Abstract
Background
Origanum vulgare Linn has traditionally been used in the treatment of urolithiasis. Therefore, we investigated the crude extract of Origanum vulgare for possible antiurolithic effect, to rationalize its medicinal use.
Methods
The crude aqueous-methanolic extract of Origanum vulgare (Ov.Cr) was studied using the in vitro and in vivo methods. In the in vitro experiments, supersaturated solution of calcium and oxalate, kidney epithelial cell lines (MDCK) and urinary bladder of rabbits were used, whereas, in the in vivo studies, rat model of urolithiasis was used for the study of preventive and curative effect.
Results
In the in vitro experiments, Ov.Cr exhibited a concentration-dependent (0.25-4 mg/ml) inhibitory effect on the slope of nucleation and aggregation and also decreased the number of calcium oxalate monohydrate crystals (COM) produced in calcium oxalate metastable solutions. It also showed concentration-dependent antioxidant effect against DPPH free radical and lipid peroxidation induced in rat kidney tissue homogenate. Ov.Cr reduced the cell toxicity using MTT assay and LDH release in renal epithelial cells (MDCK) exposed to oxalate (0.5 mM) and COM (66 μg/cm2) crystals. Ov.Cr relaxed high K+ (80 mM) induced contraction in rabbit urinary bladder strips, and shifted the calcium concentration-response curves (CRCs) towards right with suppression of the maximum response similar to that of verapamil, a standard calcium channel blocker. In male Wistar rats receiving lithogenic treatment comprising of 0.75% ethylene glycol in drinking water given for 3 weeks along with ammonium chloride (NH4Cl) for the first 5 days, Ov.Cr treatment (10-30 mg/kg) prevented as well as reversed toxic changes including loss of body weight, polyurea, crystalluria, oxaluria, raised serum urea and creatinine levels and crystal deposition in kidneys compared to their respective controls.
Conclusion
These data indicating the antiurolithic activity in Ov.Cr, possibly mediated through inhibition of CaOx crystallization, antioxidant, renal epithelial cell protective and antispasmodic activities, rationalizes its medicinal use in urolithiasis.
Background
Urolithiasis, the formation of urinary stones, is one of the oldest known diseases. Archaeological findings give profound evidence that humans have suffered from kidney and bladder stones for centuries, even examinations of Egyptian mummies have revealed kidney and bladder stones [1].
It is the third most common problem of the urinary tract with an estimated lifetime risk of 2-5% in Asia, 8-15% in Europe and America and around 20% in the Middle East. It is associated with high rate of recurrence, which is around 10-23% per year, 50% in 5-10 years and 75% in 20 years. Once afflicted, the subsequent relapse rate is increased and the recurrence interval is shortened [2-5]. Moreover, its annual incidences are increasing and also the age of onset is decreasing, perhaps due to change in life styles, diet and climate [6].
Improvement in therapy of kidney stones with modern techniques like extracorporeal shock wave lithotripsy (ESWL), ureteroscopy (URS), and percutaneous nephrolithotomy (PNL), has revolutionized the urological practice but the recurrence in kidney stone is not altered. ESWL is less effective in calcium oxalate monohydrate (COM) and cystine stones than calcium oxalate dihydrate (COD) and uric acid stones [7]. In addition to the high cast and the issues of recurrence with these measures there are multiple side effects, which include renal damage, ESWL induced hypertension, renal impairment, sever haematuria, steinstrasse (multiple small stone blocking ureter), pancreatitis, infection and persistent residual fragments as potential nidus for new stone formation. While these complications can lead to large perfusion of the collecting system, extravasations of irrigating fluid, urosepsis, ureteral injury and delayed bleeding may also occur [8,9]. Pharmacological agents include a limited choice like citrate and thiazide diuretics, which have limited efficacy in addition to their less tolerability [10,11]. On the other hand, there is growing interest of public in herbal medicine, particularly in the treatment of urolithiasis partly because of limited choice in the pharmacotherapy. Moreover, herbal remedies are known to contain multiple constituents, acting through multiple pathways needed in urolithiasis, for example, antispasmodic, diuretic, pain relieving [12,13]. In this study, we evaluated Origanum vulgare L for its antiurolithic activity, in an attempt to rationalize its folkloric use in urolithiasis and to see if it exhibits calcium oxalate crystallization inhibitory, antioxidant, renal cell protective, antispasmodic and diuretic activities, which are likely to contribute in its antiurolithic effect. Origanum vulgare Linn (family, Lamiaceae) is distributed throughout Asia, Europe and North America [14] and is commonly known as Wild Marjoram and Winter Sweet and locally in Pakistan as Mirzanjosh, Sathra [15,16]. It is widely used in the traditional medicine as lithotriptic, diuretic and antispasmodic along with other medicinal uses, such as stimulant, expectorant, antibacterial, anticancer, anti-inflammatory, antioxidant and laxative [15-17].
Methods
Chemicals and reagents
All chemical used were of analytical grade. List and sources of chemical used in the study are provided in the additional file 1.
Animals
Experiments were performed in compliance with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council [18] and approved by the Ethical Committee for Research on Animals (ECRA) of the Aga Khan University, Karachi, Pakistan.
Wistar rats (180-220 g) of either sex used for this study were sourced locally and housed at the animal house of the Aga Khan University, kept in plastic cages (47 × 34 × 18 cm3) with saw dust (renewed after every 48 hrs), under a controlled temperature of 23-25°C and 12 hrs light-dark cycle. Animals had access to food and water ad-libitum throughout the study. However, food was withdrawn 24 hrs before and during 6 hrs of diuretic study, and while collecting 24 hrs urine samples.
Plant material and extraction
The plant of Origanum vulgare was collected from the Northern Areas of Pakistan, identified by taxonomist Prof. Jhandar shah, Vice chancellor Shaheed Benazir Bhutto University, Khyber Pakhtunkhwa, Pakistan and voucher specimen (OV-PL-02-08-72) was submitted to the herbarium of the Department of Biological and Biomedical Sciences, the Aga Khan University, Karachi. The aerial part of the plant material was cleaned of adulterants and kept soaked for three days in the aqueous-methanol (30:70) with occasional shaking, at room temperature. The filtration was carried out using a muslin cloth and then through Whatman qualitative grade 1 filter paper. This procedure was repeated twice and then all the filtrates obtained were combined and concentrated on a rotary evaporator (RE-111, Buchi, Flawil, Switzerland) accompanied with B-700 recirculation chiller and a water bath model 461 at 40°C to a thick pasty mass called as crude extract (Ov.Cr), yielding approximately 12% [19,20].
Preliminary phytochemical analysis
The crude extract of Origanum vulgare, was screen for the presence of different phytochemical groups such as alkaloids, saponins, coumarins, sterols, terpenes, tannins and flavonoids by using methods followed previous studies [21,22].
In vitro experiments
In vitro crystallization studies
Kinetic study
The effect of the test material on kinetics of calcium oxalate (CaOx) crystallization was determined by the time course measurement of turbidity changes due to the crystal nucleation and aggregation after mixing metastable solutions of calcium (Ca++) and oxalate (Ox). Stock solutions of CaCl2 (8.5 mM) and Na2C2O4 (1.5 mM), containing 200 mM NaCl and 10 mM sodium acetate were adjusted to pH 5.7 [23]. An aggregometer (Chrono-Log Corporation, USA) devised for platelet aggregation studies based on the measurement of optical density at 620 nm was used to investigate the event of CaOx crystallization [24]. The slopes of nucleation (SN) and aggregation phases (SA) were calculated using linear regression analysis. Using the slopes, the percentage inhibition was calculated as [(1-Sm/Sc) × 100], where Sm is slope in the presence of modifier; K.Cit or Ov.Cr, and Sc is slope of the control experiment.
Incubation study
To determine the effect of incubation on CaOx crystal formation, stock solutions of CaCl2 and Na2C2O4 similar to those in the kinetic study were used. CaCl2 solutions, containing different concentrations of the Ov.Cr or potassium citrate, were aliquoted (0.5 ml) to the flat-bottomed tubes in a 24 well plate (Iwaki Microplate with lid, Scitech Div., Asahi Techno Glass, Japan). To each of these tubes sodium oxalate (Na2C2O4) solution (0.5 ml) was added [25]. The plates were then incubated in a shaking water bath at the 90 oscillations/min at a temperature of 37°C for 45 minutes. Abundance and the morphology of the crystals in each tube were then observed under inverted microscope (Nikon Corporation, Tokyo, Japan).
Determination of antioxidant activity
Antioxidant potential of the test materials was estimated in vitro by free radical scavenging and lipid peroxidation inhibitory activities.
Free radical scavenging activity
For free radical scavenging activity, a 0.1 mM solution of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical in methanol was prepared and 1 ml of this solution was added to 3 ml of the test material at different concentrations prepared in methanol [26]. Solutions were incubated for 30 min at room temperature and then absorbance was measured at 517 nm. Decreasing of the DPPH solution absorbance indicates an increase of the DPPH radical-scavenging activity.
Lipid peroxidation inhibitory activity
To assess lipid peroxidation inhibitory activity, the kidneys isolated from Wistar rat were homogenized with electric homogenizer (Zero-Max®), in ice cold PBS (50 mmol/l, pH 7.4) as described earlier [27].
Cell lines (MDCK) Experiment
MDCK cells were maintained as sub-confluent monolayers at 37°C in 5% CO2. The culture were grown in 75 cm2 Falcon tissue culture flasks in a 1:1 DMEM nutrient mixture and F-12 medium (DMEM/F-12) containing 10% FBS, 2% streptomycin/penicillin, pH 7.4. The medium was changed every 3 to 4 days.
XTT assay for cell viability
Media was aliquoted to designated wells, containing confluent MDCK cells, of a 96 well plate. The XTT Cell Viability Assay Kit was used to determine the cell viability. Cells were incubated with and without (Control) plant extracts for 24 hrs. Then 50 μL of the working activation solution, prepared by mixing of one bottle of XTT solution and one of the activation reagent PMS supplied with kit, was added to all the samples and control, and incubated for 2-4 hrs. Optical density absorbency was read at 450-490 nm on a Bio-Rad 3550 microplate reader (Bio-Rad, Hercules, CA).
Lactate Dehydrogenase (LDH) Release
Media was aliquated to designated wells of a 96 well plate. The CytoTox 96 Non-Radioactive Cytotoxicity assay kit was used to determine LDH percent release. Substrate (supplied with kit) was added to all samples, positive control (MDCK cells lysed with lysis solution-supplied with kit), and blanks (acclimazation media). The plate was incubated at room temperature for 30 minutes in the dark. Stop solution (supplied with kit) was added to all samples, positive control, and blanks. Optical density absorbency was read at 490 nm on a Bio-Rad 3550 microplate reader (Bio-Rad, Hercules, CA).
Antispasmodic activity
Antispasmodic activity of the plant extract was evaluated against carbachol (CCh) and high K+ (80 mM)-induced contractions in strips of rabbit urinary bladder [28]. The whole urinary bladder of rabbit was dissected out, and divided into 3-4 vertical strips. Each preparation was mounted in a 10-20 ml tissue bath containing Kreb's-Henseleit solution, maintained at 37°C and continuously aerated with carbogen. A tension of 1 g was applied to each tissue throughout the experiment. Isometric responses were recorded on a Grass Model Polygraph and/or Power lab 4/24 data acquisition system attached to computer running Chart 5.3 software (AD Instruments, Sydney, Australia). The tissues were allowed to equilibrate for a period of about 1 hr before the addition of any drug, during which the tissue was washed with fresh bathing fluid at an interval of every 15-20 minutes.
Following the equilibrium, preparations were stabilized, by repeatedly treating with 1 μM CCh, until constant responses were recorded. Then the spasmolytic activity of the plant extract was determined by adding the plant extracts on CCh or high K+-induced sustained contraction in rabbit bladder, in a cumulative fashion to obtain concentration-dependent inhibitory response [29]. IC50 values (concentration causing 50% inhibition) were calculated from these curves and calculated as a measure of spasmolytic potency of the relaxant drugs.
Calcium channel blocking activity
The concentration-response curves (CRCs) of Ca++ were constructed in the absence and presence of increasing concentrations of test drug to confirm the Ca++ antagonist action of the test substance. The tissue was allowed to stabilize in normal Kreb's-Henseleit solution, which was then replaced with Ca++-free Kreb's-Henseleit solution containing EGTA (0.1 mM) for 30 min in order to remove Ca++ from the tissues. This solution was further replaced with K+-rich and Ca++-free Kreb's-Henseleit solution. Following an incubation period of 30 min, CRCs of Ca++ were constructed in the absence and the presence of different concentrations of the test materials [30]. Isometric responses were recorded on a Grass Model 7 Polygraph and/or Power lab 4/24 data acquisition system attached to computer running Chart 5.3 software (AD Instruments, Sydney, Australia).
In vivo experiments
Determination of diuretic activity
The diuretic activity of the test material was studied on Wistar rats of either sex (180-220 g) as described previously [31]. Animals were divided with matched body weight and sex into groups of 6 animals each. Normal and positive control groups were given by gavages saline (20 ml/kg) and standard diuretic drug: hydrochlorothiazide (HCT), 10 mg/kg of body weight, respectively. The rest of the groups were given different doses of the test material dissolved in saline. Subsequently, the animals were placed individually in metabolic and diuretic cages. The urine was collected in graduated cylinders for 6 hrs at 2 hrs intervals. Total urine excreted out was collected and the volume was determined.
Study on animal model of urolithiasis
Antiurolithic activity of the test material was determined using animal model of CaOx urolithiasis [32,33]. Male Wistar rats (weighing 180-220 g) were divided with matched body weights into groups of 6-8 animals each, which were then randomly selected to receive various treatments for preventive and curative study.
Preventive study model
To study the preventive effect of the test material, rats serving as normal control, received intra-peritoneal (i.p.) injections of normal saline (2.5 ml/kg), once in 24 hrs. Whereas, untreated group received i.p. injection of normal saline (2.5 ml/kg) along with renal CaOx crystal deposits inducing (lithogenic) treatment for 21 days. The renal CaOx deposits induction was achieved by giving water containing 0.75% (w/v) ethylene glycol (EG) and 1% (w/v) ammonium chloride (AC) for 5 days, following this the water supply was switched to 0.75% EG alone [32,33]. Treated groups received i.p. injection of the extract dissolved in saline once in 24 hrs and simultaneously received crystal deposits inducing treatment similar to the untreated group. Based on the medicinal use and literature available on Origanum vulgare, where doses of 20-60 mg/kg have been used in rats [34,35], we used log doses 10 and 30 mg/kg for its diuretic and antiurolithic effect, optimized in our pilot study. Animal weight and activity were regularly monitored to assess their overall health and those looking lethargic or who have lost excessive weight were excluded from the study. Water intake was determined and 24 hrs urine samples were collected immediately before the onset and at the end of total 21 days of treatment, for which animals were housed individually in metabolic and diuretic cages. The 3 hrs morning urine for crystalluria study was also collected before collecting 24-hrs urine at the end of 21 days of treatments. The number of the crystals/mm3 was counted under light microscope using haemocytometer.
Curative study model
In the study for the curative effect, CaOx deposits were induced in the kidneys of rats in both untreated and treated groups with EG (0.75%) and AC (1%) treatment for 21 days, following the plan as given with the study on the preventive effect. Thereafter, lithogenic treatment was withdrawn and treatment with vehicle and the test material was respectively started to the untreated and the treated groups for another period of 14 days [33]. Normal control, received no treatment for the first 21 days study period, but received i.p. injections of normal saline (2.5 ml/kg), during the next 14 days. Animal weights and their activity were regularly monitored, whereas, 24 hrs urine samples were collected immediately before and after the crystals deposits induction (lithogenic) treatment and at the end of total treatment period (35 days). 3-hrs urine sample was collected after both 21 and 35 days.
Following volume and pH determination, 24 hrs urine samples were stored at -20°C until analyzed. Blood was collected through cardiac puncture from animals under ether anaesthesia for serum separation in order to assess serum creatinine and blood urea nitrogen (BUN). Animals were sacrificed and the kidneys were fixed in 10% neutral buffered formalin, processed, embedded in paraffin wax, sectioned at 5 μm and stained with Haematoxylin and Eosin (H & E) and by Pizzolato's method, for calcium oxalate crystals [36], for microscopic examination.
Calcium oxalate crystal deposition in kidney
Crystal distribution within the kidneys was determined by using the semiquantitaive scoring by the methods used by Vanachayangkul et al., [37]. Briefly the crystal deposits in stained sections with visible in a field of 10× magnification were counted and severity grades were assigned as 0 = < 1 crystals, 1 = ≤ 10, 2 = ≤ 30, 3 = ≤ 50, 4 = ≤ 75 and 5 = > 75 crystals. Most of the crystals were located in the outer modularly and cortical region of the kidney.
Biochemical analysis of urine and serum
Urine samples for Ox, Ca++ and Mg++ , citrate, and uric acid (UA) and serum samples for creatinine and BUN contents were determined by using commercially available kits (Company names for kits are given in the additional file), while urinary inorganic phosphate and protein were determined by using the molybdenum blue reaction [38] and Lowry's [39] method respectively.
Data Analysis
The data expressed are mean ± standard error of mean (SEM) and the median effective concentration (EC50 value) with 95% confidence intervals (CI). All statistical comparisons between the groups are made by means of t- test (comparison between two groups) or One Way Analysis of Variance (ANOVA) with post hoc Dunnett's test. p value less than 0.05 is regarded as significant. CRCs were analyzed by non-linear regression using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Phytochemical Screenings
The phytochemical test showed the presence of saponins, alkaloids, coumarins, sterol, terpenes, flavonoids and tannins in Ov.Cr.
In vitro Experiment
Effect on in vitro crystallization
Kinetic study
Effect of Ov.Cr on various phases of CaOx crystallization as determined by time course measurement of turbidity under standard conditions (4.25 mM Ca++ and 0.75 mM Ox) is given in Figure 1, which shows typical tracing (Figure 1A and 1B) of the experiment in the presence of Ov.Cr and potassium citrate. In the control curve, the initial rise in turbidity; the nucleation phase, on attaining its maximum after about 150 ± 15 sec, followed by a slow decrease; the aggregation phase. Ov.Cr inhibited the SA with a median inhibitory concentration of 0.47 mg/ml (0.31 to 0.69; 95% CI), similar to potassium citrate which causes inhibition with IC50 value of 0.42 mM (0.31 to 0.58; 95% CI) as shown in Figure 1C. Ov.Cr caused 10 ± 1.73, 16 ± 2.3, 20 ± 1.7 and 35 ± 2.8% inhibition of SN at the concentration of 0.5, 1, 2 and 4 mg/ml, while the citrate caused 20 ± 1.7, 31 ± 2.31, 42 ± 4.0 and 65 ± 4.2% inhibition at a concentration of 0.5, 1, 2 and 4 mM, respectively (Figure 1D).
Figure 1.
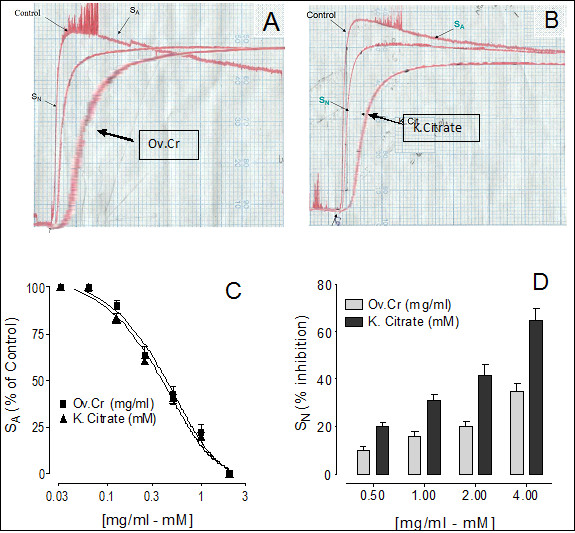
Calcium Oxalate crystallization study. Effect of Origanum vulgare (Ov.Cr) and Potassium Citrate (k-Cit) on calcium oxalate crystallization. (A) and (B) are the typical tracing of the control and in the presence of Ov.Cr and potassium citrate. Panel (C) is concentration-response curves of Ov.Cr and potassium citrate on SA of the turbidity curves, while (D) shows the % inhibition on the SN. Symbols shown are mean ± S.E.M. (n = 3). SN and SA represent slope of nucleation and slop of aggregation respectively.
Incubation study
In the incubation study, mixing the metastable solutions of Ca++ and Ox resulted in the formation of CaOx crystals, predominately of the dumbbell shaped calcium oxalate monohydrate (COM). Ov.Cr caused a concentration-dependent (1-4 mg/ml) decrease in the CaOx crystal formation (Figure 2A &2B) and also decreased the number and size of COM crystals; similarly citrate also decreased the number and size of crystals formed (Figure 2C &2D).
Figure 2.
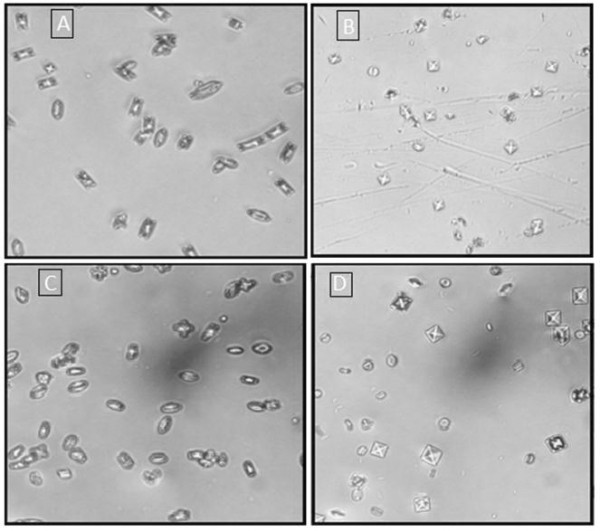
Calcium Oxalate incubation study. Representative photographs, under inverted microscope (200x), of CaOx crystals developed in the metastable solutions in the absence (A and C) and in the presence of crude extract of Origanum vulgare (Ov.Cr) 2 mg/ml (B) and 2 mM K-Citrate (D).
Antioxidant activity
Free radical scavenging activity
Ov.Cr caused inhibition of DPPH free radical with IC50 value of 6.28 μg/ml (5.41 - 7.27; 95% CI), while the control drug butylated hydroxytoluene (BHT) inhibited DPPH with IC50 value of 3.41 μg/ml (3.16 - 3.68; 95% CI) as shown in the Figure 3A.
Figure 3.
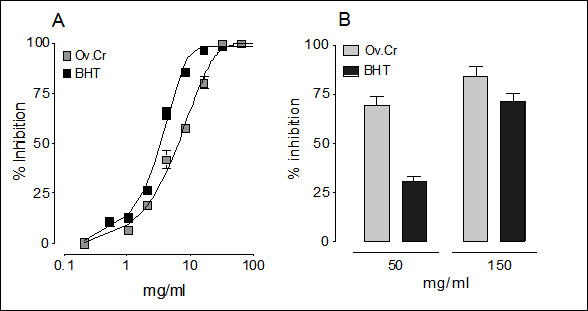
Antioxidant activity. Concentration-response curves of the free radical scavenging activity of the butylated hydroxytoluene (BHT) and Ov.Cr, while bar-chart (B) representing lipid peroxidation inhibitory activity of two different concentrations of Ov.Cr and BHT. Inhibition is measure as % of the respective control experiments. The values shown are mean ± SEM (n = 3).
Lipid peroxidation inhibitory activity
Ov.Cr inhibited the in vitro lipid peroxidation induced in rat kidney homogenate by 69.3 ± 4.7% and 84.1 ± 5.0% (Figure 4B), while BHT caused 30.8 ± 2.6 and 71.6 ± 3.8% inhibition of lipid peroxidation at 50 and 150 μg/ml, respectively (Figure 3B).
Figure 4.
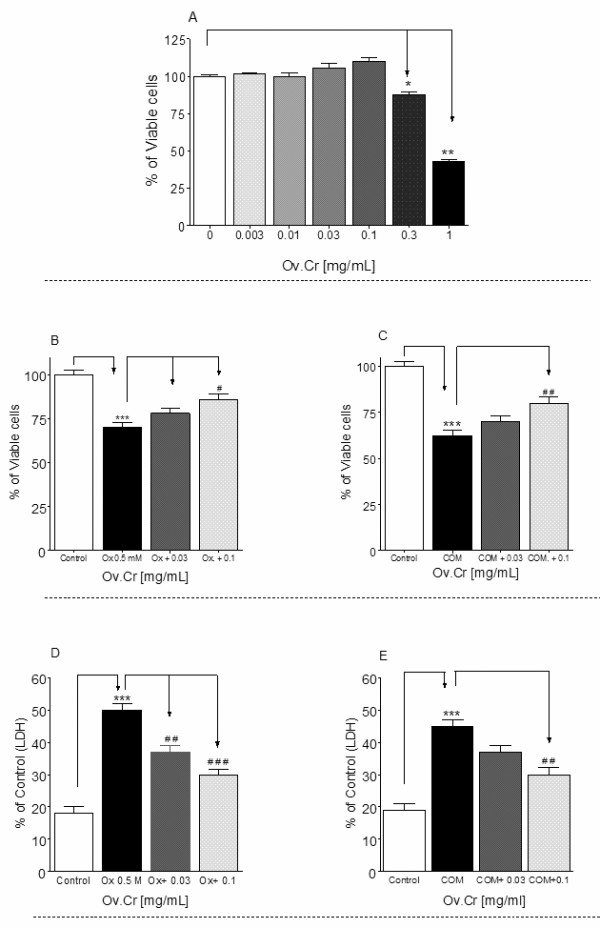
Effect on MDCK cells. Effects of various concentrations of Ov.Cr on MDCK cell survival in acclimatization media (A). B and C show the protective effect of Ov.Cr after exposure to 0.5 mM oxalate or 66 mg/cm2 COM respectively. While (D) and (E) shows the percent increase in LDH release against control by MDCK cells exposed Ox. (0.5 mM) and COM (66 μg/cm2) for 24 hrs. Data shown are mean ± SEM of two separate experiments with 3 independent replicates. * p < 0.05, ** p < 0.05 and *** p < 0.001
Effect on Kidney Epithelial Cell Lines (MDCK)
Effect on cell viability
Ov.Cr had no toxic effect on MDCK cells up to 0.1 mg/ml. However, it significantly (p < 0.01) reduced the cell viability at higher concentrations (Figure 4A).
Effect Ov.Cr on MDCK cells after exposure to Oxalate and COM crystals
The cell viability was decreased (p < 0.001) after exposure to 0.5 mM Ox or 66 μg/cm2 of COM. However, after the co-exposure of Ov.Cr, the cell viability was mildly increased at 0.03 mg/ml while significantly (p < 0.01) increased as compared to Ox or COM at a concentration of 0.1 mg/ml (Figure 4B and 4C).
Effect of Ov.Cr on cell membrane damage
LDH release was significantly increased (p < 0.001) after exposure to 0.5 mM Ox or 66 μg/cm2 COM vs. untreated control. However, co-exposure of Ov.Cr (0.1 and 0.3 mg/ml) significantly decrease the LDH release (Figure 4D and 4E).
Effect on Urinary bladder
Ov.Cr caused concentration-dependent inhibition of both CCh (1 μM) and high K+ (80 mM)-induced contractions in rabbit urinary bladder preparations (Figure 5A) with an EC50 values of 0.061 (0.04-0.08) and 0.068 mg/ml (0.05-0.08) respectively. Similarly, verapamil was found to be more potent against the K+ than the CCh-induced contraction. It relaxed both CCh and K+-induced contractions (Figure 5C) with IC50 values of 0.08 μM (0.07-0.10) and 0.04 μM (0.03-0.5) respectively. Ov.Cr (0.03-0.1 mg/m1) caused rightward shift of the Ca++ CRCs accompanied by suppression of the maximum contractile effect, similar to that caused by verapamil (0.01-0.03 μM), as shown in Figure 5B and 5D.
Figure 5.
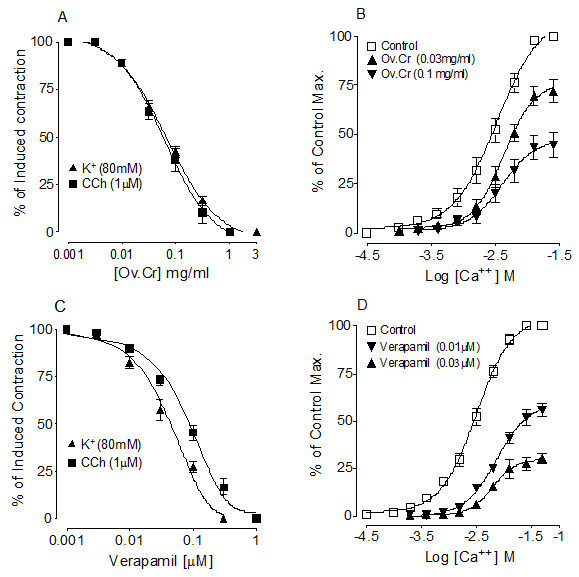
Antispasmodic effect. Concentration-response curves of crude extract of Ov.Cr (A) and verapamil (B) on K+ (80 mM) and CCh (1 μM)-induced contractions and the concentration-response curves of Ca++ constructed in the absence and presence of increasing concentrations of Ov.Cr (C) and verapamil (D) in isolated rabbit urinary bladder. The symbols represent mean ± SEM (n = 4-6).
In vivo experiments
Diuretic effect
Ov.Cr increased significantly (p < 0.05) the urine output in Wistar rats at the dose of 10 mg/kg, while there was no affect seen at lower (3 mg/kg) or higher dose (30 mg/kg). HCT (10 mg/kg) was used as reference drug, which significantly (p < 0.01) increased the urine output (Figure 6).
Figure 6.
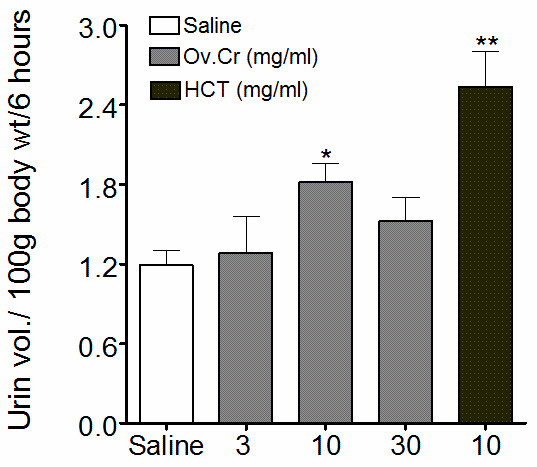
Diuretic effect. Effect of the Ov.Cr and hydrochlorothiazide (HCT) on urine volume collected in 6 hrs (values shown are means ± SEM, n = 6-8). * p < 0.05, ** p < 0.01
Effect on animal model of Urolithiasis
Preventive effect
In preventive study, all the parameters, like body weights, 24 hrs water intake, urine volume, urinary pH and composition, recorded before the treatment were not significantly different among the groups. The parameters recorded at day 0 and at the end of 3 weeks of treatment period are listed in the additional file 2.
In the 3-hrs morning urine sample of rats, significantly more and bigger CaOx crystals mostly of COD were observed in lithogenic group as compared to the saline group. Whereas, Ov.Cr significantly reduced urinary crystal count as well as decreased crystal size (Figure 7). At the end of the treatment a significant (p < 0.01 vs. Normal) loss in body weights was caused by the EG and AC consumption in the lithogenic group as compared to the normal saline group. The co-administration of Ov.Cr (10-30 mg/ml) prevented the loss in body weights of rats (p < 0.01 vs. lithogenic group). 24 hrs urine volume and water intake were higher (p < 0.01) in the lithogenic group compared to that of normal saline animals. Urine pH was also reduced, though not to a significant extent. A co-treatment with Ov.Cr significantly reduced (p < 0.05) polyurea and water intake compared to lithogenic group. Similarly, oxalate excretion was significantly increased (p < 0.01) in lithogenic animals, whereas Ca++ excretion was decreased (p < 0.05). Urine contents of citrate, phosphate, UA, Mg 2+, Na+ and K+ did not alter to a significant level. Co-administration of Ov.Cr (10-30 mg/kg) to lithogenic group significantly (p < 0.05) decreased oxalate excretion, whereas urinary excretion of citrate and Ca2+ was significantly increased (p < 0.05). EG treatment caused impairment of renal functions of the untreated rats (lithogenic group) as evident from total protein loss and raised BUN and serum creatinine (p < 0.05), which were prevented in the animals treated with Ov.Cr. (please see additional file 2).
Figure 7.
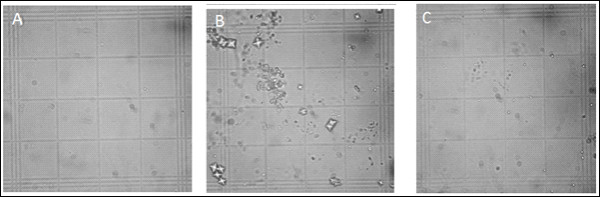
Images of Crystalluria. Images of calcium oxalate crystals in 3 hrs morning urine collected from Normal control (A), Lithogenic Control (B) and treated with crude extract of Origanum vulgare(Ov.Cr) (C), under light microscope at 400× magnification.
Kidneys excised from lithogenic group were enlarged. Histological preparations of kidneys of normal saline group did not show any crystalline deposits, whereas, a high score of crystalline deposits was observed in all regions of kidneys in the lithogenic group. However, Ov.Cr treatment significantly lowered the CaOx crystal deposits (p < 0.5) as compared to untreated (lithopgenic) group (Figure 8 and 9A).
Figure 8.
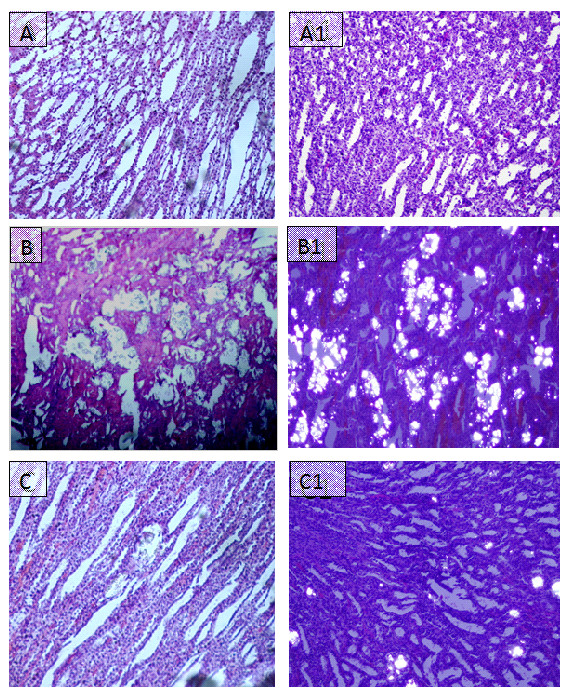
Microscopic images of Kidney sections in preventive study. Representative microscopic images of the H and E stain of the kidney sections from normal (A), Lithogenic group (B) and Treated (C) with Ov.Cr. A1, B1 and C1 show the polarized images of the sections.
Figure 9.
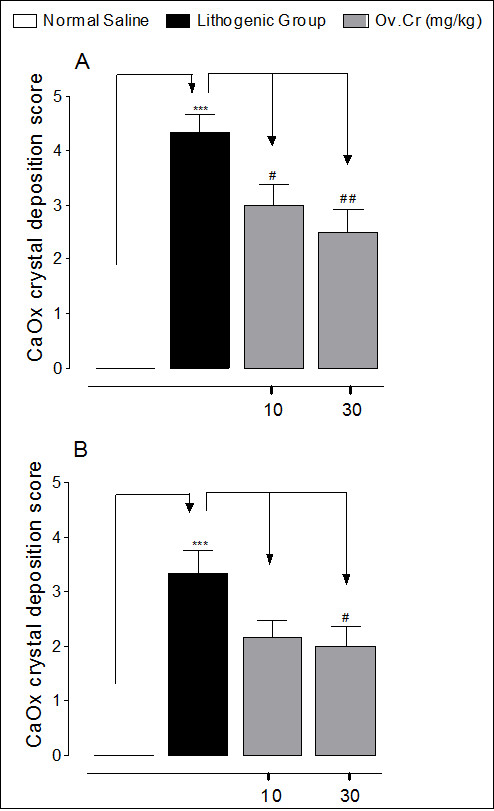
Crystals deposits score in Kidney sections of preventive and curative study. Calcium oxalate crystal deposition score after treatment with 0.75% EG, 1% NH4Cl (lithogenic group), Ov.Cr 10 and 30 mg/kg in preventive (A) and curative (B) study model. Severity grade were assigned as 0 = < 1 crystals, 1 = ≤ 10, 2 = ≤ 30, 3 = ≤ 50, 4 = ≤ 75 and 5 = > 75 crystals; data are expressed as mean ± SEM. * p < 0.05, ** p < 0.05 and *** p < 0.001 vs. Normal, # p < 0.05, ## p < 0.01 and ### p < 0.001 vs. Lithogenic group.
Curative effect
In the curative study, all the parameters were recorded before start of the treatment (day 0), after 3 weeks of the crystals deposits induction, and then two weeks after the withdrawal of the crystals deposition treatment (0.75% EG + 1% AC in drinking water). The parameters recorded for the curative study are given in the Additional file 3.
The groups, which consumed the lithogenic treatment for the first 21 days, developed the lithogenic parameters as compared to the normal saline group like in the preventive study. This was suggested by a net loss in the body weights, a significant increase in 24 hrs water intake, urine volume, oxalate, uric acid, total protein, and decreased urinary pH and Ca++ contents, impaired renal function suggested by increased serum creatinine, BUN and total urinary protein loss (Additional file 3). Post-induction treatment with Ov.Cr (10 and 30 mg/kg) reversed the loss in body weights, impaired urinary and serum functions, crystalluria and deposition of crystals in the kidney more quickly than the control group (Additional file 3).
After two weeks of Ov.Cr treatment in the curative study, renal CaOx crystal deposits were found in 4 out of 6 rats. However, they were significantly less (p < 0.5) than the untreated group (Figure 9B and Figure 10).
Figure 10.
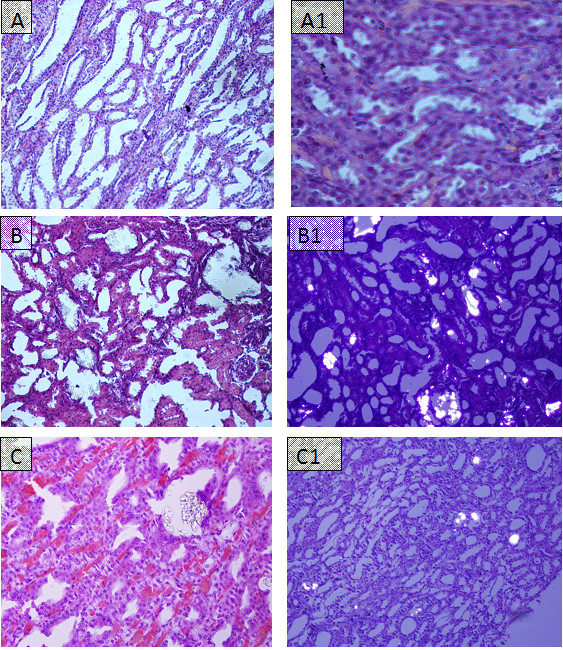
Microscopic images of Kidney sections in curative study. Representative microscopic images of the H and E of the kidney sections from normal (A), Lithogenic group (B) and Treated (C) with Ov.Cr. A1, B1 and C1 show the polarized images of the sections.
Discussion
In view of the medicinal uses of Origanum vulgare in urolithiasis, we evaluated its crude extract for the possible antiurolithic effect along with antioxidant, antispasmodic and diuretic activities using the in vitro assays and in vivo rat models.
In this study, the plant extract inhibited the CaOx crystal nucleation and aggregation in a concentration-dependent manner, similar to citrate, a well-known inhibitor of CaOx crystallization and clinically used for the management of urolithiasis [40]. Similarly, in the incubation study, Ov.Cr caused a decrease in crystal count and transformed COM to COD crystal like that of citrate and Mg2+ [41]. COM crystals are considered to be more harmful than COD because of their tendency to attach with the membrane to form aggregates [42] and are more likely to attach with the kidney epithelial cells than CaOx dehydrate, resulting in the formation of kidney stones [42,43]. Calcification is a multifactorial phenomenon [44], arising as a result of a cascade of events initiated by supersaturation, including crystal nucleation, growth, aggregation and retention [45]. Although supersaturation is not the only critical step involved in the formation of kidney stones as several studies have identified many inhibitors of calcium oxalate and calcium phosphate crystallization including ionic or macromolecules [46], yet it is the prerequisite for the crystals formation in the urinary tract [47]. Various crystal inhibitors like potassium-sodium citrate and magnesium oxide have been shown to decrease the saturation of CaOx and inhibit crystal nucleation, growth and aggregation and reduced crystallization in urine of stone forming patients [48]. Interference with crystal growth and aggregation therefore seems a possible therapeutic strategy for the prevention of recurrent stone disease. Ov.Cr inhibits CaOx crystal nucleation and aggregation along with decrease in count and morphological change, from COM to COD, in crystals. These results indicate the presence of CaOx crystal aggregation inhibitory constituent(s) in the plant.
Animal and cellular studies have revealed that oxalate, calcium oxalate and hydroxy apatite crystals cause injury to kidney cells [49,50], caused by the production of reactive species (ROS) which is considered to be the risk factor for the crystallization and crystals deposition in the kidney by promoting crystal nucleation, aggregation, retention and stone development [49,51]. Antioxidants such as vitamin E, catechin and selenium have been shown to protect against oxidative injury by oxalate and crystal deposition [52,53]. When studied for its antioxidant activity, Ov.Cr caused scavenging of DPPH free radical and inhibited ferrous-ascorbate-induced lipid peroxidation of rat kidney homogenate similar to BHT, a standard antioxidant [54], confirming its antioxidant activity [35,55,56].
Cytoprotective effect of the plant was confirmed when pre-treatment of the normal kidney epithelial (MDCK) cells with Ov.Cr significantly increased the survival rate and reduced the LDH, a marker of cell membrane damage [57], release of MDCK cells exposed to Ox and COM crystals. This protective effect could be the result of its antioxidant activity.
Antispasmodics are more commonly used in the medical expulsive therapy (MET) for the kidney stones [58]. Administration of alpha-adrenoreceptor antagonists or Ca++ channel blockers enhances the expulsion rate of crystals and reduces colic event [59]. Therefore, we evaluated the antispasmodic effect of Ov.Cr, and studied its relaxant effect against high K+ (80 mM) and CCh (1 μM) induced contraction, using rabbit urinary bladder strips. High K+ (> 30 mM) is known to cause smooth muscle contraction though opening of voltage depended L-type calcium channels, thus allowing influx of extracellular Ca++ resulting in the contraction of the smooth muscles [20,60] and a substance that relax the high K+ induced contraction is considered to be CCB [61]. Whereas, CCh is a cholinergic drug, which can induce contraction in urinary bladder through activation of muscarinic receptors, predominately M3 subtype [62]. Ov.Cr relaxed the high K+ and CCh induced contraction like that of verapamil, a standard CCB [63], indicating CCB activity, which was confirmed when pre-treatment of the tissue with the plant material shifted the Ca++ CRC to the right with suppression of the maximum response, like that of verapamil.
In the in vivo experiment, Ov.Cr caused significant increase in urine output at the dose of 10 mg/kg similar to that caused by HCT, a standard diuretic, while, the administration of next higher dose (30 mg/kg) did not caused any significant change in the diuretic effect, compared to the saline control, which could be due to the co-existence of anti-diuretic component(s) in Ov.Cr, as plant extract may exhibit multiple therapeutic activities probably on account of having a mixture of phytochemicals. The presence of synergistic and/or side-effect neutralizing effect in plants is known to exist [12], which is probably meant by nature not to allow the pharmacological effect go beyond a certain limit, beyond which it could have been harmful. Diuretics increase urine volume, which results in the reduction of supersaturation of crystals forming salts and also help in the expulsion of already formed crystals [64,65].
The antiurolithic effect of Ov.Cr was evaluated on the most commonly applied EG-induced model for urolithiasis [66-68] and male Wistar rats, which develop changes in urine electrolytes and CaOx super-saturation to a greater extent due to their greater sensitivity to EG toxicity [69], were used.
In this study, hyperoxaluria was induced by administration of EG (0.75% in drinking water) for 21 days and AC (1%) was given only for the first 5 days, as administration of AC for more than 5 days led to extreme weight loss and ultimately death of the rats [37].
In the preventive study, administration of EG and AC resulted in the increase in crystalluria with larger crystals due to hyperoxaluria, increase in water intake and urine output, which might be due to the renal impairment [68], as there was significant increase in serum creatinine, blood urea nitrogen and total protein loss in lithogenic group as compared to normal, which has been restored by the Ov.Cr treatment. Consistent with some previous reports, crystals deposition by hyperoxaluria caused an increase in oxalate and decrease in Ca2+ excretion in the lithogenic group [70,71], which was prevented by Ov.Cr. There was hypertrophy and extensive calcium oxalate crystal deposition in kidneys of lithogenic group. The renal tubules were markedly dilated, which might be due to the obstruction in distal renal tubular flow by large crystals [68]. Several in vivo and in vitro studies have demonstrated that hyperoxaluria, a major risk factor for calcium oxalate nephrolithiasis, results in production of superoxide and hydroxyl free radicals causing oxidative stress, cell membrane rupture and cell death [53,72], and leads to CaOx crystal adherence and retention in renal tubules [53,73]. This can be speculated that the inhibitory effect of Ov.Cr on calcium oxalate crystal deposition in renal tubules could have also been caused by its antioxidant activity.
In curative study, withdrawal of lithogenic treatment after 21 days evoked a spontaneous recovery of nephrolithic animals in the untreated groups. However, Ov.Cr enhanced the spontaneous recovery in the treated group than the untreated group, which was clearly shown by the gain in body weight, significant decrease in urinary oxalate, renal crystal deposition and improvement in renal functions compared to the lithogenic group.
Phytochemical screening revealed the presence of saponins, alkaloids, coumarins, sterol, terpenes, flavonoids and tannins. Different activities observed in the crude extract might be due to the presence of these phytochemicals. For example, flavonoids are known to possess antispasmodic and Ca++ channel blocking [74,75], antioxidant [76] and diuretic [77] activities. Saponins are known to possess anti-crystallization property by disaggregating the suspension of mucoproteins, promoters of crystallization [9]. However, the contribution of other phytochemicals accounting for the reported activities cannot be rule out.
The plant showed antiurolithic activity both in the in vitro and in vivo models in addition to its antioxidant, renal epithelial cell protective, antispasmodic and diuretic activities reported in this study, all of which could be beneficial in urolithiasis. The plant is also reported to possess anti-inflammatory [78] and antimicrobial [79] activities, which could also be supplementing its beneficial effect, as the infection and inflammation are likely to be associated with urolithiasis process. Thus, the herbal remedies known to contain multiple activities offer therapeutic potential particularly in the urolithiasis, where multiple targets are needed. A few studies on the effectiveness of herbal remedies in urolithiasis exists, such as Hernaira hirsute, Phylanthus niruri and Hibiscus sabdariffa, which showed promising results in the management of urolithiasis [8]. However, most of these studies were using the in vitro and/or in vivo studies with limited advancement in the possible mechanisms of their effectiveness. In this study, we used both the in vivo and in vitro models along with multiple activities, as mentioned above, giving comprehensive information on the pharmacological basis for its effectiveness in urolithiasis. The plant being of edible nature with a long history of medicinal use is considered to be relatively safe, however, detailed studies on its safety profile is needed before recommending for clinical use.
Conclusion
Together these data suggest that the presence of antiurolithic effect in Origanum vulgare against renal calcium oxalate crystal deposits is mediated possibly through a combination of CaOx crystal inhibitory, diuretic, antioxidant, antispasmodic, epithelial cell protective, hypocalciuric and hypercitrauric effects thus acting on multiple sites. This study rationalizes its medicinal use in the treatment of urolithiasis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AK carried out the draft, experimental work, data collection and evaluation, literature search and manuscript preparation. SB helped in the experimental study designing and corrected the manuscript for publication. SRK helped in the cell culture experiments and refined the manuscript for publication. AHG supervised the work and refined the manuscript for publication. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Chemicals and reagents. List of names and sources of the chemicals and reagents used in the study.
Table S1. Various parameters from different groups of rats recorded after 21 days, to study the preventive effect in animal model of urolithiasis
Table S2. Various parameters from different groups of rats, recorded after 21 and 35 days, to study the curative effect in animal model of urolithiasis
Contributor Information
Aslam Khan, Email: aslamkhan_mkd@yahoo.co.uk.
Samra Bashir, Email: samra.bashir@aku.edu.
Saeed R Khan, Email: khan@pathology.ufl.edu.
Anwar H Gilani, Email: anwar.gilani@aku.edu.
Acknowledgements
This study was supported by the Higher Education Commission (HEC) of Pakistan as (i) indigenous M.Phill/PhD and (ii) International Research Support Initiative Program (IRSIP) scholarships awarded to Aslam Khan.
References
- Lopez M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2008. [DOI] [PMC free article] [PubMed]
- Coe FL, Keck J, Norton ER. The natural history of calcium urolithiasis. JAMA. 1977;238(14):1519–1523. doi: 10.1001/jama.238.14.1519. [DOI] [PubMed] [Google Scholar]
- Bashir S, Gilani AH, Siddiqui AA, Pervez S, Khan SR, Sarfaraz NJ, Shah AJ. Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phytother Res. 2010;24(8):1250–1255. doi: 10.1002/ptr.3196. [DOI] [PubMed] [Google Scholar]
- Pak CY. Kidney stones. Lancet. 1998;351(9118):1797–1801. doi: 10.1016/S0140-6736(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367(9507):333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- Grover PK, Kim DS, Ryall RL. The effect of seed crystals of hydroxyapatite and brushite on the crystallization of calcium oxalate in undiluted human urine in vitro: implications for urinary stone pathogenesis. Mol Med. 2002;8(4):200–209. [PMC free article] [PubMed] [Google Scholar]
- Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst Rev. 2009. p. CD007044. [DOI] [PubMed]
- Butterweck V, Khan SR. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med. 2009;75(10):1095–1103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurocak S, Kupeli B. Consumption of historical and current phytotherapeutic agents for urolithiasis: a critical review. J Urol. 2006;176(2):450–455. doi: 10.1016/j.juro.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Hess B. [Pathophysiology, diagnosis and conservative therapy in calcium kidney calculi] Ther Umsch. 2003;60(2):79–87. doi: 10.1024/0040-5930.60.2.79. [DOI] [PubMed] [Google Scholar]
- Mattle D, Hess B. Preventive treatment of nephrolithiasis with alkali citrate--a critical review. Urol Res. 2005;33(2):73–79. doi: 10.1007/s00240-005-0464-8. [DOI] [PubMed] [Google Scholar]
- Gilani AH, Rahman AU. Trends in ethnopharmocology. J Ethnopharmacol. 2005;100(1-2):43–49. doi: 10.1016/j.jep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- Fleming T. PDR for herbal medicines. First. Medical Economics Company; 1998. [Google Scholar]
- Baquar SR. Medicinal and Poisonous Plants of Pakistan. Karachi: Printas; 1989. [Google Scholar]
- Usmanghani K, Saeed A, Alam MT. Indusyunic Medicine. Karachi: University of Karachi Press; 1997. [Google Scholar]
- Duke JA. Handbook of medicinal herbs. Boca Raton, LA: CRC Press; 2002. [Google Scholar]
- National Research Council, Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Williamson EM, Okpako DT, Evans FJ. Pharmacological methods in phytotherapy research: Selection, Preparation and Pharmacological Evaluation of Plant Material v.1. Chichester: John Wiley & Sons; 1996. [Google Scholar]
- Gilani AH, Mandukhail SU, Iqbal J, Yasinzai M, Aziz N, Khan A, Rehman NU. Antispasmodic and vasodilator activities of Morinda citrifolia root extract are mediated through blockade of voltage dependent calcium channels. BMC Complement Altern Med. 2010;10(1):2. doi: 10.1186/1472-6882-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani AH, Khan A, Khan AU, Bashir S, Rehman NU, Mandukhail SU. Pharmacological basis for the medicinal use of Holarrhena antidysenterica in gut motility disorders. Pharm Biol. 2010;48(11):1240–1246. doi: 10.3109/13880201003727960. [DOI] [PubMed] [Google Scholar]
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. [Google Scholar]
- Hess B, Meinhardt U, Zipperle L, Giovanoli R, Jaeger P. Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res. 1995;23(4):231–238. doi: 10.1007/BF00393304. [DOI] [PubMed] [Google Scholar]
- Ebisuno S, Komura T, Yamagiwa K, Ohkawa T. Urease-induced crystallizations of calcium phosphate and magnesium ammonium phosphate in synthetic urine and human urine. Urol Res. 1997;25(4):263–267. doi: 10.1007/BF00942096. [DOI] [PubMed] [Google Scholar]
- Guerra A, Meschi T, Allegri F, Schianchi T, Adorni G, Novarini A, Borghi L. Calcium oxalate crystallization in untreated urine, centrifuged and filtered urine and ultrafiltered urine. Clin Chem Lab Med. 2004;42(1):45–50. doi: 10.1515/CCLM.2004.009. [DOI] [PubMed] [Google Scholar]
- Huang DJ, Chen HJ, Hou WC, Lin CD, Lin YH. Active recombinant thioredoxin h protein with antioxidant activities from sweet potato (Ipomoea batatas [L.] Lam Tainong 57) storage roots. J Agric Food Chem. 2004;52(15):4720–4724. doi: 10.1021/jf0498618. [DOI] [PubMed] [Google Scholar]
- Ajith TA, Usha S, Nivitha V. Ascorbic acid and [alpha]-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta. 2007;375(1-2):82–86. doi: 10.1016/j.cca.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Borchert VE, Czyborra P, Fetscher C, Goepel M, Michel MC. Extracts from Rhois aromatica and Solidaginis virgaurea inhibit rat and human bladder contraction. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(3):281–286. doi: 10.1007/s00210-004-0869-x. [DOI] [PubMed] [Google Scholar]
- Farre AJ, Colombo M, Fort M, Gutierrez B. Differential effects of various Ca2+ antagonists. Gen Pharmacol. 1991;22(1):177–181. doi: 10.1016/0306-3623(91)90331-Y. [DOI] [PubMed] [Google Scholar]
- Van Rossum JM. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Consolini AE, Baldini OA, Amat AG. Pharmacological basis for the empirical use of Eugenia uniflora L. (Myrtaceae) as antihypertensive. J Ethnopharmacol. 1999;66(1):33–39. doi: 10.1016/S0378-8741(98)00194-9. [DOI] [PubMed] [Google Scholar]
- Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92(1):137–140. doi: 10.1046/j.1464-410X.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- Bashir S, Gilani AH. Antiurolithic effect of berberine is mediated through multiple pathways. Eur J Pharmacol. pp. 168–175. [DOI] [PubMed]
- Lemhadri A, Zeggwagh NA, Maghrani M, Jouad H, Eddouks M. Anti-hyperglycaemic activity of the aqueous extract of Origanum vulgare growing wild in Tafilalet region. J Ethnopharmacol. 2004;92(2-3):251–256. doi: 10.1016/j.jep.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Srihari T, Sengottuvelan M, Nalini N. Dose-dependent effect of oregano (Origanum vulgare L.) on lipid peroxidation and antioxidant status in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J Pharm Pharmacol. 2008;60(6):787–794. doi: 10.1211/jpp.60.6.0015. [DOI] [PubMed] [Google Scholar]
- Pizzolato P. Mercurous nitrate as a histochemical reagent for calcium phosphate in bone and pathological calcification and for calcium oxalate. Histochem J. 1971;3(6):463–469. doi: 10.1007/BF01014785. [DOI] [PubMed] [Google Scholar]
- Vanachayangkul P, Chow N, Khan SR, Butterweck V. Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urol Res. 2010. [DOI] [PMC free article] [PubMed]
- Daly JA, Ertingshausen G. Direct method for determining inorganic phosphate in serum with the "CentrifiChem". Clin Chem. 1972;18(3):263–265. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Tiselius HG. Epidemiology and medical management of stone disease. BJU Int. 2003;91(8):758–767. doi: 10.1046/j.1464-410X.2003.04208.x. [DOI] [PubMed] [Google Scholar]
- Guerra A, Meschi T, Allegri F, Prati B, Nouvenne A, Fiaccadori E, Borghi L. Concentrated urine and diluted urine: the effects of citrate and magnesium on the crystallization of calcium oxalate induced in vitro by an oxalate load. Urol Res. 2006;34(6):359–364. doi: 10.1007/s00240-006-0067-z. [DOI] [PubMed] [Google Scholar]
- Wesson JA, Worcester EM, Wiessner JH, Mandel NS, Kleinman JG. Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney international. 1998;53(4):952–957. doi: 10.1111/j.1523-1755.1998.00839.x. [DOI] [PubMed] [Google Scholar]
- Wesson JA, Ward MD. Role of crystal surface adhesion in kidney stone disease. Curr Opin Nephrol Hypertens. 2006;15(4):386–393. doi: 10.1097/01.mnh.0000232879.50716.6f. [DOI] [PubMed] [Google Scholar]
- Wang AY. Vascular and other tissue calcification in peritoneal dialysis patients. Perit Dial Int. 2009;29(Suppl 2):S9–S14. [PubMed] [Google Scholar]
- Khan SR. Animal models of kidney stone formation: an analysis. World J Urol. 1997;15(4):236–243. doi: 10.1007/BF01367661. [DOI] [PubMed] [Google Scholar]
- Marangella M, Bagnis C, Bruno M, Vitale C, Petrarulo M, Ramello A. Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol Int. 2004;72(Suppl 1):6–10. doi: 10.1159/000076583. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Vieira MA. Changes in calcium oxalate crystal morphology as a function of supersaturation. Int Braz J Urol. 2004;30(3):205–208. doi: 10.1590/s1677-55382004000300005. discussion 209. [DOI] [PubMed] [Google Scholar]
- Kato Y, Yamaguchi S, Yachiku S, Nakazono S, Hori J, Wada N, Hou K. Changes in urinary parameters after oral administration of potassium-sodium citrate and magnesium oxide to prevent urolithiasis. Urology. 2004;63(1):7–11. doi: 10.1016/j.urology.2003.09.057. discussion 11-12. [DOI] [PubMed] [Google Scholar]
- Aihara K, Byer KJ, Khan SR. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int. 2003;64(4):1283–1291. doi: 10.1046/j.1523-1755.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Escobar C, Byer KJ, Khaskheli H, Khan SR. Apatite induced renal epithelial injury: insight into the pathogenesis of kidney stones. J Urol. 2008;180(1):379–387. doi: 10.1016/j.juro.2008.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byer K, Khan SR. Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol. 2005;173(2):640–646. doi: 10.1097/01.ju.0000143190.49888.c7. [DOI] [PubMed] [Google Scholar]
- Santhosh Kumar M, Selvam R. Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem. 2003;14(6):306–313. doi: 10.1016/S0955-2863(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31(1):3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- Babich H. Butylated hydroxytoluene (BHT): A review. Environ Res. 1982;29(1):1–29. doi: 10.1016/0013-9351(82)90002-0. [DOI] [PubMed] [Google Scholar]
- Lin SP, Tsai SY, Lin YL, Kuo SC, Hou YC, Chao PD. Biotransformation and pharmacokinetics of 4-(3,4-dihydroxybenzoyloxymethyl)phenyl-O-beta-D-glucopyranoside, an antioxidant isolated from Origanum vulgare. J AgricFood Chem. 2008;56(8):2852–2856. doi: 10.1021/jf703730e. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Chiji H, Asakawa C, Amano M, Yoshihara T, Mizutani J. DPPH radical scavengers from dried leaves of oregano (Origanum vulgare) Biosci Biotechnol Biochem. 2003;67(11):2311–2316. doi: 10.1271/bbb.67.2311. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cruz E, Escartin P, Bootello A, Kreisler M, Segovia de Arana JM. Hepatocyte damage induced by lymphocytes from patients with chronic liver diseases, as detected by LDH release. Clin Exp Immunol. 1978;31(3):436–442. [PMC free article] [PubMed] [Google Scholar]
- Ohgaki K, Horiuchi K, Hikima N, Kondo Y. Facilitation of expulsion of ureteral stones by addition of alpha1-blockers to conservative therapy. Scand J Urol Nephrol. 2010;44(6):420–424. doi: 10.3109/00365599.2010.497769. [DOI] [PubMed] [Google Scholar]
- Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56(3):455–471. doi: 10.1016/j.eururo.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Bolton TB. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38(4):321–416. [PubMed] [Google Scholar]
- Brown JH Taylor P Muscarinic receptor agonists and antagonists Goodman and Gilman's manual of pharmacology and therapeutics 200611New Yark: McGraw-Hill; 183–200.21204365 [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annual Review PharmacolToxicol. 1977;17(1):149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Dasaeva LA, Shilov EM, Shatokhina SN. [Diuress for the treatment of early stages of urolithiasis] Klin Med (Mosk) 2003;81(10):50–52. [PubMed] [Google Scholar]
- Goldfarb DS, Coe FL. Oxford textbook of clinical nephrology. New Yark: Oxford University Press; 2005. In A.M. Davison, J.S. Cameron, E. Ritz, J. Grünfeld, C.G. Winearls, C. Ponticelli & C.V. The medical management of stone disease; pp. 1199–1279. [Google Scholar]
- Tsai CH, Chen YC, Chen LD, Pan TC, Ho CY, Lai MT, Tsai FJ, Chen WC. A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res. 2008;36(1):17–24. doi: 10.1007/s00240-007-0122-4. [DOI] [PubMed] [Google Scholar]
- Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. pp. 1013–1018. [DOI] [PubMed]
- Bashir S, Gilani AH. Antiurolithic effect of berberine is mediated through multiple pathways. Eur J Pharmacol. 2011;651(1-3):168–175. doi: 10.1016/j.ejphar.2010.10.076. [DOI] [PubMed] [Google Scholar]
- Li Y, McMartin KE. Strain differences in urinary factors that promote calcium oxalate crystal formation in the kidneys of ethylene glycol-treated rats. Am J Physiol Renal Physiol. 2009;296(5):F1080–1087. doi: 10.1152/ajprenal.90727.2008. [DOI] [PubMed] [Google Scholar]
- Fan J, Glass MA, Chandhoke PS. Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Scanning Microsc. 1999;13(2-3):299–306. [Google Scholar]
- Park HK, Jeong BC, Sung MK, Park MY, Choi EY, Kim BS, Kim HH, Kim JI. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol. 2008;179(4):1620–1626. doi: 10.1016/j.juro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Santhosh Kumar M, Selvam R. Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. JNut Biochem. 2003;14(6):306–313. doi: 10.1016/s0955-2863(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int. 2001;59(2):637–644. doi: 10.1046/j.1523-1755.2001.059002637.x. [DOI] [PubMed] [Google Scholar]
- Revuelta MP, Cantabrana B, Hidalgo A. Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen Pharmacol. 1997;29(5):847–857. doi: 10.1016/S0306-3623(97)00002-5. [DOI] [PubMed] [Google Scholar]
- Pietta P. In: Flavonoids in Health and Disease. Rive-Evans CV, Packer L, editor. New York: Marcel Dekker; 1998. Flavonoids in medicinal plants; pp. 61–110. [Google Scholar]
- Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy J, Venkataraman S, Meera R, Chiristina AJM, Chidambaranathan N. Physio-Phytochemical screening and Diuretic activity of leaves of Pavetta indica Linn. J Pharm Sci Res. 2010;2(8):506–512. [Google Scholar]
- Ocana-Fuentes A, Arranz-Gutierrez E, Senorans FJ, Reglero G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem Toxicol. 2010;48(6):1568–1575. doi: 10.1016/j.fct.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Sarac N, Ugur A. Antimicrobial activities of the essential oils of Origanum onites L., Origanum vulgare L. subspecies hirtum (Link) Ietswaart, Satureja thymbra L., and Thymus cilicicus Boiss. & Bal. growing wild in Turkey. J Med Food. 2008;11(3):568–573. doi: 10.1089/jmf.2007.0520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemicals and reagents. List of names and sources of the chemicals and reagents used in the study.
Table S1. Various parameters from different groups of rats recorded after 21 days, to study the preventive effect in animal model of urolithiasis
Table S2. Various parameters from different groups of rats, recorded after 21 and 35 days, to study the curative effect in animal model of urolithiasis


