]
- 99.Urbani L, Catalano G, Biancofiore G, Bindi L, Consani G, Bisa M, et al. Surgical complications after liver transplantation. Minerva Chirurgica. 2003;58(5):675–692. [PubMed] [Google Scholar]
- 100.O’Connor TP, Lewis WD, Jenkins RL. Biliary tract complications after liver transplantation. Archives of Surgery. 1995;130(3):312–317. doi: 10.1001/archsurg.1995.01430030082017. [DOI] [PubMed] [Google Scholar]
- 101.Sawyer RG, Punch JD. Incidence and management of biliary complications after 291 liver transplants following the introduction of transcystic stenting. Transplantation. 1998;66(9):1201–1207. doi: 10.1097/00007890-199811150-00015. [DOI] [PubMed] [Google Scholar]
- 102.Baccarani U, Risaliti A, Zoratti L, Zilli M, Brosola P, Vianello V, et al. Role of endoscopic retrograde cholangiopancreatography in the diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Digestive & Liver Disease. 2002;34(8):582–586. doi: 10.1016/s1590-8658(02)80092-5. [see comment] [DOI] [PubMed] [Google Scholar]
- 103.Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, et al. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointestinal Endoscopy. 2000;52(1):55–63. doi: 10.1067/mge.2000.106687. [DOI] [PubMed] [Google Scholar]
- 104.Shah JN, Ahmad NA, Shetty K, Kochman ML, Long WB, Brensinger CM, et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2004;99(7):1291–1295. doi: 10.1111/j.1572-0241.2004.30775.x. [DOI] [PubMed] [Google Scholar]
- 105.Fleck J, A., Zanotelli ML, Meine M, Brandao A, Leipnitz I, Schlindwein E, et al. Biliary tract complications after orthotopic liver transplantation in adult patients. Transplantation Proceedings. 2002;34(2):519–520. doi: 10.1016/s0041-1345(02)02615-5. [DOI] [PubMed] [Google Scholar]
- 106.Chahin NJ, De Carlis L, Slim AO, Rossi A, Groeso CA, Rondinara GF, et al. Long-term efficacy of endoscopic stenting in patients with stricture of the biliary anastomosis after orthotopic liver transplantation. Transplantation Proceedings. 2001;33(5):2738–2740. doi: 10.1016/s0041-1345(01)02174-1. [DOI] [PubMed] [Google Scholar]
- 107.Zhou G, Cai W, Li H, Zhu Y, Fung JJ. Experiences relating to management of biliary tract complications following liver transplantation in 96 cases. Chinese Medical Journal. 2002;115(10):1533–1537. [PubMed] [Google Scholar]
- 108.Park JS, Kim MH, Lee SK, Seo DW, Lee SS, Han J, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointestinal Endoscopy. 2003;57(1):78–85. doi: 10.1067/mge.2003.11. [DOI] [PubMed] [Google Scholar]
- 109.Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72(3):449–454. doi: 10.1097/00007890-200108150-00016. [see comment] [DOI] [PubMed] [Google Scholar]
- 110.Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28(3):638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 111.Bak T, Wachs M, Trotter J, Everson G, Trouillot T, Kugelmas M, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7(8):680–686. doi: 10.1053/jlts.2001.26509. [DOI] [PubMed] [Google Scholar]
- 112.Testa G, Malago M, Valentin-Gamazo C, Lindell G, Broelsch CE. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl. 2000;6(6):710–714. doi: 10.1053/jlts.2000.18706. [DOI] [PubMed] [Google Scholar]
- 113.Fan S-T, Lo C-M, Liu C-L, Yong B-H, Chan JK-F, Ng IO-L. Safety of Donors in Live Donor Liver Transplantation Using Right Lobe Grafts. Arch Surg. 2000;135(3):336–340. doi: 10.1001/archsurg.135.3.336. [DOI] [PubMed] [Google Scholar]
- 114.Settmacher U, Theruvath T, Pascher A, Neuhaus P. Living-donor liver transplantation--European experiences. Nephrol Dial Transplant. 2004;19(Suppl 4):iv16–21. doi: 10.1093/ndt/gfh1036. [DOI] [PubMed] [Google Scholar]
- 115.Parolin MB, Lazzaretti CT, Lima JH, Freitas AC, Matias JE, Coelho JC. Donor quality of life after living donor liver transplantation. Transplant Proc. 2004;36(4):912–913. doi: 10.1016/j.transproceed.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 116.Ghobrial RM, Saab S, Lassman C, Lu DS, Raman S, Limanond P, et al. Donor and recipient outcomes in right lobe adult living donor liver transplantation. Liver Transpl. 2002;8(10):901–909. doi: 10.1053/jlts.2002.35548. [DOI] [PubMed] [Google Scholar]
- 117.Malago M, Testa G, Frilling A, Nadalin S, Valentin-Gamazo C, Paul A, et al. Right living donor liver transplantation: an option for adult patients: single institution experience with 74 patients. Ann Surg. 2003;238(6):853–862. doi: 10.1097/01.sla.0000098619.71694.74. discussion 862-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garcia-Retortillo M, Forns X, Llovet JM, Navasa M, Feliu A, Massaguer A, et al. Hepatitis C recurrence is more severe after living donor compared to cadaveric liver transplantation. Hepatology. 2004;40(3):699–707. doi: 10.1002/hep.20357. [DOI] [PubMed] [Google Scholar]
- 119.Russo MW, Galanko J, Beavers K, Fried MW, Shrestha R. Patient and graft survival in hepatitis C recipients after adult living donor liver transplantation in the United States. Liver Transpl. 2004;10(3):340–346. doi: 10.1002/lt.20090. [DOI] [PubMed] [Google Scholar]
- 120.Brown RS., Jr. Is recurrence of hepatitis C worse after living donor or deceased donor liver transplantation? Liver Transpl. 2004;10(10):1256. doi: 10.1002/lt.20284. [DOI] [PubMed] [Google Scholar]
- 121.Sugawara Y, Kaneko J, Akamatsu N, Kishi Y, Hata S, Kokudo N, et al. Living donor liver transplantation for end-stage hepatitis C. Transplant Proc. 2004;36(5):1481–1482. doi: 10.1016/j.transproceed.2004.04.076. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez-Luna H, Vargas HE, Sharma P, Ortiz J, De Petris G, Balan V, et al. Hepatitis C virus recurrence in living donor liver transplant recipients. Dig Dis Sci. 2004;49(1):38–41. doi: 10.1023/b:ddas.0000011599.78222.9e. [DOI] [PubMed] [Google Scholar]
- 123.Ohkubo M, Nagino M, Kamiya J, Yuasa N, Oda K, Arai T, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239(1):82–86. doi: 10.1097/01.sla.0000102934.93029.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hisatsune H, Yazumi S, Egawa H, Asada M, Hasegawa K, Kodama Y, et al. Endoscopic management of biliary strictures after duct-to-duct biliary reconstruction in right-lobe living-donor liver transplantation. Transplantation. 2003;76(5):810–815. doi: 10.1097/01.TP.0000083224.00756.8F. [DOI] [PubMed] [Google Scholar]
- 125.Taber DJ, Dupuis RE, Fann AL, Andreoni KA, Gerber DA, Fair JH, et al. Tacrolimus dosing requirements and concentrations in adult living donor liver transplant recipients. Liver Transpl. 2002;8(3):219–223. doi: 10.1053/jlts.2002.30885. [DOI] [PubMed] [Google Scholar]
- 126.Russo MW, Brown RS., Jr. Is the cost of adult living donor liver transplantation higher than deceased donor liver transplantation? Liver Transpl. 2004;10(3):467–468. doi: 10.1002/lt.20102. [DOI] [PubMed] [Google Scholar]
- 127.Longworth L, Bryan S. An empirical comparison of EQ-5D and SF-6D in liver transplant patients. Health Econ. 2003;12(12):1061–1067. doi: 10.1002/hec.787. [DOI] [PubMed] [Google Scholar]
- 128.Walter M, Dammann G, Papachristou C, Pascher A, Neuhaus P, Danzer G, et al. Quality of life of living donors before and after living donor liver transplantation. Transplant Proc. 2003;35(8):2961–2963. doi: 10.1016/j.transproceed.2003.10.048. [DOI] [PubMed] [Google Scholar]


