Abstract
Currently, four species of the lacertid lizard genus Adolfus are known from Central and East Africa. We sequenced up to 2,825 bp of two mitochondrial (16S and cyt b) and two nuclear (c-mos and RAG1) genes from 41 samples of Adolfus (representing every species), two species each of Gastropholis and Holaspis, and in separate analyses combined this data with GenBank sequences of all other Eremiadini genera and four Lacertini outgroups. Data from DNA sequences were analyzed with maximum parsimony (PAUP), maximum-likelihood (RAxML) and Bayesian inference (MrBayes) criteria. Results demonstrated that Adolfus is not monophyletic: A. africanus (type species), A. alleni and A. jacksoni are sister taxa, whereas A. vauereselli and a new species from the Itombwe Plateau of Democratic Republic of the Congo are in a separate lineage. Holaspis and Gastropholis were recovered in separate clades. Based on this molecular data, relatively substantial sequence divergence and multiple morphological differences, we describe a new genus of lacertid for the lineage including A. vauereselli and the new Itombwe species. The recognition of this new, endemic genus underscores the conservation importance of the Albertine Rift, especially the Itombwe Plateau, a unique region that is severely threatened by unchecked deforestation, mining and poaching.
ADDITIONAL KEYWORDS: Itombwe Plateau, Albertine Rift, Afromontane, Systematics, Lizard, Endemism, Conservation
INTRODUCTION
Meadow and forest lizards of the lacertid genus Adolfus are currently known from Central and East Africa, including A. africanus (mid- to low elevation forests from Cameroon to Kenya), A. alleni (montane moorlands of Kenya and Uganda), and A. jacksoni and A. vauereselli, which are both known from mid- to high elevation forests in countries surrounding the Albertine Rift (Spawls et al., 2002; Köhler et al., 2003). Adolfus are medium-sized (total size to 25.6 cm), relatively slim lizards, and tend to be good climbers on standing and fallen timber, rocky walls, holes and crevices (A. africanus is also known to climb twiggy and herbaceous plants), but tend to hunt on the ground (Arnold, 1989a, 1998; Spawls et al., 2002). Recent work on this genus has included aspects of reproduction (A. jacksoni, Goldberg, 2009), endoparasites (A. jacksoni, Goldberg & Bursey, 2009), geographic distribution (A. africanus, Köhler et al., 2003), and morphology and color pattern (A. jacksoni, Poblete, 2002).
The taxonomic status and affinities of the currently recognized species of Adolfus have changed considerably over time. The genus Adolfus was first proposed by Sternfeld (1912) for the taxon A. fridericianus, which was presumably in honor of Adolphus Frederick, Duke of Mecklenburg, who led the German East Africa Expedition in 1907–08 when the specimens were collected (Frederick, 1910). In his opus on the Family Lacertidae, Boulenger (1920) considered Adolfus fridericianus to be a synonym of Algiroides africanus (= Algyroides africanus), a species he described in 1906, and recognized Algiroides alleni, Lacerta jacksonii (a species he described in 1899), and L. vauereselli. Based on morphological characters, Arnold (1973) resurrected the genus Adolfus for A. africanus, A. alleni and A. vauereselli, and noted a close relationship between this genus and Bedriagaia, Gastropholis and Lacerta jacksoni. In morphology-based parsimony and compatibility analyses, Arnold (1989a) transferred Lacerta jacksoni to the genus Adolfus, synonymized Bedriagaia with Gastropholis, recognized a clade called the “Equatorial African group” including Adolfus, Gastropholis and Holaspis (a well-supported clade recovered in a later morphology-based phylogeny by Harris, Arnold & Thomas, 1998), and discussed the problematic relationship of Holaspis to the paraphyletic genus Adolfus; if the latter two genera were to be joined, Holaspis would have priority. Arnold (1989a,b) admitted that Adolfus was poorly defined, and considered A. jacksoni to be the most plesiomorphic member of the Equatorial African clade. In a more extensive morphological analysis of the entire Family Lacertidae, Arnold (1989b) grouped the Equatorial African Clade with Lacerta jayakari (now Omanosaura jayakari), Lacerta australis (now Australolacerta australis) and several other genera (e.g., Tropidosaura, Poromera, Nucras) in an “Ethiopian and advanced Saharo-Eurasian forms” (ESE) group, which was later included in an “Armatured Clade” (Afrotropical species plus Eremias, Acanthodactylus, Mesalina and Ophisops) in recognition of the members’ unique supporting structure of the male hemipenis (Arnold, 1986a, 1998; Harris et al., 1998). Mayer & Benyr (1994) used an albumin-based analysis of most lacertid genera to imply paraphyly of the ESE group, with some of the Saharo-Eurasian genera grouping with European lacertids. Based on a combination of morphology and mtDNA data that contradicted several findings of Mayer & Benyr (1994), Harris et al. (1998) assigned the subfamily Eremiainae (Szczerbak, 1975) to the Armatured Clade.
More recent analyses of lacertids with mitochondrial data have done little to clarify the position of Adolfus in relation to other members of the Equatorial African clade, or the ESE group as a whole. Although Fu (1998) recovered a monophyletic “African clade” in a mitochondrial phylogeny of lacertids, no members of the Equatorial African clade were included. Harris (1999) combined the mitochondrial data of Fu (1998) and Harris et al. (1998) with some new data to produce a phylogeny of Lacertidae, but support for the ESE clade (still recognized as Eremiainae) was weak; two samples of Adolfus (A. africanus and A. jacksoni) were not supported as sister taxa. Fu (2000) published another phylogeny of Lacertidae with six mitochondrial genes (4.7 kb of DNA data), with most trees supporting the monophyly of the ESE clade, but with the exception of three closely related genera (Nucras, Latastia and Heliobolus), relationships among ESE genera were unclear, and the monophyly of two samples of Adolfus (A. jacksoni and A. vauereselli) was again not supported.
Mayer & Pavlicev (2007) published the first lacertid phylogeny based on nuclear data (c-mos and RAG1), and recovered two clades within a well-supported ESE (Eremiainae) group: clade B1, mainly from sub-Saharan Africa, including Poromera, Nucras, Latastia, Philochortus, Pseuderemias, Heliobolus, Tropidosaura, Pedioplanis, Ichnotropis and Meroles; and clade B2, mainly from the Saharo-Eurasian region, including Ophisops, Omanosaura, Acanthodactylus, Eremias, Mesalina, Adolfus and Holaspis, with the latter two Central African genera as well-supported sister taxa. Arnold, Arribas & Carranza (2007) re-analyzed the datasets of Harris et al. (1998) and Fu (2000), and published yet another lacertid phylogeny based on two mitochondrial genes (12S and cyt b). Although their main focus was not on the ESE group, they redefined the Eremiainae as the tribe Eremiadini, and placed the North African monotypic genus Atlantolacerta as the most basal member of the Eremiadini. Pavlicev & Mayer (2009) criticized the dataset of the latter study as “relatively short mitochondrial sequences when all taxa are considered,” rejected the tribe Eremiadini (instead recognizing it as subfamily Eremiadinae), but confirmed the placement of Atlantolacerta as the most basal member of the group. Hipsley et al. (2009) used mitochondrial and nuclear data from several previous studies to confirm the main findings of Mayer and Pavlicev (2007), but continued to recognize the tribe Eremiadini (sensu Arnold et al., 2007) and revised the date of its origin to the mid- to late Eocene, when the group could have invaded northwestern Africa via small island chains.
Three of the four species of Adolfus can be found in eastern Democratic Republic of the Congo (DRC), which harbors a panoply of habitats ranging from lowland rainforest to alpine grassland (Bastin et al., 2004; Vande weghe, 2004). Based on fieldwork in the poorly known Itombwe Plateau (eastern DRC) by EG, CK and MMA, we collected several specimens of an Adolfus that does not fit the description of any currently recognized species. To clarify the position of the Itombwe population to other Adolfus, we sequenced multiple genes from several members of the Equatorial African group of lacertids (Adolfus, Gastropholis and Holaspis), and discovered that the Itombwe population is a new species belonging to a lineage that deserves recognition as a distinct genus. We follow the General Lineage Species Concept (de Queiroz, 1998, 1999), an extension of the Evolutionary Species Concept (Wiley, 1981), which provides a consistent philosophical framework for taxonomic decisions, and rejects the premise of subspecies as natural groups. Our species recognition criteria (de Queiroz, 2007; Wiens and Penkrot, 2002) correspond in part to traditional morphological species, which are diagnosed by unique morphological characters, size and color pattern. We utilize a molecular estimate of phylogenetic relationships that is based on multiple, unlinked markers from multiple individuals within species to guide species delimitation and diagnosis, and identify relevant comparisons for species diagnoses (Barraclough & Davies, 2005; Brown et al., 2009).
MATERIALS AND METHODS
DNA EXTRACTION, PCR AMPLIFICATION AND SEQUENCING
Two mitochondrial (16S and cyt b) and two nuclear (c-mos and RAG1) genes were sequenced from all genera in the Equatorial African Group, including 41 samples of all species of Adolfus, Holaspis guentheri, H. laevis, Gastropholis prasina, G. vittatus, and five outgroup taxa, including: Acanthodactylus erythrurus (clade B2 of Mayer and Pavlicev, 2007), the basal-most member of Eremiadini (Atlantolacerta andreanskyi, Arnold et al., 2007), and three Lacertini genera (Iberolacerta cyreni, Podarcis muralis, Timon tangitanus). Some samples (e.g., Adolfus alleni) did not amplify for all genes; all sequences were deposited in GenBank (Appendix I). Genomic DNA was isolated from alcohol-preserved liver or muscle tissue samples with the Qiagen DNeasy tissue kit (Qiagen Inc., Valencia, CA, USA). We used 25 µl PCR reactions with gene-specific primers (Table 1) with an initial denaturation step of 95°C for 2 min, followed by denaturation at 95°C for 35s, annealing at 50°C for 35s, and extension at 72°C for 95s with 4s added to the extension per cycle for 32 (mitochondrial genes) or 34 (nuclear genes) cycles. Amplicons were visualized on a 1.5% agarose gel stained with SYBR Safe DNA gel stain (Invitrogen Corporation, Carlsbad, CA, USA), and target products were purified with AMPure magnetic bead solution (Agencourt Bioscience, Beverly, MA, USA) and sequenced with BigDye® Terminator Cycle Sequencing Kits (Applied Biosystems, Foster City, CA, USA). Sequencing reactions were purified with CleanSeq magnetic bead solution (Agencourt Bioscience) and sequenced with an ABI 3130xl automated sequencer at the DNA Core Facility at the University of Texas at El Paso (UTEP). Forward and reverse sequence contigs for each sample were assembled and edited using SeqMan (DNAStar, Maison, WI, USA) to ensure accuracy. Four samples of Adolfus showed evidence of pseudogenes (i.e., six codon insertion relative to all other lacertids with a reading frame shift) for c-mos, including A. jacksoni (CAS 201598), A. vauereselli (UTEP 20294, 20296), and the new species (UTEP 20263); Pavlicev and Mayer (2006) also reported c-mos pseudogenes in three species of Lacerta. Our pseudogene sequences were excluded from the data set of this study.
Table 1.
Primer sequences used in this study.
| Name | Source | Sequence | Gene |
|---|---|---|---|
| 16SA-L | Palumbi et al. (1991) | 5’-CGCCTGTTTATCAAAAACAT-3’ | 16S |
| 16SB-H | Palumbi et al. (1991) | 5’-CCGGTCTGAACTCAGATCACGT-3’ | 16S |
| CytbF700 | Bauer et al. (2007) | 5'-CTTCCAACACCAYCAAACATCTCAGCATGATGAAA-3' | cyt b |
| CytbR700 | Bauer et al. (2007) | 5'-ACTGTAGCCCCTCAGAATGATATTTGTCCTCA-3' | cyt b |
| Hcmos3 | Mayer and Pavlicev (2007) | 5’-GGTGATGGCAAATGAGTAGAT-3’ | c-mos |
| L-1zmos | Mayer and Pavlicev (2007) | 5’-CTAGCTTGGTGTTCTATAGACTGG-3’ | c-mos |
| Hcmos1 | Mayer and Pavlicev (2007) | 5’-GCAAATGAGTAGATGTCTGCC-3’ | c-mos |
| R13 | Groth and Barrowclough (1999) | 5'- TCTGAATGGAAATTCAAGCTGTT-3' | RAG1 |
| R18 | Groth and Barrowclough (1999) | 5'-GATGCTGCCTCGGTCGGCCACCTTT-3' | RAG1 |
| RAG1f700 | Bauer et al. (2007) | 5'-GGAGACATGGACACAATCCATCCTAC-3' | RAG1 |
| RAG1r700 | Bauer et al.(2007) | 5'-TTTGTACTGAGATGGATCTTTTTGCA-3' | RAG1 |
| RAG-R1 | Mayer and Pavlicev (2007) | 5’-AAAATCTGCCTTCCTGTTATTG-3’ | RAG1 |
| RAG-fo | Mayer and Pavlicev (2007) | 5’-GAAAAGGGCTACATCCTGG-3’ | RAG1 |
| RAG-re | Mayer and Pavlicev (2007) | 5’-CCAGTTATTGCTTTTACAGTTC-3’ | RAG1 |
SEQUENCE ALIGNMENT AND PHYLOGENETIC ANALYSES
An initial alignment of each gene was produced in MEGALIGN (DNA Star) with the Clustal W algorithm, and manual adjustments were made in MacClade 4.08 (Maddison & Maddison, 2005). Protein-coding genes were translated to amino acids with MacClade to confirm conservation of the amino acid reading frame, ensure alignment and check for premature stop codons. No ambiguously aligned regions were observed, and as a result, no data were excluded from phylogenetic analyses. Phylogenetic relationships among the samples were assessed with maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI) optimality criteria in the programs PAUP* 4.0b10 (Swofford, 2002), RAxML (Stamatakis, 2006) and MrBayes 3.1 (Ronquist & Huelsenbeck, 2003), respectively. For MP analyses, the heuristic search algorithm was used with 100 random-addition replicates, accelerated character transformation and tree bisection-reconnection branch swapping, zero-length branches collapsed to polytomies, and gaps treated as missing data; we used non-parametric bootstraps (1,000 pseudoreplicates) to assess node support in resulting topologies from these parsimony searches (Felsenstein, 1985). The Akaike Information Criterion (Posada & Buckley, 2004) in jModelTest (Posada, 2008) was used to find the model of evolution that best fit the data for subsequent BI analyses. RAxML analyses were executed with partitioned datasets (one for 16S, and one for each codon position of all other protein-coding genes), and 100 replicate ML inferences were peformed for each analysis. Each analysis was initiated with a random starting tree, included the GTRGAMMA option (−m) and employed the rapid hill-climbing algorithm (− x) (Stamatakis et al., 2007). Clade support was assessed with 1,000 bootstrap replicates, with the rapid-hill climbing algorithm (Stamatakis, Hoover & Rougemont, 2008). Phylogenetic trees were visualized with FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Partitioned Bayesian analyses were conducted with default priors. Analyses were initiated with random starting trees and run for 10,000,000 generations; Markov chains were sampled every 1000 generations. Convergence was checked by importing the trace files (p files) from the MrBayes output to the computer program Tracer v1.3 (http://tree.bio.ed.ac.uk/software/tracer/), which plots the likelihood values against generation number. Once the graphical plot leveled off, convergence had been met; we conservatively discarded 25% of trees as “burn in.” Four separate analyses with two independent chains were executed to check for convergence of log-likelihoods in stationarity (Huelsenbeck & Ronquist, 2001; Leaché & Reeder, 2002). To test the monophyly of polyphyletic lineages recovered in our phylogenetic analyses of the four-gene dataset, we used the Shimodaira-Hasegawa (SH) and approximately unbiased (AU) tests as implemented in CONSEL V0.1i (Shimodaira & Hasegawa, 2001; Shimodaira, 2002). We tested the hypothesis of zero-length branches for polyphyletic lineages of the Equatorial African lacertids by comparing the likelihood of the optimal ML tree from the four-gene dataset to the likelihood of the optimal tree with one branch collapsed with the “describe trees” function in PAUP* (sensu Poe & Chubb, 2004), and a Bonferroni-corrected p value of 0.025.
Combining data from multiple mitochondrial genes is appropriate because the entire animal mitochondrial genome is inherited as a single unit, and different mitochondrial genes are not independent estimates of organismal phylogeny (Moore, 1995; Page, 2000). We combined mitochondrial and nuclear gene datasets if there was no strong bootstrap support for conflicting nodes (exceeding 70% for MP analyses [Hillis & Bull, 1993] and 95% for ML and BI analyses [Leaché & Reeder, 2002; Wilcox et al., 2002]) when these datasets were analyzed independently. After preliminary analyses confirmed there was no conflict between mitochondrial and nuclear gene datasets (data not shown), we conducted two analyses: (1) c-mos and a 1,012-bp fragment of RAG1 (primers from Mayer and Pavlicev, 2007) for samples from this study and previously sequenced lacertids from GenBank (Appendix 1) with Gallotia as the outgroup; and (2) both mitochondrial (16S and cyt b) genes, c-mos and a 1,394-bp fragment of RAG1 (primers from Groth & Barrowclough [1999] and Bauer et al., [2007]) for every sample from this study (hereafter referred to as the four-gene dataset) with three Lacertini outgroups.
MORPHOLOGY
Specimens examined for this study (Appendix 2) were preserved in 10% buffered formalin in the field, and transferred to 70% ethanol at the conclusion of each expedition. Tissues were harvested before formalin fixation from the liver or hind limb muscle of lizards, and preserved in 95% ethanol. Institutional abbreviations are listed at http://www.asih.org/codons.pdf. The senior author recorded morphometric data from these preserved specimens with vernier calipers to the nearest 0.1 mm under a stereomicroscope. Color descriptions are based on preserved specimens, field notes, and color digital images in life. Sex was determined by direct examination of gonads, or from the presence of everted hemipenes as noted in field notes. X-rays for descriptions of the post-cranial skeleton were taken with a Kodak Image Station In-Vivo FX (Carestream Health, Inc., Rochester, NY, USA) under the following conditions: f-stop: 8.0; FOV: 198 mm; focal plane: 0; exposure time: 288 sec; Kilovolt Potential Energy: 35; filter: 600WB.
Meristic and mensural characters were chosen from lacertid studies by Arnold (1989b) and Lue and Lin (2008). Measurements were taken on the right side of the lizard and include: snout–vent length (SVL, from tip of snout to anterior margin of vent); tail length (TL, from posterior margin of vent to tail tip, measured only from specimens with complete and original tails); head length (HL, from tip of snout to anterior margin of ear opening); maximum head width (HW, measured at the broadest point); head height (HH, measured at the jaw rictus); skull length (SKL, from tip of snout to posterior margin of occipital); snout–eye length (SEL, from tip of snout to anterior margin of eye); mouth length (ML); snout–arm length (SAL, from tip of snout to anterior margin of forelimb); axilla–groin distance (AGD, from posterior edge of forelimb insertion to anterior edge of hind limb insertion); humerus length (HML); radius–ulna length (RUL); femur length (FL); tibia–fibula length (TFL); and longest toe length (LTL, length of fourth toe on hind limb).
Meristic data were taken from the right side of each lizard, except for femoral pore counts if field/museum tags were tied to the right leg. Definition of scales follow those of Arnold (1989b) and Arnold et al. (2007), and include: chin shields (CS); femoral pores (FP); supralabials (SL); infralabials (IL); supraoculars (SO): supraciliaries (SC); supraciliary granules (SG); supratemporals (ST); anterior dorsal scale rows (ADS, counted transversely at posterior insertion of forelimbs); posterior dorsal scale rows (PDS, counted transversely at anterior insertion of hind limbs); dorsal scale rows at midbody (DSR, counted transversely at midpoint between fore- and hind limbs); dorsal scale numbers (DSN, counted longitudinally from posterior margin of occipital to posterior margin of hind limbs); ventral rows (VR, counted transversely at midbody); ventral scale numbers (VN, counted longitudinally from posterior margin of collars to anteior margin of preanal scales, took average from the middle two rows); caudal scales (CDS, counted around the tail at the position of the 11th and 15th scale to avoid the difference between males and females); and subdigital lamellae on fingers (SDF1 to SDF5) and toes (SDT1 to SDT5).
RESULTS
MOLECULAR PHYLOGENETICS
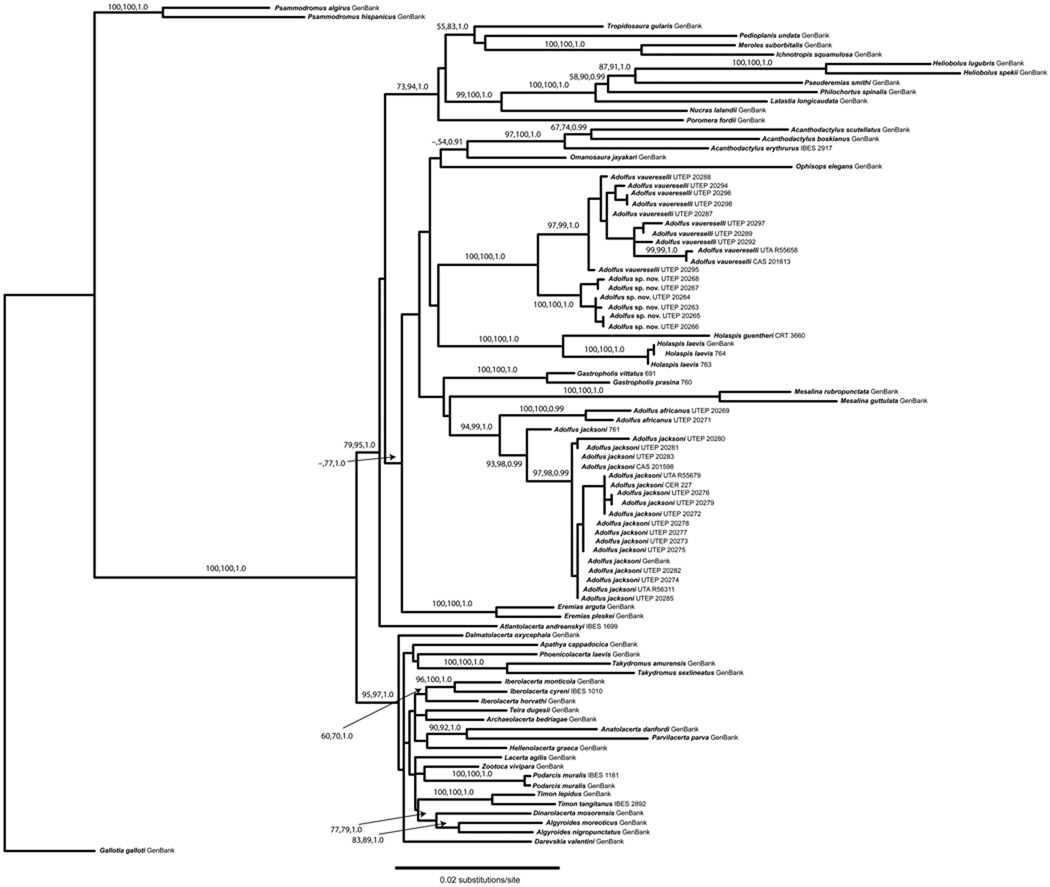
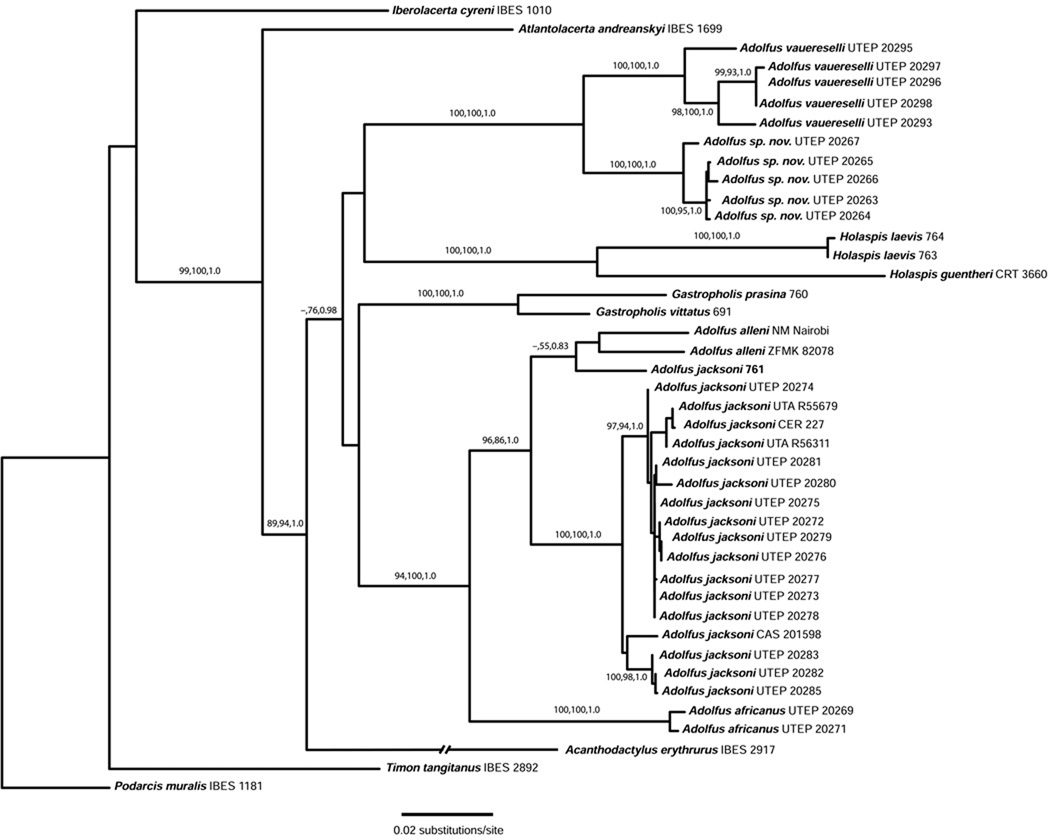
Relationships among members of the Equatorial African Group of lacertid lizards are shown in Figures 1–2; MP, ML and BI analyses produced nearly identical topologies for each dataset, with only minor differences in bootstrap support for each analysis. For the four-gene dataset (Fig. 2), we noted a six-codon deletion in the RAG1 gene (between positions 134–151) in multiple samples of Adolfus africanus and A. jacksoni.
Figure 1.
Maximum likelihood phylogeny (RAxML tree) of lacertid lizards in the Equatorial African Group, based on the combined nuclear c-mos/RAG1 dataset from this study and Genbank samples from Mayer and Pavlicev (2007). Bootstrap and posterior probability values for each well-supported node are listed in the order: maximum parsimony/maximum likelihood/Bayesian inference.
Figure 2.
Maximum likelihood phylogeny (RAxML tree) of the Equatorial African clade of lizards based on the combined 16S, cyt b, c-mos and RAG1 genes. Bootstrap and posterior probability values for each well-supported node are listed in the order: maximum parsimony/maximum likelihood/Bayesian inference.
The following models of nucleotide substitution were selected by jModeltest for BI analyses: 16S (GTR + I + G); cyt b 1st codon (TIM2ef + I); cyt b 2nd codon (GTR + I); cyt b 3rd codon (GTR + I + G); c-mos 1st codon (HKY + G); c-mos 2nd codon (TIM3 + G); c-mos 3rd codon (TrN + G); RAG1 1st codon (TrN + I); RAG1 2nd codon (TPM1uf + G); RAG1 3rd codon (TPM3uf + I + G). The MP analysis of the c-mos/RAG1 dataset (Fig. 1), included 1605 base pairs (933 constant, 429 parsimony-informative) and resulted in 28,908 most parsimonious trees (length = 1511, CI = 0.574, RI = 0.768); the ML analysis likelihood score was –11052.633819. The MP analysis of the four-gene dataset (Fig. 2), included 2,825 base pairs (2,185 constant, 444 parsimony-informative) and resulted in 5,368 most parsimonious trees (length = 1588, CI = 0.520, RI = 0.777); the ML analysis likelihood score was –11185.625563.
The c-mos/RAG1 tree (Fig. 1) showed strong support for a monophyletic Eremiadini, and a well-supported clade of Ethiopian lacertids (corresponding to clade B1 of Mayer and Pavlicev, 2007). The remaining Eremiadini lineages were recovered with the following well-supported clades: Eremias (two species), Acanthodactylus (three species), Adolfus vauereselli + A. sp. nov. (Itombwe Plateau), and Adolfus africanus + A. jacksoni. The four-gene dataset (Fig. 2) also shows well-supported clades for Adolfus vauereselli + A. sp. nov. (Itombwe Plateau), and Adolfus africanus + A. alleni + A. jacksoni, with both of these lineages included in a clade with Acanthodactylus, Gastropholis and Holaspis, and a well-supported sister relationship of all of these taxa to Atlantolacerta, again confirmed as the most basal member of Eremiadini.
Among genera of previously recognized lacertids, uncorrected p sequence divergence for the c-mos/RAG1 dataset (Table 2) ranged from 2.4% (Ichnotropis vs. Meroles) to 8.5% (Heliobolus vs. Ophisops). Among previously recognized genera of the Equatorial African Group, uncorrected p sequence divergence for the c-mos/RAG1 dataset ranged from 2.2–3.7% (Adolfus sensu strico vs. Gastropholis) to 3.5–4.1% (Adolfus sensu stricto vs. Holaspis); divergences between the two well-supported lineages of Adolfus (A. africanus + A. alleni + A. jacksoni vs. A. vauereselli + A. sp. nov. [Itombwe Plateau]) ranged from 2.7–3.4% (Table 2). Uncorrected p sequence divergence for the c-mos/RAG1 dataset ranged from 0.0–0.1% within populations of Adolfus vauereselli and A. sp. (Itombwe Plateau), but ranged from 1.3–2.2% between these well-supported taxa; equivalent 16S mitochondrial data ranged from 0.0–1.5% within populations of each taxon to 5.9–6.3% between these taxa (data not shown). Among the two disjunct, montane populations of A. alleni, cyt b divergence (the only gene that amplified for both samples) was 10.9% (data not shown). Hypothesis tests that constrained the monophyly of Adolfus were not significantly different from our preferred tree (AU: p = 0.381; SH: p = 0.382). Tests for zero-length branches for the lineage containing Holaspis + Adolfus vauereselli + A. sp. nov. (p = 0.263) and the lineage containing Gastropholis + A. africanus + A. alleni + A. jacksoni (p = 0.139) were not significantly different from zero.
Table 2.
Uncorrected p sequence divergence (c-mos/RAG1 dataset) for selected samples of Adolfus and other Eremiadini genera included in this study. When more than one sample was sequenced for a given species, specific locality information is provided for the sample included in this table.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Acanthodactylus erythrurus | — | ||||||||||||||||||||||
| 2 Adolfus africanus (Hombo, DRC) | 0.04 219 | — | |||||||||||||||||||||
| 3 Adolfus jacksoni (Rukiva, Rwanda) | 0.04 085 | 0.02 244 | — | ||||||||||||||||||||
| 4 Adolfus jacksoni (Arusha, Tanzania) | 0.03 840 | .019 26 | 0.00 835 | — | |||||||||||||||||||
| 5 Adolfus vauereselli(Kahuzi Biega NP, DRC) | 0.04 440 | 0.02 735 | 0.03 468 | 0.03 390 | — | ||||||||||||||||||
| 6 Adolfus sp. nov. (HOLOTYPE) | 0.03 952 | 0.03 264 | 0.03 085 | 0.03 144 | 0.01 610 | — | |||||||||||||||||
| 7 Atlantolacerta andreanskyi | 0.03 963 | 0.03 011 | 0.02 969 | 0.02 696 | 0.03 358 | 0.03 337 | — | ||||||||||||||||
| 8 Eremias arguta | 0.04 087 | 0.03 452 | 0.03 015 | 0.02 817 | 0.03 466 | 0.03 271 | 0.03 095 | — | |||||||||||||||
| 9 Gastropholis vittatus | 0.04 130 | 0.03 077 | 0.02 514 | 0.02 183 | 0.03 854 | 0.03 323 | 0.03 082 | 0.03 206 | — | ||||||||||||||
| 10 Heliobolus lugubris | 0.07 169 | 0.06 906 | 0.06 469 | 0.06 277 | 0.07 254 | 0.06 668 | 0.06 189 | 0.06 529 | 0.06 534 | — | |||||||||||||
| 11 Holaspis laevis (Usambaras, Tanzania) | 0.05 157 | 0.04 085 | 0.03 455 | 0.03 519 | 0.04 305 | 0.03 964 | 0.03 788 | 0.03 955 | 0.03 833 | 0.07 220 | — | ||||||||||||
| 12 Ichnotropis squamulosa | 0.05 354 | 0.05 375 | 0.05 100 | 0.04 813 | 0.05 742 | 0.05 487 | 0.04 363 | 0.05 160 | 0.05 039 | 0.07 489 | 0.05 350 | — | |||||||||||
| 13 Latastia longicaudata | 0.06 032 | 0.06 008 | 0.05 526 | 0.05 187 | 0.06 104 | 0.05 789 | 0.05 115 | 0.05 587 | 0.05 340 | 0.05 022 | 0.06 403 | 0.06 421 | — | ||||||||||
| 14 Meroles suborbitalis | 0.05 024 | 0.04 919 | 0.04 710 | 0.04 420 | 0.05 211 | 0.04 968 | 0.04 229 | 0.04 771 | 0.04 838 | 0.07 282 | 0.05 148 | 0.02 392 | 0.05 964 | — | |||||||||
| 15 Mesalina guttulata | 0.05 473 | 0.05 182 | 0.05 150 | 0.04 872 | 0.05 979 | 0.05 602 | 0.05 244 | 0.05 336 | 0.05 216 | 0.08 035 | 0.05 964 | 0.06 860 | 0.07 282 | 0.06 591 | — | ||||||||
| 16 Nucras lalandii | 0.05 089 | 0.05 177 | 0.04 775 | 0.04 485 | 0.05 343 | 0.04 974 | 0.04 357 | 0.04 648 | 0.04 776 | 0.05 967 | 0.05 465 | 0.05 478 | 0.04 396 | 0.05 276 | 0.06 721 | — | |||||||
| 17 Omanosaura jayakari | 0.03 586 | 0.03 258 | 0.03 078 | 0.03 008 | 0.03 209 | 0.03 021 | 0.03 158 | 0.03 076 | 0.03 205 | 0.06 842 | 0.03 829 | 0.04 784 | 0.05 399 | 0.04 457 | 0.05 524 | 0.04 648 | — | ||||||
| 18 Ophisops elegans | 0.05 285 | 0.05 491 | 0.05 214 | 0.05 054 | 0.05 140 | 0.04 847 | 0.05 303 | 0.04 896 | 0.05 342 | 0.08 475 | 0.05 712 | 0.06 608 | 0.07 156 | 0.06 089 | 0.06 905 | 0.06 281 | 0.04 708 | — | |||||
| 19 Pedioplanis undata | 0.05 859 | 0.05 364 | 0.04 838 | 0.04 734 | 0.05 393 | 0.05 226 | 0.04 677 | 0.04 896 | 0.04 967 | 0.07 156 | 0.05 650 | 0.05 478 | 0.05 964 | 0.05 210 | 0.07 282 | 0.05 340 | 0.04 457 | 0.06 717 | — | ||||
| 20 Philochortus spinalis | 0.06 041 | 0.05 757 | 0.05 785 | 0.05 515 | 0.06 237 | 0.05 757 | 0.05 246 | 0.05 781 | 0.05 472 | 0.05 089 | 0.06 660 | 0.06 234 | 0.03 958 | 0.05 906 | 0.07 164 | 0.05 153 | 0.05 654 | 0.07 664 | 0.05 904 | — | |||
| 21 Poromera fordii | 0.04 717 | 0.04 981 | 0.04 900 | 0.04 734 | 0.04 941 | 0.04 908 | 0.04 292 | 0.04 583 | 0.04 836 | 0.06 340 | 0.05 399 | 0.05 602 | 0.05 399 | 0.05 461 | 0.06 591 | 0.04 646 | 0.04 143 | 0.06 152 | 0.05 461 | 0.05 529 | — | ||
| 22 Pseuderemias smithi | 0.06 599 | 0.06 073 | 0.05 402 | 0.05 318 | 0.06 040 | 0.05 600 | 0.05 304 | 0.05 713 | 0.05 278 | 0.04 772 | 0.06 530 | 0.06 734 | 0.03 893 | 0.06 404 | 0.07 534 | 0.04 837 | 0.05 462 | 0.07 785 | 0.05 713 | 0.04 147 | 0.05 839 | — | |
| 23 Tropidosaura gularis | 0.04 273 | 0.04 338 | 0.04 083 | 0.03 838 | 0.04 438 | 0.04 153 | 0.03 532 | 0.03 955 | 0.03 959 | 0.06 215 | 0.04 457 | 0.03 966 | 0.04 959 | 0.03 766 | 0.05 838 | 0.04 020 | 0.03 515 | 0.05 524 | 0.03 766 | 0.05 090 | 0.03 892 | 0.05 086 | — |
TAXONOMIC IMPLICATIONS
Our molecular datasets indicate Adolfus is polyphyletic (with weak support) with regard to Acanthodactylus, Gastropholis and Holaspis (Figs. 1–2), there is a six-codon deletion in the RAG1 gene for the lineage including A. alleni, A. africanus and A. jacksoni, and c-mos/RAG1 uncorrected p sequence divergence between the two well-supported Adolfus lineages is equal to or exceeds divergences noted for previously recognized lacertid genera (Table 2; Mayer & Pavlicev, 2007). Although our hypothesis tests that constrained the monophyly of Adolfus were not significant, these results are not surprising given the zero-length branches separating the lineages of Equatorial African lacertids. Because there are numerous mensural, meristic and qualitative differences between the well-established genera of Equatorial African lacertids (Table 3; Arnold, 1989a), and considerable taxonomic instability would be created by grouping this diverse assemblage of lizards into one genus, we recognize each well-supported lineage of Adolfus as a distinct genus. Accounts for both genera are provided below, and follow the format of Arnold et al. (2007).
Table 3.
Comparison of selected mensural, meristic and qualitative diagnostic characters for genera in the Equatorial African group of lacertid lizards. SVL/TL given as percentage data. Data are from this study, Arnold (1989b), Kroniger & in den Bosch (2001), Schmidt (1919) and Spawls et al. (2002). + = present, − = absent. — = data not available.
| Character | Adolfus | Congolacerta gen. nov. | Gastropholis | Holaspis |
|---|---|---|---|---|
| Adult SVL (mm) | 55–84 | 50–58 | 80–110 | 38–52 |
| SVL/TL | 49–60 | 44–52 | 42–45 | 71–93 |
| Ventral Scale Count (transversely) | 6 | 6 | 10–14 | 6 |
| Femoral pores | 11–19 | 11–16 | 13–15 | 18–24 |
| Frontoparietal scales | + | + | + | − |
| Vertebral series of enlarged scales | − | − | − | + |
| Tail strongly depressed and fringed laterally | − | − | − | + |
| Tail prehensile | − | − | + | − |
| Ventrals keeled | − | − | + | − |
| Dorso-anterior border of quadrate bone | rounded | rounded | angular | rounded |
| Size of long free ribs immediately posterior to thoracic ribs | moderately elongated | very elongated | moderately elongated | very elongated |
| Posterior border of medial loop of clavicle | present and slender | present and thickened | present and slender | absent |
| Intramuscular portion of hemipenial armature | not deeply cleft | deeply cleft anteriorly | not deeply cleft | deeply cleft anteriorly |
| Shape of hemipenial clavulae | simple | complexly lobed | simple | simple |
| Female genital sinus | unlobed | bilobed | unlobed | unlobed |
| Habitat | forest and clearings, grassland | forest clearings, grassland | forest canopy | forest |
| Clutch size | 3–5 | — | 5 | 2 |
| Ventral coloration | yellow, orange, green or blue | yellow or unpigmented | yellow-green | orange to orange-gray |
Our data also suggest species diversity within Adolfus sensu stricto is currently underestimated. The sequence divergence (cyt b) between the samples of A. alleni from the Aberdares and Mt. Kenya suggest these populations are not conspecific, and Arnold (1989a: table 2) provided mensural and meristic data that showed marked differences between populations from Mt. Kenya, Mt. Elgon, and the Aberdares. Loveridge (1957) did not recognize any of these populations as taxonomically distinct, but additional sampling is needed before taxonomic recognition of these populations would be warranted. Further study is also needed on the Arusha, Tanzania population of A. jacksoni, which has a color pattern that is noticeably different from populations in the Albertine Rift (see also Poblete, 2002; Spawls et al., 2002).
Adolfus
Type species
Adolfus africanus (Boulenger, 1906) [=Adolfus fridericianus Sternfeld, 1912].
Synonymy
-
-
Algiroides Duméril & Bibron, 1839 (part); Boulenger, 1906. Proceedings of the Zoological Society of London 1906:570 [Algiroides africanus]; Barbour, 1914. Proceedings of the New England Zoological Club, Boston 4:97 [Algiroides alleni].
-
-
Lacerta Linnaeus, 1758 (part); Boulenger, 1899. Proceedings of the Zoological Society of London 1899:96 [Lacerta jacksoni]; Lönnberg in Sjöstedt, 1907. Wissenschaftliche Ergebnisse der Swedischen Zoologischen Expedition nach dem Kilimandjaro, dem Meru und den umgebenden Massai-steppen Deutsch-Ostafrikas 4:5 [Lacerta jacksoni kibonotensis]; Boulenger, 1920. Monograph of the Lacertidae. Vol. 1:295. [Lacerta jacksonii].
Content
Adolfus africanus (Boulenger, 1906); Adolfus alleni (Barbour, 1914); Adolfus jacksoni (Boulenger, 1899).
Distribution
Western Cameroon east to southern Sudan, Uganda, Kenya, and Tanzania, and south to northwestern Zambia (Köhler et al., 2003), with isolated montane populations in the Aberdare Mountains, Mt. Kenya and Mt. Elgon (Spawls et al., 2002).
Diagnosis
Several mensural, meristic and qualitative characters that diagnose Adolfus are shown in Tables 3–5, including: relatively large SVL (55–84 mm); dorso-anterior border of quadrate bone rounded; size of long free ribs immediately posterior to thoracic ribs moderately enlarged; posterior border of medial loop of clavicle present and slender; small post-femoral mite pockets absent (except in A. jacksoni); intramuscular portion of hemipenial armature not deeply cleft; shape of hemipenial clavulae simple; female genital sinus unlobed; habitat in forest, forest clearings and grasslands; clutch size 3–5; and ventral coloration yellow, blue, orange or green.
Table 5.
Meristic characters of adult species in the genera Adolfus and Congolacerta gen. nov. Data are averages ± one standard deviation, with ranges in parentheses. Abbreviations are explained in the Materials and Methods; m = male, f = female, n = unknown gender. Data for A. alleni are taken from single individuals from Mt. Elgon (Uganda, 1n) and the Aberdare Mountains (Kenya, 1m), which are likely not conspecific.
| Characters | A. africanus (4 m, 3 f) |
A. alleni (1n, 1m) |
A. jacksoni (10m, 6f) | C. asukului (3 m, 1f) | C. vauereselli (6 m, 5 f) |
|---|---|---|---|---|---|
| CS | 6 | 6, 5 | 6 | 6 | 6 |
| FP | 15.29 ± 1.11 (14–17) | 11, 11 | 17.27 ± 1.28 (15–19) | 13.25 ± 2.22 (11–16) | 10.0 ± 1.00 (8–11) |
| SL | 7.14 ± 0.38 (7–8) | 6, 5 | 6.13 ± 0.34 (6–7) | 7 | 6.36 ± 0.51 (6–7) |
| IL | 6 | 4, 5 | 6 | 6 | 5.91 ± 0.30 (5–6) |
| SO | 4 | 3, 4 | 4.06 ± 0.25 (4–5) | 3.25 ± 0.50 (3–4) | 4.09 ± 0.54 (3–5) |
| SC | 6 | 5, 3 | 5.00 ± 0.37 (4–6) | 4.75 ± 0.50 (4–5) | 5.55 ± 0.82 (4–7) |
| SG | 6.43 ± 0.79 (6–8) | 0, 0 | 3.53 ± 1.06 (2–5) | 3.75 ± 0.50 (3–4) | 6.36 ± 1.21 (4–8) |
| ST | 4.86 ± 0.90 (4–6) | 2, 3 | 4.81 ± 0.91 (3–6) | 4.50 ± 1.00 (3–5) | 3.20 ± 0.63 (2–4) |
| ADS | 49.29 ± 10.03 (36–60) | 31, 35 | 61.06 ± 6.61 (51–74) | 63.00 ± 4.36 (60–68) | 63.36 ± 7.55 (47–73) |
| PDS | 25.43 ± 2.82 (20–28) | 19, 22 | 40.06 ± 2.08 (37–44) | 34.25 ± 2.50 (31–37) | 39.82 ± 4.14 (32–44) |
| DSR | 24.14 ± 1.22 (23–26) | 19, 22 | 40.25 ± 2.38 (35–44) | 31.25 ± 2.22 (28–33) | 39.64 ± 5.41 (31–48) |
| DSN | 48.00 ± 3.46 (42–53) | 48, 46 | 95.38 ± 4.51 (90–105) | 81.00 ± 5.42 (73–85) | 73.18 ± 9.39 (54–84) |
| VR | 6 | 6, 6 | 6 | 6 | 6 |
| VN | 23.14 ± 0.90 (22–24) | 26, 25 | 27.34 ± 2.37 (24.5–31.5) | 25.88 ± 1.65 (24–28) | 22.55 ± 0.96 (21.5–24.0) |
| CDS- 11th scale | 15.43 ± 0.98 (14–16) | 21, 21 | 24.31 ± 1.25 (22–27) | 23.25 ± 1.71 (21–25) | 19.67 ± 2.50 (16–24) |
| CDS- 15th scale | 15.14 ± 1.07 (14–16) | 21, 21 | 24.00 ± 1.27 (21–26) | 23.50 ± 1.73 (21–25) | 18.13 ± 1.81 (16–21) |
| SDF1 | 8.14 ± 1.07 (7–10) | 7, 6 | 8.25 ± 0.58 (7–9) | 6.50 ± 0.58 (6–7) | 7.64 ± 0.67 (7–9) |
| SDF2 | 13.17 ± 0.98 (12–14) | 10, 9 | 13.31 ± 0.87 (12–15) | 10.75 ± 0.50 (10–11) | 11.55 ± 0.82 (11–13) |
| SDF3 | 16.71 ± 1.38 (15–18) | 14, 12 | 18.00 ± 1.16 (16–20) | 14.33 ± 1.16 (13–15) | 15.00 ± 1.55 (13–17) |
| SDF4 | 16.71 ± 0.76 (16–18) | 12, 12 | 19.44 ± 1.37 (17–22) | 15.50 ± 0.58 (15–16) | 16.91 ± 0.94 (16–19) |
| SDF5 | 11.57 ± 0.54 (11–12) | 8, 9 | 12.38 ± 1.03 (11–14) | 10.00 ± 1.41 (9–12) | 10.46 ± 0.69 (9–11) |
| SDT1 | 8.29 ± 0.76 (7–9) | 8, 6 | 8.69 ± 1.08 (6–10) | 7 | 8.09 ± 0.54 (7–9) |
| SDT2 | 12.67 ± 1.03 (11–14) | 11, 10 | 13.44 ± 0.89 (11–15) | 10.75 ± 0.50 (10–11) | 11.46 ± 1.13 (10–13) |
| SDT3 | 16.00 ± 0.58 (15–17) | 15, − | 18.79 ± 1.37 (17–21) | 15.25 ± 0.50 (15–16) | 15.27 ± 1.49 (12–17) |
| SDT4 | 19.00 ± 0.58 (18–20) | 19, 18 | 23.64 ± 1.69 (21–27) | 19.25 ± 0.50 (19–20) | 20.18 ± 1.99 (17–22) |
| SDT5 | 13.71 ± 0.76 (13–15) | 12, 11 | 16.13 ± 0.92 (15–17) | 12.50 ± 0.58 (12–13) | 13.73 ± 1.27 (12–16) |
Description
Size and proportions
Relatively large member of the Equatorial African group of lizards (55–84 mm SVL), with no sexual dimorphism and a long tail (SVL/TL = 49–60%; Tables 4–5) that is cylindrical without lateral fringes.
Table 4.
Measurements (in mm) of adult species in the genera Adolfus and Congolacerta gen. nov. Data are averages ± one standard deviation, with ranges in parentheses. Abbreviations and measurements are explained in the Materials and Methods. SVL/TL given as percentage data; m = adult male, f = adult female, n = unknown gender. Data for A. alleni are taken from single individuals from Mt. Elgon (Uganda, 1n) and the Aberdare Mountains (Kenya, 1m), which are likely not conspecific.
| Characters | A. africanus (4 m, 3 f) |
A. alleni (1n, 1m) |
A. jacksoni (10 m, 6f) | C. asukului (3 m, 1f) | C. vauereselli (6 m, 5 f) |
|---|---|---|---|---|---|
| SVL (m) | 56.95 ± 1.13 (55.7–58.4) | 48.0, 46.0 | 70.46 ± 6.42 (64.0–84.3) | 55.40 ± 2.52 (53.7–58.3) | 53.42 ± 2.15 (50.0–55.5) |
| SVL (f) | 60.90 ± 0.95 (59.9–61.8) | — | 70.28 ± 5.46 (62.3–76.6) | 51.9 | 55.46 ± 3.89 (50.0–60.4) |
| TL (m) | 104.6, 113.6 | — | 101.3, 112.6 | 111.4 | 114.74 ± 3.89 (50.0–60.4) |
| TL (f) | 103.0 | — | — | — | 112.10 ± 4.10 (109.2–115.0) |
| SVL/TL | 54.93 ± 5.55 (49.0–60.0) | — | 56.8, 63.3 | 52.3 | 46.95 ± 2.66 (44.4–51.7) |
| HL | 14.36 ± 0.61 (13.4–15.1) | 10.0, 10.3 | 16.33 ± 2.07 (12.4–20.2) | 11.35 ± 2.53 (8.4–14.0) | 12.61 ± 0.82 (11.1–13.8) |
| HW | 8.74 ± 0.44 (8.2–9.5) | 6.4, 6.4 | 10.63 ± 1.61 (7.8–14.2) | 8.18 ± 1.43 (6.5–10.0) | 8.12 ± 1.41 (6.8–11.9) |
| HH | 6.21 ± 0.20 (6.0–6.6) | 5.3, 5.6 | 7.19 ± 1.24 (5.6–9.4) | 8.18 ± 3.12 (4.8–11.3) | 5.57 ± 0.45 (5.0–6.5) |
| SKL | 14.06 ± 0.57 (13.1–14.7) | 9.7, 11.0 | 16.75 ± 2.32 (12.5–20.5) | 12.58 ± 1.48 (10.4–13.7) | 12.57 ± 0.77 (11.2–13.8) |
| SEL | 6.31 ± 0.43 (5.9–7.0) | 3.7, 4.4 | 6.50 ± 0.83 (5.3–7.7) | 4.90 ± 0.62 (4.0–5.4) | 5.51 ± 0.47 (4.7–6.1) |
| ML | 11.14 ± 0.48 (10.7–11.8) | 8.4, 8.9 | 12.10 ± 1.45 (9.3–14.1) | 10.33 ± 1.46 (8.2–11.5) | 10.11 ± 0.74 (8.7–11.0) |
| SAL | 20.76 ± 1.62 (19.0–23.7) | 17.9, 17.8 | 25.74 ± 3.69 (19.5–32.0) | 19.47 ± 2.63 (16.2–22.1) | 19.98 ± 1.90 (16.4–22.5) |
| AGD | 26.39 ± 2.64 (23.0–30.4) | 23.3, 22.7 | 32.10 ± 4.22 (25.9–40.2) | 27.83 ± 0.95 (26.8–28.9) | 24.91 ± 2.38 (21.8–29.5) |
| HML | 7.47 ± 0.85 (6.6–8.9) | 4.8, 4.5 | 7.53 ± 1.11 (5.5–9.2) | 5.48 ± 0.76 (4.4–6.1) | 7.00 ± 1.31 (6.0–10.4) |
| RUL | 7.84 ± 0.22 (7.5–8.1) | 4.9, 4.6 | 7.73 ± 0.97 (6.0–9.1) | 5.73 ± 1.17 (4.2–6.9) | 7.99 ± 1.35 (6.2–10.7) |
| FL | 10.06 ± 0.51 (9.3–11.0) | 5.9, 6.4 | 10.11 ± 0.96 (8.6–11.9) | 6.93 ± 1.10 (5.3–7.7) | 8.69 ± 1.10 (7.2–10.6) |
| TFL | 10.20 ± 0.51 (9.3–10.9) | 6.0, 6.2 | 10.01 ± 1.27 (7.6–11.7) | 7.10 ± 1.29 (5.2–8.1) | 9.06 ± 0.88 (8.2–10.3) |
| LTL | 10.40 ± 0.84 (9.3–11.7) | 6.4, 6.5 | 10.51 ± 1.10 (8.6–12.3) | 7.78 ± 1.09 (6.3–8.9) | 8.76 ± 0.99 (6.8–9.8) |
Skull
Premaxilla without anterior boss; postfrontal and postorbital bones fused; shape of squamosal bone slender; squamosal and parietal not in contact; dorso-anterior border of quadrate bone rounded; temporal osteoderms absent (except in A. alleni, which is variable); maxilla not extending to coronoid notch; and 14 scleral ossicles in each eye (Arnold, 1989a).
Post-cranial skeleton
Average number of presacral vertebrae in males 26–27 (except A. africanus, which is 25 or less); 7–9 long free dorsal ribs immediately posterior to thoracic ribs (except A. africanus, which is 6–7); moderately elongated long free dorsal ribs immediately posterior to thoracic ribs; posterior border of medial loop of clavicle present and slender; and transverse process of anterior autotomic caudal vertebrae directed roughly laterally (Arnold, 1989a).
Scaling
Contact between postnasal and supranasal scales below level of nostril absent; two loreal scales on each side (except A. alleni, which has one); supraciliary granules present (except A. alleni); lower eyelid opaque and covered with relatively small scales; parietal scales without lateral corner erosion; temporal scaling relatively fine (except A. alleni, which is very coarse, with 13 or fewer scales on each side, excluding the supratemporals and tympanic); keeling on temporal scales absent (A. alleni), present (A. africanus) or variable (A. jacksoni); keeling on collar scales absent (except A. africanus, which is variable); granules beneath collar scattered or absent (except A. jacksoni, which has many); dorsal scales more or less uniform in size (except A. africanus, which has flank scales that are distinctly smaller than the mid-dorsals); micro-ornamentation of dorsal scales smooth (except for A. africanus, which has pustullate scales with minute tubercles); flank scales in close contact; six or eight longitudinal rows of ventral body scales (except A. africanus, which has four complete rows and an outer row on each side that is strongly reduced anteriorly); keeling on ventrals absent (except A. africanus, which has keeling on the outer longitudinal row); preanal scale entire and without keeling; no keeling on scales beneath limbs; row of femoral pores long, extending almost to knee (except A. africanus, which has a shortened row of femoral pores, well separated from the knee); scales bearing femoral pores not or only slightly projecting, close together in males; hind toes without fringes; no pad of spinous scales on dorsum of tail base (Arnold, 1989a). In contrast to the latter author, we observed gular folds (as indicated by a heavy crease between the ear openings on the throat of adult animals) in A. jacksoni; the character was noted as absent in A. alleni and A. jacksoni, and variable in A. africanus by Arnold (1989a).
Coloring
Adolfus africanus: the entire head is metallic copper bronze with a continuous mid-dorsal band of the same color and width of the head continuing to the end of the tail. Within the mid-dorsal band are numerous randomly distributed black spots, usually beginning near the origin of the forelimbs and extending slightly beyond the base of the tail. A longitudinal series of white round spots border the mid-dorsal metallic band laterally; these coalesce into thin narrow stripes on the tail. The lateral sides of the body have dark brown bands originating on the side of the head and extending posteriorly onto the tail; some specimens have additional, diffuse rounded white spots aligned along the lower edge of the dark lateral band. Venter immaculate lime green. Adolfus alleni: ground color brown or olive, with a broad or fine dark vertebral stripe. Two black-edged, lime-green or red-brown dorsolateral stripes extend from the posterior edge of the parietals to about the hind limb insertions, and may continue as brown lines onto the tail. The lateral sides of the body are rufous or light brown; the belly varies from orange or orange-pink to blue. Adolfus jacksoni: brown to olive on the dorsum of the head, with a continuous mid-dorsal band of the same color (occasionally light green) and width of the head continuing to the end of the tail. Within the band are randomly scattered black spots or oblique black dashes. The lateral sides of the body are much darker than the dorsum, usually brown but sometimes black, and usually contain several series of white or blue, black-edged ocelli, the uppermost and most lateral of which are usually arranged in longitudinal rows and may comprise scattered blue and black scales. The venter is sometimes spotted but more frequently immaculate, and varies from yellow to dull blue (Spawls et al., 2002), or bright orange in breeding males from Tanzania (WRB, pers. obs.). Poblette (2002) described a Kenyan specimen with an “army green” dorsum with black, irregular medial dots and flanks with black lateral stripes that were spotted with a luminescent cyan color.
Distinctive internal features
Tongue surface mainly squamate; tongue color in alcohol dark; a continuous ulnar nerve present but connected to the brachial trunk by a bridge in the lower arm (except A. africanus, which has a variable ulnar nerve pattern); exit of oviducts into genital sinus dorsal; female genital sinus unlobed (Arnold, 1989a).
Hemipenis
Size relatively large; intramuscular portion of hemipenial armature not deeply cleft; medial side of hemipenial armature not reduced; size of hemipenial clavulae large; shape of hemipenial clavulae simple (Arnold, 1989a).
Ecology
Adolfus africanus is known from primary Guineo-Congolean forest (580–2200 m) and has been observed basking in dappled sunlight on fallen tree limbs, trunks and exposed roots within a few meters of ground clearings in forest (only a few were observed on tree trunks above 3 m from the ground), suggesting this species is primarily an inhabitant of undergrowth (Spawls et al., 2002; Köhler et al., 2003). It has been collected in highly disturbed forest in northeastern DRC (EG, CK and MMA pers. obs.) and Kenya (Köhler et al., 2003). Adolfus alleni is known from alpine moorland, heather and Hagenia-Hypericum zones from 2,700–4,500 m, and is more terrestrial than other members of the genus, living in tussock grass and open patches in between (Spawls et al., 2002). Adolfus jacksoni is known from clearings, forest edges, gallery forest, and disturbed habitats, even occurring in the middle of the city of Bukavu (DRC) on slopes that have been cleared of forest for centuries (EG, CK and MMA, pers. obs., Schaller, 1964), and in suburban gardens in Arusha, Tanzania (WRB, pers. obs). The species has been recorded from 450–3,000 m (Spawls et al., 2002).
Reproduction
No reproductive data are available for Adolfus africanus or A. alleni, but A. jacksoni has been observed nesting communally in crevices on exposed vertical road cut walls, and lays clutches of 3–5 eggs (Spawls et al., 2002). Goldberg (2009) confirmed the range of clutch size for A. jacksoni as 3–5 eggs (mean = 4.1 ± 0.90 standard deviation), noted reproductively active males and females at opposite ends of the year (February–March and September), and documented evidence of multiple clutches in females.
Remarks
Several morphological features (e.g., osteology, hemipenis) are shared with Gastropholis, but not other Equatorial African genera (Table 3), lending support for the weakly supported placement of Gastropholis as sister to Adolfus in our phylogenetic analyses (Figs. 1–2).
Congolacerta
Greenbaum, Villanueva, Kusamba, Aristote & Branch gen. nov.
Type species
Lacerta vauereselli Tornier, 1902.
Etymology
A feminine name derived from Democratic Republic of the Congo, where the genus occurs along most of the eastern montane border (Albertine Rift), and lacerta, a lizard.
Synonymy
-
-
Lacerta Linnaeus, 1758 (part); Tornier, 1902. Zoologische Anzeiger 25:701. [Lacerta vauereselli].
-
-
Algiroides Duméril & Bibron, 1839 (part); Peracca, 1917. Atti della Reale Accademia delle Scienze di Torino 52:351 [Algiroides boulengeri].
-
-
Adolfus Sternfeld, 1912 (part); Arnold, 1973. Bulletin of the British Museum (Natural History), Zoology 25:357 [Adolfus vauereselli].
Content
Congolacerta asukului sp. nov. (described below); C. vauereselli (Tornier, 1902).
Distribution
Occurs from the Blue Mountains (west of Lake Albert in DRC) along the Albertine Rift and its foothills through Uganda, Rwanda and Tanzania as far south as the Kabobo Plateau at the border of South Kivu and Katanga Provinces, DRC (Spawls et al., 2002; Appendix 2).
Diagnosis
Several mensural, meristic and qualitative characters that diagnose Congolacerta are shown in Tables 3–5, including: modest SVL (50–58 mm); dorso-anterior border of quadrate bone rounded; size of long free ribs immediately posterior to thoracic ribs very elongated; posterior border of medial loop of clavicle present and thickened; small to very small postfemoral mite pockets present (Arnold, 1986b); intramuscular portion of hemipenial armature deeply cleft anteriorly; shape of hemipenial clavulae complexly lobed; female genital sinus bilobed; habitat forest clearings and grasslands; and ventral coloration usually unpigmented (C. vauereselli) or yellow with black or brown blotches (C. asukului).
Description
Size and proportions
Relatively modest-sized member of the Equatorial African group of lizards (50–58 mm SVL), with no sexual dimorphism and a modest-sized tail (SVL/TL = 44–52%; Tables 4–5) that is cylindrical without lateral fringes.
Skull
Congolacerta vauereselli premaxilla without anterior boss; postfrontal and postorbital bones fused; shape of squamosal bone slender; squamosal and parietal not in contact; dorso-anterior border of quadrate bone rounded; temporal osteoderms absent; maxilla not extending to coronoid notch; and 14 scleral ossicles in each eye (Arnold, 1989a).
Post-cranial skeleton
Average number of presacral vertebrae in males 25 or less (both species); Congolacerta vauereselli has 6–7 long free dorsal ribs immediately posterior to thoracic ribs; very elongated long free dorsal ribs immediately posterior to thoracic ribs, about twice the length of other free dorsal ribs; posterior border of medial loop of clavicle present and thickened; and transverse process of anterior autotomic caudal vertebrae directed roughly laterally (Arnold, 1989a).
Scaling
Contact between postnasal and supranasal scales below level of nostril absent; two loreal scales on each side; supraciliary granules present; lower eyelid opaque and covered with relatively small scales; parietal scales without lateral corner erosion; temporal scaling relatively fine; keeling on temporal scales variable, but usually absent; keeling on collar scales absent; granules beneath collar scattered or absent; dorsal scales somewhat enlarged; micro-ornamentation of dorsal scales smooth; flank scales in close contact; four complete rows of ventral body scales and an outer row on each side that is strongly reduced anteriorly; keeling on ventrals absent; preanal scale entire and without keeling; no keeling on scales beneath limbs; row of femoral pores long, extending almost to knee (C. asukului) or shortened row of femoral pores, well separated from the knee (C. vauereselli); scales bearing femoral pores not or only slightly projecting, close together in males; hind toes without fringes; no pad of spinous scales on dorsum of tail base (Arnold, 1989a). In contrast to the latter author, we did not observe a gular fold on any specimens of C. vauereselli, and only faint indications of a gular fold on three adult specimens of C. asukului.
Coloring
Congolacerta vauereselli: the dorsum of the head is light yellow to copper bronze with a continuous mid-dorsal band of the same color and width of the head continuing to the end of the tail. Within the mid-dorsal band are small dark brown to black spots, sometimes forming a vertebral stripe. The lateral sides of the body are reddish brown, edged in black above, with one or two series of white, black-edged ocellar spots. A cream or white streak extends from the cheek to the side of the neck and passes through the ear opening. Venter usually immaculate and unpigmented. Coloring of C. asukului is generally similar to that of C. vauereselli (one major exception is yellow ventral pigmentation with black or brown blotches), and details are given in the species description below.
Distinctive internal features
Congolacerta vauereselli tongue surface mainly squamate; tongue color in alcohol dark; a “Varanidae” ulnar nerve pattern with no continuous independent ulnar nerve and all fibers to lower limb passing through the branchial trunk; exit of oviducts into genital sinus dorsal; female genital sinus bilobed (Arnold, 1989a).
Hemipenis
Congolacerta vauereselli size relatively large; intramuscular portion of hemipenial armature very deeply cleft anteriorly; medial side of hemipenial armature not reduced; size of hemipenial clavulae large; shape of hemipenial clavulae complexly lobed (Arnold, 1989a).
Ecology
Congolacerta vauereselli is found in clearings and openings within Guineo-Congolian forests from 1,000–2,675 m. Little is known of its natural history, but based on observations made in Bwindi National Park (Uganda), Spawls et al. (2002) suggested it is likely similar to Adolfus africanus. Congolacerta asukului is known from high elevations (> 2,650 m) grasslands of the Itombwe Plateau, and has been found in small burrows among tussocks of grass.
Reproduction
No reproductive data are available for either species of Congolacerta.
Remarks
Several mensural, meristic, qualitative and molecular divergence characters distinguish the Itombwe population of Congolacerta from its congener C. vauereselli. The Itombwe population is described as a new species below.
Congolacerta asukului
Greenbaum, Villanueva, Kusamba, Aristote & Branch sp. nov.
Asukulu’s grass lizard
Holotype
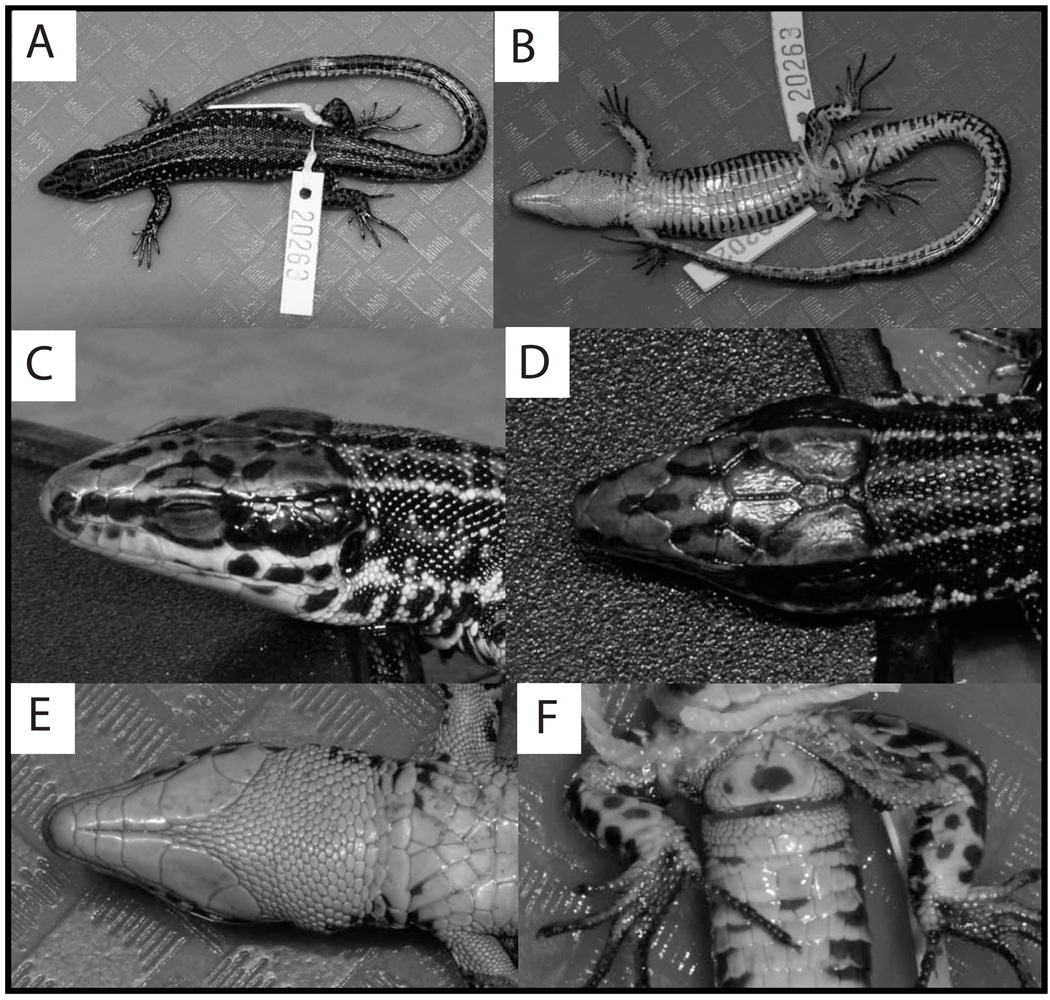
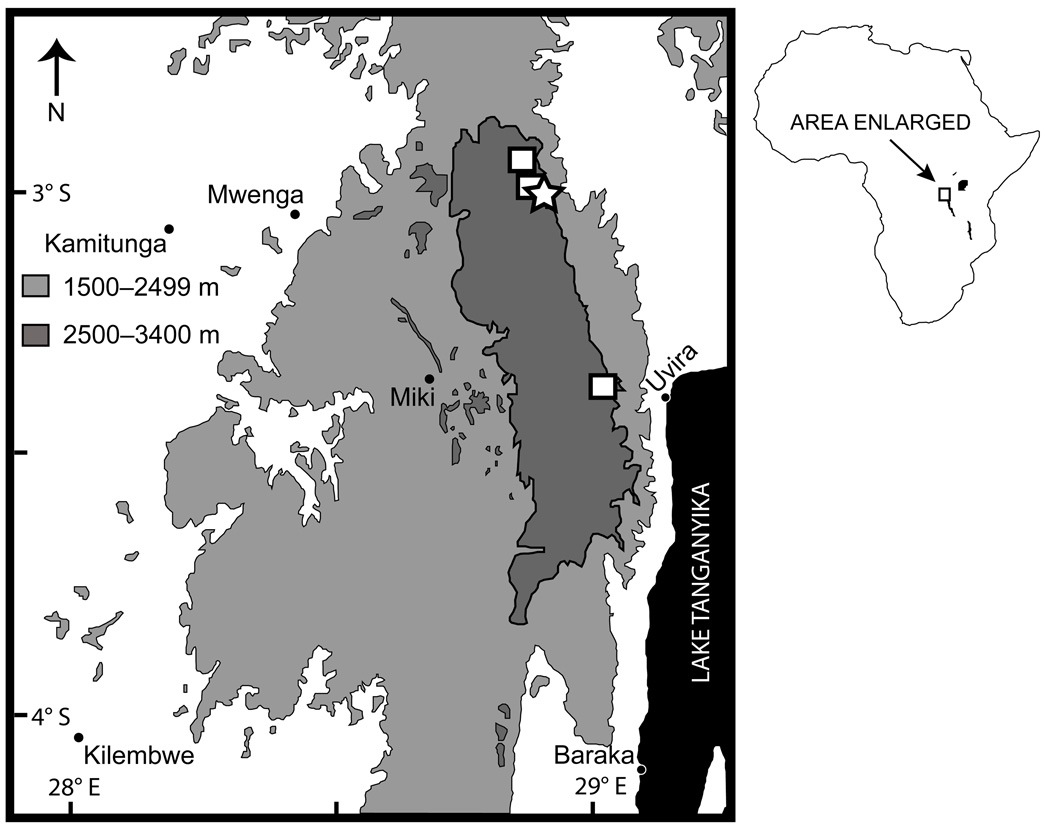
UTEP 20263 (field no. EBG 2025, Figs. 3 A,B, 4), an adult male, from footpath south of Rurambo village, Itombwe Plateau, South Kivu Province (SKP), DRC (02.99437°S, 28.87620°E, 2,876 m; see Figs. 6–7). Collected ca. 08:00 hrs on 23 May 2009 by MMA, EG, CK, Wandege Mastaki Monigan, Maurice Luhumio and Asukulu M’Mema.
Figure 3.
Photographs of Congolacerta in life. Dorsal (A) and ventral (B) view of C. asukului holotype UTEP 20263 (adult male, 58.3 mm SVL), dorsal view (C) of C. asukului paratype UTEP 20265 (adult male, 53.7 mm SVL), dorsal view (D) of C. asukului paratype UTEP 20267 (subadult male, 42.7 mm SVL), and dorsal (E) and ventral (F) view of C. vauereselli UTEP 20289 (adult male, 54.4 mm SVL).
Figure 4.
Photographs of the holotype of Congolacerta asukului (UTEP 20263, adult male, 58.3 mm SVL) after preservation. Dorsal (A) and ventral (B) view of whole specimen, lateral (C), dorsal (D) and ventral (E) view of head, and ventral view of cloacal region (F) illustrating femoral pores.
Figure 6.
Map of the Itombwe Plateau, showing collection localities for Congolacerta asukului sp. nov. (open squares). The type locality is indicated by a star symbol.
Figure 7.
Photograph of the type locality of Congolacerta asukului sp. nov., showing grassland habitat with rocky outcrops.
Paratopotype
UTEP 20264 (field no. EBG 2028), a subadult male, with same date, locality, collectors, and circumstances of capture as holotype.
Other paratypes
UTEP 20265 (field no. EBG 2082, Fig. 3 C), an adult male, collected by MMA, EG and CK 25 May 2009 at Komesha village, Itombwe Plateau, SKP, DRC (03.0870°S, 28.8101°E, 2,891 m); UTEP 20266 (field no. EBG 2114), an adult female, collected by MMA, EG and CK 26 May 2009 at Mugegema village, Itombwe Plateau, SKP, DRC (03.06940°S, 28.76813°E, 2,765 m); UTEP 20267–68 (field nos. EBG 1715–16), one adult male and one subadult male, collected by EG, WMM, MMA, CK, ML, and AM 30 June 2008 at Ruhuha, Itombwe Plateau, SKP, DRC (03.37871°S, 29.01293°E, 2,886 m).
Diagnosis
Congolacerta asukului can be distinguished from all other species in the Equatorial African group of lacertids by the following combination of characters: (1) medium body size (SVL 53.7–58.3 for adult males; 51.9 in one adult female); (2) dorsum brown, rusty brown or tan with several dark brown to black blotches forming a vertebral line from occipital to first quarter of tail, and a dark brown line with cream or grayish white blotches extending from lateral side of rostral through eye and flanks to lateral side of tail; (3) moderate numbers of femoral pores (11–16); (4) low numbers of supraciliary granules (3–4); (5) moderate numbers of dorsal scale rows at midbody (28–33); (6) moderate numbers of dorsal scales in a longitudinal row from occipital to posterior insertion of hind limb (73–85); (7) high numbers of ventral scales from collar to preanal (24–28); (8) high numbers of caudal scale rows at 15th scale (21–25); (9) smooth dorsal scales; and (10) yellow ventral coloration with black or brown blotches.
Differential diagnosis from similar species
Because the genera Adolfus and Congolacerta have similar external morphology, the new species is diagnosed from all species in each genus. Congolacerta asukului differs from its partially sympatric and phenotypically similar congener C. vauereselli (Fig. 3 E,F) by a higher SVL/TL ratio of 52.3 (vs. 44.4–51.7), a smaller HML (4.4–6.1 vs. 6.0–10.4), a smaller TFL (5.2–8.1 vs. 8.2–10.3), a higher number of femoral pores (11–16 vs. 8–11), a smaller number of supraciliary granules (3–4 vs. 4–8; Fig. 5), a smaller number of dorsal scale rows at midbody (28–33 vs. 31–48), a higher number of VN (24–28 vs. 21.5–24), a higher number of caudal scales at the 15th scale row (21–25 vs. 16–21), a smaller number of subdigital lamellae on digits 1 (6–7 vs. 7–9), 2 (10–11 vs. 11–13), and 4 (15–16 vs. 16–19), dorsal scale keeling (smooth vs. keeled), ventral pigmentation (yellow with black or brown blotches vs. usually unpigmented), and habitat (montane grassland vs. forest clearings and openings). Algiroides boulengeri, Peracca, 1917, described from Fort Portal, Uganda (east of Ruwenzori Mountains) was synonymized with C. vauereselli by Loveridge (1957:229), with which it shares keeled dorsal scales and a strip of metallic bronze in the middle 7–8 longitudinal scale rows (Peracca, 1917), and is clearly not conspecific with Congolacerta asukului. Most examined specimens of C. vauereselli have unpigmented venters, but UTEP 20295 (adult male) from the Kabobo Plateau (most basal population of this species in all analyses, Figs. 1–2) has a yellow venter.
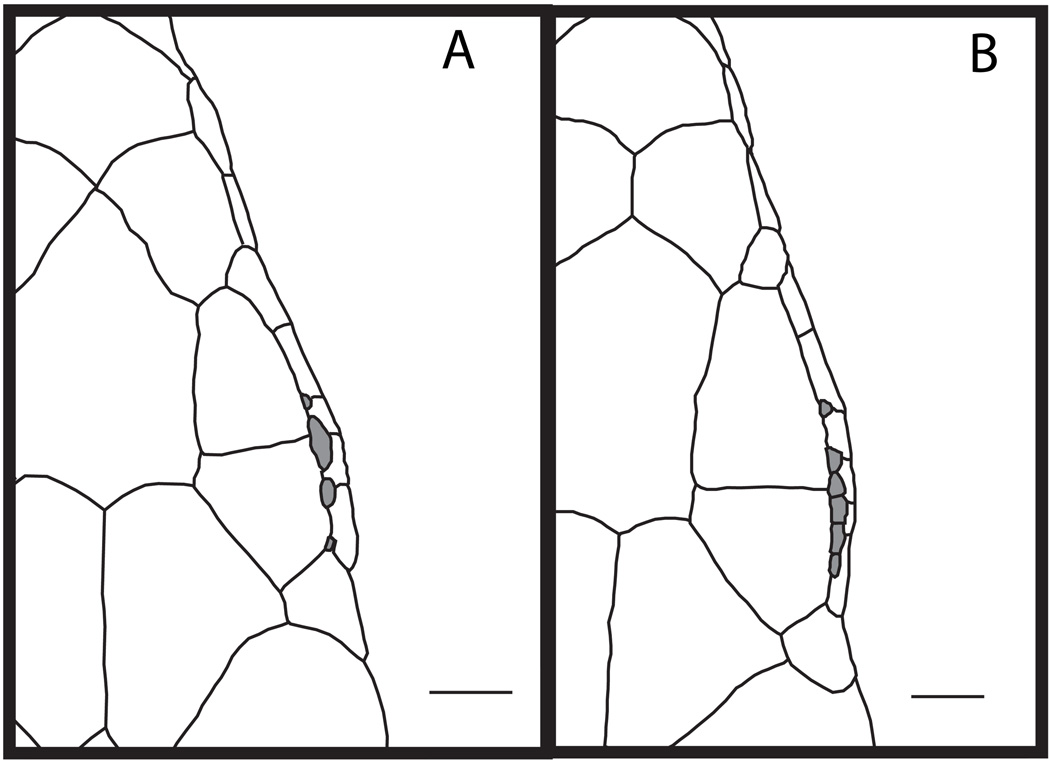
Figure 5.
Dorsal views of the heads of Congolacerta asukului sp. nov. (holotype, UTEP 20263) and C. vauereselli (UTEP 20291). Supraciliary granules are shaded in gray. Scale bars = 1 mm.
The new species differs from Adolfus africanus in having a smaller SEL (4.0–5.4 vs. 5.9–7.0), a smaller HML (4.4–6.1 vs. 6.6–8.9), a smaller RUL (4.2–6.9 vs. 7.5–8.1), a smaller FL (5.3–7.7 vs. 9.3–11.0), a smaller TFL (5.2–8.1 vs. 9.3–10.9), a smaller LTL (6.3–8.9 vs. 9.3–11.7), a smaller number of supraciliaries (4–5 vs. 6), a smaller number of supraciliary granules (3–4 vs. 6–8), a higher number of dorsal scale rows at midbody (28–33 vs. 23–26), a higher number of DSN (73–85 vs. 42–53), a higher number of VN (24–28 vs. 22–24), a higher number of caudal scales at the 11th and 15th scale rows (21–25 vs. 14–16), fewer numbers of subdigital lamellae for each digit (Table 5), dorsal scale keeling (smooth vs. strongly keeled), ventral coloration (yellow with black or brown blotches vs. green), and habitat (montane grassland vs. forest clearings and openings).
The new species differs from our two examined specimens of Adolfus alleni in having two loreals (vs. one), supraciliary granules (present vs. absent), dorsal scale shape (rhombic vs. lanceolate), dorsal scale keeling (smooth vs. strongly keeled), granules beneath the collar (present vs. absent), and ventral coloration (yellow with black or brown blotches vs. orange, orange-pink or blue). The new species also has larger numbers of anterior, posterior and mid-dorsal scale rows, femoral pores, and subdigital lamellae under Toe IV (Table 5). Arnold (1989a: table 2) noted several interesting mensural, meristic and qualitative differences among three allopatric populations of A. alleni, which suggests that our two samples from Mt. Elgon (Uganda) and the Aberdare Mountains (Kenya) are likely not conspecific (Appendix 2).
The new species differs from Adolfus jacksoni in having a smaller SVL (53.7–58.3 vs. 64.0–84.3), smaller SEL (4.0–5.4 vs. 5.3–7.7), smaller FL (5.3–7.7 vs. 8.6–11.9), a smaller number of femoral pores (11–16 vs. 15–19), a smaller number of PDS (31–37 vs. 37–44), a smaller number of DSR (28–33 vs. 35–44), a smaller number of DSN (73–85 vs. 90–105), a smaller number of subdigital lamellae of several fingers and toes (Table 5), flank color pattern (spots vs. ocelli), and habitat (montane grassland vs. forest clearings and openings). It is not known to tolerate anthropogenically disturbed habitats.
Description of holotype
Measurements of the holotype are provided in Table 6. Rostral separated from frontonasal by supranasals; nostril surrounded by supranasal, postnasal and first supralabial; supralabials seven (fourth largest) and infralabials six on each side; supraoculars three on each side, the posteriormost ones much smaller than others; supraciliaries five on each side, first supraciliary largest and continuing to dorsum of head to contact first supraocular (likely fused to former first supraocular), relative lengths 1 > 2 > 5 > 4 > 3; second supraciliary in contact with first supraocular, posterior three supraciliaries separated from posterior supraoculars by two (left) or four (right) supraciliary granules; postnasal one, followed by two loreals at each side, anterior loreal smaller than posterior one; two prefrontals separated by medial contact of frontal and frontonasal; frontal hexagonal, contacting supranasals, postnasals, anteior loreals, prefrontals and frontal; frontoparietals two and connected; parietals two, separated by two interparietals (anterior interparietal largest) and occipital; supratemporals five on each side, the first one largest; temporal scales non-imbricate, much larger than scales posterior to ear opening; six pairs of chin shields, anteriormost three pairs in contact medially; faint indication of gular fold; collar with seven plates, granules present beneath collar; dorsal scales on body enlarged, imbricate, smooth and rhombic, extending anteriorly beyond forelimbs on to neck, slightly larger than lateral scales at midbody, much larger than lateral scales near limb insertions; 61 anterior dorsal scale rows, 37 posterior dorsal scale rows, 32 scale rows at midbody; 85 scales counted longitudinally from occipital to the posterior margin of hind limb on middle-left and middle-right rows, respectively; lateral body scales at midbody smooth and rhombic, arranged in disorder; lateral body scales at limb insertions small, smooth and granular, arranged in disorder; small post-femoral mite pockets present (containing larvae of trombiculid mites); ventral scales rectangular, smooth, in six longitudinal rows at midbody, median and outer longitudinal rows smaller than others, outermost rows incomplete and smooth; 26 (left) and 25 (right) scales counted longitudinally from collars to preanal on middlemost two rows; preanal single, ovoid, enlarged and smooth; 14 femoral pores on each side; scales on anterior and dorsal surfaces of forelimbs enlarged, smooth and imbricate; scales on posterior and ventral surfaces of forelimbs mostly small and granular, with two rows of enlarged, smooth and imbricate scales; two rows of enlarged, smooth and imbricate scales on antero-ventral side of hind limbs, the other areas with small, smooth granular scales; relative lengths of appressed fingers IV > III > II = V > I; subdigital lamellae seven (left) – seven (right), 11–11, 14–15, 16–16, 10–9 on fingers I, II, III, IV and V, respectively; relative lengths of appressed toes IV > III > V > II > I; subdigital lamellae eight (left) – seven (right), 11–11, 15–16, 19–19, 13–13 on toes I, II, III, IV and V, respectively; tail long (191% of SVL) and complete, covered with strongly keeled scales on lateral and dorsal sides, in 33 rows at base, decreased to 24 rows at 15th scale.
Table 6.
Continuous (mm) and meristic variation among six specimens of Congolacerta asukului sp. nov Abbreviations and measurements are explained in the Materials and Methods; m = adult male, f = adult female, s = subadult male, SD = standard deviation. Tail length (TL) data is not provided for specimens with broken or regenerated tails.
| Character | EBG 2025, m | EBG 1716, m | EBG 2082, m | EBG 2114, f | EBG 1715, s | EBG 2028, s | Average | SD |
|---|---|---|---|---|---|---|---|---|
| SVL | 58.3 | 54.2 | 53.7 | 51.9 | 42.7 | 47.2 | 51.33 | 5.56 |
| TL | 111.4 | — | — | — | — | — | — | — |
| HL | 14.0 | 12.8 | 8.4 | 10.2 | 9.9 | 11.3 | 11.10 | 2.04 |
| HW | 10.0 | 8.0 | 8.2 | 6.5 | 6.3 | 6.9 | 7.65 | 1.39 |
| SAL | 22.1 | 21.0 | 18.6 | 16.2 | 15.6 | 16.8 | 18.38 | 2.67 |
| AGD | 28.3 | 27.3 | 26.8 | 28.9 | 20.4 | 23.6 | 25.88 | 3.26 |
| HML | 6.1 | 5.5 | 5.9 | 4.4 | 4.9 | 4.5 | 5.22 | 0.72 |
| RUL | 6.9 | 5.5 | 6.3 | 4.2 | 4.1 | 5.1 | 5.35 | 1.12 |
| FL | 7.3 | 7.4 | 7.7 | 5.3 | 5.6 | 5.6 | 6.48 | 1.09 |
| TFL | 8.1 | 7.6 | 7.5 | 5.2 | 6.6 | 6.8 | 6.97 | 1.03 |
| FP | 14 | 16 | 12 | 11 | 15 | 13 | 13.50 | 1.87 |
| SO | 3 | 4 | 3 | 3 | 4 | 3 | 3.33 | 0.52 |
| SC | 5 | 5 | 5 | 4 | 6 | 4 | 4.83 | 0.75 |
| SG | 4 | 4 | 3 | 4 | 1 | 3 | 3.17 | 1.17 |
| ST | 5 | 5 | 5 | 3 | 4 | 5 | 4.50 | 0.84 |
| DSR | 32 | 32 | 28 | 33 | 32 | 32 | 31.50 | 1.76 |
| DSN | 85 | 83 | 73 | 83 | 79 | 82 | 80.83 | 4.31 |
| VN | 25.5 | 26 | 24 | 28 | 23 | 24.5 | 25.17 | 1.75 |
| SDF4 | 16 | 15 | 15 | 14 | 15 | 16 | 15.17 | 0.75 |
| SDT4 | 19 | 19 | 20 | 19 | 19 | 20 | 19.33 | 0.52 |
Coloration in life
From photographs of holotype before preservation (Fig. 3A,B). Dorsal ground color brown to rusty brown with several dark brown to black blotches on the head scales, mid-dorsal area (forming a vertebral line from occipital to first ¼ of tail), limbs and tail. A narrow line of cream to white spots forms a border between the dorsum and flanks from the parietals to the insertion of the hind limb. A dark brown line with cream blotches extends from the lateral side of the rostral through the eye and flanks to the lateral side of the tail. Lateral side of snout and neck (below dark brown line) cream with dark brown blotches. Chin shields white, remainder of venter light yellow anteriorly, becoming increasingly darker yellow posteriorly, with dark brown to black blotches on the lateral sides of the venter and limbs. Coloration in preservative (70% ethanol) similar to coloration in life.
Variation
Variation of mensural and meristic data in the paratypes of Congolacerta asukului are shown in Table 6. The holotype is the only specimen with separated prefrontals; the prefrontals are in broad contact in UTEP 20267–68 and 20266, and the prefrontals are in narrow contact in EBG 2028 and 2082. Three specimens (UTEP 20265–67) have only one interparietal; the interparietal in UTEP 20266 is unusually elongate, and is about 2.5 times longer than wide. Coloration in life of UTEP 20265 (Fig. 3C) differed from the holotype in having a tan dorsal ground color, and grayish white flanks below the dark brown lateral stripe. Coloration in life of UTEP 20267 (Fig. 3D) differed from the holotype in having rusty brown head plates, and an olive brown ground color on the remainder of the dorsum.
Ecology and natural history
Congolacerta asukului is a diurnal species that occurs in high-elevation (> 2,650 m) grasslands (often near rocky outcrops) of the Itombwe Plateau (Figs. 6–7) in the Albertine Rift Montane Forest ecoregion as defined by Burgess et al. (2004). At least two individuals were observed basking on rocks, and one individual was captured after it retreated to a small burrow among tussocks of grass. Laurent (1964) described this habitat as high-elevation meadows that are common between swamps, rivers and subalpine scrub forests, which are dominated by tree heathers and other Ericacea (Doumenge, 1998).
Etymology
The new species is named in honor of Itombwe native Asukulu M’Mema, an aspiring zoologist and conservationist who was our colleague and guide through the Itombwe Plateau in 2008 and 2009, when all the known specimens of C. asukului were collected. Asukulu was killed by militia during his investigation into the killing of a gorilla on August 22, 2009 in Mulombozi village (on the route between Miki and Mwenga) in the western slopes of the Itombwe Plateau. For his service to conservation in Africa, he was posthumously honored with the Medail de Bravoure from the Alexander Abraham Foundation in 2010.
Conservation
Congolacerta asukului is currently known from four localities, which have a total extent of occurrence of about 550 square kilometers. Because of mining activity, widespread cattle/goat grazing (EG, CK and MMA, pers. obs.) and dry-season burning of grasslands for agriculture (CK, pers. obs.), the area, extent and quality of the grassland habitat is declining. Applying the IUCN Red List criteria to C. asukului results in a classification of endangered, EN B1ab(iii).
DISCUSSION
Using the Bayesian phylogenetic analysis program TreeTime, Hipsley et al. (2009) estimated that the common ancestor of Eremiadini lacertids dispersed to northwestern Africa via small island chains from Europe in the mid- to late Eocene, substantially earlier than the previous estimates of ca. 14 Myr by Pavlicev & Mayer (2009). Separation of the “Saharo-Eurasian” and “Ethiopian” lineages (equivalent to clades B1 and B2 of Mayer & Pavlicev, 2007) occurred shortly thereafter. Given the pattern we recovered for the Equatorial African group of lacertids near the base of the Eremiadini clade, it is likely that the genera Adolfus, Congolacerta, Gastropholis and Holaspis diverged from each other shortly after the group’s arrival in Central Africa, either in the late Eocene or the Miocene. The Miocene is a relatively dry period marked by expansion of grasslands (Lovett et al., 2005), and is synchronous with orogeny of the Albertine Rift, which began forming via doming in the early Miocene (Vande weghe, 2004; Partridge, 2010).
Because Congolacerta asukului is known from a small area (550 km2) at the highest elevations of the Itombwe Plateau, potential negative effects from global warming are of paramount concern. In general, the African continent warmed by 0.5° C since 1900, but global climate models for future temperature and precipitation trends are problematic (Hulme et al., 2005). Hernes et al. (1995) and Ringius et al. (1996) constructed climate change scenarios that predicted equatorial African countries would warm by 1.4° C by the 2050’s. Hulme et al. (2001) also constructed climate change scenarios that predicted a 0.2–0.5° C/decade increase in temperature, with the least amount of warming in equatorial latitudes and coastal environments. These authors examined the inter-model range of their global climate models to assess their levels of agreement, and found the smallest range over northern Africa and the equator, suggesting warming estimates for equatorial Africa are relatively robust. However, estimates of the magnitude and direction of future rainfall changes in Africa are not precise, because models have not accounted for the roles of land-cover change, and dust and biomass aerosols in inducing regional climate changes (Hulme et al., 2005). This is especially relevant to Itombwe, where human activity during Africa’s World War (Prunier, 2008) destroyed large areas of natural habitat on the plateau (EG, CK and MMA pers. obs.).
Alward, Detling & Milchunas (1999) noted that average annual minimum temperatures (TMIN) have increased at twice the rate of average annual maximum temperatures at a global scale. These authors analyzed a 23-year dataset from a montane grassland long-term ecological research site in Colorado (USA), and demonstrated that increased TMIN was correlated with decreased net primary production by the dominant C4 grass, rendering the habitat more susceptible to invasion by exotic species and less tolerant of drought and grazing. If similar effects are occurring at Itombwe, it is likely that the high-elevation grassland habitat will be devastated, possibly leading to the extinction of Congolacerta asukului. Moreover, in a study that focused on the potential effects of global climate change on Mexican lizards, Sinervo et al. (2010) noted extinction risk was significantly related to low latitudinal and altitudinal range limits, where thermal physiology and/or ecological interactions limit species, especially viviparous species in cool, montane habitats. Although C. asukului is likely oviparous, it is restricted to a limited latitudinal and elevation range in a cool, montane habitat, rendering it especially vulnerable to extinction risk from global warming.
Recognition of the genus Congolacerta adds another distinct evolutionary lineage to a long list of taxa that are endemic to the Albertine Rift mountains in eastern DRC, Uganda, Rwanda, Burundi and Tanzania (Plumptre et al., 2007). Overall, the Albertine Rift contains more than half of continental Africa’s bird species (Omari et al., 1999), and nearly 40% of its mammal species (Plumptre et al., 2007), including endangered mountain gorillas (Gorilla beringei; Schaller, 1964; Eckhart and Lanjouw, 2008). Many of these high-elevation forests are threatened by deforestation, and on a global scale, montane forests make up 12% of existing tropical forests worldwide, but are being cleared at twice the rate of the global average (Sodhi, Brook & Bradshaw, 2007).
Among other Albertine Rift sites, Itombwe (under consideration for a national park) has the most threatened species of amphibians (Laurent, 1964, 1983; Evans et al., 2008; Stuart et al., 2008; Roelke et al., in press), and the second-highest number of endemic amphibians (most not recorded for 60 years), rendering it among the most important sites for amphibian conservation in continental Africa (Burgess et al., 2004). Two amphibian genera, Laurentophryne, Tihen, 1960 and Chrysobatrachus, Laurent, 1951, are endemic to Itombwe, and the latter genus is only found above 2,400 m, with a peak in activity during the dry season (Laurent, 1951, 1964). Congolacerta asukului underscores the high levels of reptile endemism of the plateau—Itombwe also contains large numbers of endemic and threatened plants, butterflies, birds, and mammals (Louette, 1990; Omari et al., 1999; Plumptre et al., 2003). This diversity is remarkable given the superficial herpetological exploration of the plateau; Laurent (1954) mentioned only two reptiles from the upper plateau of Itombwe (2,500–3,000 m), including taxa in the lizard genera Trioceros (Chamaeleonidae) and Leptosiaphos (Scincidae).
In October, 2006, parts of the Itombwe Plateau were recognized as the Réserve Naturelle d’Itombwe (RNI), by decree of the Ministry of Environment, Conservation of Nature and Tourism (CK, pers. comm.). An administration with a managing warden was established for RNI in 2008, but armed militias have impeded conservation efforts (EG, CK and MMA, pers. obs., and see C. asukului etymology above). The reserve faces severe anthropogenic pressure from a growing human population (Barnes & Lahm, 1997), and park rangers are not yet established to ameliorate poaching and deforestation (elephants were likely extirpated recently), which is currently not monitored. Based on preliminary vegetation surveys, Doumenge (1998) remarked that Itombwe is, “undoubtedly the second most important, if not the most important, location of highland forests in continental Africa.” Because multiple, additional new species of amphibians and reptiles await description (EG, unpubl. data), it is likely that Itombwe’s importance as a center of endemism and conservation concern will increase as biological exploration continues.
ACKNOWLEDGEMENTS
Fieldwork by the senior author in DRC was funded by the Percy Sladen Memorial Fund, an IUCN/SSC Amphibian Specialist Group Seed Grant, research funds from the Department of Biology at Villanova University, a National Geographic Research and Exploration Grant (no. 8556-08), and University of Texas at El Paso (UTEP); EG, CK and MMA thank our field companions Wandege Mastaki Moninga, Maurice Luhumyo, John and Felix Akuku, and the late Asukulu M’Mema. Baluku Bajope and Muhimanyi Manunu of CRSN provided project support and permits, and we thank the Institut Congolais pour la Conservation de la Nature for permits to work in protected areas. We thank Bob Drewes and Jens Vindum of CAS, Corey Roelke of UTA, Aaron Bauer and Perry (JR) Wood of Villanova University, Kevin de Queiroz and Addison Wynn of USNM, Zoltan Nagy of the Royal Belgian Institute of Natural Sciences, Jos Kielgast of the Zoological Museum of Copenhagen and Salvador Carranza of Consejo Superior de Investigaciones Científicas for the loan of specimens and tissues. Marion Rohrleitner translated German publications, and David C. Blackburn provided comments on a preliminary draft of the manuscript. We are especially grateful to Tony Gamble, who provided many useful comments on the manuscript and assistance with statistical analyses. Technicians Ana Betancourt, Omar Hernandez and Carolina Lema of the UTEP DNA Analysis Core Facility (funded with NIH grant #5G12RR008124) sequenced samples for this study; x-ray equipment was partially supported by a grant from the National Center for Research Resources (#5G12RR008124) to the UTEP Border Biomedical Research Center. The herpetological research of WRB is supported by National Research Foundation (South Africa) core funding.
APPENDIX 1
List of specimens, geographical origin, voucher number, and GenBank accession numbers for mitochondrial and nuclear gene sequences used in this study. DRC = Democratic Republic of the Congo; CER = Corey E. Roelke field series; IBE-S and Sa field numbers were kindly provided by Salvador Carranza; CRT field number was kindly provided by Zoltan Nagy and Jos Kielgast; NM Nairobi and field numbers without letters, William R. Branch tissue collection.
| Species | Geographic origin | Museum/field voucher number | 16S | cyt b | c-mos | RAG1 |
|---|---|---|---|---|---|---|
| Acanthodactylus boskianus | Egypt (animal trade) | — | — | — | EF632251 | EF632206 |
| Acanthodactylus erythrurus | Morocco: Foret de Cedres (Azrou) | IBES 2917 | HQ605790 | HQ605832 | HQ605874 | HQ616540 |
| Acanthodactylus scutellatus | Egypt: Abu Simbel | — | — | — | EF632252 | EF632207 |
| Adolfus africanus | DRC: South Kivu Province, Hombo | UTEP 20269 | HQ605799 | HQ605846 | HQ605887 | HQ616552 |
| Adolfus africanus | DRC: South Kivu Province, Mashaba | UTEP 20271 | HQ605828 | HQ605870 | HQ605911 | HQ616576 |
| Adolfus alleni | Kenya: Aberdares | NM Nairobi | — | HQ605841 | HQ605882 | — |
| Adolfus alleni | Kenya: Mt. Kenya | ZFMK 82078 | HQ605779 | HQ605840 | — | — |
| Adolfus jacksoni | DRC: South Kivu Province, Lwiro | UTEP 20276 | HQ605792 | HQ605842 | HQ605883 | HQ616548 |
| Adolfus jacksoni | DRC: South Kivu Province, Bitale | UTEP 20279 | HQ605793 | HQ605843 | HQ605884 | HQ616549 |
| Adolfus jacksoni | Uganda: Kabale District, Bwindi Impenetrable National Park | CAS 201598 | HQ605794 | HQ605844 | HQ605885 | HQ616550 |
| Adolfus jacksoni | Rwanda: North Province, Ruhengeri | CER 227 | HQ605826 | HQ605868 | HQ605909 | HQ616574 |
| Adolfus jacksoni | Rwanda: North Province, Ruhengeri | UTA R55679 | HQ605827 | HQ605869 | HQ605910 | HQ616575 |
| Adolfus jacksoni | Rwanda: North Province, Buhanga Forest | UTA R56311 | HQ605825 | HQ605867 | HQ605908 | HQ616573 |
| Adolfus jacksoni | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20272 | HQ605813 | HQ605858 | HQ605899 | HQ616564 |
| Adolfus jacksoni | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20273 | HQ605814 | HQ605859 | HQ605900 | HQ616565 |
| Adolfus jacksoni | DRC: Orientale Province, Aboro | UTEP 20283 | HQ605806 | HQ605852 | HQ605893 | HQ616558 |
| Adolfus jacksoni | DRC: South Kivu Province, Itombwe Plateau | UTEP 20280 | HQ605809 | HQ605854 | HQ605895 | HQ616560 |
| Adolfus jacksoni | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20274 | HQ605816 | HQ605861 | HQ605902 | HQ616567 |
| Adolfus jacksoni | DRC: Orientale Province, Aboro | UTEP 20285 | HQ605823 | HQ605866 | HQ605907 | HQ616572 |
| Adolfus jacksoni | DRC: Orientale Province, Aboro | UTEP 20282 | HQ605811 | HQ605856 | HQ605897 | HQ616562 |
| Adolfus jacksoni | DRC: South Kivu Province, Lwiro | UTEP 20275 | HQ605815 | HQ605860 | HQ605901 | HQ616566 |
| Adolfus jacksoni | DRC: South Kivu Province, Lwiro | UTEP 20277 | HQ605817 | HQ605862 | HQ605903 | HQ616568 |
| Adolfus jacksoni | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20278 | HQ605818 | HQ605863 | HQ605904 | HQ616569 |
| Adolfus jacksoni | DRC: South Kivu Province, Bukavu | UTEP 20281 | HQ605805 | HQ605851 | HQ605892 | HQ616557 |
| Adolfus jacksoni | Rwanda: Rukiva | — | — | — | EF632253 | EF632208 |
| Adolfus jacksoni | Arusha, Tanzania | 761 | HQ605785 | HQ605839 | HQ605881 | HQ616547 |
| Algyroides fitzingeri | Italy: Nuoro: Sardinia, Lula, direction to Conca e Crapa, monte Turuddo | Sa 35 | HQ605789 | — | — | — |
| Algyroides moreoticus | Greece: Korinthia, Feneos | — | — | — | EF632254 | EF632209 |
| Algyroides nigropunctatus | Greece: Preveza, Parga | — | — | — | EF632255 | EF632210 |
| Anatolacerta danfordi | Turkey: Icel, Camliyayla | — | — | — | DQ461743 | EF632224 |
| Apathya cappadocica | Turkey: Kayseri, Mt. Ercyas | — | — | — | EF632268 | EF632223 |
| Archaeolacerta bedriagae | France: Corsica | — | — | — | EF632256 | EF632211 |
| Atlantolacerta andreanskyi | Morocco: Oukaimeden | IBES 1699 | HQ605787 | HQ605830 | HQ605872 | HQ616538 |
| Congolacerta asukului | DRC: South Kivu Province, Itombwe Plateau | UTEP 20268 | HQ605801 | — | HQ616585 | HQ616577 |
| Congolacerta asukului | DRC: South Kivu Province, Itombwe Plateau | UTEP 20265 | HQ605804 | HQ605850 | HQ605891 | HQ616556 |
| Congolacerta asukului | DRC: South Kivu Province, Itombwe Plateau | UTEP 20264 | HQ605803 | HQ605849 | HQ605890 | HQ616555 |
| Congolacerta asukului | DRC: South Kivu Province, Itombwe Plateau | UTEP 20263 | HQ605802 | HQ605848 | HQ605889 | HQ616554 |
| Congolacerta asukului | DRC: South Kivu Province, Itombwe Plateau | UTEP 20267 | HQ605800 | HQ605847 | HQ605888 | HQ616553 |
| Congolacerta asukului | DRC: South Kivu Province, Itombwe Plateau | UTEP 20266 | HQ605808 | HQ605853 | HQ605894 | HQ616559 |
| Congolacerta vauereselli | DRC: South Kivu/Katanga Province border, Kabobo Plateau | UTEP 20295 | HQ605810 | HQ605855 | HQ605896 | HQ616561 |
| Congolacerta vauereselli | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20289 | HQ605795 | — | HQ616586 | HQ616578 |
| Congolacerta vauereselli | DRC: South Kivu Province, Itombwe Plateau | UTEP 20293 | HQ605798 | HQ605845 | HQ605886 | HQ616551 |
| Congolacerta vauereselli | DRC: Uganda: Kabale District, Bwindi Impenetrable National Park | CAS 201613 | HQ605797 | — | HQ616587 | HQ616579 |
| Congolacerta vauereselli | Rwanda: North Province, Bisate | UTA R55658 | HQ605824 | — | HQ616592 | HQ616584 |
| Congolacerta vauereselli | DRC: South Kivu Province, Itombwe Plateau | UTEP 20292 | HQ605796 | — | HQ616588 | HQ616580 |
| Congolacerta vauereselli | DRC: Orientale Province, Aboro | UTEP 20297 | HQ605822 | HQ605865 | HQ605906 | HQ616571 |
| Congolacerta vauereselli | DRC: Orientale Province, Aboro | UTEP 20296 | HQ605812 | HQ605857 | HQ605898 | HQ616563 |
| Congolacerta vauereselli | DRC: South Kivu Province, Itombwe Plateau | UTEP 20294 | HQ605807 | — | HQ616589 | HQ616581 |
| Congolacerta vauereselli | DRC: Orientale Province, Aboro | UTEP 20298 | HQ605821 | HQ605864 | HQ605905 | HQ616570 |
| Congolacerta vauereselli | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20288 | HQ605820 | — | HQ616591 | HQ616583 |
| Congolacerta vauereselli | DRC: South Kivu Province, Kahuzi-Biega National Park | UTEP 20287 | HQ605819 | — | HQ616590 | HQ616582 |
| Dalmatolacerta oxycephala | Croatia: Hvar Island | — | — | — | EF632271 | EF632228 |
| Darevskia valentini | Armenia: Rasdan | — | — | — | EF632257 | EF632212 |
| Dinarolacerta mosorensis | Montenegro: Durmitor Mts. | — | — | — | EF632270 | EF632227 |
| Eremias arguta | Ukraine | — | — | — | EF632258 | EF632213 |
| Eremias pleskei | Armenia: Ararat region | — | — | — | EF632259 | EF632214 |
| Gallotia galloti | Spain: Tenerife Island | — | — | — | EF632260 | EF632215 |
| Gastropholis prasina | Tanzania: East Usambaras | 760 | HQ605781 | HQ605835 | HQ605877 | HQ616543 |
| Gastropholis vittatus | Tanzania: Amani, East Usambaras (captive specimen) | 691 | HQ605780 | HQ605834 | HQ605876 | HQ616542 |
| Heliobolus lugubris | Namibia: Haruchas | — | — | — | EF632261 | EF632216 |
| Heliobolus spekii | Tanzania: Lake Natron | — | — | — | EF632262 | EF632217 |
| Hellenolacerta graeca | Greece: Lakonia, Monemvasia | — | — | — | EF632269 | EF632225 |
| Holaspis guentheri | DRC: Orientale Province: Yaekela near Yangambi, Congo River | CRT 3660 | HQ605784 | HQ605838 | HQ605880 | HQ616546 |
| Holaspis laevis | Tanzania: E. Usambaras | 763 | HQ605783 | HQ605837 | HQ605879 | HQ616545 |
| Holaspis laevis | Tanzania: Handeni | 764 | HQ605782 | HQ605836 | HQ605878 | HQ616544 |
| Holaspis laevis | Tanzania: Usambara Mts. | — | — | — | EF632263 | EF632218 |
| Iberolacerta cyreni | Spain: Avila: Track to Laguna Grande de Gredos | IBES 1010 | HQ605788 | HQ605831 | HQ605873 | HQ616539 |
| Iberolacerta horvathi | Austria: Carinthia, Rattendorf | — | — | — | EF632264 | EF632219 |
| Iberolacerta monticola | Portugal: Sierra Estrela | — | — | — | EF632265 | EF632220 |
| Ichnotropis squamulosa | Mozambique (animal trade) | — | — | — | EF632266 | EF632221 |
| Lacerta agilis | Austria: Lower Austria, Weitra | — | — | — | EF632267 | EF632222 |
| Latastia longicaudata | Eritrea: Nakfa | — | — | — | EF632272 | EF632229 |
| Meroles suborbitalis | Namibia: Rosh Pinah | — | — | — | EF632273 | EF632230 |
| Mesalina guttulata | Tunisia: Tamerza | — | — | — | EF632274 | EF632231 |
| Mesalina rubropunctata | Egypt: Hurghada | — | — | — | EF632275 | EF632232 |
| Nucras lalandii | South Africa: Stellenbosch | — | — | — | EF632276 | EF632233 |
| Omanosaura jayakari | United Arab Emirates: Fujayrah | — | — | — | EF632277 | EF632234 |
| Ophisops elegans | Greece: Evros, Gianuli | — | — | — | EF632278 | EF632235 |
| Parvilacerta parva | Turkey: Malatya | — | — | — | EF632279 | EF632236 |
| Pedioplanis undata | Namibia: Nauchas | — | — | — | EF632280 | EF632237 |
| Philochortus spinalis | Eritrea: Ghinda | — | — | — | EF632281 | EF632238 |
| Phoenicolacerta laevis | Cyprus: Pafos | — | — | — | DQ461715 | EF632226 |
| Podarcis muralis | France: Languedoc-Roussillon: Lac du Salut de Vesoles | IBES 1181 | HQ605791 | HQ605833 | HQ605875 | HQ616541 |
| Podarcis muralis | Austria: Lower Austria, Gumpoldskirchen | — | — | — | EF632282 | EF632239 |
| Poromera fordii | Cameroon: Mt. Nlonako | — | — | — | EF632283 | EF632240 |
| Psammodromus algirus | Spain: Lerida | — | — | — | EF632284 | EF632241 |
| Psammodromus hispanicus | Spain: Barcelona | — | — | — | EF632285 | EF632242 |
| Pseuderemias smithi | Kenya: Lake Turkana | — | — | — | EF632286 | EF632243 |
| Takydromus amurensis | Russia: Amur Region | — | — | — | EF632287 | EF632244 |
| Takydromus sexlineatus | Indonesia (animal trade) | — | — | — | EF632288 | EF632245 |
| Teira dugesii | Portugal: Madeira Island | — | — | — | EF632289 | EF632246 |
| Timon lepidus | Spain: Alicante | — | — | — | EF632290 | EF632247 |
| Timon tangitanus | Morocco: Foret de Cedres (Azrou) | IBES 2892 | HQ605786 | HQ605829 | HQ605871 | HQ616537 |
| Tropidosaura gularis | South Africa: SW-Cape | — | — | — | EF632291 | EF632248 |
| Zootoca vivipara | Austria: Lower Austria, Schneeberg | — | — | — | EF632292 | EF632249 |
APPENDIX 2
SPECIMENS EXAMINED
Adolfus africanus
CAS 54812 (male), Democratic Republic of the Congo (DRC), Medje; CAS 176853 (female), Uganda, Rukungiri District, Impenetrable (Bwindi) Forest Reserve, Buhoma Rd, 2 km S of forest reserve boundary, 1.0098°S, 29.6207E, 1,585m; CAS 176858 (female), Uganda, Rukungiri District, Impenetrable (Bwindi) Forest Reserve, Ngoto Swamp, 0.8855°S, 29.7295°E, 1,280 m; CAS 204389 (male), Uganda, Toro District, Kibale National Park, Makerere University Biological Field Station; UTEP 20269 (male), UTEP 20270 (female), DRC, South Kivu Province, Hombo, 1.8446°S, 28.4435°E, 800 m; UTEP 20271 (male), DRC, South Kivu Province, Mashaba village near Irangi, 1.8745°S, 28.4518°E, 800 m.
Adolfus alleni
CAS 162680, Uganda, Mt. Elgon, Arugot, 2,896 m; USNM 49411 (male), Kenya, Aberdare Range summit, 0.3167°S, 36.6167°E.
Adolfus jacksoni
CAS 122729 (male), Kenya, Western Province, Kakamega District, Kakamega Forest, near Kaimosi; CAS 152783 (male), Kenya, Nyanza Province, Kisumu District, Chemelil; CAS 201598 (female), Uganda, Kabale District, Bwindi Impenetrable National Park, Institute for Tropical Forest Conservation (Ruhizha), 1.0466°S, 29.7746°E, 2,362 m; CAS 201610 (female), Uganda, Kabale District, Bwindi Impenetrable National Park, Kabale-Kayonza rd, 1.0436°S, 29.7682°E, 2,347 m; CAS 204386 (male), Uganda, Masaka District, Lake Nabagabo; UTEP 20272 (male), UTEP 20273 (male), DRC, South Kivu Province, Kahuzi-Biega National Park, Tshivanga, 2.3128°S, 28.7552°E, 2,200 m; UTEP 20274 (subadult), DRC, South Kivu Province, Mbayo, 2.2663°S, 28.7838°E, 1,943 m; UTEP 20275 (male), South Kivu Province, Lwiro, 2.2457°S, 28.8126°E, 1,678 m; UTEP 20276 (subadult), UTEP 20277 (male), DRC, South Kivu Province, Lwiro, 2.2409°S, 28.8496°E, 1,530 m; UTEP 20278 (female), DRC, South Kivu Province, Kahuzi-Biega National Park, Mugaba, 2.2675°S, 28.6621°E, 2,264 m; UTEP 20279 (subadult), DRC, South Kivu Province, Bitale, 2.2078°S, 28.6296°E, 1,770 m; UTEP 20280 (female), DRC, South Kivu Proince, Itombwe Plateau, Kizuka, 03.0066°S, 28.7501°E, 2,450 m; UTEP 20281 (subadult), DRC, South Kivu Province, Bukavu, 02.5066°S, 28.8558°E, 1,529 m; UTEP 20282 (female), UTEP 20283 (male), UTEP 20284 (female), DRC, Orientale Province, Aboro, 2.0164°N, 30.8336°E, 2,088 m; UTEP 20285 (male), UTEP 20286 (male), DRC, Orientale Province, summit of Mt. Aboro, 2.0056°N, 30.5190°E, 2,445 m.
Congolacerta vauereselli
CAS 201613 (subadult), CAS 201614 (female), Uganda, Kabale District, Bwindi Impenetrable National Park, Mubwindi Swamp, NW end, S trail, 1.0710°S, 29.7536°E; CAS 204387 (female), Uganda, Toro District, Kibale National Park, Makerere University Biological Field Station, Kanyawara, Lower Camp; UTEP 20287 (subadult), DRC, South Kivu Province, Kahuzi-Biega National Park, Bwindi, 2.2769°S, 28.6613°E, 2,333 m; UTEP 20288 (male), DRC, South Kivu Province, Kahuzi-Biega National Park, Chinya, 2.2739°S, 28.6600°E, 2,297 m; UTEP 20289 (male), UTEP 20290 (female), UTEP 20291 (male), DRC, South Kivu Province, Kahuzi-Biega National Park, Mugaba, 2.2675°S, 28.6621°E, 2,264 m; UTEP 20292 (subadult), DRC, South Kivu Province, Itombwe Plateau, Bishaka, 03.3410°S, 28.7944°E, 2,208 m; UTEP 20293 (subadult), DRC, South Kivu Province, Itombwe Plateau, ca. Miki, 3.3746°S, 28.6426°E, 1,799 m; UTEP 20294 (female), DRC, South Kivu Province, Itombwe Plateau, Mugegema, 03.0618°S, 28.7786°E, 2,675 m; UTEP 20295 (male), DRC, South Kivu/Katanga Province border, Kabobo Plateau near Kilwemapante, 5.0538°S, 28.9917°E, 1,993 m; UTEP 20296 (female), UTEP 20297 (male), UTEP 20298 (female), UTEP 20299 (male), DRC, Orientale Province, Aboro, 2.0164°N, 30.8336°E, 2,088 m.
REFERENCES
- Alward RD, Detling JK, Milchunas DG. Grassland vegetation changes and nocturnal global warming. Science (Washington, DC) 1999;283:229–231. doi: 10.1126/science.283.5399.229. [DOI] [PubMed] [Google Scholar]
- Arnold EN. Relationships of the Palaearctic lizards assigned to the genera Lacerta, Algyroides and Psammodromus (Reptilia: Lacertidae) Bulletin of the British Museum (Natural History), Zoology series. 1973;25:291–366. [Google Scholar]
- Arnold EN. The hemipenis of lacertid lizards (Reptilia: Lacertidae): structure, variation and systematic implications. Journal of Natural History. 1986a;20:1221–1257. [Google Scholar]
- Arnold EN. Mite pockets of lizards, a possible means of reducing damage by ectoparasites. Biological Journal of the Linnean Society. 1986b;29:1–21. [Google Scholar]
- Arnold EN. Systematics and adaptive radiation of Equatorial African lizards assigned to the genera Adolfus, Bedriagaia, Gastropholis, Holaspis and Lacerta (Reptilia: Lacertidae) Journal of Natural History. 1989a;23:525–555. [Google Scholar]
- Arnold EN. Towards a phylogeny and biogeography of the Lacertidae: relationships within an Old-World family of lizards derived from morphology. Bulletin of the British Museum (Natural History), Zoology series. 1989b;55:209–257. [Google Scholar]
- Arnold EN. Structural niche, limb morphology and locomotion in lacertid lizards (Squamata, Lacertidae); a preliminary survey. Bulletin of the Natural History Museum of London, Zoology. 1998;64:63–89. [Google Scholar]
- Arnold EN, Arribas O, Carranza S. Systematics of the Palaearctic and Oriental lizard tribe Lacertini (Squamata: Lacertidae: Lacertinae), with descriptions of eight new genera. Zootaxa. 2007;1430:1–86. [Google Scholar]
- Barnes RFW, Lahm SA. An ecological perspective on human densities in the central African forests. Journal of Applied Ecology. 1997;34:245–260. [Google Scholar]
- Barraclough TG, Davies TJ. Predicting future speciation. In: Purvis A, Gittleman JL, Brooks T, editors. Phylogeny and Conservation. Conservation Biology 8. Cambridge, UK: Cambridge University Press; 2005. pp. 400–418. [Google Scholar]
- Bastin Y, Beeckman H, Cornelissen E, Fernandez Alonso M, Gansemans J, Huysmans C, Janssens B, Kennes E, Lavreau J, Louette M, Maniacky J, Omasombo J, Tack L, Tréfois P, Van Bockhaven V, Van Schuylenbergh P, Vanhee H. Nature and Culture in the Democratic Republic of Congo. Tervuren, Belgium: Royal Museum for Central Africa; 2005. [Google Scholar]
- Bauer AM, de Silva A, Greenbaum E, Jackman TR. A new species of day gecko from high elevation in Sri Lanka, with a preliminary phylogeny of Sri Lankan Cnemaspis (Reptilia: Squamata: Gekkonidae) Mitteilungen aus dem Museum für Naturkunde in Berlin. Zoologische Reihe. 2007;83:22–32. (Sonderheft) [Google Scholar]
- Boulenger GA. Monograph of the Lacertidae. Volume I. London, United Kingdom: Trustees of the British Museum (Natural History); 1920. [Google Scholar]
- Brown RM, Siler CD, Diesmos AC, Alcala AC. Philippine frogs of the genus Leptobrachium (Anura; Megophryidae): phylogeny-based species delimitation, taxonomic review, and descriptions of three new species. Herpetological Monographs. 2009;23:1–44. [Google Scholar]
- Burgess N, Hales JD, Underwood E, Dinerstein E, Olson D, Itoua I, Schipper J, Ricketts T, Newman K. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment. Washington, Covelo and London: Island Press, World Wildlife Fund; 2004. [Google Scholar]
- de Queiroz K. The general lineage concept of species, species criteria, and the process of speciation. In: Howard DJ, Berlocher SH, editors. Endless Forms: Species and Speciation. New York, NY: Oxford University Press; 1998. pp. 57–75. [Google Scholar]
- de Queiroz K. The general lineage concept of species and the defining properties of the species category. In: Wilson RA, editor. Species: New Interdisciplinary Essays. Cambridge, MA: Massachusetts Institute of Technology Press; 1999. pp. 49–89. [Google Scholar]
- de Queiroz K. Species concepts and species delimitation. Systematic Biology. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Doumenge C. Forest diversity, distribution, and dynamique in the Itombwe Mountains, South-Kivu, Congo Democratic Republic. Mountain Research and Development. 1998;18:249–264. [Google Scholar]
- Eckhart G, Lanjouw A. Mountain Gorillas: Biology, Conservation and Coexistence. Baltimore, MD: The Johns Hopkins University Press; 2008. [Google Scholar]
- Evans BJ, Carter TF, Tobias ML, Kelley DB, Hanner R, Tinsley RC. A new species of clawed frog (genus Xenopus) from the Itombwe Massif, Democratic Republic of the Congo: implications for DNA barcodes and biodiversity conservation. Zootaxa. 2008;1780:55–68. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Frederick A. In the Heart of Africa. London, New York, Toronto and Melbourne: Cassell and Company, Ltd; 1910. [Google Scholar]
- Fu J. Toward the phylogeny of the family Lacertidae: Implications from mitochondrial DNA 12S and 16S gene sequences (Reptilia: Squamata) Molecular Phylogenetics and Evolution. 1998;9:118–130. doi: 10.1006/mpev.1997.0456. [DOI] [PubMed] [Google Scholar]
- Fu J. Toward the phylogeny of the family Lacertidae—Why 4708 base pairs of mtDNA sequences cannot draw the picture. Biological Journal of the Linnean Society. 2000;71:203–217. [Google Scholar]
- Goldberg SR. Reproduction of Jackson’s Forest Lizard, Adolfus jacksoni (Squamata: Lacertidae) African Herp News. 2009;48:5–7. [Google Scholar]
- Goldberg SR, Bursey CR. Adolfus jacksoni. Endoparasites. African Herp News. 2009;48:16–17. [Google Scholar]
- Groth JG, Barrowclough GF. Basal divergences in birds and the phylogenetic utility of the nuclear RAG-1 gene. Molecular Phylogenetics and Evolution. 1999;12:115–123. doi: 10.1006/mpev.1998.0603. [DOI] [PubMed] [Google Scholar]
- Harris DJ. Molecular systematics and evolution of lacertid lizards. Natura Croatica. 1999;8:161–180. [Google Scholar]
- Harris DJ, Arnold EN, Thomas RH. Relationships of lacertid lizards (Reptilia: Lacertidae) estimated from mitochondrial DNA sequences and morphology. Proceedings of the Royal Society B, Biological Sciences. 1998;265:1939–1948. doi: 10.1098/rspb.1998.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernes H, Dalfelt A, Bernsten T, Holtsmark B, Naess LO, Selrod R, Aaheim HA. Climate Strategy for Africa. University of Oslo, Norway: CICERO Report 1995; 1995. p. 3. [Google Scholar]
- Hillis DM, Bull JJ. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology. 1993;42:182–192. [Google Scholar]
- Hipsley CA, Himmelmann L, Metzler D, Müller J. Integration of Bayesian molecular clock methods and fossil-based soft bounds reveals early Cenozoic origin of African lacertid lizards. BMC Evolutionary Biology. 2009;9:151. doi: 10.1186/1471-2148-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hulme M, Doherty RM, Ngara T, New MG, Lister D. African climate change: 1900–2100. Climate Research. 2001;17:145–168. [Google Scholar]
- Hulme M, Doherty R, Ngara T, New M. Global warming and African climate change: a reassessment. In: Low PS, editor. Climate Change and Africa. Cambridge, UK: Cambridge University Press; 2005. pp. 29–40. [Google Scholar]
- Köhler J, Wagner P, Visser S, Böhme W. New country records of Adolfus africanus (Sauria: Lacertidae) – a rain forest lizard with disjunct distribution? Salamandra. 2003;39:241–248. [Google Scholar]
- Kroniger M, in den Bosch HAJ. Biological data on Holaspis guentheri laevis Werner, 1895 obtained from vivarium keeping. Podarcis. 2001;2:72–80. [Google Scholar]
- Laurent R. Deux reptiles et onze batraciens nouveaux d’Afrique centrale. Revue de zoologie et de botanique africaines. 1951;44:360–381. [Google Scholar]
- Laurent RF. Aperçu de la biogéographie des batraciens et des reptiles de la région des grand lacs. Bulletin de la Société Zoologique de France. 1954;79:290–310. [Google Scholar]
- Laurent RF. Adaptive modifications in frogs of an isolated highland fauna in Central Africa. Evolution. 1964;18:458–467. [Google Scholar]
- Laurent RF. About the herpetofauna of Central African montane forest. In: Rhodin AGJ, Miyata K, editors. Advances in Herpetology and Evolutionary Biology: Essays in Honor of Ernest E. Williams. Cambridge, MA: Museum of Comparative Zoology; 1983. [Google Scholar]
- Leaché AD, Reeder T. Molecular systematics of the fence lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Systematic Biology. 2002;51:44–68. doi: 10.1080/106351502753475871. [DOI] [PubMed] [Google Scholar]
- Louette M. A new species of nightjar from Zaïre. Ibis. 1990;132:349–353. [Google Scholar]
- Lovett JC, Marchant R, Taplin J, Küper W. The oldest rainforests in Africa: stability of resilience for survival and diversity? In: Purvis A, Gittleman JL, Brooks T, editors. Phylogeny and Conservation. New York, NY: Cambridge University Press; 2005. [Google Scholar]
- Loveridge A. Check list of the reptiles and amphibians of East Africa (Uganda; Kenya; Tanganyika; Zanzibar) Bulletin of the Museum of Comparative Zoology. 1957;117:153–362. + xxxvi. [Google Scholar]
- Lue K-Y, Lin S-M. Two new cryptic species of Takydromus (Squamata: Lacertidae) from Taiwan. Herpetologica. 2008;64:379–395. [Google Scholar]
- Maddison DR, Maddison WP. MacClade: Analysis of Phylogeny and Character Evolution. Sunderland, MA: Sinauer Associates, Inc.; 2005. [Google Scholar]
- Mayer W, Benyr G. Albumin-Evolution und Phylogenese in der Familie Lacertidae (Reptilia: Sauria) Annalen des Naturhistorischen Museums in Wien. 1994;96B:621–648. [Google Scholar]
- Mayer W, Pavlicev M. The phylogeny of the family Lacertidae (Reptilia) based on nuclear DNA sequences: convergent adaptations to arid habitats within the subfamily Eremiainae. Molecular Phylogenetics and Evolution. 2007;44:1155–1163. doi: 10.1016/j.ympev.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Moore WS. Inferring phylogenies from mtDNA variation: Mitochondrial gene trees versus nuclear gene trees. Evolution. 1995;49:718–726. doi: 10.1111/j.1558-5646.1995.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Omari I, Hart JA, Butynski TM, Birhashirwa NR, Upoki A, M’Keyo Y, Bengana F, Bashonga M, Bagurubumwe N. The Itombwe Massif, Democratic Republic of Congo: biological surveys and conservation with an emphasis on Grauer's gorilla and birds endemic to the Albertine Rift. Oryx. 1999;33:301–322. [Google Scholar]
- Page RDM. Extracting species trees from complex gene trees: Reconciled trees and vertebrate phylogeny. Molecular Phylogenetics and Evolution. 2000;14:89–106. doi: 10.1006/mpev.1999.0676. [DOI] [PubMed] [Google Scholar]
- Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G. The Simple Fool’s Guide to PCR. Version 2. Honolulu, Hawaii: The University of Hawaii; 1991. [Google Scholar]
- Partridge TC. Tectonics and geomorphology of Africa during the Phanerozoic. In: Werdelin L, Sanders WJ, editors. Cenozoic Mammals of Africa. Berkeley, Los Angeles and London: University of California Press; 2010. pp. 3–17. [Google Scholar]
- Pavlicev M, Mayer W. Multiple copies of coding as well as pseudogene c-mos sequence exist in three lacertid species. Journal of Experimental Zoology Part B Molecular and Developmental Evolution. 2006;306B:539–550. doi: 10.1002/jez.b.21110. [DOI] [PubMed] [Google Scholar]
- Pavlicev M, Mayer W. Fast radiation of the subfamily Lacertinae (Reptilia: Lacertidae): History or methodical artefact? Molecular Phylogenetics and Evolution. 2009;52:727–734. doi: 10.1016/j.ympev.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Peracca MG. Sopra una nuova specie di Lacertidae del gen. “Algiroides” dell’Uganda. Atti della R. Accademia delle Scienze di Torino. Classe di Scienze fisiche, matematiche e naturali. 1917;52:351–354. [Google Scholar]
- Plumptre AJ, Behangana M, Davenport TRB, Kahindo C, Kityo R, Ndomba E, Nkuutu D, Owiunji I, Ssegawa P, Eilu G. The Biodiversity of the Albertine Rift. Albertine Rift Technical Reports No 3. Wildlife Conservation Society; 2003. [Google Scholar]
- Plumptre AJ, Davenport TRB, Behangana M, Kityo R, Eilu G, Ssegawa P, Ewango C, Meirte D, Kahindo C, Herremans M, Kerbis Peterhans J, Pilgrim JD, Wilson M, Languy M, Moyer D. The biodiversity of the Albertine Rift. Biological Conservation. 2007;134:178–194. [Google Scholar]
- Poblete GH. Adolfus jacksoni. Morphology. African Herp News. 2002;34:23–24. [Google Scholar]
- Poe S, Chubb AL. Birds in a bush: five genes indicate explosive evolution of avian orders. Evolution. 2004;58:404–415. [PubMed] [Google Scholar]
- Posada D. jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- Prunier G. Africa's World War: Congo, the Rwandan Genocide, and the Making of a Continental Catastrophe. New York, NY: Oxford University Press; 2008. [Google Scholar]
- Ringuis L, Downing TE, Hulme M, Waughray D, Selrod R. Climate Change in Africa: Issues and Regional Strategy. Oslo, Norway: CICERO Report No. 1996; 1996. p. 8. [Google Scholar]
- Roelke CE, Greenbaum E, Kusamba C, Aristote MM, Smith EN. Systematics and conservation status of two distinct Albertine Rift treefrogs, Leptopelis karissimbensis and L. kivuensis (Anura: Arthroleptidae) Journal of Herpetology. In press. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schaller GB. The Year of the Gorilla. Chicago, IL: The University of Chicago Press; 1964. [Google Scholar]
- Schmidt KP. Contributions to the herpetology of the Belgian Congo based on the collection of the American Museum Congo Expedition, 1909–1915. Part I. Turtles, Crocodiles, Lizards, and Chameleons. Bulletin of the American Museum of Natural History. 1919;39:385–624. [Google Scholar]
- Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Systematic Biology. 2002;51:492–508. doi: 10.1080/10635150290069913. [DOI] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 2001;17:1246–1247. doi: 10.1093/bioinformatics/17.12.1246. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, Villagrán-Santa Cruz M, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro R, Gadsden H, Avila LJ, Morando M, De La Riva IJ, Sepulveda PV, Rocha CFD, Ibargüengoytía N, Puntriano CA, Massot M, Lepetz V, Oksanen TA, Chapple DG, Bauer AM, Branch WR, Clobert J, Sites JW., Jr Erosion of lizard diversity by climate change and altered thermal niches. Science (Washington, DC) 2010;328:894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- Sodhi NS, Brook BW, Bradshaw CJA. Tropical Conservation Biology. Malden, MA: Blackwell Publishing; 2007. [Google Scholar]
- Spawls S, Howell K, Drewes R, Ashe J. A Field Guide to the Reptiles of East Africa: Kenya, Tanzania, Uganda, Rwanda and Burundi. San Diego, CA: Academic Press; 2002. [Google Scholar]
- Stamatakis A. RAXML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Blagojevic F, Nikolopoulos D, Antonopoulos C. Exploring new search algorithms and hardware for phylogenetics: RAxML meets the IBM cell. Journal of VLSI Signal Processing. 2007;48:271–286. [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Sternfeld R. Wissenschaftliche Ergebnisse der Deutschen Zentral-Afrika-Expedition 1907–1908 unter Führung Adolf Friedrichs, Herzogs zu Mecklenberg. Band IV, Zoologie II. Leipzig, Germany: Klinkhardt & Biermann; 1912. Reptilia; pp. 197–279. + figs. 1–4 + pl. VI–IX. [Google Scholar]
- Stuart SN, Hoffman M, Chanson JS, Cox NA, Berridge RJ, Ramani P, Young BE, editors. Threatened Amphibians of the World. Barcelona, Spain: Lynx Editions, Gland, Switzerland: IUCN, Arlington, VA: Conservation International; 2008. [Google Scholar]
- Swofford DL. PAUP*4.0b10. Phylogenetic Analysis Using Parsimony [*and Other Methods] Sunderland, MA: Sinauer Associates, Inc.; 2002. [Google Scholar]
- Szczerbak NN. Katalog afrikanskih Jashchurok. Kiev. 1975 [Google Scholar]
- Vande weghe JP. Forests of Central Africa: Nature and Man. Pretoria, South Africa: Ecofac, Protea Book House; 2004. [Google Scholar]
- Wiens JJ, Penkrot TA. Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus) Systematic Biology. 2002;51:69–91. doi: 10.1080/106351502753475880. [DOI] [PubMed] [Google Scholar]
- Wilcox TP, Zwickl DJ, Heath TA, Hillis DM. Phylogenetic relationships of the dwarf boas and a comparison of Bayesian and bootstrap support measures of phylogenetic support. Molecular Phylogenetics and Evolution. 2002;25:361–371. doi: 10.1016/s1055-7903(02)00244-0. [DOI] [PubMed] [Google Scholar]
- Wiley EO. Phylogenetics: The Theory and Practice of Phylogenetic Systematics. New York, NY: John Wiley and Sons; 1981. [Google Scholar]