Abstract
Objective:
To review general principles of brain development, identify basic principles of brain plasticity, and discuss factors that influence brain development and plasticity.
Method:
A literature review of relevant English-language manuscripts on brain development and plasticity was conducted.
Results:
Brain development progresses through a series of stages beginning with neurogenesis and progressing to neural migration, maturation, synaptogenesis, pruning, and myelin formation. Eight basic principles of brain plasticity are identified. Evidence that brain development and function is influenced by different environmental events such as sensory stimuli, psychoactive drugs, gonadal hormones, parental-child relationships, peer relationships, early stress, intestinal flora, and diet.
Conclusions:
The development of the brain reflects more than the simple unfolding of a genetic blueprint but rather reflects a complex dance of genetic and experiential factors that shape the emerging brain. Understanding the dance provides insight into both normal and abnormal development.
Keywords: brain development, cerebral plasticity, environmental stimulation, epigenetics
Résumé
Objectifs:
Présenter les grandes lignes du développement cérébral; expliquer le principe de la plasticité du cerveau; exposer les facteurs qui influencent son développement et sa plasticité.
Méthodologie:
Analyse de la littérature publiée en anglais sur le développement et la plasticité du cerveau.
Résultats:
Le cerveau se développe par étapes, la première étant la neurogénèse, suivie de la migration des neurones, de la maturation, de la synaptogénèse, de l’élagage synaptique et de la myélinisation. Les auteurs présentent huit principes fondamentaux de la plasticité du cerveau. Ils constatent que le développement et le fonctionnement du cerveau sont influencés par divers facteurs environnementaux comme les stimuli sensoriels, les substances psychoactives, les hormones gonadales, les relations parent-enfant, les relations avec les pairs, le stress dans la petite enfance, la flore intestinale et le régime alimentaire.
Conclusion:
Le développement du cerveau va au-delà de la construction de la carte génétique; certaines interactions complexes entre facteurs génétiques et expérimentaux agissent sur le cerveau en formation. Comprendre ces interactions permettra d’étudier le développement normal ou anormal du cerveau.
Keywords: développement cerebral, plasticité du cerveau, environnementaux comme les stimuli, épigénétique
The development of the brain reflects more than the simple unfolding of a genetic blueprint but rather reflects a complex dance of genetic and experiential factors that shape the emerging brain. Brains exposed to different environmental events such as sensory stimuli, drugs, diet, hormones, or stress thus may develop in very different ways. The goal of the current article is to review the ways the developing brain can be sculpted by a wide range of pre- and postnatal factors. We begin with an overview of brain development, followed by a brief review of principles of brain plasticity and finally a consideration of how factors influence brain development and adult behaviour. Because most of what we know about brain plasticity and behaviour in development comes from studies of the laboratory rat our discussion will focus on the rat but will consider humans when possible. In addition, the discussion will be biased towards plasticity in cerebral structures because most of what we know about modulation of brain development is based upon studies of cerebral development. There is little reason to believe, however, that other brain structures will not be changed in similar ways.
Brain Development
Some 2000 years ago the Roman philosopher Seneca proposed that a human embryo is an adult in miniature and thus the task of development is simply to grow bigger. This idea was so appealing that it was widely believed until well into the 19th century. It became obvious in the early 20th century that brain development reflected a series of stages that we can now see as being broadly divided into two phases. In most mammals the first reflects a genetically determined sequence of events in utero that can be modulated by maternal environment. The second phase, which is both preand postnatal in humans, is a time when the connectivity of the brain is very sensitive not only to the environment but also to the patterns of brain activity produced by experiences. More importantly, however, it is now recognized that epigenetic changes, which can be defined as changes in developmental outcomes, including regulation of gene expression, are based upon mechanisms other than DNA itself (Blumberg, Freeman, & Robinson, 2010). For example, gene expression can be altered by specific experiences, and this in turn can lead to organizational changes in the nervous system.
Stages of brain development
Table 1 outlines the general stages characteristic of brain development in all mammals. Cells that are destined to produce the nervous system begin to form about three weeks after fertilization in humans. These cells form the neural tube, which is the brain’s nursery and is later called the subventricular zone. Cells that are destined to form the cerebrum begin division at about six weeks of age and by about 14 weeks the cerebrum looks distinctly human, although it does not begin to form sulci and gyri until about seven months. Most neurogenesis is complete by five months, with one important exception being cells in the hippocampus, which continues to form neurons throughout life. There are about ten billion cells needed to form the human cerebral cortex in each hemisphere. These cells are formed rapidly and it is estimated that at its peak, there are about 250,000 neurons formed per minute. It is obvious that any brain perturbation at this time could have significant consequences.
Table 1.
Stages of brain development
|
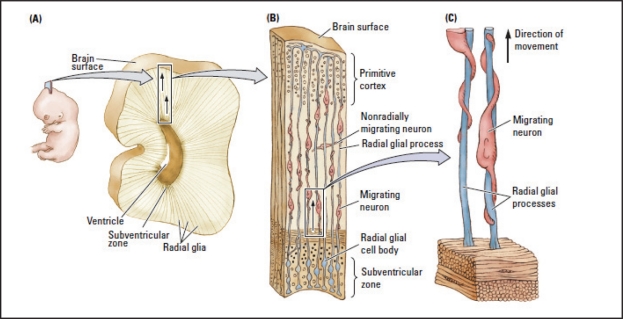
Once the neurons are formed, they begin to migrate along fibrous pathways formed by radial glial cells, which extend from the subventricular zone to the surface of the cerebral cortex (Figure 1). The subventriucular zone appears to contain a primitive map of the cortex that predisposes the cells formed in a particular subventricular region to migrate to a certain cortical location. As cells migrate they have an unlimited cell-fate potential but as they reach their destination the interaction of genes, maturation, and environmental influences increasingly steer them toward differentiating into a particular cell type. Once cells reach their final destination they begin to mature by: (1) growing dendrites to provide surface area for synapses with other cells; and, (2) extending axons to appropriate targets to initiate synapse formation.
Figure 1.
Cells migrate from the subventricular zone along radial glia to their eventual adult location (Kolb & Whishaw, 2009).
The formation of dendrites begins prenatally in humans but continues for a long time after birth. Dendrites in newborn babies begin as individual processes protruding from the cell body and over the next two years these processes are elaborated and spines, which are the location of most excitatory synapses, are formed. Dendritic growth is slow, on the order of micrometers per day. Axons grow about 1000 times faster, namely about one mm per day. This differential growth rate is important because the faster growing axons can contact target cells before the dendrites of that cell are completely formed. As a result, axons can influence dendritic differentiation and the formation of cerebral circuits.
Synapse formation in the human cerebral cortex poses a formidable challenge, with a total of more than 100,000 trillion (1014). This enormous number could not possibly be determined by a genetic program, but rather only the general outlines of neural connections in the brain will be genetically predetermined. The vast array of synapses is thus guided into place by a variety of environmental cues and signals. As we shall see, the manipulation of different types of cues and signals can produce dramatic differences in cerebral circuitry.
Owing to the uncertainty in the number of neurons that will reach their appropriate destination and the appropriateness of the connections that they form, the brain overproduces both neurons and connections during development, with the peak of synapse formation being between one and two years, depending upon the region of cortex. Just like a sculptor who creates a statue with a block of stone and a chisel to remove the unwanted pieces, the brain has a parallel system in which unneeded cells and connections are removed by cell death and synaptic pruning. The metaphorical chisels in the brain can be of many forms, including some type of epigenetic signal, a wide range of experiences, gonadal hormones, and even stress.
The effect of this cell loss and synaptic pruning can be seen in changes in cortical thickness over time. That is, the cortex actually becomes measurably thinner in a caudal-rostral gradient beginning around age two and continuing until at least 20 years of age. It is possible to correlate cortical thinning with behavioral development. For example, the results of MRI studies of changes in cortical thickness have shown that increased motor dexterity is associated with a decrease in cortical thickness in the hand region of the left motor cortex in right-handers (O’Hare & Sowell, 2008). One exception to the thinner is better rule is seen in the development of some, but not all, language processes. Thus, MRI studies have shown a thickening of the left inferior frontal cortex (roughly Broca’s area) is associated with enhanced phonological processing (i.e., the understanding of speech sounds). This unique association between cortical thickness and behavior is not characteristic of language functions in general, however. For example, vocabulary development is correlated with decreased cortical thickness in diffuse cortical regions (O’Hare & Sowell, 2008).
The relation between cortical thickness and behavioural development is likely an explanation for the variance in the development of behavioural skills in children. For example, the delayed development of language in children with normal intelligence and motor dexterity (about 1% of children) could be the result of slower than normal changes in cortical thickness. Why this might be is unknown.
The final stage of brain development is glial development to form myelin. The birth of astrocytes and oligodendrocytes begins after most neurogenesis is complete and continues throughout life. Although CNS axons can function before myelination, normal adult function is attained only after myelination is complete, which is after 18 years of age in regions such as the prefrontal, posterior parietal, and anterior temporal cortex.
Brain development, therefore, is composed of a cascade of events beginning with mitosis and ending with myelin formation. The effect of brain perturbations and experiences will therefore vary with the precise stage of brain development. We should not be surprised, for example, that experiences and/or perturbations during mitosis would have quite different effects than similar events during synaptogenesis or later during pruning. Experiences are essentially acting on very different brains at different stages of development.
Special features of brain development
Two features of brain development are especially important for understanding how experiences can modify cortical organization. First, the cells lining the subventricular zone are stem cells that remain active throughout life. These stem cells can produce neural or glial progenitor cells that can migrate into the cerebral white or gray matter, even in adulthood. These cells can remain quiescent in these locations for extended periods but can be activated to produce either neurons and/or glia. The role of these cells is poorly understood at present but they likely form the basis of at least one form of postnatal neurogenesis, especially after injury (e.g., Gregg, Shingo, & Weiss, 2001; Kolb et al., 2007). In addition, the mammalian brain, including the primate brain, can generate neurons in adulthood that are destined for the olfactory bulb, hippocampal formation, and possibly other regions (e.g., Eriksson et al., 1998; Gould, Tanapat, Hastings, & Shors, 1999; Kempermann & Gage, 1999). The functional role of these cells is still controversial but their generation can be influenced by many factors including experience, drugs, hormones, and injury.
The second special feature is that dendrites and spines show remarkable plasticity in response to experience and can form synapses in hours and possibly even minutes after some experiences (e.g., Greenough & Chang, 1989). On the surface, this would appear to be at odds with the process of overproduction of synapses followed by synaptic pruning described earlier. A key point is that although synaptic pruning is an important feature of brain development, the brain does continue to form synapses throughout the lifetime and in fact these synapses are necessary for learning and memory processes. Greenough, Black and Wallace (1987) have argued that there is a fundamental difference between the processes governing the formation of synapses in early brain development and those during later brain development and adulthood. Specifically, they argue that the early forming synapses are “expecting” experiences, which act to prune them back. They call these synapses “experience-expectant” and note that they are found diffusely throughout the cerebrum. In contrast, later synapse formation is more focal and localized to regions involved in processing specific experiences. They label these synapses as “experience-dependent.” One curious aspect of experience-dependent effects on synapses is that not only do specific experiences lead to selective synapse formation but also to selective synaptic loss. Thus, experiences are changing neural networks by both adding and pruning synapses. This leads us to the issue of brain plasticity.
General Principles of Plasticity in Normal Brain
Before we address the experiences that influence brain plasticity, we must briefly review several key principles of plasticity in the normal brain.
1. Changes in the brain can be shown at many levels of analysis
A change in behavior must certainly result from some change in the brain but there are many ways to investigate such changes. Changes may be inferred from global measures of brain activity, such as in the various forms of in vivo imaging, but such changes are far removed from the molecular processes that drive them. Global changes presumably reflect synaptic changes but synaptic changes result from more molecular changes such as modifications in channels, gene expression, and so on. The problem in studying brain plasticity is to choose a surrogate marker that best suits the question being asked. Changes in calcium channels may be perfect for studying synaptic changes at specific synapses that might be related to simple learning but are impractical for understanding sex differences in language processing. The latter might best be studied by in vivo imaging or postmortem analysis of cell morphology (e.g., Jacobs & Scheibel, 1993). The appropriate level must be targeted at the research question at hand. Studies investigating strategies for stimulating functional improvement after injury most commonly use anatomical (cell morphology and connectivity), physiological (cortical stimulation), and in vivo imaging. Each of these levels can be linked to behavioral outcomes in both human and nonhuman studies whereas more molecular levels have proven to be much more difficult to relate to behavior, and especially mental behavior.
2. Different measures of neuronal morphology change independently of each other and sometimes in opposite directions
There has been a tendency in the literature to see different neuronal changes as surrogates for one another. One of the most common is to assume that changes in spine density reflect changes in dendritic length and vice versa. This turns out not to be the case as the two measures can vary independently and sometimes in opposite directions (e.g., Comeau, McDonald, & Kolb, 2010; Kolb, Cioe, & Comeau, 2008). Furthermore, cells in different cortical layers, but in the same presumptive columns, can show very different responses to the same experiences (e.g., Teskey, Monfils, Silasi, & Kolb, 2006).
3. Experience-dependent changes tend to be focal
Although there is a tendency to think of plastic changes in response to experiences as being widespread across the brain, this is rarely the case. For example, psychoactive drugs may produce large behavioural changes and have widespread acute effects on neurons, but the chronic plastic changes are surprisingly focal and largely confined to the prefrontal cortex and nucleus accumbens (e.g., Robinson & Kolb, 2004). As a result, researchers need to carefully think about where the best places are to look after specific experiences. A failure to find synaptic changes that correlate with behavioural change is not evidence of the absence of changes.
4. Plastic changes are time-dependent
Perhaps the largest changes in synaptic organization can be seen in response to placing lab animals in complex (so-called “enriched”) environments. Thus, there are widespread changes throughout sensory and motor cortex. These changes appear to defy the principle of experience-dependent changes being focal but the generality of the changes is likely due to the global nature of the experiences including experiences as diffuse as visual, tactile, auditory, olfactory, motor, and social experiences. But these plastic changes are not all permanent and they may change dramatically over time.
For example, when rats are placed in complex environments there is a transient increase in dendritic length in the prefrontal cortex that can be seen after four days of complex housing but has disappeared after 14 days. In contrast, there are no obvious changes in sensory cortex after four days but clear, and seemingly permanent, changes after 14 days (Comeau et al., 2010).
The possibility that there are different chronic and transient experience-dependent changes in cerebral neurons is consistent with genetic studies showing that there are different genes expressed acutely and chronically in response to complex environments (e.g. Rampon et al., 2000). The difference in how transient and persistent changes in neuronal networks relate to behavior is unknown.
5. Experience-dependent changes interact
Humans have a lifetime of experiences beginning prenatally and continuing until death. These experiences interact. For example, we have shown in laboratory rats that if animals are exposed to psychomotor stimulants either as juveniles or in adulthood, later experiences have a much-attenuated (or sometimes absent) effect. For example, when rats are given methylphenidate as juveniles or amphetamine as adults and then sometime later are placed in complex environments or trained on learning tasks, the later experience-dependent changes are blocked. What is surprising is that although the drugs do not show any obvious direct effect on sensory cortical regions, prior exposure prevents the expected changes in these regions (e.g., Kolb, Gibb, & Gorny, 2003a). These drug-experience interactions are not unidirectional however. When pregnant rats are given a mild stressor for 20 minutes twice a day during the period of maximal cerebral neurogenesis in their offspring (embryonic days 12–18), their offspring show stress-related changes in spine density in the prefrontal cortex (PFC) but no drug-related effects (Muhammad & Kolb, in press a). It is not clear why there is a complete absence of drug-related effects or what this will mean for addiction but it does show that experiences interact in their effects on the brain.
7. Plastic changes are age-dependent
It is generally presumed that the developing brain will be more responsive to experiences than the adult or senescent brain. This is most certainly correct but there is another important wrinkle: there are qualitatively different changes in the brain in response to what appears to be the same experience at different ages. For example, when weanling, adult, or senescent rats were placed in a complex environment, all groups showed large synaptic changes but they were surprisingly different. Specifically, whereas we anticipated an increase in spine density in response to complex housing, this was only true in adult and senescent rats. Rats placed in the environments as juveniles showed a decrease in spine density (Kolb et al., 2003a). A similar drop in spine density was found in later studies in which newborn rats were given tactile stimulation with a soft brush for 15 minutes, three times daily over the first ten days of life but not if the stimulation is in adulthood (Gibb, Gonzalez, Wagenest, & Kolb, 2010; Kolb & Gibb, 2010). The age-dependent nature of synaptic change is clearly important for understanding how experiences change the brain.
8. Not all plasticity is good
Although the general gist of the literature is that plastic changes in the brain support improved motor and cognitive functions, plastic changes can interfere with behavior too. A good example is the drug-induced changes seen in response to psychomotor stimulants (e.g., Robinson & Kolb, 2004). It is reasonable to propose that some of the maladaptive behavior of drug addicts could result from drug-related changes in prefrontal neuronal morphology. There are many other examples of pathological plasticity including pathological pain (Baranauskas, 2001), pathological response to sickness (Raison, Capuron, & Miller, 2006), epilepsy (Teskey, 2001), schizophrenia (Black et al., 2004), and dementia (Mattson, Duan, Chan, & Guo, 2001).
Although there are not many studies of pathological plasticity in the developing brain, an obvious example is fetal alcohol spectrum disorder. Another example is the effects of severe prenatal stress, which has been shown to markedly reduce the complexity of neurons in the prefrontal cortex (e.g., Murmu et al., 2006) and in turn can affect normal cognitive and motor functions both in development and in adulthood (e.g., Halliwell, 2011). Although the mechanisms underlying these changes are poorly understood it is known that early postnatal stress can alter gene expression in the brain (Weaver et al., 2004; Weaver, Meaney, & Szf, 2006).
Factors Influencing Brain Development
When researchers began to study experience-dependent changes in the developing brain in the 1950s and 1960s, there was a natural assumption that changes in brain development would only be obvious in response to rather large changes in experience, such as being raised in darkness. Over the past 20 years it has become clear that even fairly innocuous-looking experiences can profoundly affect brain development and that the range of experiences that can alter brain development is much larger than had been once believed (see Table 2). We will highlight some of the most well-studied effects.
Table 2.
Factors influencing brain development and function
| Sensory and motor experience |
| Psychoactive drugs |
| Gonadal hormones |
| Parent-child relationships |
| Peer relationships |
| Stress |
| Intestinal flora |
| Diet |
1. Sensory and motor experiences
The simplest way to manipulate experience across ages is to compare brain structure in animals living in standard laboratory caging to animals placed either in severely impoverished environments or so-called enriched environments. Raising animals in deprived environments such as in darkness, silence, or social isolation clearly retards brain development. For example, dog puppies raised alone show a wide range of behavioural abnormalities, including a virtual insensitivity to painful experiences (Hebb, 1949). Similarly, raising animals as diverse as monkeys, cats, and rodents in the dark severely interferes with development of the visual system. Perhaps the best-known deprivation studies are those of Weisel and Hubel (1963) who sutured one eyelid of kittens closed and later showed that when the eye was opened there was an enduring loss of spatial vision (amblyopia) (e.g., Giffin & Mitchell, 1978). It has only been recently, however, that investigators considered the opposite phenomenon, namely giving animals enriched visual experiences to determine if vision could be enhanced. In one elegant study, Prusky et al. (Prusky, Silver, Tschetter, Alam, & Douglas, 2008) used a novel form of visual stimulation in which rats were placed in a virtual optokinetic system in which vertical lines of differing spatial frequency moved past the animal. If the eyes are open and oriented towards the moving grating, it is impossible for animals, including humans, to avoid tracking the moving lines, if the spatial frequency is within the perceptual range. The authors placed animals in the apparatus for about two weeks following the day of eye opening (postnatal day 15). When tested for visual acuity in adulthood, the animals showed about a 25% enhancement in visual acuity relative to animals without the early treatment. The beauty of the Prusky study is that improved visual function was not based upon specific training, such as in learning a problem, but occurred naturally in response to enhanced visual input.
We have attempted to enhance tactile experience using a procedure first devised by Schanberg and Field (1987). In these studies infant rats were given tactile stimulation with a small brush for 15 minutes three times per day for 10–15 days beginning at birth. When the infants were studied in adulthood they showed both enhanced skilled motor performance and spatial learning as well as changes in synaptic organization across the cerebral cortex (e.g., Kolb & Gibb, 2010). Although the precise mechanism of action of the tactile stimulation is not known, we have shown that the tactile stimulation leads to an increase in the production of a neurotrophic factor, fibroblast growth factor-2 (FGF-2) in both skin and brain (Gibb, 2004). FGF-2 is known to play a role in normal brain development and can stimulate recovery from perinatal brain injury (e.g., Comeau, Hastings, & Kolb, 2007). FGF-2 expression is also increased in response to a variety of treatments including enriched housing and psychoactive drugs, both of which stimulate plastic changes in the brain (see below).
Another way to enhance sensory and motor functions is to place animals in complex environments in which there is an opportunity for animals to interact with a changing sensory and social environment and to engage in far more motor activity than regular caging. Such studies have identified a large range of neural changes associated with this form of “enrichment.” These include increases in brain size, cortical thickness, neuron size, dendritic branching, spine density, synapses per neuron, glial numbers and complexity, and vascular arborization (e.g. Greenough & Chang, 1989; Siervaag & Greenough, 1987). The magnitude of these changes should not be underestimated. For example, in our own studies of the effects of housing young rats for 60 days in enriched environments, we reliably observe changes in overall brain weight on the order of 7–10% (e.g., Kolb, 1995). This increase in brain weight reflects increases in the number of glia and blood vessels, neuron soma size, dendritic elements, and synapses. It would be difficult to estimate the total number of increased synapses but it is probably on the order of 20% in the cortex, which is an extraordinary change. Importantly, although such studies show experience-dependent changes at any age, there are two unexpected wrinkles. First, adult rats at any age show a large increase in dendritic length and spine density across most of the cerebral cortex whereas juvenile rats show a similar increase in dendritic length but a decrease in spine density. That is, the young animals show a qualitatively different change in the distribution of synapses on pyramidal neurons compared to older animals (Kolb et al., 2003a). Second, when pregnant dams were placed in complex environments for eight hours a day prior to their pregnancy and then throughout the three-week gestation, analysis of the adult brains of their infants showed a decrease in dendritic length and an increase in spine density. Thus, not only is there an effect of prenatal experience but the effect was qualitatively different from experience either in the juvenile period or in adulthood. Curiously, all of the changes in response to the complex housing lead to enhanced cognitive and motor functions.
There are three clear messages from these studies. First, a wide range of sensory and motor experiences can produce long-lasting plastic changes in the brain. Second, the same experience can alter the brain differently at different ages. Third, there is no simple relationship between the details of synaptic plasticity and behaviour during development. What is certain, however, is that these early experiences have a powerful effect on brain organization both during development and in adulthood.
2. Psychoactive drugs
It has long been known that early exposure to alcohol is deleterious for brain development, but it has only recently been shown that other psychoactive drugs, including prescription drugs, can dramatically alter brain development. Robinson and Kolb (2004) found that exposure to psychomotor stimulants in adulthood produced large changes in the structure of cells in PFC and nucleus accumbens (NAcc). Specifically, whereas these drugs (amphetamine, cocaine, nicotine) produced increases in dendritic length and spine density in medial prefrontal cortex (mPFC) and NAcc, there was either a decrease in these measures in orbital frontal cortex (OFC), or in some cases, no change. They subsequently showed that virtually every class of psychoactive drugs also produces changes in PFC, and that the effects are consistently different in the two prefrontal regions. Given that the developing brain is often exposed to psychoactive drugs, either in utero or during postnatal development, we asked what effects these drugs would have on cortical development.
Our first studies looked at the effects of amphetamine or methylphenidate given during the juvenile period (e.g., Diaz, Heijtz, Kolb, & Forssberg, 2003). Both drugs altered the organization of the PFC. The dendritic changes were associated with abnormal play behaviour in the drug-treated rats, as they displayed reduced play initiation compared to saline-treated playmates as well as impaired performance on a test of working memory. Psychomotor stimulants thus appear to alter the development of the PFC and this is manifested in behavioural abnormalities on prefrontal-related behaviours later in life.
Children may also be exposed to prescription medications either in utero or postnatally. Three commonly prescribed classes of drugs are antipsychotics, antidepressants, and anxiolytics. All three have dramatic effects on cortical development. Frost, Cerceo, Carroll, and Kolb (2009) analyzed dendritic architecture in adult mice treated with paradigmatic typical- (haloperidol) or atypical (olanzapine) antipsychotic drugs at developmental stages corresponding to fetal (postnatal days 3–10) or fetal and early childhood (postnatal days 3–20) stages in humans. Both drugs produced reductions in dendritic length, dendritic branching complexity, and spine density in both medial prefrontal and orbital cortex. In a subsequent study using rats the authors showed impairments in PFC-related neuropsychological tasks such as working memory.
In a parallel set of studies we have looked at the effect of prenatal exposure to diazepam or fluoxetine in rats (Kolb, Gibb, Pearce, & Tanguay, 2008). Both drugs affected brain and behavioural development, but in opposite ways. Prenatal diazepam increased dendritic length and spine density in pyramidal cells in the parietal cortex and this was associated with enhanced skilled motor functions. In contrast, fluoxetine decreased dendritic measures and this was correlated with impaired spatial learning deficits in adulthood.
One additional question is whether early exposure to psychoactive drugs might alter brain plasticity later in life. We had previously shown that if adult rats are given amphetamine, cocaine, or nicotine and then later placed in complex environments, neuronal plasticity was blocked (Hamilton & Kolb, 2005; Kolb, Gorny, Samaha, & Robinson, 2003b). In a subsequent study we gave juvenile rats methylphenidate and then in adulthood we placed these animals in complex environments and, once again, we found that the early drug exposure blocked the expected experience-dependent changes in the cortex (Comeau & Kolb, 2011). Furthermore, in a parallel study we showed that juvenile methylphenidate exposure impaired performance on neuropsychological tasks sensitive to prefrontal functioning.
In sum, exposure to both prescription drugs and drugs of abuse has a profound effect on prefrontal development and prefrontal-related behaviours. These effects appear to be long lasting or permanent and can influence brain plasticity in adulthood. The unexpected serious effects of prescription drugs on brain and behavioral development are undoubtedly important in human infant brain development. It is clearly not a simple call on whether pregnant mothers with serious depression, psychosis, or anxiety-disorders should be prescribed medications given that these behavioral conditions are likely to affect brain development in the infant and especially to the extent that there are pathological mother-infant interactions. The research does suggest, however, that such medications should be used in as low an effective dose as can be used and not simply for their “calming” effects on mothers with mild anxiety.
3. Gonadal hormones
The most obvious effect of exposure to gonadal hormones during development is the differentiation of genitals that begins prenatally. In this case the production of testosterone by males leads to the development of male genitalia. Later in life, both estrogen and testosterone affect receptors in many regions of the body, including the brain. MRI studies of human brain development have shown large differences in the rate of brain development in the two sexes (O’Hare & Sowell, 2008). Specifically, the total volume of brain reaches asymptote in females around age 11 and 15 in males and females respectively. But there is more to sexual dimorphism in brain than rate of maturation. For example, Kolb and Stewart (1991) showed in rats that neurons in the mPFC had larger dendritic fields in males and that neurons in OFC had larger cells in females. These differences vanished when animals were gonadectomized at birth. Similarly, Goldstein et al. (2001) did a comprehensive evaluation of the volume of 45 different brain regions from MRI scans of healthy adult subjects. There were sex differences in volume, relative to total cerebral volume, and this was especially true in PFC: females had a relatively larger volume of dorsolateral PFC whereas males had a relatively larger volume of OFC. This sexual dimorphism is correlated with relatively high regional levels of sex steroid receptors during early life in laboratory animals. It thus appears in both humans and laboratory animals that gonadal hormones alter cortical development. This is particularly important when we consider that the effects of other experiences such as exposure to complex housing or psychomotor stimulants are also sexually dimorphic. It seems likely that many other developmental experiences may differentially alter the female and male brains, although few studies have actually made this comparison.
4. Parent-child relationships
Mammalian infants that are born in an immature state face a significant challenge in early life. They are dependent upon their parents and they must learn to identify, remember, and prefer their caregivers. Although we now know that young animals (and even prenatal animals) can learn more than previously recognized (see review by Hofer & Sullivan, 2008), there is little doubt that the parent-child relationships are critical and that they play a key role in brain development. Differences in the pattern of early maternal-infant interactions can initiate long-term developmental effects that persist into adulthood (Myers, Brunelli, Squire, Shindledecker, & Hofer, 1989). For example, rodent studies have shown that the time spent in contact, the amount of maternal licking and grooming, and the time the mothers spend in a highly stimulating high-arched resting position correlate with a variety of somatic and behavioural differences. Over the past decade Meaney and his colleagues (e.g. Cameron et al., 2005) have been able to show these rodent maternal-infant interactions systematically modify the development of the hypothalamic-adrenal stress response and a variety of emotional and cognitive behaviours in adulthood. These changes are correlated with changes in hippocampal cell membrane corticosterone receptors, which in turn are controlled by changes in gene expression (Weaver et al., 2006).
The effects of variations in maternal care are not restricted to the hippocampus, however, and may be quite widespread. For example, Fenoglio, Chen and Barum (2006) have shown that enhanced maternal care during the first week of life produced enduring changes in cell signaling pathways in the hypothalamus and amgydala (also see review by Fenoglio, Bruson, & Barum, 2006).
We are unaware of similar studies looking at neocortical, and especially prefrontal, plasticity in response to differences in maternal-infant interactions, but such changes seem likely. We have shown, for example, that daily maternal separation, which is the procedure that was used to increase maternal-infant interactions in the Fenoglio et al. (2006) study, does increase dendritic length and spine density in both mPFC and OFC in adult rats (Muhammad & Kolb, 2011).
5. Peer relationships
Peer relationships have been known to influence adult behavior since the studies of Harlow (e.g., Harlow & Harlow, 1965). One of the most powerful peer relationships is play, which has been shown to be important for the development of adult social competence (e.g., Pellis & Pellis, 2010). The frontal lobe plays an essential role in play behaviour. An infant injury to the mPFC and OFC compromise play behavior, although in different ways (e.g., Pellis et al., 2006). In view of such results, we hypothesized that the development, and subsequent functioning, of the two prefrontal regions would be differentially altered if play behaviour was manipulated in development. Juvenile rats were thus given the opportunity to play with 1 or 3 adult rats or with 1 or 3 other juvenile animals. There was virtually no play with the adult animals but play behaviour was increased the more juvenile animals that were present. Analysis of cells in the PFC showed that neurons of the OFC responded to the number of peers present, and not whether or not play occurred, whereas the neurons of mPFC responded to the amount of play but not the number of conspecifics (Bell, Pellis, & Kolb, 2010). We have subsequently shown in a series of studies that a variety of early experiences alter rat play behaviour, including prenatal stress, postnatal tactile stimulation, and juvenile exposure to methylphenidate (e.g., Muhammad, Hossain, Pellis, & Kolb, 2011) and, in each case, there are abnormalities in prefrontal development. There may be an important lesson here when we consider conditions in which human childhood play is not normal, such as in autism or attention-deficit hyperactivity disorder (ADHD). The abnormalities in play behaviour may influence prefrontal development and later adult behaviour.
6. Early stress
There is enormous literature collected over the past 60 years showing the effects of stress on brain and behaviour in adults but it is only more recently that the role of perinatal stress in infants has been appreciated. It is now known that both gestational and infant stress predisposes individuals for a variety of maladaptive behaviours and psychopathologies. For example, prenatal stress is a risk factor in the development of schizophrenia, ADHD, depression, and drug addiction (Anda et al., 2006; van den Bergh & Marcoen, 2004). Experimental studies with lab animals have confirmed these findings with the overall results being that perinatal stress, in rodents as well as non-human primates, produced behavioural abnormalities such as elevated and prolonged stress response, impaired learning and memory, deficits in attention, altered exploratory behaviour, altered social and play behaviour, and an increased preference for alcohol (e.g., review by Weinstock, 2008).
The plastic changes in the synaptic organization of brains of perinatally-stressed animals are less well studied, however, and the effects appear to be related to the details of the stressful experience. For example, Murmu et al. (2006) reported that moderate prenatal stress during the third week of gestation resulted in decreased spine density and dendritic length in both the mPFC and OFC of adult degus. In contrast, Muhammad & Kolb (2011) found that mild prenatal stress during the second week of gestation decreased spine density in mPFC but had no effect in OFC and increased spine density in NAcc of adult rats. Analysis of dendritic length showed a somewhat different pattern as there was an increase in dendritic length in mPFC and NAcc but a decrease in OFC. Curiously, Mychasiuk, Gibb and Kolb (2011) found that mild stress during the second gestational week increased spine density in both mPFC and OFC when the brains were examined in juvenile, rather than adult rats. Taken together these studies show that differences in the timing of prenatal stress and the age at which the brain is examined result in differing plastic changes in neuronal circuits. One thing that is clear, however, is that the effects of prenatal stress appear to be different from those of adult stress. For example, Liston et al. (2006) first showed that adult stress led to a decrease in dendritic branching and spine density in mPFC but an increase in OFC.
We are aware of only one study looking at the effects of early postnatal stress (maternal separation) on synaptic organization in adult brains. Thus, Muhammad & Kolb (2011) found that maternal separation increased spine density in mPFC, OFC, and NAcc in adult rats. What is yet to be determined following either prenatal or infant stress is how these differences in synaptic changes relate to later behaviour or how plastic the neurons will be in response to other experiences such as complex housing, play, or infant-parent relationships. Such studies are sure to be the grist of future studies.
7. Intestinal flora
Immediately after birth, mammals are rapidly populated by a variety of indigenous microbes. These microbes influence development of many body functions. For example, gut microbiota have systemic effects on liver function (e.g., Björkholm et al., 2009). Because there is a known relation between neurodevelopmental disorders such as autism and schizophrenia and microbioal pathogen infections during the perinatal period (e.g., Finegold et al., 2002; Mittal, Ellman, & Cannon, 2008), Diaz Heijtz et al. (in press) wondered if such infections could alter brain and behavioural development. They do. The authors compared measures of both motor behaviour and brain in mice that developed with or without normal gut mircrobiota. The authors found that gut bacteria influence signaling pathways, neurotransmitter turnover, and the production of synaptic-related proteins in cortex and striatum in developing mice and these changes were associated with changes in motor functions. This is an exciting finding because it provides insight into the way that infections during development could alter brain development and subsequent adult behaviour.
8. Diet
There is extensive literature on the effects of protein and/or caloric restricted diets on brain and behavioural development (e.g., Lewis, 1990) but much less is known about the effects of enhanced diets on brain development. It is generally presumed that the body heals better when it is given good nutrition so it is reasonable to predict that brain development might be facilitated by vitamin and/or mineral supplements. Dietary choline supplementation during the perinatal period produces a variety of changes both to behavior and brain (Meck & Williams, 2003). For example, perinatal choline supplementation leads to enhanced spatial memory in various spatial navigation tests (e.g., Meck & Williams, 2003; Tees, & Mohammadi, 1999) and increases the levels of nerve growth factor (NGF) in the hippocampus and neocortex (e.g., Sandstrom, Loy, & Williams, 2002). Halliwell, Tees and Kolb (2011) did similar studies and found that choline supplementation increased dendritic length across the cerebral cortex and in hippocampal CA1 pyramidal neurons.
Halliwell (2011) has also studied the effects of the addition of a vitamin/mineral supplement to the food of lactating rats. She chose to use a diet supplement that has been reported to improve mood and aggression in adults and adolescents with various disorders (Leung, Wiens & Kaplan, 2011) and decreased anger, activity levels and social withdrawal in autism with an increase in spontaneity (Mehl-Madrona, Leung, Kennedy, Paul, & Kaplan, 2010). Analysis of the adult offspring of lactating rats fed the same supplement found an increase in dendritic length in neurons in mPFC and parietal cortex but not in OFC. In addition, the diet was effective in reversing the effects of mild prenatal stress on the reduction of dendritic length in OFC.
Much is left to be learned about the effects of both dietary restriction and supplementation on the development of neuronal networks and behaviour. Both procedures do alter brain development but as in many of the other factors discussed here, we do not have a clear picture of how the early experiences will interact with later experiences, such as psychoactive drugs, to alter brain and behaviour.
Conclusions
Our understanding of the nature of normal brain development has advanced a long way in the past 30 years but we are just beginning to understand some of the factors that modulate this development. Understanding this modulation will be essential for us to begin to unravel the puzzles of neurodevelopmental disorders and to initiate early treatments to block or reverse pathological changes. An obvious complication is that experiences are not singular events but rather as we go through life, experiences interact to alter both behaviour and brain, a process often referred to as metaplasticity.
As we discussed the various experience-dependent changes in the developing brain we have used the “developing brain” as though it were a single time. This is obviously not so and there is little doubt that we will eventually find that there are critical windows of time in which the developing brain is more (or less) responsive than at other times. In addition, it is likely that different cerebral regions will show different critical windows. We have found, for example, that if the motor cortex is injured in early adolescence there is a poor outcome relative to the same injury at late adolescence (Nemati & Kolb, 2010). Curiously, however, the reverse is true for injury to the prefrontal cortex. Sorting out the areal-dependent critical windows will be a challenge for the next decade.
We have focused here on measures of synaptic plasticity but we certainly recognize that plastic changes in brain organization can be studied at many other levels. Ultimately the fundamental mechanism of synaptic change will be found in gene expression. The difficulty is that it is likely that experiences that alter behaviour significantly will be related to changes in dozens or hundreds of genes. The challenge is to identify the changes that are most closely associated with the observed behavioural changes.
Acknowledgements / Conflicts of Interest
We wish to thank both NSERC and CIHR for their long-term support for the studies related to our work discussed in this review. We also thank Cathy Carroll, Wendy Comeau, Dawn Danka, Grazyna Gorny, Celeste Halliwell, Richelle Mychasiuk, Arif Muhammad, and Kehe Xie for their many contributions to the studies.
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G. Pain-induced plasticity in the spinal cord. In: Shaw CA, McEachern J, editors. Toward a Theory of Neuroplasticity. Philadelphia, PA: Psychology Press; 2001. pp. 373–386. [Google Scholar]
- Bell HC, Pellis SM, Kolb B. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortex. Behavioural Brain Research. 2010;207:7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. American Journal of Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Freeman JH, Robinson SR. A new frontier for developmental behavioral neuroscience. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York, NY: Oxford University Press; 2010. pp. 1–6. [Google Scholar]
- Björkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS ONE. 2009;4:e6958. doi: 10.1371/journal.pone.0006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Carine P, Fish EW, Ozaki-Kuroda K, Meaney M. The programming of individual differences in defensive responses and reproductive strategies in a rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Comeau W, Hastings E, Kolb B. Differential effect of pre and postnatal FGF-2 following medial prefrontal cortical injury. Behavioural Brain Research. 2007;180:18–27. doi: 10.1016/j.bbr.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Comeau WL, McDonald R, Kolb B. Learning-induced alterations in prefrontal cortical circuitry. Behavioural Brain Research. 2010;214:91–101. doi: 10.1016/j.bbr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Comeau W, Kolb B. Juvenile exposure to methylphenidate blocks later experience-dependent plasticity in adulthood. 2011. Manuscript in submission.
- Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? European Journal of Neuroscience. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Science (USA) doi: 10.1073/pnas.1010529108. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfi lieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Frontiers in Neuroendocrinology. 2006;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. Journal of Neuroscience. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, Kaul A. Gastrointestinal microflora studies in late-onset autism. Clinical Infectious Diseases. 2002;35(Suppl 1):S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- Frost DO, Cerceo S, Carroll C, Kolb B. Early exposure to Haloperidol or Olanzapine induces long-term alterations of dendritic form. Synapse. 2009;64:191–199. doi: 10.1002/syn.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R. Perinatal experience and recovery from brain injury. 2004. Unpublished PhD thesis, University of Lethbridge, Canada.
- Gibb R, Gonzalez CLR, Wegenast W, Kolb B. Tactile stimulation facilitates recovery following cortical injury in adult rats. Behavioural Brain Research. 2010;214:102–107. doi: 10.1016/j.bbr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Giffin F, Mitchell DE. The rate of recovery of vision after early monocular deprivation in kittens. Journal of Physiology. 1978;274:511–537. doi: 10.1113/jphysiol.1978.sp012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Cainess VS, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: A possible role in learning. Trends in Cognitive Science. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Developmental Psychobiology. 1987;22:727–252. doi: 10.1002/dev.420220707. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Chang FF. Plasticity of synapse structure and pattern in the cerebral cortex. In: Peters A, Jones EG, editors. Cerebral Cortex, Vol 7. New York, NY: Plenum Press; 1989. pp. 391–440. [Google Scholar]
- Gregg CT, Shingo T, Weiss S. Neural stem cells of the mammalian forebrain. Symposium of the Society of Experimental Biology. 2001;53:1–19. [PubMed] [Google Scholar]
- Halliwell CI. Treatment Interventions Following Prenatal Stress and Neonatal Cortical Injury. 2011. Unpublished PhD thesis, University of Lethbridge, Canada.
- Halliwell C, Tees R, Kolb B. Prenatal choline treatment enhances recovery from perinatal frontal injury in rats. 2011. Manuscript in submission.
- Hamilton D, Kolb B. Nicotine, experience, and brain plasticity. Behavioral Neuroscience. 2005;119:355–365. doi: 10.1037/0735-7044.119.2.355. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schier A, Harlow HF, Stollnitz F, editors. Behaviour of nonhuman primates. Vol. 2. New York, NY: Academic Press; 1965. [Google Scholar]
- Hebb DO. The Organization of Behavior. New York, NY: McGraw-Hill; 1949. [Google Scholar]
- Hofer MA, Sullivan RM. Toward a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2008. pp. 787–806. [Google Scholar]
- Jacobs B, Scheibel AB. A quantitative dendritic analysis of Wernicke’s area in humans. I. Lifespan changes. Journal of Comparative Neurology. 1993;327:383–396. doi: 10.1002/cne.903270107. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Scientific American. 1999;280(5):48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kolb B. Brain plasticity and behavior. Mahwah, NJ: Erlbaum; 1995. [Google Scholar]
- Kolb B, Cioe J, Comeau W. Contrasting effects of motor and visual learning tasks on dendritic arborization and spine density in rats. Neurobiology of Learning and Memory. 2008;90:295–300. doi: 10.1016/j.nlm.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Tactile stimulation facilitates functional recovery and dendritic change after neonatal medial frontal or posterior parietal lesions in rats. Behavioural Brain Research. 2010;214:115–120. [Google Scholar]
- Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary with age and sex. Neurobiology of Learning and Memory. 2003a;79:1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proceedings of the National Academy of Science (USA) 2003b;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Pearce S, Tanguay R. Prenatal exposure to prescription medications alters recovery following early brain injury in rats. Society for Neuroscience Abstracts. 2008;349:5. [Google Scholar]
- Kolb B, Morshead C, Gonzalez C, Kim N, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. Journal of Cerebral Blood Flow and Metabolism. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. Journal of Neuroendocrinology. 1991;3:95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. 6th edition. New York, NY: Worth; 2009. [Google Scholar]
- Leung BM, Wiens KP, Kaplan BJ. Does prenatal micronutrient supplementation improve children’s mental development? A systematic review. BCM Pregnancy Childbirth. 2011;11:1–12. doi: 10.1186/1471-2393-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PD. Nutrition and anatomical development of the brain. In: van Gelder NM, Butterworth RF, Drujan BD, editors. (Mal)Nutrition and the Infant Brain. New York, NY: Wiley-Liss; 1990. pp. 89–109. [Google Scholar]
- Liston C, Miller MM, Godwater DS, Radley JJ, Rocher AB, Hof PR, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Chan SL, Guo Z. In: Toward a Theory of Neuroplasticity. Shaw CA, McEachern J, editors. Philadelphia, PA: Psychology Press; 2001. pp. 402–426. [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neuroscience and Biobehavioral Reviews. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Mehl-Madrona L, Leung B, Kennedy C, Paul S, Kaplan BJ. Micronutrients versus standard medication management in autism: A naturalistic case-control study. Journal of Child Adolescent Psychopharmacology. 2010;20:95–103. doi: 10.1089/cap.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: The role of obstetric complications. Schizophrenia Bulletin. 2008;34:1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Hossain S, Pellis S, Kolb B. Tactile stimulation during development attenuates amphetamine sensitization and alters neuronal morphology. Behavioral Neuroscience. 2011;125:161–174. doi: 10.1037/a0022628. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Mild prenatal stress modulates behaviour and neuronal spine density without affecting amphetamine sensitization. Developmental Neuroscience. doi: 10.1159/000324744. (in press). [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behavioural Brain Research. 2011;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Murmu M, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes in spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Gibb R, Kolb B. Prenatal bystander stress induces neuroanatomical changes in the prefrontal cortex and hippocampus of developing rat offspring. Brain Research. 2011;1412:55–62. doi: 10.1016/j.brainres.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Squire JM, Shindledecker R, Hofer MA. Maternal behavior of SHR rats in its relationship to offspring blood pressure. Developmental Psychobiology. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- Nemati F, Kolb B. Motor cortex injury has different behavioral and anatomical effects in juvenile and adolescent rats. Behavioral Neuroscience. 2010;24:612–622. doi: 10.1037/a0020911. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Sowell ER. Imaging developmental changes in gray and white matter in the human brain. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2008. pp. 23–38. [Google Scholar]
- Pellis SM, Hastings E, Takeshi T, Kamitakahara H, Komorowska J, Forgie ML, Kolb B. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and non-playful social contexts. Behavioral Neuroscience. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- Pellis S, Pellis V. The Playful Brain. New York, NY: Oneworld Publications; 2010. [Google Scholar]
- Prusky GT, Silver BD, Tschetter WW, Alam NM, Douglas RM. Experience-dependent plasticity from eye opening enables lasting, visual cortex-dependent enhancement of motion vision. Journal of Neuroscience. 2008;28:9817–9827. doi: 10.1523/JNEUROSCI.1940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockart DJ, Schultz PG, Hu Y. Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Science (USA) 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increase NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Research. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Schanberg SM, Field TM. Sensory deprivation stress and supplemental stimulation in the rat pup and preterm human neonate. Child Development. 1987;58:1431–1447. [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. A multivariate statistical summary of synaptic plasticity measures in rats exposed to complex, social and individual environments. Brain Research. 1987;441:386–392. doi: 10.1016/0006-8993(88)91420-5. [DOI] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Developmental Psychobiology. 1999;35:226–240. [PubMed] [Google Scholar]
- Teskey GC, Monfils MH, Silasi G, Kolb B. Neocortical kindling is associated with opposing alterations in dendritic morphology in neocortical layer V and striatum from neocortical layer III. Synapse. 2006;59:1–9. doi: 10.1002/syn.20215. [DOI] [PubMed] [Google Scholar]
- Teskey GC. Using kindling to model the neuroplastic changes associated with learning and memory, neuropsychiatric disorders, and epilepsy. In: Shaw CA, McEachern JC, editors. Toward a theory of neuroplasticity. Philadelphia, PA: Taylor and Francis; 2001. pp. 347–358. [Google Scholar]
- van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Meaney M, Szf M. Maternal care effects on the maternal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Science (USA) 2006;103:3480–3486. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]