Abstract
Na-coupled HCO3 transporters (NCBTs) play important roles in brain pH regulation. One NCBT, the Na-driven Cl-HCO3 exchanger (SLC4A8 or NDCBE), appears to be the major regulator of intracellular pH (pHi), at least in some hippocampal pyramidal neurons. NDCBE is widely expressed throughout the central nervous system in rodent brain. In a previous study, it has been demonstrated that CCH decreases the abundance of NBCn1 and NBCn2 proteins in four regions of the mouse brain: cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC). Here we report the effect of CCH (11% O2) on the expression of NDCBE protein in mouse brain. Neonates (beginning at age P2) or adult mice (beginning at P90) were subjected to either normoxia or CCH for durations of 14 or 28 days. Membrane-protein levels were assessed by western blotting using our polyclonal antibody directed against NDCBE. In neonates, CCH significantly decreased NDCBE expression in HC after 14 days and SCX after 28 days, but had no significant effect for other combinations of region/duration. In adults, however, CCH significantly decreased (by 20–50%) the expression of NDCBE in all four brain regions, both with 14 and 28 days’ duration. Thus, the mouse brain exhibits marked developmental differences in the response of NDCBE protein expression to CCH. We hypothesize that decreases in adult NDCBE protein levels, which are probably out of proportion to the decreases in other proteins, may be part of an adaptive response that reduces energy consumption and/or stabilizes brain pHi. The smaller or absent responses in the young animals could be related to neonatal hypoxia tolerance.
Keywords: acid-base transporter, hippocampus, bicarbonate, neonates
1. INTRODUCTION
Due to its very high energy demands, the mammalian brain is particularly sensitive to hypoxia—a low-oxygen status in tissue. Even though it constitutes only ~2% of body weight, the brain accounts for ~20% of the body’s energy consumption (Buzsaki et al., 2007; Mellergard and Siesjo, 1998). pH is a key parameter in the central nervous system (CNS), with changes in pH affecting a range of neuronal parameters, including neuronal excitability, synaptic transmission, and ion-channel activity. The effect of hypoxia on extra-as well as intracellular pH (pHi) in the CNS is complex (for review, see ref. Yao and Haddad, 2004). Depending on the experiment conditions and the preparation, hypoxia can cause a fall (Melzian et al., 1996; Roberts, Jr. et al., 2000; von Hanwehr et al., 1986), or a rise (Cowan and Martin, 1995; Mitsufuji et al., 1995; Yao et al., 2001), or a fall followed by a rise (Diarra et al., 1999; Melzian et al., 1996; Sheldon and Church, 2002) of pHi in the CNS.
The brain achieves pH homeostasis by employing multiple acid-base transport mechanisms including: (1) several Na-H exchangers (NHEs) in the solute carrier 9 (SLC9) family (for reviews, see refs. Pedersen, 2006; Slepkov et al., 2007); (2) some members of SLC26 family capable of Cl-HCO3 exchange (for reviews, see ref. Dorwart et al., 2008); (3) Na-coupled HCO3− transporters (NCBTs) of the SLC4 family including the electrogenic Na/HCO3 cotransporters NBCe1 and NBCe2, the electroneutral NBCs NBCn1 and NBCn2, and the Na+-driven Cl-HCO3 exchanger NDCBE, which is electroneutral. Thus, it is reasonable to hypothesis that changes in the expression of acid-base transporters underlie—at least in part—the aforementioned pH changes. A recent study on cultured rat hippocampal astrocytes illustrates the complexity of the changes (Bevensee and Boron, 2008): acute hypoxia stimulates SITS-insensitive acid-extrusion (e.g., NHE activity) and acid loading, but inhibits SITS-sensitive acid-extrusion (e.g., NCBT activity). Moreover, in a previous study, Chen at al. demonstrated that chronic continuous hypoxia (CCH) significantly decreases the expression of NBCn1 and NBCn2 in mouse brain of neonates as well as adults (Chen et al., 2007).
NDCBE was the first acid-base transporter known to be involved in the regulation of pHi (Boron and De Weer, 1976a; Boron and De Weer, 1976b; Boron and Russell, 1983; Russell and Boron, 1976; Thomas, 1976a; Thomas, 1976b; Thomas, 1977). Based upon pHi measurement, NDCBE appears to be the major acid-extrusion mechanism in freshly dissociated hippocampal CA1 neurons with a low initial pHi (Schwiening and Boron, 1994). By immunocytochemistry, NDCBE protein is expressed in pyramidal neurons in hippocampus and in Purkinje cells in cerebellum of human (Damkier et al., 2007) and mouse (Chen et al., 2008a). Moreover, immunostaining in mouse brain sections shows that NDCBE is widely expressed in neurons of cerebral cortex, other areas of the cerebellum, substantia nigra, and medulla (Chen et al., 2008a).
We hypothesized that CCH—as it does for NBCn1 and NBCn2—reduces the expression of NDCBE protein in the brain of both neonatal and adult mice. To address our hypothesis, we subjected neonates (postnatal day 2 or P2) as well as adult (P90) mice to CCH (14 or 28 days’ duration). By western blotting with our newly available antibody (Chen et al., 2008a), we examined the expression of NDCBE in four mouse brain regions—cerebral cortex (CX), subcortex (SCX), cerebellum (CB) and hippocampus (HC). We made the surprising observation that, in neonatal mice, CCH significantly decreased the expression of NDCBE protein in only two combinations of region/duration: HC after 14 days’ treatment and SCX after 28 days’ treatment. However, in adult mice, CCH consistently caused a significant decrease (by 20–50%) in the expression of NDCBE protein in all four examined brain regions, for durations of both 14 and 28 days. We conclude that, in response to CCH, the ability of the mouse brain to regulate the protein levels of NDCBE is under developmental control.
2. RESULTS
2.1 Neonates: Hypoxia of 14 days’ duration
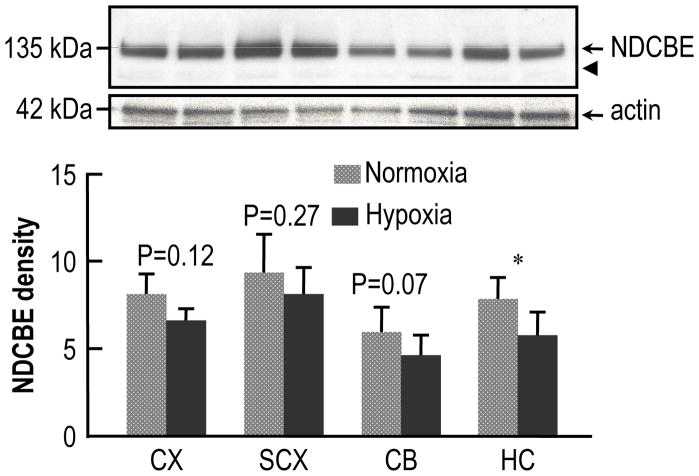
The upper panel of Fig. 1 shows a typical western blot representing the expression of NDCBE in different regions of mouse brain after 14 days of normoxia or CCH, starting at age P2. Throughout this study, we loaded the various lanes of the gels with equal amounts of total protein. As a loading marker for paired hypoxic and normoxic samples, we probed the blot with monoclonal mouse anti-actin antibody (middle panel of Fig. 1). Note that the actin signal varies in different regions of the normoxic brain, and may also vary with age in a single brain region.1 Therefore, in assessing the relative abundance of NDCBE protein in different brain regions, we did not normalize the NDCBE protein level to actin level, but rather to total-protein level, as in a previous study (Chen et al., 2007). We used the same strategy throughout the present study (Fig. 2–Fig. 4). The lower panel of Fig. 1 summarizes the densitometric data obtained from 5 such experiments like the one shown in the upper panel. Chronic continuous hypoxia caused significant decrease (lowering the mean by 26%) in the expression of NDCBE in HC. However, although the trend was always to decrease the mean intensity, CCH did not produce significant changes in CX, SCX, or CB.
Fig. 1.
Effect of chronic hypoxia (14 days’ duration) on the expression of NDCBE protein in the brains of neonatal mice. The mice were placed in the chamber at age P2. The upper panel shows typical western blots of NDCBE and actin in four brain regions—one pair of bands each for (from left to right) cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC). In each pair of bands, the left one represents normoxia, and the right one, hypoxia. The arrowhead represents the expected molecular weight of the unglycosylated protein (i.e., ~116 kDa). We used actin (middle panel) only as a loading marker, and not to normalize the NDCBE data (see text). The lower panel shows a summary of densitometry data from experiments like that in the upper panel. Each bar represents 5 groups of mice (8 mice per group). We made statistical comparisons between adjacent bars using paired two-tailed student’s T-tests (* P<0.05).
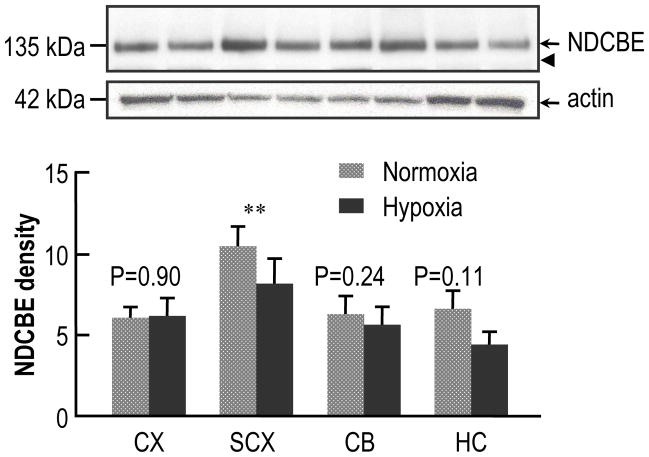
Fig. 2.
Effect of chronic hypoxia (28 days’ duration) on the expression of NDCBE protein in the brains of neonatal mice. The mice were placed in the chamber at age P2. The upper panel shows typical western blots of NDCBE and actin in four brain regions—one pair of bands each for (from left to right) cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC). In each pair of bands, the left one represents normoxia, and the right one, hypoxia. The arrowhead represents the expected molecular weight of the unglycosylated protein (i.e., ~116 kDa). We used actin (middle panel) only as a loading marker, and not to normalize the NDCBE data (see text). The lower panel shows a summary of densitometry data from experiments like that in the upper panel. Each bar represents 6 groups of mice (8 mice per group). We made statistical comparisons between adjacent bars using paired two-tailed student’s T-tests (** P<0.01).
Fig. 4.
Effect of chronic hypoxia (28 days’ duration) on the expression of NDCBE protein in the brains of adult mice. The mice were placed in the chamber at age P90. The upper panel shows typical western blots of NDCBE and actin in four brain regions—one pair of bands each for (from left to right) cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC). In each pair of bands, the left one represents normoxia, and the right one, hypoxia. The arrowhead represents the expected molecular weight of the unglycosylated protein (i.e., ~116 kDa). We used actin (middle panel) only as a loading marker, and not to normalize the NDCBE data (see text). The lower panel shows a summary of densitometry data from experiments like that in the upper panel. Each bar represents 5 groups of mice (5 mice per group). We made statistical comparisons between adjacent bars using paired two-tailed student’s T-tests (* P<0.05, ** P<0.01).
2.2 Neonates: Hypoxia of 28 days’ duration
The upper panel of Fig. 2 represents a typical western blot showing the expression of NDCBE in four regions of mouse brain after 28 days’ of normoxia or CCH, starting at age of P2. Actin staining provides a reference for total-protein loading (middle panel of Fig. 2). The lower panel of Fig. 2 summarizes the densitometric data from 6 such experiments like the one shown in the upper panel. CCH caused a significant decrease (lowering the mean by 22%) in NDCBE protein levels in SCX. Although the trend was for CCH to have no effect or modestly decrease the mean, the effects were not significant in CX, CB, or HC.
2.3 Adults: Hypoxia of 14 days’ duration
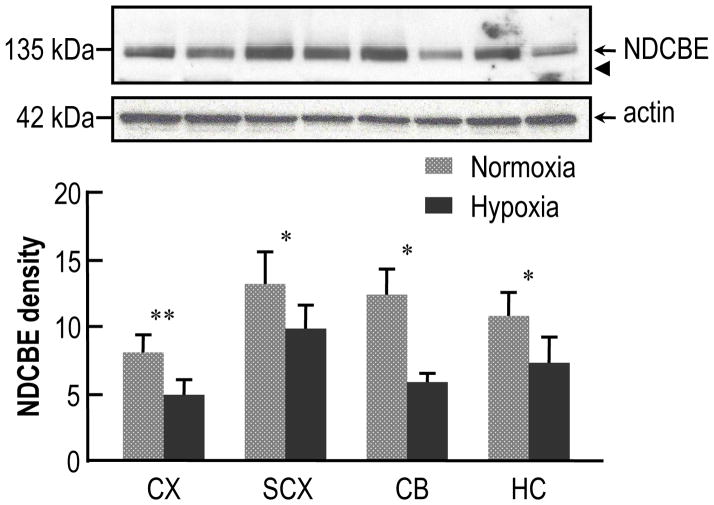
Fig. 3 shows the effect of CCH of 14 days’ duration, starting at age of P90, on the expression of NDCBE in adult mouse brain. The upper panel of Fig. 3 is a typical western blot showing NDCBE protein levels in different brain regions after either normoxia or hypoxia. The middle panel shows the blot for actin. The lower panel of Fig. 3 summarizes the densitometric data collected from five such experiments like the one shown in the upper panel. Unlike the case for neonates, here in adults NDCBE protein levels are significantly decreased under hypoxic conditions in all four regions, with means decreased by 50% in CX, 33% in SCX, 42% in CB and 45% in HC.
Fig. 3.
Effect of chronic hypoxia (14 days’ duration) on the expression of NDCBE protein in the brains of adult mice. The mice were placed in the chamber at age P90. The upper panel shows typical western blots of NDCBE and actin in four brain regions—one pair of bands each for (from left to right) cerebral cortex (CX), subcortex (SCX), cerebellum (CB), and hippocampus (HC). In each pair of bands, the left one represents normoxia, and the right one, hypoxia. The arrowhead represents the expected molecular weight of the unglycosylated protein (i.e., ~116 kDa). We used actin (middle panel) only as a loading marker, and not to normalize the NDCBE data (see text). The lower panel shows a summary of densitometry data from experiments like that in the upper panel. Each bar represents 5 groups of mice (5 mice per group). We made statistical comparisons between adjacent bars using paired two-tailed student’s T-tests (* P<0.05, ** P<0.01).
2.4 Adults: Hypoxia of 28 days’ duration
Fig. 4 shows the expression of NDCBE in the brains of mice subjected to normoxia or CCH for 28 days, starting at age of P90. The upper panel represents a typical western blot showing NDCBE protein levels in different brain regions after either normoxia or hypoxia. The middle panel shows the blot for actin. The lower panel of Fig. 4 summarizes data collected from five such experiments like the one shown in the upper panel. Again, CCH of 28 days’ duration significantly decreases the expression of NDCBE in all four regions of mouse brain, decreasing the mean by 40% in CX, 26% in SCX, 52% in CB, and by 32% in HC.
2.5 Lack of effect of CCH on NDCBE glycosylation
NCBT proteins have several potential glycosylation sites on the extracellular loop between putative transmembrane segments 5 and 6. Previous work demonstrated that virtually all NDCBE expressed in mouse brain cortex has an apparent MW of ~135 kDa and is N-glycosylated (Chen et al., 2008a). In the present study, we find that NDCBE has an apparent MW of ~135 kDa for all ages, all four brain regions, and for normoxia and CCH (see Fig. 1 through Fig. 4). The predicted MW of unglycosylated NDCBE is ~116 kDa (arrowhead in each upper panel). Thus, our results suggest that the NDCBE protein expressed in the four mouse-brain region—for both neonates and adults, under both normoxia and CCH conditions—are virtually all N-glycosylated.
3. DISCUSSION
3.1 Time course of the spatial distribution of NDCBE in the normoxic mouse brain
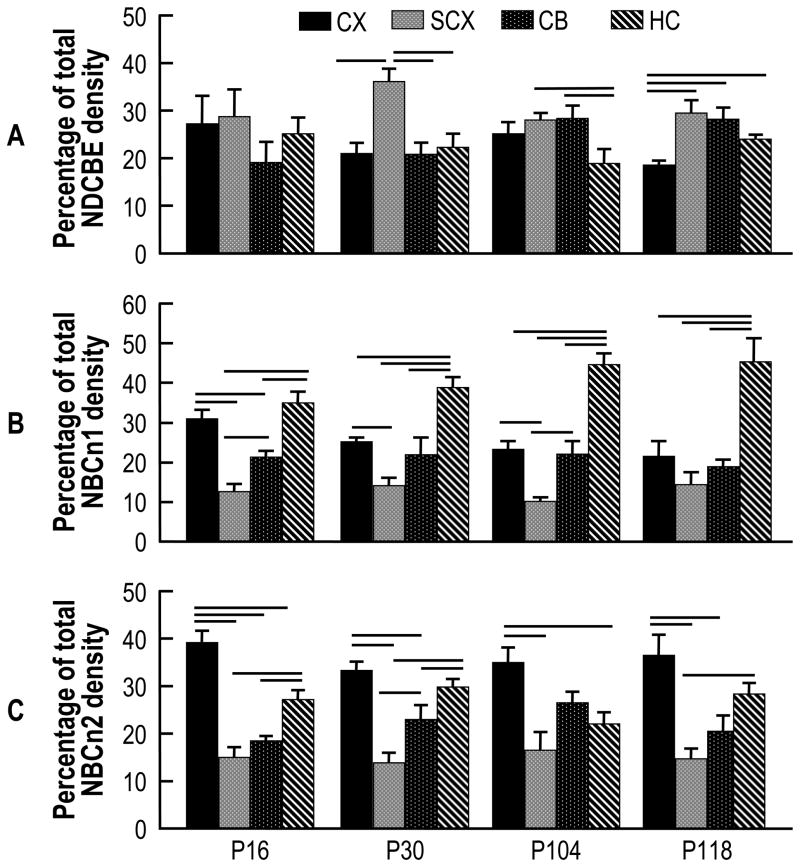
By immunocytochemistry, we had previously demonstrated that NDCBE is widely expressed throughout the adult mouse brain (Chen et al., 2008a). Although our standard method for dissecting brain tissue does not exclude the choroid plexus (Chen et al., 2008b), NDCBE is not expressed in the choroid plexus (Chen et al., 2008a). Fig. 5A summarizes the relative distribution of NDCBE in different mouse-brain regions as a function of development. Generally, NDCBE is rather evenly distributed among the four brain regions, and this stability is maintained as a function of age. However, NDCBE expression is relatively high in SCX at P30. Moreover, NDCBE expression is relatively low in HC at P104, and in CX at P118.
Fig. 5.
Time course of the relative levels of NDCBE protein in four brain regions of normoxic mouse. A, NDCBE. In the case of P16, for each experiment summarized in Fig. 1, we summed the total densities of the four brain regions under normoxic conditions, and then determined the fraction of total density contributed by each region (totaling 100%). The four bars at P16 represent the means ± SE of these data. We similarly computed the values for P30 (from data that contributed to Fig. 2), P104 (Fig. 3), and P118 (Fig. 4). We also reproduce, from a previous paper (Chen et al., 2007), comparable bar graphs that show the relative levels of NBCn1 (panel B) and NBCn2 (panel C). Horizontal lines indicate a significant difference, as assessed by an ANOVA analysis (p<0.05), between bars in the same age group.
For convenience of comparison, we also present analogous data on NBCn1 (Fig. 5 B) and NBCn2 (Fig. 5C) from a previous paper (Chen et al., 2007). In the case of NBCn1, expression at P16 is most abundant in CX and HC, with the relative fraction in HC increasing dramatically with development (Fig. 5B). The relatively abundant expression of NBCn1 in HC is consistent with a recent study on mice with the slc4a7 gene disrupted by insertion of the LacZ gene; this work shows that the NBCn1 promoter is most active in hippocampus (Boedtkjer et al., 2008). Finally, in the case of NBCn2, expression is highest in CX, and the expression pattern remains stable with development (Fig. 5C).
Our results indicate that the three electroneutral Na-coupled HCO3− transporters—NDCBE, NBCn1, and NBCn2—have distinct spatial and temporal expression patterns in mouse brain. Functional experiments employing pHi measurements indicate that Na-driven Cl-HCO3 exchange is the dominant acid-extrusion mechanism in freshly dissociated, low-pHi rat HC pyramidal neurons (Schwiening and Boron, 1994). The expression pattern of NDCBE protein in mouse brain suggests that NDCBE plays an important role in pH regulation throughout the brain.
3.2 Effect of CCH on the expression of NDCBE in neonatal vs adult mouse brain
Compared to adult mammals, neonates exhibit reduced sensitivity to hypoxia, referred to as neonatal hypoxia tolerance (Adolph, 1969; Britton and Kline, 1945; Duffy et al., 1975; Glass et al., 1944; Thurston and McDougal, Jr., 1969; for review see ref. Singer, 1999). This neonatal tolerance is related to the low metabolic rate in brain (for review, see ref. Singer, 1999), evidence for which is markedly lower rates of brain glucose and O2 consumption during the early postnatal period (Novotny, Jr. et al., 2001).
As part of an effort to understand the importance of developmental status for the response of acid-base to hypoxia, we studied the effect of CCH on the expression of the three known electroneutral Na-coupled HCO3− transporters. In the previous study on NBCn1 and NBCn2 (Chen et al., 2007), we found that—in both neonates and adults—CCH caused a statistically significant decrease in the expression of NBCn1 and NBCn2 proteins in nearly every combination of brain region/hypoxia duration. CCH reduced the abundance of NBCn1 protein by 20–50%, and the abundance of NBCn2 protein by 15–40%. In the present study, we extend our observation to NDCBE, the major acid-extrusion mechanism in brain neurons.
Neonates
The present study shows that—in neonates—CCH has much smaller effects on the expression of NDCBE protein than on either NBCn1 or NBC-n2. We find that 14 days’ CCH caused a statistically significant decrease in NDCBE protein only in hippocampus, although the tendency was for CCH to decrease NDCBE expression in other regions. After 28 days’ CCH, NDCBE protein abundance was significantly decreased only in SCX.
Adults
The present study reveals that—in adults—the effects of CCH on NDCBE expression are very similar to those on NBCn1 and NBCn2. Namely, in adults, NDCBE protein levels are decreased in all four brain regions by 33–50% after 14 days’ CCH, and by 26–52% after 28 days’ CCH. Thus, CCH has a much smaller effect on the expression of NDCBE protein in neonates than in adults.
Thus, in contrast to the two electroneutral Na/HCO3 cotransporters (i.e., NBCn1 and NBCn2), which exhibited no developmental differences in the response to CCH (Chen et al., 2007), adaptations in NDCBE levels exhibit major differences between neonates and adult mice.
Our CCH protocol causes a decrease in body weight (Chen et al., 2007), and thus presumably a decrease in overall brain protein levels. Because we loaded an equal amount of total protein onto each lane, we can conclude that CCH led to a preferential decrease in NDCBE protein (i.e., out of proportion to decreases in other proteins).
In brain, NDCBE is mainly expressed in neurons (Chen et al., 2008a; Damkier et al., 2007) and appears not to be expressed at substantial levels in astrocytes (Chen et al., 2008a). We do not know if the CCH-induced changes in NDCBE expression occur in particular populations of neurons or generally in the brain. Also, because the necessary antibodies are not available, we do not know the extent to which the observed effects of CCH relate to NDCBE-A vs -B. Moreover, no antibodies exist to detect two new splice variants, NDCBE-C and -D, which are truncated at their N terminus (Parker et al., 2008). Finally, we do not know the degree to which the CCH-induced changes in the expression of NDCBE protein are a result of pathology or an adaptive response to hypoxia or to other changes (e.g., respiratory alkalosis) secondary to the hypoxia.
3.3 Physiological significance
A major challenge during CCH—especially for tissues with a high energy utilization—is a substantial decrease in energy production (for reviews, see refs. Hardie et al., 2006; Ramamurthy and Ronnett, 2006). The tendency for CCH to lead to a decrease in the expression of NDCBE protein, as observed in the present study—as well as a decrease in NBCn1 and NBCn2, as observed in the previous study (Chen et al., 2007)—might be beneficial for mice subjected to CCH. In the previous study (Chen et al., 2007), the authors hypothesized that decreases in NBCn1 and NBCn2 could potentially reduce the brain energy consumption by: (1) contributing to an overall reduction in protein synthesis, (2) reducing Na+ influx (i.e., reducing Na+ extrusion by the Na+-K+ pump), and/or (3) lowering (or minimizing a rise in) neuronal pHi, thereby decreasing neuronal firing.
Recent work bears on the third hypothesis. First, Kanaan et al (Kanaan et al., 2007) demonstrated that chronic hypercapnia (which causes respiratory acidosis) leads to a rise in the expression of acid extruders (which tend to raise pHi) in the brain, but a fall in the expression of acid loaders (which tend to lower pHi)—presumably stabilizing pHi. Second, knocking out HCO3− transporters causes predictable effects on neuronal excitability. Jacobs et al (Jacobs et al., 2008) demonstrated that disrupting the slc4a10 gene encoding NBCn2—an acid-extruder—increases seizure threshold and increases the survivability of the mouse in seizures induced by proconvulsant substances. On the other hand, disrupting the slc4a3 gene encoding the Cl-HCO3 exchanger AE3—an acid-loader—decreases seizure threshold (Hentschke et al., 2006). These knockout studies suggest that inhibiting acid extrusion decreases, whereas inhibiting acid loading increases, the tendency of neurons to fire action potentials.
It is reasonable to hypothesize that decreases in protein levels of NDCBE observed in the present study—or of NBCn1 and NBCn2 in the previous study (Chen et al., 2007)—is part of an adaptive response to hypoxia in which a decrease in net acid-extrusion rate leads to a decrease in pHi and thus a decrease in neuronal firing and a decrease in energy consumption. However, as discussed previously (Chen et al., 2007), if the hypothesized decrease in the activities of acid-extruding processes (e.g., NDCBE, NBCn1, and NBCn2) were accompanied by decreases in the activities of acid-loading processes, then steady-state pHi could well be stable even though net energy consumption would fall. Note that the price paid by such a parallel inhibition of acid extrusion and acid loading would be a slower recovery of pHi from acute acid or alkali loads (Boron, 2004). A second possibility—not mutually exclusive—is that the fall in the protein levels of acid extruders is an adaptive response to an imperfectly compensated respiratory alkalosis. Finally, it is possible that the decreased protein levels of acid extruders are not adaptive, but pathological sequelae of hypoxia.
It is interesting that the effects of CCH on neonates were smaller or absent. It is well established that neonates are much less sensitive to hypoxia than adults. During the maturation of the central nervous system, the metabolic rates and energy consumption rates increase substantially (for review see ref. Singer, 1999). However, the mechanism of neonatal hypoxia tolerance is not well understood. If the adult NDCBE response to hypoxia is an adaptive mechanism to reduce energy consumption, then our data would be consistent with the hypothesis that neonates are able to have a different programmed response to hypoxia because they have an intrinsically lower energy demand. If the adult NDCBE response is an adaptive response to stabilize pHi, then it is possible that neonates have smaller imperfectly compensated respiratory alkalosis. Finally, if the adult NDCBE response is part of the pathological sequelae of hypoxia, then our data are consistent with the hypothesis that neonates have less hypoxia-induced pathology.
3.4 Summary
We demonstrate that, in neonates, chronic continuous hypoxia of 14 or 28 days’ duration causes significant decrease in NDCBE protein levels in only two combinations of region/duration. However, in adults, CCH causes substantial decrease in NDCBE protein levels in all four studied brain regions—cerebral cortex, subcortex, cerebellum, and hippocampus. Thus, the effects of CCH on the expression of NDCBE protein in mouse brain exhibits striking developmental differences.
4. EXPERIMENTAL PROCEDURE
4.1 Antibodies
At the time that we performed the present experiments, the only two published variants of NDCBE were mouse NDCBE-A (Wang et al., 2001) and human NDCBE-B (Grichtchenko et al., 2001), which differ in their carboxy termini. Therefore, in the present study, we used a previously described (Chen et al., 2008a) rabbit polyclonal antibody that is directed against the first 18 residues at the N terminus of human NDCBE-B, which is highly homologous to that of mouse NDCBE-A. A very recent paper (Parker et al., 2008) has described two novel NDCBE splice variants that, compared to NDCBE-A and -B, are truncated at their N termini: NDCBE-C (with the same C terminus as -A) and NDCBE-D (with the same C terminus as -B). To our knowledge, no antibody exists that would detect either of these new splice variants. Antibodies are not available to distinguish between the alternative carboxy termini that were identified previously. Moreover, because the alternative carboxyl termini of NDCBE-A and NDCBE-B are homologous to splice variants of other NCBTs, developing such antibodies would be problematic. Monoclonal mouse anti-actin antibody (catalog #MAB1501), HRP-conjugated goat anti-mouse (catalog #AP124P), and HRP-conjugated goat anti-rabbit (catalog #AP132P) secondary antibodies were all purchased from Chemicon International (Temecula, CA, USA).
4.2 Chronic exposure of mice to hypoxia
Animal treatment was performed as described previously (Chen et al., 2007). Briefly, CD1 mice (Charles River Laboratories, Raleigh, NC) were subjected to either normoxia or chronic hypoxia in normobaric chambers (OxyCycler, Reming Bioinstruments, Redfield, NY, USA) according to protocols approved by the Animal Care and Use Committees at Albert Einstein College of Medicine and University of California at San Diego. The inspired O2 concentration was monitored by a computer-controlled system. In each experiment, the mice—at the postnatal age of 2 days (P2) in the case of “neonates”, or at the age of 90 days (P90) in the case of “adults”—were randomly assigned to normoxic and hypoxic groups. The mice were subjected to either normoxia or chronic continuous hypoxia (11% O2) for either 14 days or 28 days. The mice were then anaesthetized with halothane by inhalation and sacrificed. Brain tissues from cerebral cortex (CX), subcortex (SCX), cerebellum (CB) and hippocampus (HC) were dissected on ice according to the anatomical definition previously described (Douglas et al., 2003). Briefly, the CX includes the superficial lobes of the cerebrum except for the hippocampus. The SCX represents the regions subcortical regions of the cerebrum, such as basal ganglia, thalamus, hypothalamus, as well as medulla. The tissues were immediately frozen at −80 °C until processed for membrane proteins.
4.3 Preparation of membrane proteins
All the following experimental procedures were performed on tissues from one group of control mice subjected to normoxia and from a matched group of mice subjected (at the same time as the controls) to chronic continuous hypoxia. Frozen mouse-brain tissues were pooled (8 for neonates or 5 for adult mice) and placed in ice-cold Na-Phosphate buffer (in mM: 7.5 NaH2PO4, 250 sucrose, 5 EDTA, 5 EGTA, pH 7.0) containing 1% protease inhibitor cocktail for mammalian tissues (Catalog #P8340, Sigma-Aldrich, MO). The tissues were homogenized by 10 strokes of a teflon pestle probe on a Glas-Col® Homogenizer (Glas-Col, Terre Haute, IN, USA). The homogenate were then centrifuged at 3,000 × g for 15 min at 4 °C to remove cell debris. The supernatant were ultracentrifuged at 100,000 × g for 1 hr at 4°C. The pellet was resuspended in protein-suspension buffer (in mM: 20 Tris-HCl, 5 EDTA, pH 8.0) containing 5% SDS. Total-protein concentration was measured using the BCATM Protein Assay Reagent (Catalog #23228 and #23224, Pierce, Rockford, IL, USA) according to the manufacturer’s instructions, and the membrane-protein preparations were stored in aliquots at −80 °C until use.
4.4 Western Blotting
Total membrane proteins were separated on SDS-polyacrylamide gel and transferred onto Immuno-BlotTM PVDF membrane (Bio-Rad, Hercules, CA). The blots were blocked at 4°C overnight in TBST (in mM: 10 Tris-HCl, 150 NaCl, 0.1% Tween 20, pH7.5) containing 5% Nestle powdered milk. The blots were then incubated with polyclonal rabbit anti-NDCBE (at a dilution of 1:2000) and monoclonal mouse anti-actin (at a dilution of 1:10,000) in TBST containing 1% powdered milk at room temperature (RT) for 2 hours, and then washed five times for 6 min with TBST. Afterward, the blots were incubated with secondary antibody in TBST containing 1% powdered milk at RT for 1 hour, and then washed with TBST × 6 min a total of five times. Chemiluminescent detection was performed by using ECL plusTM Western Blotting Detection Reagents (Amersham Biosciences, Piscataway, NJ, USA) together with X-ray film.
4.5 Statistics Analysis
Densitometric data of western blots are presented as means ± SE. A paired two-tailed student’s T-test was performed to compare the NDCBE density from paired groups of mice simultaneously subjected to normoxia or chronic continuous hypoxia. P<0.05 was considered statistically significant. To compare the relative distribution of NDCBE in different brain regions, we performed a one-way ANOVA and Student-Newman-Keuls (SNK) Multiple Comparison, using KaleidaGraph (Version 4, Synergy Software). The densitometric data of NDCBE from two of the five control groups of adult subjected to normoxia 14 days’ duration were reported previously (Chen et al., 2008a).
Acknowledgments
We thank Drs. Michelle L. Kelly, Bruce A. Davis, Amjad Kanaan as well as Ms Christina Lavoie and Ms Jenifer Salvato for help to collect and process tissue samples. We also thank Ms Orit Gavrialov and Ms Shirley Reynolds for their help with animal husbandry and care. Some of the work was carried out at the Albert Einstein College of Medicine and University of California at San Diego.
Footnotes
In addition, we noted that actin signals consistently appeared to be stronger in the lateral vs the middle lanes. In a control study, we loaded equal amount of the same sample in each lane, and observed that although the NDCBE signal was consistent across lanes, the actin signal was strongest in the lateral lanes.
DISCLOSURE
This work was supported by Program Project Grant HD32573 from the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolph EF. Regulations during survival without oxygen in infant mammals. Respir Physiol. 1969;7 (3):356–368. doi: 10.1016/0034-5687(69)90019-x. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Boron WF. Effects of acute hypoxia on intracellular-pH regulation in astrocytes cultured from rat hippocampus. Brain Res. 2008;1193:143–152. doi: 10.1016/j.brainres.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+, HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol. 2008;294 (2):C591–603. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- Boron WF. Regulation of intracellular pH. Adv Physiol Educ. 2004;28(4):160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Active proton transport stimulated by CO2/HCO3− blocked by cyanide. Nature. 1976a;259:240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J Gen Physiol. 1976b;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Russell JM. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983;81:373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton SW, Kline RF. Age, sex, carbohydrate, adrenal cortex and other factors in anoxia. Am J Physiol. 1945;46 (145):190–202. doi: 10.1152/ajplegacy.1945.145.2.190. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56 (5):771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Choi I, Haddad GG, Boron WF. Chronic continuous hypoxia decreases the expression of SLC4A7 (NBCn1) and SLC4A10 (NCBE) in mouse brain. Am J Physiol Regul Integr Comp Physiol. 2007;293 (6):R2412–R2420. doi: 10.1152/ajpregu.00497.2007. [DOI] [PubMed] [Google Scholar]
- Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF. Expression and localization of Na-driven Cl-HCO3- exchanger (SLC4A8) in rodent CNS. Neuroscience. 2008a;153 (1):162–174. doi: 10.1016/j.neuroscience.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Kelly ML, Rojas JD, Parker MD, Gill HS, Davis BA, Boron WF. Use of a new polyclonal antibody to study the distribution and glycosylation of the sodium-coupled bicarbonate transporter NCBE in rodent brain. Neuroscience. 2008b;151 (2):374–385. doi: 10.1016/j.neuroscience.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AI, Martin RL. Simultaneous measurement of pH and membrane potential in rat dorsal vagal motoneurons during normoxia and hypoxia: a comparison in bicarbonate and HEPES buffers. J Neurophysiol. 1995;74(6):2713–2721. doi: 10.1152/jn.1995.74.6.2713. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4 derived Na+ dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293 (5):R2136–46. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience. 1999;93 (3):1003–1016. doi: 10.1016/s0306-4522(99)00230-4. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23 (2):104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na+/H+ exchangers and HCO3-dependent transporters in mouse CNS. J Appl Physiol. 2003;95(1):292–299. doi: 10.1152/japplphysiol.01089.2002. [DOI] [PubMed] [Google Scholar]
- Duffy TE, Kohle SJ, Vannucci RC. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem. 1975;24(2):271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Glass HG, Snyder FF, Webster E. The rate of decline in resistance to anoxia of rabbits, dogs and guinea pigs from the onset of viability to adult life. Am J Physiol. 1944;140:609–615. [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276(11):8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574 (Pt 1):7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol Cell Biol. 2006;26(1):182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci U S A. 2008;105 (1):311–316. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan A, Douglas RM, Alper SL, Boron WF, Haddad GG. Effect of chronic elevated carbon dioxide on the expression of acid-base transporters in the neonatal and adult mouse. Am J Physiol Regul Integr Comp Physiol. 2007;293 (3):R1294–R1302. doi: 10.1152/ajpregu.00261.2007. [DOI] [PubMed] [Google Scholar]
- Mellergard P, Siesjo BK. In: pH and Brain Function. Kaila K, Ransom BR, editors. 1998. pp. 67–91. [Google Scholar]
- Melzian D, Scheufler E, Grieshaber M, Tegtmeier F. Tissue swelling and intracellular pH in the CA1 region of anoxic rat hippocampus. J Neurosci Methods. 1996;65 (2):183–187. doi: 10.1016/0165-0270(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Mitsufuji N, Yoshioka H, Tominaga M, Okano S, Nishiki T, Sawada T. Intracellular alkalosis during hypoxia in newborn mouse brain in the presence of systemic acidosis: a phosphorus magnetic resonance spectroscopic study. Brain & Development. 1995;17 (4):256–260. doi: 10.1016/0387-7604(95)00053-e. [DOI] [PubMed] [Google Scholar]
- Novotny EJ, Jr, Ariyan C, Mason GF, O’Reilly J, Haddad GG, Behar KL. Differential increase in cerebral cortical glucose oxidative metabolism during rat postnatal development is greater in vivo than in vitro. Brain Res. 2001;888(2):193–202. doi: 10.1016/s0006-8993(00)03051-1. [DOI] [PubMed] [Google Scholar]
- Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl-HCO3 exchanger variants -A, -C and -D. Physiol Genomics. 2008 doi: 10.1152/physiolgenomics.90259.2008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF. The Na+/H+ exchanger NHE1 in stress-induced signal transduction: implications for cell proliferation and cell death. Pflugers Arch. 2006;452(3):249–259. doi: 10.1007/s00424-006-0044-y. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574 (Pt 1):85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EL, Jr, He J, Chih CP. Rat hippocampal slices need bicarbonate for the recovery of synaptic transmission after anoxia. Brain Res. 2000;875(1–2):171–174. doi: 10.1016/s0006-8993(00)02587-7. [DOI] [PubMed] [Google Scholar]
- Russell JM, Boron WF. Role of chloride transport in regulation of intracellular pH. Nature. 1976;264:73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurons from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. J Physiol (Lond) 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C, Church J. Intracellular pH response to anoxia in acutely dissociated adult rat hippocampal CA1 neurons. J Neurophysiol. 2002;87(5):2209–2224. doi: 10.1152/jn.2002.87.5.2209. [DOI] [PubMed] [Google Scholar]
- Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol. 1999;123 (3):221–234. doi: 10.1016/s1095-6433(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J. 2007;401 (3):623–633. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976a;262:54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol (Lond) 1976b;255:715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol (Lond) 1977;273:317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston JH, McDougal DB., Jr Effect of ischemia on metabolism of the brain of the newborn mouse. Am J Physiol. 1969;216 (2):348–352. doi: 10.1152/ajplegacy.1969.216.2.348. [DOI] [PubMed] [Google Scholar]
- von Hanwehr R, Smith ML, Siesjo BK. Extra- and intracellular pH during near-complete forebrain ischemia in the rat. J Neurochem. 1986;46:331–339. doi: 10.1111/j.1471-4159.1986.tb12973.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Conforti L, Petrovic S, Amlal H, Burnham CE, Soleimani M. Mouse Na+:HCO3- Cotransporter Isoform NBC-3 (kNBC-3): Cloning, expression, and renal distribution. Kidney Int. 2001;59(4):1405–1414. doi: 10.1046/j.1523-1755.2001.0590041405.x. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu XQ, Douglas RM, Haddad GG. Role of Na+/H+ exchanger during O2 deprivation in mouse CA1 neurons. Am J Physiol Cell Physiol. 2001;281(4):C1205–C1210. doi: 10.1152/ajpcell.2001.281.4.C1205. [DOI] [PubMed] [Google Scholar]
- Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium. 2004;36 (3–4):247–255. doi: 10.1016/j.ceca.2004.02.013. [DOI] [PubMed] [Google Scholar]