Abstract
Purpose
To describe the prevalence and pattern of dyslipidemia in children with chronic kidney disease (CKD).
Methods
391 children aged 1–16 yrs underwent measurement of serum triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C). GFR by plasma disappearance of iohexol and urine protein/creatinine ratio (Up/c) were concomitantly measured. Multivariate analysis adjusted for age, gender, body mass index (BMI), GFR, and Up/c.
Results
The median GFR and age were 43 ml/min/1.73m2 and 12 years. Proteinuria was nephrotic range (Up/c > 2) in 12% and BMI exceeded the 95th percentile in 15%.
32% of the cohort had TG > 130 mg/dL. 21% had HDL-C < 40 mg/dl, and 16% had non-HDL-C > 160 mg/dL. Lower GFR was associated with higher TG, lower HDL-C and higher non-HDL-C.
Overall, 45% of the cohort had dyslipidemia, defined as one or more abnormal lipid measure; 45% of those had combined dyslipidemia. Nephrotic range proteinuria was associated with dyslipidemia and combined dyslipidemia to an equal and large degree: odds ratio (OR) of 4.16 (95% CI: 1.96, 8.79). Compared to children with GFR > 50 ml/min/1.73m2, children with GFR < 30 ml/min/1.73m2 had an OR of 2.9 (95% CI: 1.47, 5.70) for any dyslipidemia and an OR of 8.58 (95% CI: 3.70, 19.88) for combined dyslipidemia.
Conclusions
Among children with moderate CKD, dyslipidemia is common and is associated with lower GFR, nephrotic proteinuria, and non-renal factors including age and obesity.
Introduction
Individuals with chronic kidney disease (CKD) suffer an exceptionally high burden of atherosclerotic cardiovascular disease (ASCVD).(1) Dyslipidemia, a known risk factor for atherosclerosis, is frequent among both adults and children with CKD.(2, 3) In addition, there is evidence to suggest that dyslipidemia contributes to the initiation and progression of CKD itself.(4–9) While CKD patients are commonly burdened with multiple cardiovascular risk factors, dyslipidemia is an important focus of clinical CKD research since it is both highly prevalent and a potentially modifiable exposure.
Accelerated atherosclerosis in children with CKD is likely. Within the general population, atherosclerosis begins during childhood and dyslipidemia is a risk factor for its development.(10–12) Young adult survivors of childhood end-stage renal disease (ESRD) experience an extremely high rate of premature mortality due to ASCVD, their principal cause of death.(13–16) However, compared to the adult population, data about dyslipidemia in children with CKD remain scarce.
The primary aim of this cross-sectional investigation was to describe the prevalence and pattern of dyslipidemia in children with moderate CKD using data collected on participants in the Chronic Kidney Disease in Children (CKiD) Study. We also aimed to identify clinical and laboratory factors associated with dyslipidemia in children with CKD.
Results
As of May 2009, 574 children had completed a baseline study visit in CKiD. A subset of 427 children with known age, sex, race and CKD diagnosis had complete lipid and GFR. After excluding 11 children currently taking lipid lowering medication and 25 children known not to be fasting, 391 children remained for analysis. In 29% (n=112) of this group, fasting status was not recorded while the remainder (71%, n=279) of the children were confirmed to be fasting. Of all GFR measures, 94% (n=368) were direct (iGFR) by plasma iohexol disappearance.
Characteristics of the study population are shown in Table 1, including overall lipid measures. In general, the population had a slight male predominance, was mostly Caucasian, and was skewed toward overweight. A significant minority (31%) had moderate or nephrotic range proteinuria.
Table 1.
Study Population Characteristics, N=391
| Characteristic | median [IQR] or %(n) |
|---|---|
| Age, years | 12 [8,15] |
| Male | 60% (236) |
| Race | |
| White | 71% (277) |
| Black | 15% (57) |
| Other/mixed | 15% (57) |
| Hispanic Ethnicity* | 14% (55) |
| GFR, ml/min/1.73m²† | 43 [32,55] |
| Glomerular CKD | 19% (76) |
| Proteinuria (Up/c, mg/mg)* | 0.4 [0.2,1.2] |
| Normal, Up/c < 0.2 | 27% (103) |
| Mild, 0.2 ≤ Up/c < 1.0 | 42% (160) |
| Moderate, 1.0 ≤ Up/c < 2.0 | 19% (72) |
| Nephrotic, Up/c ≥ 2.0 | 12% (44) |
| BMI percentile‡* | 61[32,85] |
| Overweight, 85 < BMI percentile‡ ≤ 95 | 10% (37) |
| Obese, BMI percentile‡ > 95 | 15% (57) |
| Triglycerides, mg/dL | 106 [75, 141] |
| Total cholesterol, mg/dL | 174 [154, 194] |
| HDL – cholesterol, mg/dL | 47 [40, 55] |
| non-HDL - cholesterol, mg/dL | 126 [107, 147] |
Missing data: Hispanic ethnicity, n=3; Up/c, n=12; BMI percentile, n=16
n=368 (94%) directly measured by iohexol plasma disappearance; n=23 (6%) based on estimating equation.
Age and gender-specific BMI percentiles were calculated using 2000 CDC standard growth charts for United States children(57)
Triglycerides
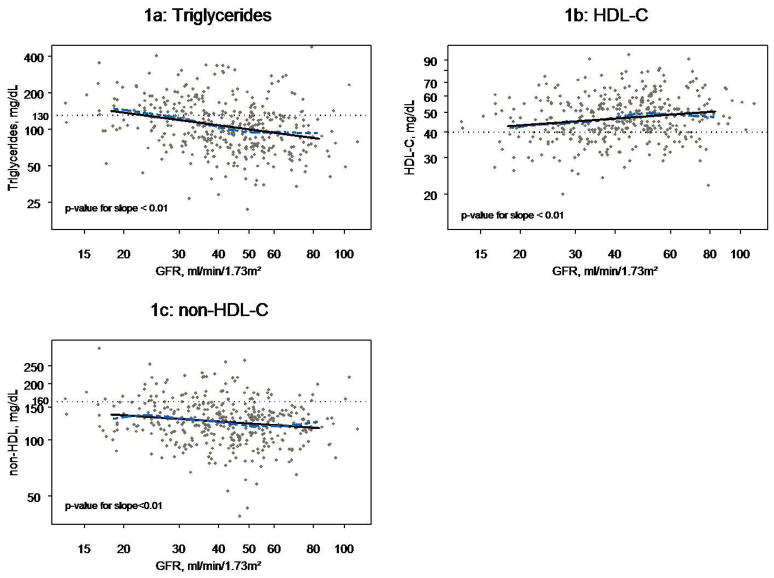
Figure 1a shows the relationship between TG levels and GFR. Univariately, log(TG) and GFR displayed a linear relationship with TG levels increasing on average 8% (95% CI: 5%, 11%) for every 10 ml/min/1.73m2 decrease in GFR (Table 2a).While a relationship was suggested between elevated TG and subnephrotic proteinuria, this was clearer in subjects with nephrotic range proteinuria, who displayed TG levels on average 55% higher (95% CI: 32, 84) than those with normal Up/c. The prevalence of hypertriglyceridemia in children with nephrotic range proteinuria was 61%, as compared to 21%, 30% and 24% in children with normal, mild, and moderate proteinuria, respectively.
Figure 1.
Figures 1a, 1b, and 1c: Scatterplots of Triglycerides, HDL-C, and non-HDL-C, respectively, by GFR for N=391 children with moderate CKD overlayed with lowess smoothing curve (dashed line) and linear regression line (solid line). All axes are log scale.
Table 2a.
Results of Uni- and multi-variate linear regression models of log-transformed Serum Total Triglycerides (TG) on select clinical CKD and anthropometric measures, N=391
| % Change in TG* | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted† | |||
| Est. (95% CI) | p-value | Est. (95% CI) | p-value | |
| Age, per 3 yr. increase | 5% (2,9) | <0.01 | 4% (0,7) | 0.03 |
| Male | 0% (−9,11) | 0.98 | 1% (−8,11) | 0.84 |
| GFR, per 10 ml/min/1.73m2 decrease | 8% (5,11) | <0.01 | 7% (4,10) | <0.01 |
| Proteinuria (vs. Normal,Up/c<0.2) | ||||
| mild, 0.2≤ Up/c <1.0 | 21% (7,36) | <0.01 | 18% (5,33) | <0.01 |
| moderate, 1.0≤ Up/c <2.0 | 15% (0,32) | 0.06 | 3% (−11,19) | 0.69 |
| nephrotic, Up/c ≥ 2.0 | 55% (32,84) | <0.01 | 34% (13,59) | <0.01 |
| BMI percentile (vs. BMI percentile≤85) | ||||
| Overweight, 85<BMI percentile ≤95 | 18% (0,40) | 0.04 | 29% (10,51) | <0.01 |
| Obese, BMI percentile>95 | 28% (12,47) | <0.01 | 30% (13,48) | <0.01 |
Percent change of lipid level = (eβjΔxj − 1) × 100 where xj and βj for j = 1, 2, 3, …, k are the k independent variables and regression coefficients, respectively.
Adjusted for all variables shown. Based on N=364 due to missing data (n=12 missing Up/c; n=16 missing BMI percentile).
After multivariate adjustment, higher TG levels continued to be associated with increased age, decreased GFR, proteinuria, and being either overweight or obese (Table 2a). Children who were overweight or obese, or had nephrotic proteinuria had TG levels on average 30% higher than those of children with normal weight and normal Up/c, a magnitude of association greater than that for a 30 ml/min/1.73m2 decrease in GFR (23%, not shown in table). Higher TG levels were associated with mild proteinuria (18% increase in TG) and nephrotic proteinuria (34% increase in TG), but not with moderate proteinuria (3% increase in TG). Restricting analysis to the n=279 children who self-reported overnight fasting did not qualitatively change the reported associations.
Cholesterol
The cohort demonstrated modest hypercholesterolemia: 21% of the cohort had total cholesterol (TC) greater than 200 mg/dL; 21% had HDL-C less than 40 mg/dL, and 16% had non-HDL-C greater than 160 mg/dL.
HDL-C Analysis
As shown in Figure 1b, a linear relationship was observed between log(HDL-C) and GFR. After multivariate adjustment, lower HDL-C levels were associated with increased age, decreased GFR, mild proteinuria, and obesity (Table 2b). In general, the magnitudes of association were smaller between HDL-C and the set of modeled risk factors than those observed with TG. Each 10 ml/min/1.73 m2 decrease of GFR was associated with a 3% decrease in HDL-C (95% CI: −4%, −1%). Compared to those children without proteinuria, HDL-C levels were 7% lower (95% CI −13%, −2%) in children with mild proteinuria, while children with moderate or nephrotic range proteinuria showed no decrease in HDL-C levels. Obese children had average HDL-C levels 14% (95% CI: −20%, −8%) lower than those neither overweight nor obese.
Table 2b.
Results of Uni- and multi-variate linear regression models of log-transformed HDL-C on select clinical CKD and anthropometric measures, N=391
| % Change in HDL-C* | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted† | |||
| Est. (95% CI) | p-value | Est. (95% CI) | p-value | |
| Age, per 3 yr. increase | −5% (−6, −3) | <0.01 | −5% (−7, −3) | <0.01 |
| Male | −3% (−7,2) | 0.28 | −5% (−9,0) | 0.06 |
| GFR, per 10 ml/min/1.73m2 decrease | −2% (−4, −1) | <0.01 | −3% (−4, −1) | <0.01 |
| Proteinuria (vs. Normal,Up/c<0.2) | ||||
| mild, 0.2≤ Up/c <1.0 | −7% (−13, −1) | 0.02 | −7% (−13, −2) | 0.01 |
| moderate, 1.0≤ Up/c <2.0 | −1% (−8,7) | 0.88 | 4% (−3,12) | 0.31 |
| nephrotic, Up/c ≥ 2.0 | −5% (−13,4) | 0.29 | 3% (−6,12) | 0.51 |
| BMI percentile (vs. BMI percentile≤85) | ||||
| Overweight, 85<BMI percentile ≤95 | −3% (−11,6) | 0.51 | −7% (−14,1) | 0.09 |
| Obese, BMI percentile>95 | −13% (−19, −6) | <0.01 | −14% (−20, −8) | <0.01 |
Non-HDL-C and TC Analysis
There was a borderline statistically significant and quantitatively smallassociation between higher non-HDL-C and lower GFR (Figure 1c). After multivariate adjustment, for each 10 ml/min/1.73 m2 lower GFR, non-HDL-C was approximately 2% greater (95% CI: 0%, 3%) as shown in (Table 2c). No relationship was observed between TC and GFR (not shown). In contrast, compared to normal Up/c, nephrotic range proteinuria was associated with a 31% (95% CI: 18%, 44%) increase in non-HDL-C and a 23% (95% CI: 14%, 32%) increase in TC (not shown).
Table 2c.
Results of Uni- and multi-variate linear regression models of log-transformed non-HDL-C on select clinical CKD and anthropometric measures, N=391
| % Change in non-HDL-C* | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted† | |||
| Est. (95% CI) | p-value | Est. (95% CI) | p-value | |
| Age, per 3 yr. increase | 1% (−1,3) | 0.19 | 0% (−2,2) | 0.84 |
| Male | 0% (−5,6) | 0.96 | −1% (−6,5) | 0.81 |
| GFR, per 10 ml/min/1.73m2 decrease | 2% (1,4) | <0.01 | 2% (0,3) | 0.05 |
| Proteinuria (vs. Normal,Up/c<0.2) | ||||
| mild, 0.2≤ Up/c <1.0 | 7% (0,14) | 0.05 | 6% (−1,14) | 0.07 |
| moderate 1.0≤ Up/c <2.0 | 4% (−4,12) | 0.43 | 2% (−7,10) | 0.72 |
| nephrotic, Up/c ≥ 2.0 | 32% (21,45) | <0.01 | 31% (18,44) | <0.01 |
| BMI percentile (vs. BMI percentile ≤85) | ||||
| Overweight, 85<BMI percentile ≤95 | 4% (−5,14) | 0.40 | 7% (−3,17) | 0.16 |
| Obese, BMI percentile>95 | 13% (5,22) | <0.01 | 12% (4,21) | <0.01 |
Dyslipidemic Profiles
One third (32%) of children had elevated TG, while 21% had low HDL-C and 16% had high non-HDL-C. Of the 45% with dyslipidemia, 45% displayed combined (multiple-marker) dyslipidemia, defined as the presence of 2 or more lipid abnormalities, as shown in Table 3.(17, 18) While both low HDL-C and high non-HDL-C were univariately associated with hypertriglyceremia (Chi-square p-value<0.01 for both), the joint bivariate distribution of the two abnormal cholesterol markers did not differ by hypertriglyeremia status (p=0.16 by Breslow-Day Test for homogeneity of odds ratios) suggesting that neither abnormal cholesterol marker was more likely to be associated with hypertriglyeremia than the other.
Table 3.
Prevalence of Dyslipidemia Markers, N=391
| Number of markers of Dyslipidemia | TG >130 mg/dl | HDL-C <40 mg/dl | non-HDL-C >160 mg/dl | % (n) | % (n) | |
|---|---|---|---|---|---|---|
| 0/3 | no | no | no | 55% (214) | 55% (214) |
|
|
| ||||||
| 1/3 | yes | no | no | 12% (47) | 25 % (98) | |
| no | yes | no | 8% (31) | |||
| no | no | yes | 5% (20) | |||
|
| ||||||
| 2/3 | yes | yes | no | 9% (37) | 16 % (64) | |
| yes | no | yes | 7% (27) | |||
| no | yes | yes | 0 % (0) | |||
|
| ||||||
| 3/3 | yes | yes | yes | 4% (15) | 4% (15) | |
|
| ||||||
| Totals by Marker | 32 % (126) | 21% (83) | 16% (62) | |||
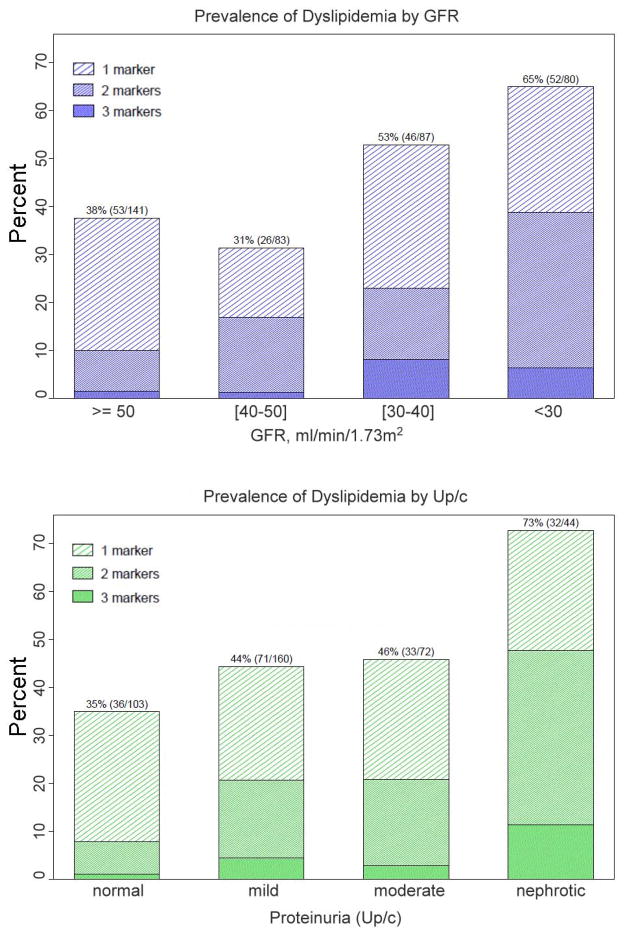
Analysis of the prevalence of dyslipidemia profiles is presented in Figures 2a, 2b and Table 4. As shown in Figure 2a, lower levels of GFR were associated not only with an increased prevalence of dyslipidemia as defined by the presence of at least one abnormal lipid measure, but also with an increased prevalence of combined dyslipidemia (multiple abnormal lipid measures) (p<0.01 for differences in the distribution of number of dyslipidemia markers by GFR category based on Chi-Square Test of Association for an R x C contingency table). Similarly, increased prevalence of dyslipidemia and combined dyslipidemia was also seen with increasing levels of proteinuria (Figure 2b; p<0.01 for differences in the distribution of number of dyslipidemia markers by level of proteinuria based on Chi-Square Test of Association for an R x C contingency table).
Figure 2.
Figures 2a and 2b: Prevalence of Dyslipidemia and combined dyslipidemia by (a) GFR and (b) Up/c for N=391 children with moderate CKD.
Table 4.
Adjusted odds ratios (95% CI) for the prevalence of dyslipidemia markers* using Partial Proportional Odds Model for ordinal response variables, N=364
| Variable | Adjusted OR (95% CI) † |
||
|---|---|---|---|
| 1+ markers vs. 0 markers | 2+ markers vs. <2 markers | p-value for difference in ORs | |
| GFR, ml/min/1.73m2 | |||
| ≥50 | 1.00 (ref) | 1.00 (ref) | - |
| [40–50) | 0.66 (0.34, 1.28) | 2.16 (0.87, 5.36) | 0.002 |
| [30–40) | 1.65 (0.92, 2.97) | 3.21 (1.47, 7.01) | 0.091 |
| <30 | 2.90 (1.47, 5.70) | 8.58 (3.70, 19.88) | 0.007 |
| Proteinuria, Up/c | |||
| normal, Up/c<0.2 | 1.00 (ref) | 1.00 (ref) | - |
| mild, 0.2≤ Up/c <1.0 | 1.95 (1.14, 3.33) | 1.95 (1.14, 3.33) | na‡ |
| moderate, 1.0≤ Up/c <2.0 | 1.33 (0.68, 2.58) | 1.33 (0.68, 2.58) | na‡ |
| nephrotic, Up/c ≥ 2.0 | 4.16 (1.96, 8.79) | 4.16 (1.96, 8.79) | na‡ |
| Age, per 3 year increase | 1.27 (1.07, 1.50) | 1.27 (1.07, 1.50) | na‡ |
| Male vs. female | 1.18 (0.76, 1.85) | 1.18 (0.76, 1.85) | na‡ |
| BMI percentile | |||
| Normal, BMI percentile≤85 | 1.00 (ref) | 1.00 (ref) | - |
| Overweight, BMI percentile (85–95] | 2.82 (1.31, 6.03) | 6.75 (2.78, 16.35) | 0.022 |
| Obese, BMI percentile >95 | 2.43 (1.33, 4.45) | 5.38 (2.64, 10.97) | 0.022 |
– Markers of dyslipidemia are: TG>130 mg/dL, HDL-C<40 mg/dL, non-HDL-C>160 mg/dL
– All models contain the variables shown in the table.
– na= not applicable; modeled with a single estimate (proportional odds).
The results of the multivariate PPOM estimating associations between the prevalence of dyslipidemic measure and GFR, proteinuria, age, sex and BMI are displayed in Table 4. The adjusted relative odds of having combined dyslipidemia (≥2 vs. <2 lipid abnormalities) for different levels of proteinuria, increasing age and female sex, respectively, were similar to those for having any level of dyslipidemia (≥1 vs. 0 lipid abnormalities) and were therefore modeled using a single estimate (i.e., proportional odds was met). Compared to normal proteinuria, nephrotic proteinuria was strongly associated with a) dyslipidemia in general, and to an equal degree with b) combined dyslipidemia (OR=4.16, 95% CI: (1.96, 8.79) for both estimates). Mild proteinuria and increasing age demonstrated similar relationships with dyslipidemia, though to a lesser degree than nephrotic range proteinuria.
Lower GFR was associated with dyslipidemia. However, unlike with proteinuria, GFR displayed non-proportional odds with respect to overall and combined dyslipidemia (Table 4): lower GFR was more strongly associated with combined dyslipidemia than with overall dyslipidemia. While children with GFR<30 were about 3 times more likely to have any dyslipidemia than children with GFR≥50 ml/min/1.73m2, they were 8 to 9 times more likely to have combined dyslipidemia than children with GFR≥50 ml/min/1.73m2. Likewise, while children with GFR 30 to 40 ml/min/1.73m2 did not have statistically significant higher odds of dyslipidemia compared to children with GFR≥50 ml/min/1.73m2, they did have higher odds of having combined dyslipidemia (OR=3.21, 95% CI (1.47, 7.01)). Being overweight or obese also displayed non-proportional odds with respect to dyslipidemia outcomes. While overweight or obese individuals were 2–3 times more likely to have any dyslipidemia than normal weight children, they were 5 to 7 times more likely to have combined dyslipidemia.
Discussion
This cross-sectional analysis of baseline lipid data from the CKiD cohort demonstrates that among children with moderate CKD, dyslipidemia is common and independently associated with lower GFR and nephrotic range proteinuria. In particular, we report a high prevalence of hypertriglyceridemia, increased non-HDL-C, reduced HDL-C, and combined dyslipidemias, particularly with lower GFR. Nephrotic range proteinuria is associated with increased TG and non-HDL-C without significant effect on HDL-C. While these findings are compatible with data from adult populations with CKD,(19) this report is a significant contribution beyond the adult literature because the majority of participants have primary renal disease and few co-existing conditions; hence, the dyslipidemic effects of CKD are more clearly delineated. The clinical significance of the high prevalence of “atherogenic dyslipidemia”(20–22) in this pediatric population lies in the potential risks of premature morbidity and mortality due to accelerated ASCVD as well as the potential to accelerate CKD progression.
The primary mechanisms of dyslipidemia in CKD are thought to involve impaired TG lipolysis, associated with increased Apolipoprotein C-III (an inhibitor of lipoprotein lipase) and reduced insulin sensitivity in the vascular endothelium of skeletal muscle and other major sites of TG (fatty acid) energy utilization.(3) As a result, chylomicron and VLDL remnants (in our study, these are best represented by non-HDL-C) are increased and there is secondary TG enrichment of all of the lipoprotein fractions. Triglyceride enrichment of HDL mediated by cholesterol ester transfer protein (CETP) is of particular relevance as it simultaneously depletes cholesterol from HDL. Furthermore, TG enrichment of HDL leads to its accelerated degradation, noting that impaired maturation of HDL may also contribute to lower HDL-C. Conversely, impaired hepatic clearance of non-HDL lipoproteins results in prolonged circulation, and therefore higher serum concentrations. Our findings are consistent with these proposed mechanisms both in terms of individual lipid abnormalities and combined dyslipidemia.
It is note-worthy that while combined dyslipidemias were present in only 20% in our overall study population, among those children with dyslipidemia, almost half had combined dyslipidemia. Even after adjusting for age, sex, proteinuria level and BMI, lower kidney function was strongly associated with combined dyslipidemia. The excess burden of combined dyslipidemia was present among children with GFR in the 30–40 ml/min/1.73m2 range but more evident among children with GFR<30 ml/min/1.73m2: they were 3 times more likely to have some profile of dyslipidemia (65% prevalence) and nearly 9 times more likely (39% prevalence) to have combined dyslipidemia. This suggests that declining GFR progressively elicits and intensifies abnormal lipoprotein metabolism, both in terms of the proportion expressing dyslipidemia and the degree to which it is expressed. It is important to highlight that the presented odds of dyslipidemia and combined dyslipidemia are not in relation to healthy children but rather to other children in the cohort with CKD and GFR > 50 ml/min/1.73m2. Therefore, as striking as the relative odds of dyslipidemia are, these findings likely under represent the odds of dyslipidemia in relation to the general population.
Little information exists about the dyslipidemic effects of proteinuria in the sub-nephrotic range and our results are somewhat unexpected. Compared to no proteinuria, mild proteinuria (0.2 ≤ Up/c < 1.0) was associated with higher TG but the next higher level of proteinuria (1.0 ≤ Up/c < 2.0) was not. Lower HDL-C was associated with mild proteinuria despite the fact HDL-C was not affected by nephrotic range proteinuria. We interpret these findings with caution, considering these are sub-group analyses and there is potential for unknown confounders despite multivariate adjustment. At the same time, analysis of proteinuria as a categorical variable is useful as this approach provides results which are both clinically applicable and which quantitatively acknowledge significant elements of nonlinearity that exist between lipid levels and continuous Up/c values (not shown).
It is worthwhile to consider potential mechanisms to explain the dyslipidemic effects of the various levels of proteinuria. The lipid profile of nephrotic syndrome reflects a composite and extreme expression of multiple concomitant disturbances of lipoprotein metabolism(23) and we can hypothesize that intermediate levels of proteinuria elicit graded and differential expression of those pathophysiological mechanisms. For example, lipolysis may be inhibited with only mild proteinuria, leading to increased TG and resulting in decreased HDL-C via (cholesterol ester transfer protein (CETP)-mediated) exchange of cholesteryl esters (CE) into ApoB lipoproteins in exchange for TG. Decreased lecithin-cholesterol acyltransferase (LCAT) activity could also reduce HDL-C. Hepatic expression of the scavenger receptor class B member 1 (SCARB1, also known as SR-B1, a receptor for HDL that mediates the selective uptake of HDL-C) might be diminished by increasing degrees of proteinuria, reducing reverse cholesterol transport and restoring HDL-C levels. The latter might sustain CE-TG exchange, facilitating TG catabolism via the action of hepatic lipase on TG-enriched HDL, offering a potential explanation of the null effect of moderate proteinuria on TG. With nephrotic range proteinuria, perhaps the hepatic lipase pathway is down-regulated or becomes fully saturated, allowing unmitigated elevation of TG despite preserved HDL-C.
A general limitation of defining dyslipidemia in children is a lack of a comprehensive normative set of data that includes TG levels in young (< 12 years) children and significant variability in lipid values with respect to gender, age, and ethnicity. Furthermore, because ASCVD does not occur in the general population of children, levels associated with “risk” must be inferred from adult populations. We adopted conservative cut-points to define “dyslipidemia” with respect to these considerations; as such, our results may further underestimate the prevalence of dyslipidemia, particularly in younger children and among African American children in the cohort, in whom TG levels are generally lower. Our analysis however, did not rely only on these cut points; the variation of lipids with GFR and proteinuria was independent of such considerations. Another limitation of this report is the lack of comprehensive lipoprotein analysis. Alterations in lipoprotein particle number, differences in apolipoprotein content and biophysical abnormalities of lipoproteins such as small dense LDL (low density lipoproteins) or altered oxidation status are relevant examples of expected abnormalities in CKD. As discussed below, the performance of the direct HDL-C assay could hypothetically be affected by those unique abnormalities, noting that the other techniques suitable for automation and small sample volumes are also subject to this problem. For example, a mean difference of 3.5 mg/dl HDL-C between an unrelated homogenous assay and a precipitation technique was reported in nephrotic subjects.(24) Stored biorepository samples, samples obtained at future visits, and analysis of important ancillary measures like insulin will provide opportunity for further investigation of these important physiological and technical issues. Finally, the cross-sectional design of the analysis does not permit us to draw conclusions about the temporal relationship between dyslipidemia and CKD progression. The longitudinal design of CKiD is ideal for addressing this limitation, and ongoing study goals include describing the evolution of dyslipidemic profiles over time and in relation to CKD progression.
The CKiD study has many other strengths, including a large sample size, precise measurement of GFR by iohexol clearance, and standardized demographic, clinical and laboratory measures. These aspects of the study, in addition to the previously mentioned advantage of studying lipoprotein physiology in children with primary CKD and little co-morbidity greatly enhance the significance of the findings.
Discussion of the risk of atherosclerosis in the CKiD cohort requires a long-term perspective about the lifetime of children with CKD. Unfortunately, CKD remains an irreversible and progressive condition. Historically, the majority of children with significant CKD develop ESRD--half within five years, 70% within ten years.(25, 26) Functional abnormalities of the vascular endothelium have implicated ASCVD in children with moderate (stage 3–4) CKD and ESRD.(27–36) Subclinical ASCVD during childhood has been demonstrated by vascular imaging, in small autopsy series, and by sampling vasculature at the time of kidney transplant during the course of childhood CKD/ESRD.(35–42) The expected remaining lifetime after ESRD in the 0–14 year age group is 30 years(43) and the principal cause of death during young adulthood is premature cardiovascular disease, including ASCVD.(13–16) Dyslipidemia and other CVD risk factors are a research focus because they are deep-rooted barriers to improving long-term survival in this population.
This study does not address treatment of dyslipidemia among children with CKD. Surprisingly, while the dyslipidemic profile itself is amenable to pharmaceutical treatment in CKD and ESRD, this has not been convincingly demonstrated to reduce mortality. Recently, two large clinical trials (“SHARP” and “AURORA”) were initiated to answer the question of whether LDL lowering therapy benefits patients with CKD or ESRD.(44, 45) Recently, the results of AURORA indicated that rosuvastatin has no effect on ASCVD-related mortality in hemodialysis patients. SHARP is still ongoing. Two other studies addressing the issue of whether statin therapy slows progression of CKD issue are underway.(46, 47) These studies should help fill the gap in evidence-based treatment recommendations for adult CKD patients and even greater uncertainty about potential use of pharmaceutical agents to treat dyslipidemia among children.
Current guidelines for management of dyslipidemias in patients with severe CKD from the 2003 National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (KDOQI)(2) do encompass children from the onset of puberty. Given the high prevalence of dyslipidemia in the CKiD cohort, our findings support the recommendation to screen children with CKD for dyslipidemia.
Methods
The CKiD Study is a multicenter prospective observational cohort study of CKD in children. The design and methods have been previously published; the study protocol was approved by the Institutional Review Board of each participating center.(48) Briefly, children with moderate CKD (estimated creatinine clearance 30–90 ml/min/1.73m2 as calculated by the original Schwartz equation(49, 50)) and between the ages of 1 and 16 years were eligible to enter the study following signed written consent. Exclusion criteria included prior malignancy, transplantation, dialysis within 3 months, and a limited number of other conditions.
Data was collected through the second study visit, when serum triglycerides (TG), total cholesterol (TC), and HDL cholesterol (HDL-C) were first measured in CKiD participants. Samples were drawn after an overnight fast of at least 10 hours. Free access to water was allowed.
Blood was drawn into a serum separator tube and allowed to clot at room temperature for 30 minutes before centrifugation and serum separation. Serum was shipped to the central CKiD laboratory by next day mail or (if the next day was not a working laboratory day) refrigerated at 4º C and shipped the next available day. All lipid measurements were automated (Seimens Advia® 2400 analyzer). Serum TG and TC measurement followed routine enzymatic methods; HDL-C was analyzed by the Bayer® Clinical Method for ADVIA® 2400 Direct HDL Cholesterol (D-HDL) V 1.00.00 technique. Briefly, this involves detection of HDL-C following selective elimination of cholesterol from chylomicron and VLDL-remnants from the detection reactions.(51) Since older techniques have slightly different performances in subjects with CKD, it should be noted that CKD-specific validation of this method is lacking. However, this homogenous HDL-C technique is not subject to interference by TG < 1700 mg/dL or anemia, and is commercially certified via comparison protocols against multiple other HDL-C techniques. In all uses, analyzer calibration was confirmed by known standards. Non-HDL cholesterol was calculated as the difference between TC and HDL-C.
The following cut-points were used to define the presence of dyslipidemia: TG>130 mg/dL, HDL-C<40 mg/dL, and non-HDL-C>160 mg/dL. These cut-points were based upon:1) normative NHANES data available for children 12 and older, 2) normative data of the Lipid Research Clinic data set, and 3) levels frequently considered atherogenic in adults. (22, 52, 53)
For the majority of study participants, direct measurement of GFR was accomplished by the plasma disappearance of iohexol (Ominipaque, GE Healthcare, Princeton, NJ) method (iGFR), as detailed previously.(54) In the event iGFR was not available, GFR was estimated (eGFR) using a published estimating equation developed within this study population.(55) In this paper, the pooled grouping of iGFR and eGFR is referred to as GFR. Total urine protein and urine creatinine were measured in a first morning urine sample and expressed as the protein:creatinine ratio (Up/c) in units of mg/mg. Details of the techniques used to measure Up/c in CKiD have been published.(56) Proteinuria was categorized as normal (Up/c<0.2), mild (0.2≤Up/c<1.0), moderate (1.0≤Up/c<2.0), or nephrotic (Up/c≥2.0).
Non-laboratory data was obtained concomitantly using standardized forms and physical exam. Data collected and used in the current analysis includes age, sex, race, ethnicity, height, weight and primary CKD diagnosis. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Age and gender-specific BMI percentiles were calculated using 2000 Centers for Disease Control and Prevention (CDC) standard growth charts for United States children.(57) Overweight was defined as a BMI between the 85th and 95th percentiles, obesity as a BMI > 95th percentile. CKD diagnosis was classified as glomerular or non-glomerular; a more detailed description of this classification for specific diagnoses is published.(56)
Analysis was restricted to individuals with known age, sex, race, GFR, and CKD diagnosis as well as complete lipid measures. Excluded from the analysis were children receiving lipid-lowering medication and those who were known to have been non-fasting at the time the blood sample was obtained.
Statistical Analysis
Clinical and demographic characteristics for the study population were summarized using medians and interquartile ranges (IQR) for continuous variables and frequencies and percentages for categorical variables. Of primary interest was a comprehensive description of the relationship between the lipid concentrations and the major markers of CKD, namely GFR and proteinuria. Other variables of interest as well as potential confounders (selected a priori of analysis) included overweight status, age and sex.
Scatterplots of the log-transformed lipid measures on log-transformed GFR were developed and overlayed with lowess smoothing curves. Univariate and multivariate linear regression models were used to quantify the association between the respective log-transformed lipid levels and the risk factors of interest. These models took the form:
where α is the intercept, xj and βj for j = 1,2,3,…, k are the independent variables and regression coefficients, respectively, and e is the error assumed to be normally distributed with mean zero and variance σ2.(58) Each of the regression coefficients, βj, estimates average changes in the log-transformed value of the lipid marker for a unit change in the respective independent variable, xj. As such, the antilog of βjΔxj can be interpreted as a proportional lipid level (i.e. percent change) associated with a change in the independent variable, Δxj. That is,
Specifically, for categorical independent variables, we used Δxj = 1 representing the effect of being in a given category relative to the reference category; to describe the effect of continuous independent variables, we used Δxj = 3 years for age and Δxj = −10 for GFR (i.e., a decrease of 10 ml/min/1.73 m2).
The lipid markers were analyzed as log-transformed values to meet the assumption that the residuals of the regression models would be normally distributed. The validity of this assumption was confirmed by fitting a generalized gamma distribution and assessing whether the shape parameter estimate was statistically different from zero(59).
A second goal was to describe the prevalence and co-prevalence of the three separate markers of dyslipidemia in children with CKD. Prevalence of dyslipidemia by individual marker (high TG, low HDL, and high non-HDL) was summarized to show how the three markers jointly distribute themselves. Statistical independence of the three markers was assessed using Pearson Chi-Square tests for independence and the Breslow-Day Test for homogeneity of odds ratios.
A third goal was to describe the association of the number of dyslipidemia markers presented by a child with the aforementioned CKD risk factors of interest. In this analysis, a subject’s GFR was classified as ≥50, (50-40], (40-30] or <30 ml/min/1.73m2. Chi-Square Tests of Association were performed on 4x4 contingency tables to test for differences in the distribution of number of dyslipidemia markers (0,1,2, or 3) in subjects across a) the four categories of GFR, and b) the four categories of proteinuria. Because a relatively small number of children had three markers of dyslipidemia, subjects were classified as having zero, one, or two or more dyslipidemia markers for subsequent analysis. Using the SAS GENMOD procedure, multivariate partial proportional odds models (PPOM)(60, 61) were fit to this ordinal categorical variable with the same set of independent (risk factor) variables as the lipid specific linear regression models described above. As an extension of the more commonly used proportional odds model (cumulative logit), the PPOM allows for non-proportional odds as the binary cutoff of the ordinal outcome variable is moved from ≥ 1 vs. 0 markers to ≥2 vs. <2 markers. In other words, for a given risk factor (e.g. GFR<30 vs. GFR≥50), the relative odds of having combined dyslipidemia (≥2 markers) may be substantially stronger than the relative odds associated with having any dyslipidemia (≥1 markers). The PPOM allows for such non-linearity where it is warranted, thus allowing for a better fit of the data.
All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC); accompanying figures were created using S-Plus 8.0 (Insightful Corp., Seattle, WA). Significance tests with a p-value less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Supplemental Table 1: The Multicenter CKiD Investigators
Data in this article were collected by the CKiD study with clinical coordinating centers (principal investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, PhD), and data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, PhD) with the Central Biochemistry Laboratory at the University of Rochester (George J. Schwartz, MD). The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurologic Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, and UO1-DK-66116). The CKiD study has been supported by multiple participating institutional General Clinical Research Centers and Clinical Translational Research Centers.The CKID web site is located at http://www.statepi.jhsph.edu/ckid.
Support for this project at The Mount Sinai School of Medicine General Clinical Research Center was supported by the Grant Number MO1-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH
Footnotes
Disclosure:
None of the authors’ have relationships with companies that may have a financial interest in the information contained in the manuscript.
Preliminary results of this study were presented as a poster at the 2008 annual meeting of the American Society of Nephrology/World Congress of Nephrology in Philadelphia PA.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. Am J Kidney Dis. 2003;41:I–IV. S1–91. [PubMed] [Google Scholar]
- 3.Saland JM, Ginsberg HN. Lipoprotein metabolism in chronic renal insufficiency. Pediatr Nephrol. 2007;22:1095–1112. doi: 10.1007/s00467-007-0467-5. [DOI] [PubMed] [Google Scholar]
- 4.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Oikawa S, Sato H, Sasaki J. Lipoprotein glomerulopathy: renal lipidosis induced by novel apolipoprotein E variants. Nephron. 1999;83:193–201. doi: 10.1159/000045511. [DOI] [PubMed] [Google Scholar]
- 6.Moorhead JF, Chan MK, El Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 7.Diamond JR, Karnovsky MJ. A putative role of hypercholesterolemia in progressive glomerular injury. Annu Rev Med. 1992;43:83–92. doi: 10.1146/annurev.me.43.020192.000503. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Coresh J, Smith JC, Eckfeldt J, et al. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsson O, Attman PO, Knight-Gibson C, Larsson R, et al. Complex apolipoprotein B-containing lipoprotein particles are associated with a higher rate of progression of human chronic renal insufficiency. J Am Soc Nephrol. 1998;9:1482–1488. doi: 10.1681/ASN.V981482. [DOI] [PubMed] [Google Scholar]
- 10.Kwiterovich PO., Jr Recognition and management of dyslipidemia in children and adolescents. J Clin Endocrinol Metab. 2008;93:4200–4209. doi: 10.1210/jc.2008-1270. [DOI] [PubMed] [Google Scholar]
- 11.McMahan CA, Gidding SS, Viikari JS, Juonala M, et al. Association of Pathobiologic Determinants of Atherosclerosis in Youth risk score and 15-year change in risk score with carotid artery intima-media thickness in young adults (from the Cardiovascular Risk in Young Finns Study) Am J Cardiol. 2007;100:1124–1129. doi: 10.1016/j.amjcard.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahan CA, Gidding SS, Malcom GT, Tracy RE, et al. Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics. 2006;118:1447–1455. doi: 10.1542/peds.2006-0970. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: Bethesda, MD: USRDS Coordinating Center; 2008. http://www.usrds.org, 2008. [Google Scholar]
- 14.Groothoff JW, Gruppen MP, Offringa M, Hutten J, et al. Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61:621–629. doi: 10.1046/j.1523-1755.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 15.Gruppen MP, Groothoff JW, Prins M, van der Wouw P, et al. Cardiac disease in young adult patients with end-stage renal disease since childhood: a Dutch cohort study. Kidney Int. 2003;63:1058–1065. doi: 10.1046/j.1523-1755.2003.00814.x. [DOI] [PubMed] [Google Scholar]
- 16.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 17.Chow S, Rodgers P. Applet for Drawing 3-Set Proportional Area Venn Diagrams. Vol. 2009 [Google Scholar]
- 18.Chow S, Rodgers P. Constructing area-proportional venn and euler diagrams with three circles. Euler Designs Workshop; 2005; 2005. [Google Scholar]
- 19.Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis. 1998;32:S142–156. doi: 10.1053/ajkd.1998.v32.pm9820472. [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg HN. New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation. 2002;106:2137–2142. doi: 10.1161/01.cir.0000035280.64322.31. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM. Atherogenic dyslipidemia: lipoprotein abnormalities and implications for therapy. Am J Cardiol. 1995;75:45B–52B. doi: 10.1016/0002-9149(95)80011-g. [DOI] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 23.Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63:1964–1976. doi: 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 24.Simo JM, Castellano I, Ferre N, Joven J, et al. Evaluation of a homogeneous assay for high-density lipoprotein cholesterol: limitations in patients with cardiovascular, renal, and hepatic disorders. Clin Chem. 1998;44:1233–1241. [PubMed] [Google Scholar]
- 25.North American Pediatric Renal Trials and Collaborative Studies Annual Report. 2008. [Google Scholar]
- 26.Ardissino G, Dacco V, Testa S, Bonaudo R, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382–387. doi: 10.1542/peds.111.4.e382. [DOI] [PubMed] [Google Scholar]
- 27.Kari JA, Donald AE, Vallance DT, Bruckdorfer KR, et al. Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int. 1997;52:468–472. doi: 10.1038/ki.1997.354. [DOI] [PubMed] [Google Scholar]
- 28.Bennett-Richards KJ, Kattenhorn M, Donald AE, Oakley GR, et al. Oral L-arginine does not improve endothelial dysfunction in children with chronic renal failure. Kidney Int. 2002;62:1372–1378. doi: 10.1111/j.1523-1755.2002.kid555.x. [DOI] [PubMed] [Google Scholar]
- 29.Lilien MR, Stroes ES, Op’t Roodt J, de Jongh S, et al. Vascular function in children after renal transplantation. Am J Kidney Dis. 2003;41:684–691. doi: 10.1053/ajkd.2003.50131. [DOI] [PubMed] [Google Scholar]
- 30.Lilien MR, Koomans HA, Schroder CH. Hemodialysis acutely impairs endothelial function in children. Pediatr Nephrol. 2005;20:200–204. doi: 10.1007/s00467-004-1718-3. [DOI] [PubMed] [Google Scholar]
- 31.Covic A, Mardare N, Gusbeth-Tatomir P, Brumaru O, et al. Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant. 2006;21:729–735. doi: 10.1093/ndt/gfi196. [DOI] [PubMed] [Google Scholar]
- 32.Shroff RC, Donald AE, Hiorns MP, Watson A, et al. Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol. 2007;18:2996–3003. doi: 10.1681/ASN.2006121397. [DOI] [PubMed] [Google Scholar]
- 33.Hussein G, Bughdady Y, Kandil ME, Bazaraa HM, et al. Doppler assessment of brachial artery flow as a measure of endothelial dysfunction in pediatric chronic renal failure. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 34.Briese S, Claus M, Querfeld U. Arterial stiffness in children after renal transplantation. Pediatr Nephrol. 2008;23:2241–2245. doi: 10.1007/s00467-008-0894-y. [DOI] [PubMed] [Google Scholar]
- 35.Muscheites J, Meyer AA, Drueckler E, Wigger M, et al. Assessment of the cardiovascular system in pediatric chronic kidney disease: a pilot study. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-0906-y. [DOI] [PubMed] [Google Scholar]
- 36.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, et al. Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation. 2004;110:97–101. doi: 10.1161/01.CIR.0000133412.53089.26. [DOI] [PubMed] [Google Scholar]
- 37.Litwin M, Wuhl E, Jourdan C, Trelewicz J, et al. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol. 2005;16:1494–1500. doi: 10.1681/ASN.2004110932. [DOI] [PubMed] [Google Scholar]
- 38.Milliner DS, Zinsmeister AR, Lieberman E, Landing B. Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int. 1990;38:931–936. doi: 10.1038/ki.1990.293. [DOI] [PubMed] [Google Scholar]
- 39.Pennisi AJ, Heuser ET, Mickey MR, Lipsey A, et al. Hyperlipidemia in pediatric hemodialysis and renal transplant patients. Associated with coronary artery disease. Am J Dis Child. 1976;130:957–961. doi: 10.1001/archpedi.1976.02120100047007. [DOI] [PubMed] [Google Scholar]
- 40.Nayir A, Bilge I, Kilicaslan I, Ander H, et al. Arterial changes in paediatric haemodialysis patients undergoing renal transplantation. Nephrol Dial Transplant. 2001;16:2041–2047. doi: 10.1093/ndt/16.10.2041. [DOI] [PubMed] [Google Scholar]
- 41.Oh J, Wunsch R, Turzer M, Bahner M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 42.Goodman WG, Goldin J, Kuizon BD, Yoon C, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Renal Data System. Bethesda, MD: USRDS Coordinating Center UR; 2007. USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Includes Special Request, Table H.31 Combined Transplant and Dialysis Groups) http://www.usrds.org. [Google Scholar]
- 44.Baigent C, Landry M. Study of Heart and Renal Protection (SHARP) Kidney Int Suppl. 2003:S207–210. doi: 10.1046/j.1523-1755.63.s84.4.x. [DOI] [PubMed] [Google Scholar]
- 45.Fellstrom B, Holdaas H, Jardine AG, Rose H, et al. Effect of rosuvastatin on outcomes in chronic haemodialysis patients: baseline data from the AURORA study. Kidney Blood Press Res. 2007;30:314–322. doi: 10.1159/000106803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Statins in Proteinuric Nephropathies Study NCT00199927 referenced online at Clinicaltrials.gov.
- 47.Fassett RG, Ball MJ, Robertson IK, Geraghty DP, et al. The Lipid lowering and Onset of Renal Disease (LORD) Trial: a randomized double blind placebo controlled trial assessing the effect of atorvastatin on the progression of kidney disease. BMC Nephrol. 2008;9:4. doi: 10.1186/1471-2369-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 50.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 51.Warnick GR, Nauck M, Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin Chem. 2001;47:1579–1596. [PubMed] [Google Scholar]
- 52.Christensen B, Glueck C, Kwiterovich P, Degroot I, et al. Plasma cholesterol and triglyceride distributions in 13,665 children and adolescents: the Prevalence Study of the Lipid Research Clinics Program. Pediatr Res. 1980;14:194–202. doi: 10.1203/00006450-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz GJ, Furth S, Cole SR, Warady B, et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz GJ, Munoz A, Schneider MF, Mak RH, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong CS, Pierce CB, Cole SR, Warady BA, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 58.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312:1079. doi: 10.1136/bmj.312.7038.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 60.Peterson B, Harrell FE. Partial Proportional Odds Models For Ordinal Response Variables. Applied Statistics-Journal Of The Royal Statistical Society Series C. 1990;39:205–217. [Google Scholar]
- 61.Stokes M, Davis C, Koch G. Categorical Data Analysis Using the SAS System. 2. SAS Institute, Inc and John Wiley and Sons, Inc; Cary, NC: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.