Abstract
Background
Promising results have been reported for a urine circulating cathodic antigen (CCA) test for the diagnosis of Schistosoma mansoni. We assessed the accuracy of a commercially available CCA cassette test (designated CCA-A) and an experimental formulation (CCA-B) for S. mansoni diagnosis.
Methodology
We conducted a cross-sectional survey in three settings of Côte d'Ivoire: settings A and B are endemic for S. mansoni, whereas S. haematobium co-exists in setting C. Overall, 446 children, aged 8–12 years, submitted multiple stool and urine samples. For S. mansoni diagnosis, stool samples were examined with triplicate Kato-Katz, whereas urine samples were tested with CCA-A. The first stool and urine samples were additionally subjected to an ether-concentration technique and CCA-B, respectively. Urine samples were examined for S. haematobium using a filtration method, and for microhematuria using Hemastix dipsticks.
Principal Findings
Considering nine Kato-Katz as diagnostic ‘gold’ standard, the prevalence of S. mansoni in setting A, B and C was 32.9%, 53.1% and 91.8%, respectively. The sensitivity of triplicate Kato-Katz from the first stool and a single CCA-A test was 47.9% and 56.3% (setting A), 73.9% and 69.6% (setting B), and 94.2% and 89.6% (setting C). The respective sensitivity of a single CCA-B was 10.4%, 29.9% and 75.0%. The ether-concentration technique showed a low sensitivity for S. mansoni diagnosis (8.3–41.0%). The specificity of CCA-A was moderate (76.9–84.2%); CCA-B was high (96.7–100%). The likelihood of a CCA-A color reaction increased with higher S. mansoni fecal egg counts (odds ratio: 1.07, p<0.001). A concurrent S. haematobium infection or the presence of microhematuria did not influence the CCA-A test results for S. mansoni diagnosis.
Conclusion/Significance
CCA-A showed similar sensitivity than triplicate Kato-Katz for S. mansoni diagnosis with no cross-reactivity to S. haematobium and microhematuria. The low sensitivity of CCA-B in our study area precludes its use for S. mansoni diagnosis.
Author Summary
We aimed to assess the accuracy of a commercially available rapid diagnostic test for the detection of an infection with the blood fluke Schistosoma mansoni in urine. In total, 446 school children from three different settings of south Côte d'Ivoire provided three stool and three urine samples. Stool samples were examined with the widely used Kato-Katz technique and analyzed with a microscope for S. mansoni eggs. Urine samples were examined with a filtration method for S. haematobium eggs and with a rapid diagnostic test for S. mansoni that is based on detecting circulating cathodic antigens (CCA). We used a commercially available test (designated CCA-A) and an experimental formulation (CCA-B). Examination of nine Kato-Katz thick smears per child revealed a prevalence of S. mansoni in the three settings of 32.9%, 53.1%, and 91.8%. The sensitivity of triplicate Kato-Katz from the first stool sample was comparable to a single CCA-A (47.9–94.2% vs. 56.3–89.6%), and significantly higher than the sensitivity of a single CCA-B test (10.4–75.0%). CCA-A showed a considerably lower specificity than CCA-B (76.9–84.2% vs. 96.7–100%). In the settings studied in south Côte d'Ivoire, the CCA-A test holds promise for the diagnosis of S. mansoni, whereas results with CCA-B were suboptimal.
Introduction
There is growing awareness, political commitment, and financial resources to control neglected tropical diseases (NTDs) [1]–[3]. Preventive chemotherapy, that is the repeated large-scale administration of drugs to at-risk populations, has become the key strategy for the control of several NTDs, including schistosomiasis [3]–[5]. Although the issue of diagnosis has received only token attention in the current era of preventive chemotherapy, its importance must be emphasized for rapid identification of high-risk communities warranting regular treatment, appraisal of drug efficacy, monitoring progress of control interventions, and improved patient management [6]–[8]. With regard to intestinal schistosomiasis due to Schistosoma mansoni and S. japonicum, the Kato-Katz technique is the most widely used diagnostic approach in epidemiological surveys [8], [9]. Although the Kato-Katz technique is relatively simple to perform, it requires a minimum of equipment (i.e., microscope, chemicals, and test kit material) and well-trained laboratory technicians [10]. Moreover, a shortcoming of the Kato-Katz technique is the only low-to-moderate sensitivity for S. mansoni diagnosis in low endemicity areas [11]–[13]. Hence, multiple Kato-Katz thick smears are required to enhance sensitivity [14], but this poses operational challenges and strains financial resources.
The detection of circulating antigen of S. mansoni in urine has been suggested as an alternative to the Kato-Katz technique [15]–[17]. Indeed, both circulating anodic antigen (CAA) and circulating cathodic antigen (CCA) can be detected in sera and urine of individuals infected with S. mansoni [18]. Both antigen-detecting assays are sensitive and specific and correlate with the presence and intensity of infection [19]. Antigen detection in urine using a rapid diagnostic test (RDT) based on an enzyme-linked immunosorbent assay (ELISA) technique is potentially useful and non-invasive and could change the management of infected individuals, particularly at the peripheral level in endemic countries where microscopes and qualified laboratory technicians are often not available [6], [7]. A point-of-contact (POC) CCA urine test has been developed for the diagnosis of S. mansoni [15], which is now commercially available as a RDT in cassette form. In view of promising results obtained thus far [17], [20], [21], the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) initiated a multi-country study to assess the accuracy of a commercially available CCA cassette test for the diagnosis of S. mansoni.
The study reported here is part of this multi-country evaluation. We assessed the accuracy of a commercially available urine CCA cassette test (designated CCA-A) for S. mansoni diagnosis. Additionally, we employed an experimental formulation of the test (CCA-B). Nine Kato-Katz thick smears from each participant served as diagnostic ‘gold’ standard. In addition, our team employed the ether-concentration method on sodium acetate-acetic acid-formalin (SAF)-fixed stool samples for the diagnosis of S. mansoni, urine filtration for the identification of S. haematobium eggs, and Hemastix dipsticks for the detection of microhematuria in urine. The study was carried out in south Côte d'Ivoire, in three settings where S. mansoni is endemic at different levels, whereas S. haematobium co-exists in one of the settings.
Methods
Ethics Statement
The study protocol was approved by the institutional research commission of the Swiss Tropical and Public Health Institute (Basel, Switzerland) and was cleared by the ethics committees of Basel (EKBB; reference no. 377/09) and Côte d'Ivoire (reference no. 1993 MSHP/CNER). District health and education authorities, village chiefs, parents/legal guardians, and participating children were informed about the purpose and procedures of the study. Parents/legal guardians provided written informed consent for their children to participate. Additionally, all children assented orally. Participation was voluntary and children could withdraw at any time without further obligation. All parasitological results were coded and treated confidentially. At the end of the study, children attending the schools involved in this study were treated with praziquantel (single 40 mg/kg oral dose) and albendazole (single 400 mg oral dose) free of charge, irrespective of the child's helminth infection status [22].
Study Area and Population
In October/November 2010, we carried out a cross-sectional survey in three epidemiological settings in the district of Azaguié, south Côte d'Ivoire. Azaguié is located approximately 40 km north of Abidjan, the economic capital of Côte d'Ivoire. The settings were selected after a pre-screening done in 10 schools. For the pre-screening, in each school, 25 children were randomly selected. All children attending grades 3–5 (CE1, CE2, and CM1) were given a unique number, lots including all numbers were closed and placed in a box, and finally 25 lots per school were drawn. The selected children provided a single stool and a single urine sample, which were examined for S. mansoni with triplicate Kato-Katz thick smears and S. haematobium with a single filtration, respectively. Based on this pre-screening, we selected the following sites, according to SCORE guidelines: setting A, low S. mansoni endemicity (i.e., prevalence: 10–24%); setting B, moderate S. mansoni endemicity (prevalence: 25–49%); and setting C, co-endemic for S. mansoni and S. haematobium.
Sample Size
According to the literature, a single Kato-Katz thick smear for diagnosis of S. mansoni in low endemicity settings has a sensitivity of only 20–30% [23], [24]. However, since our study was to be carried out in both low and moderate endemicity settings, we assumed that a single Kato-Katz thick smear has a maximum sensitivity of 60%. The sensitivity of the CCA test is reported to be 80% or higher [15], [25]. Using these sensitivity estimates, a significance level of 5%, and a power of 80%, our sample size of complying children was calculated at 90. Assuming a compliance of 70% for the submission of each of three requested stool samples, the number of children to be included in each study setting was at least 199. To achieve this sample size, we selected by computer-based randomization 220 children aged 8–12 years from readily available school lists of Abbé-Begnini (setting A), Azaguié Gare (setting B), and M'Bromé/Makouguié (setting C).
Field Procedures
The purpose and procedures of the study were explained to the village authorities, the school directors, and the teachers of the selected schools. Teachers were invited to prepare class lists, including names, sex, and age of the children attending grades 3–5. Next, the study was explained to the children in lay terms and they were provided with an information and consent sheet with further details of the study and children and parents' rights. Children who submitted a written informed consent from their parents/guardians and assented orally themselves were given a 125 ml plastic container labeled with a unique identifier (ID). Children were invited to return the containers filled with a fresh lime-sized morning stool sample the following day. Upon collection of the filled container, a new empty container was handed out for stool collection on the next day. This procedure was repeated over a week until most children had submitted a total of three stool samples. Each day, between 10:00 and 12:00 hours, participating children were provided with another empty container labeled with the respective ID for collection of urine samples.
Laboratory Procedures
Stool and urine samples were transferred to a laboratory at the Université de Cocody and processed the same day. From each stool sample, triplicate Kato-Katz thick smears were prepared, using 41.7 mg templates, following standard protocols [9]. In brief, triplicate Kato-Katz thick smears were prepared on microscope slides, labeled with a child's ID plus letter A, B, or C. Slides were allowed to clear for at least 30 min before quantitative examination under a microscope by experienced laboratory technicians. The number of S. mansoni and other helminth eggs (e.g., Ascaris lumbricoides, hookworm, and Trichuris trichiura) was counted and recorded for each species separately. For quality control, 10% of the Kato-Katz thick smears were re-examined by a senior technician.
In addition, from the second day stool sample, ∼1 g of feces was weighed into plastic vials containing 10 ml of a SAF solution. Within 8 weeks, the SAF-fixed stool samples were processed with the ether-concentration method, following a standard protocol [12], [26]. In brief, the stool-SAF solution was rigorously shaken and then poured through medical gauze placed on a plastic funnel into a conical glass tube. The conical tubes were centrifuged for 1 min at 500× g. Subsequently, the supernatant was discarded and 7 ml of 0.85% sodium chloride (NaCl) solution and 2–3 ml ether were added to the pellet. Tubes were closed with a rubber stopper, manually shaken for ∼30 sec and then centrifuged for 5 min at 500× g. This procedure leads to the separation of the suspension in four layers. The three top layers were discarded and the complete sediment layer was placed on a microscope slide, covered with a slip and subsequently examined under a microscope for helminth eggs (i.e., S. mansoni and soil-transmitted helminths) and intestinal protozoon cysts.
All urine samples were subjected to CCA-A (batch 32727) on the day of sample collection. The first urine sample was additionally subjected to CCA-B (batch 32686). Both CCA urine cassette assays were obtained from Rapid Medical Diagnostics (Pretoria, South Africa) and performed at ambient temperature, following the manufacturer's instructions. Briefly, one drop of urine was added to the well of the testing cassette and allowed to absorb. Once fully absorbed, one drop of buffer (provided with the CCA test kits) was added. The test results were read 20 min after adding the buffer. In case the control bands did not develop, the test was considered as invalid. Valid tests were scored as either negative or positive, the latter further stratified into 1+, 2+, or 3+ according to the visibility of the color reaction. All tests were read independently by two blinded investigators and in case of discordant results discussed with a third independent investigator until agreement was reached.
In addition to the CCA cassettes, each urine sample was subjected to a filtration method for S. haematobium egg counts and to a Hemastix dipstick (Siemens Healthcare Diagnostics GmbH; Eschborn, Germany) for microhematuria assessment on the day of sample collection. In brief, samples were shaken, and 10 ml of urine filtered through a 13-mm diameter small meshed filter (20 µm; Sefar AG; Heiden, Switzerland), which was then placed on a labeled slide and examined under a microscope for S. haematobium eggs [8]. For appraisal of microhematuria, a Hemastix dipstick was soaked in urine, left in the open air for 1 min, before scoring according to the manufacturer's instructions.
Statistical Analysis
Data were entered twice in a Microsoft Excel spreadsheet, transferred in EpiInfo version 6.4 (Centers for Disease Control and Prevention; Atlanta, GA, USA) and validated. Statistical analyses were done with STATA version 10 (Stata Corp.; College Station, TX, USA).
Only those children who had complete data records were included in the final analysis (i.e., nine Kato-Katz thick smears, a single ether-concentration, three CCA-A, one CCA-B, three urine filtrations, and three Hemastix dipsticks). To obtain a standardized measure of infection intensity, expressed as eggs per gram of stool (EPG), for each individual, we calculated the arithmetic mean S. mansoni fecal egg counts (FECs) from the nine Kato-Katz thick smears and multiplied by a factor 24. Infection intensity of S. mansoni was classified into light (1–99 EPG), moderate (100–399 EPG), and heavy (≥400 EPG). Egg counts of S. haematobium were utilized to stratify into light (1–49 eggs/10 ml of urine) and heavy infection intensities (≥50 eggs/10 ml of urine) [4].
The strength of agreement between nine Kato-Katz thick smears and triplicate CCA-A, one CCA-B, and one ether-concentration for each endemicity setting was assessed by kappa statistics (κ), as follows: κ<0 indicating no agreement, κ = 0–0.2 indicating poor agreement, κ = 0.21–0.4 indicating fair agreement, κ = 0.41–0.6 indicating moderate agreement, κ = 0.61–0.8 indicating substantial agreement, and κ = 0.81–1.0 indicating almost perfect agreement [27], [28].
As proposed by the SCORE secretariat, the results from nine Kato-Katz thick smears were considered our ‘gold’ standard. We determined the sensitivity (proportion of true-positives detected by the test) and specificity (proportion of true-negatives detected by the test) of single and multiple tests. As with some of our previous work, we used a second ‘gold’ standard by considering a positive test result (regardless of the test) as true-positive [29], [30]. Hence, we combined results from all tests (i.e., nine Kato-Katz thick smears plus triplicate CCA-A, one CCA-B, and one ether-concentration) and therefore maximized specificity.
We employed an ordinal logistic regression approach, which is an extension of the general linear model to ordinal categorical outcomes to assess the correlation between CCA-A and CCA-B color reaction categories and S. mansoni FECs. The arithmetic mean FEC of three Kato-Katz thick smears per stool sample per day served as continuous explanatory variable, whereas the color reaction of the CCA test was considered as categorical outcome. This statistical procedure was also used to compare between the CCA test results considered as categorical outcome, and different infection intensity categories of S. mansoni (i.e., light, moderate, and heavy) utilized as categorical explanatory variables.
A logistic regression was performed to assess the association between CCA-A and CCA-B test results, expressed as binary outcome variable (negative/positive) with S. haematobium egg count as continuous explanatory variable and mircohematuria as categorical explanatory variable among children without a S. mansoni infection.
Non-overlapping 95% confidence intervals (CI) or p-values≤0.05 were considered as statistical significance.
Results
Study Adherence
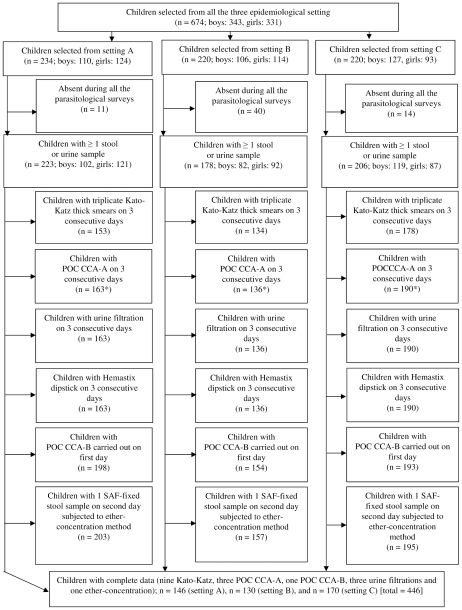
Figure 1 shows the adherence of school children to provide multiple stool and urine samples for a suite of diagnostic tests for detection of S. mansoni and S. haematobium infection. Overall, 674 school children aged 8–12 years were enrolled with slightly more boys than girls (343 vs. 331). The number of children in settings A, B and C was 234, 220 and 220, respectively. At least one stool or one urine sample was provided by 223, 178 and 206 children in settings A, B and C, respectively. Overall, 465 children submitted three stool samples, which were subjected to triplicate Kato-Katz thick smears. Results from a single ether-concentration method were available for 555 children. Three CCA-A test results were available for 489 children, whereas 545 children had the first urine sample additionally subjected to a CCA-B test. Finally, three urine filtrations for S. haematobium diagnosis and three Hemastix dipstick tests for appraisal of microhematuria were done for 489 children.
Figure 1. STARD-flowchart showing study participation, stratified by epidemiological setting.
Flowchart detailing the study participation and adherence of children for multiple stool and urine submissions for diagnosis of S. mansoni and S. haematobium infections in Azaguié, south Côte d'Ivoire, in October and November 2010. According to nine Kato-Katz thick smear examinations, the prevalence of S. mansoni in setting A, B and C was 32.9%, 53.1% and 91.8%, respectively. Of note, S. haematobium is co-endemic in setting C.
Results on three stool samples (examined with nine Kato-Katz thick smears and a single ether-concentration) and three urine samples (examined with three CCA-A, one CCA-B, three urine filtrations and three Hemastix dipsticks) were available from a total of 446 children. Among them 48.7% (n = 217) were boys and the median age of the cohort was 10 years. All further analysis focused on this cohort of children.
S. mansoni and S. haematobium Infection
Table 1 shows the number of children examined and those positive for S. mansoni and S. haematobium, as assessed by different diagnostic approaches, stratified by study setting.
Table 1. Prevalence of S. mansoni and S. haematobium according to different diagnostic approaches, stratified by epidemiological setting.
| Diagnostic approach | Setting A | Setting B | Setting C | ||||||
| No. of children tested | No. of children positive | % positive (CI*) | No. of children tested | No. of children positive | % positive (CI*) | No. of children tested | No. of children positive | % positive (CI*) | |
| S. mansoni diagnosis | |||||||||
| Nine Kato-Katz thick smears | 146 | 48 | 32.9 (25.2–40.6) | 130 | 69 | 53.1 (44.4–61.8) | 170 | 156 | 91.8 (87.6–95.9) |
| Three CCA-A** | 146 | 49 | 33.8 (26.0–41.6) | 130 | 60 | 48.8 (39.8–57.7) | 170 | 141 | 85.9 (80.6–91.3) |
| One CCA-B* | 146 | 5 | 3.4 (0.3–4.7) | 130 | 22 | 17.2 (10.6–23.8) | 170 | 117 | 68.8 (61.8–75.9) |
| One ether-concentration | 146 | 6 | 4.1 (0.8–7.4) | 130 | 9 | 6.9 (2.5–11.3) | 170 | 65 | 38.2 (30.8–45.6) |
| S. haematobium diagnosis | |||||||||
| Three Hemastix dipsticks (excluding trace results) | 113 | 6 | 5.3 (1.0–9.5) | 86 | 5 | 6.7 (1.4–12.1) | 92 | 41 | 44.6 (32.2–54.9) |
| Three urine filtrations | 146 | 6 | 4.1 (0.8–7.4) | 130 | 1 | 0.8 (0.0–2.2) | 170 | 111 | 65.3 (58.1–72.1) |
The study was carried out in three epidemiological settings of south Côte d'Ivoire in October and November 2010. Triplicate Kato-Katz thick smears from the first collected stool sample, nine Kato-Katz thick smears from three stool samples, one CCA-A from the first collected urine sample, three CCA-A tests from three urine samples, one CCA-B test from the first collected urine sample, and one ether-concentration test on SAF-fixed stool samples from the second collected stool sample were used for the diagnosis of S. mansoni. Three urine filtrations were employed for S. haematobium diagnosis and three Hemastix dipsticks were used for microhematuria appraisal.
*Exact 95% confidence interval.
**In settings A, B and C, there were 2, 6 and 7 tests considered invalid, and hence not taken into account for prevalence calculations.
Kato-Katz technique and ether-concentration method
In setting A, B, and C, according to nine Kato-Katz thick smears examined per child, the observed prevalence of S. mansoni was 32.9%, 53.1% and 91.8%, respectively. In settings A and B, most of the infections were of light intensity (96.2% and 68.1%, respectively), whereas in setting C, three-quarter of the children had moderate or heavy infection intensities (76.2%). The lowest arithmetic mean FEC was found in setting A (17.4 EPG, 95% CI: 0–38.9 EPG) and the highest arithmetic mean FEC in setting C (482.8 EPG, 95% CI: 388.1–577.4 EPG). In setting B, the arithmetic mean FEC was 62.4 EPG (95% CI: 36.2–88.5 EPG).
Considerably lower S. mansoni prevalence estimates were obtained after subjecting a single stool sample to an ether-concentration method, 4.1%, 6.9% and 38.2% in setting A, B and C, respectively.
CCA test results
In setting A, B, and C, the respective prevalence of S. mansoni based on triplicate CCA-A tests were 33.8%, 48.8%, and 85.9%. Out of the 570 CCA-A tests performed on three consecutive days in setting A, a total of 141 showed a positive color reaction, most of which were classified as 1+ (n = 104, 73.8%). Reactions of 2+ and 3+ were seen in 24 (17.0%) and 13 (9.2%) of the tests, respectively. In setting B, 460 CCA-A tests were performed. Overall, 169 tests showed a positive reaction: 99 were judged 1+ (58.6%), 44 considered 2+ (26.0%) and the remaining 26 as 3+ (15.4%). Finally, in setting C, 76 (15.8%), 150 (31.1%) and 256 (53.1%) of the CCA-A tests performed were classified as 1+, 2+ and 3+, respectively.
The prevalence of S. mansoni according to a single CCA-B in setting A, B and C was 3.4%, 17.2% and 69.8%, respectively. In setting A, the five positive CCA-B tests were all judged 1+. In setting B, one CCA-B test result was considered 2+, whereas the remaining 21 test results were classified as 1+. Finally, in setting C, there were 116 CCA-B test results considered as 1+ (87.2%) and 17 as 2+ (12.8%).
Urine filtration and Hemastix results
In settings A, B and C, according to triplicate urine filtrations, the prevalence of S. haematobium was 4.1%, 0.8% and 65.3% in setting A, B and C, respectively. With regard to Hemastix dipstick results, after exclusion of trace results, the prevalence of microhematuria in settings A, B and C was 5.3%, 6.7% and 44.6%. Traces of microhematuria were additionally found in 33, 34 and 78 urines tested in setting A, B and C, respectively.
Association between Kato-Katz and CCA Test Results
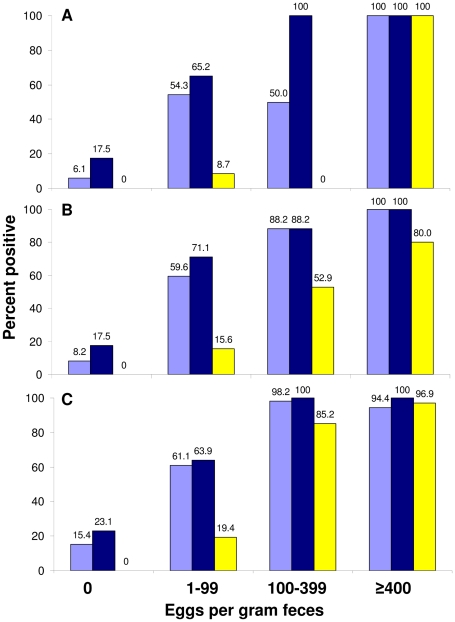
As indicated in Table 2, our ordinal logistic regression analysis showed that for an increase of S. mansoni infection intensity by 1 EPG, the likelihood of a stronger color reaction of the CCA-A (odds ratio (OR) = 1.07) and the CCA-B (OR = 1.03) is significant (both p<0.001). When S. mansoni FECs were not considered as continuous, but stratified according to pre-set thresholds into no, light, moderate and heavy infection intensity, we found that for each increase in infection intensity category, the likelihood of a stronger color reaction of both CCA-A (OR = 36.5) and CCA-B (OR = 25.2) is highly significant (both p<0.001). Figure 2 shows the correlation between infection intensity classes according to pre-set thresholds [22] and the percentage of infected individuals as determined by a single or triplicate CCA-A and a single CCA-B.
Table 2. Correlation of CCA test with schistosome infection and microhematuria (n = 526).
| Test | Association | Adjusted OR (95% CI) | P-value |
| CCA-A | |||
| (color categories) | S. mansoni egg count | 1.07 (1.05, 1.09) | <0.001 |
| S. mansoni infection categories | 36.50 (27.35, 48.77) | <0.001 | |
| CCA-A (pos/neg) | S. haematobium egg count | 1.09 (0.97, 1.21) | 0.121 |
| Hematuria trace | 0.39 (0.09, 1.64) | 0.200 | |
| Hematuria moderate (2+) | 1.09 (0.17, 7.00) | 0.923 | |
| Hematuria heavy (3+) | 0.90 (0.12, 6.99) | 0.195 | |
| CCA-B | |||
| (color categories) | S. mansoni egg count | 1.03 (1.01, 1.04) | <0.001 |
| S. mansoni infection categories | 25.20 (15.83, 39.95) | <0.001 | |
| CCA-B (pos/neg) | S. haematobium egg count | NA* | |
| Hematuria trace | NA* | ||
| Hematuria moderate (2+) | NA* | ||
| Hematuria heavy (3+) | NA* |
*NA: not applicable due to the small number of children without S. mansoni infection.
Ordinal logistic regression was used to assess the correlation between CCA test categories (0, 1+, 2+, and 3+) as outcome and S. mansoni egg count or S. mansoni infection categories (low, moderate, and heavy) as explanatory variable. Category “low” was used as baseline for comparison of other categories.
Logistic regression was applied to assess the correlation between CCA test results expressed as binary variables (positive/negative) and S. haematobium egg counts and microhematuria categories (trace, 1+, 2+, and 3+). Category “1+” was used as baseline for comparison of other categories.
Figure 2. Correlation between Kato-Katz and CCA for S. mansoni diagnosis.
Figure showing the correlation between the prevalence and intensity (stratified by intensity class) of S. mansoni infections, as determined by a single or triplicate CCA-A (light blue and dark blue bar, respectively), and a single CCA-B (yellow bar), stratified by study setting. According to nine Kato-Katz thick smear examinations, the prevalence of S. mansoni in setting A, B and C was 32.9%, 53.1% and 91.8%, respectively. In setting C, S. haematobium is co-endemic.
Diagnosis Accuracy of Different Tests
Agreement of the diagnostic assays
Table 3 shows the agreement between different diagnostic approaches and our first ‘gold’ standard (i.e., nine Kato-Katz thick smears derived from three stool samples) for the diagnosis of S. mansoni, stratified by study setting. The agreement between nine Kato-Katz thick smears and (i) a single Kato-Katz from the first stool sample was fair in setting A (κ = 0.31) and moderate in settings B and C (κ = 0.44–0.45), (ii) triplicate Kato-Katz from a single stool sample was moderate in settings A (κ = 0.55), and substantial in settings B and C (κ = 0.73), (iii) a single CCA-A was moderate in settings A, B and C (κ = 0.49–0.60), (iv) three CCA-A tests was moderate in settings A and C ( κ = 0.49–0.51) and substantial in setting B (κ = 0.61), (v) a single CCA-B was poor in setting A (κ = 0.14) and fair in settings B and C (κ = 0.26–0.33), and (vi) a single ether-concentration method was poor for all three settings (κ = 0.08–0.12).
Table 3. Agreement between different techniques for the diagnosis of S. mansoni.
| Diagnostic approach | Test result | Setting A | Setting B | Setting C | |||||||||
| Nine Kato-Katz thick smears | Nine Kato-Katz thick smears | Nine Kato-Katz thick smears | |||||||||||
| Positive | Negative | K* | P-values | Positive | Negative | K* | P-values | Positive | Negative | K* | P-values | ||
| One Kato-Katz thick smear | Positive | 12 | 0 | 32 | 0 | 129 | 0 | ||||||
| Negative | 36 | 98 | 0.31 | <0.001 | 37 | 61 | 0.45 | <0.001 | 27 | 14 | 0.44 | <0.001 | |
| Three Kato-Katz thick smears | Positive | 23 | 0 | 51 | 0 | 147 | 0 | ||||||
| Negative | 25 | 98 | 0.55 | <0.001 | 18 | 61 | 0.73 | <0.001 | 9 | 14 | 0.73 | <0.001 | |
| One CCA-A | Positive | 27 | 6 | 48 | 5 | 138 | 2 | ||||||
| Negative | 21 | 92 | 0.54 | <0.001 | 21 | 56 | 0.60 | <0.001 | 16 | 11 | 0.49 | <0.001 | |
| Three CCA-A | Positive | 32 | 17 | 51 | 9 | 138 | 3 | ||||||
| Negative | 16 | 80 | 0.49 | <0.001 | 15 | 48 | 0.61 | <0.001 | 13 | 10 | 0.51 | <0.001 | |
| One CCA-B | Positive | 5 | 0 | 20 | 2 | 117 | 0 | ||||||
| Negative | 43 | 98 | 0.14 | 0.001 | 47 | 59 | 0.26 | <0.001 | 39 | 14 | 0.33 | <0.001 | |
| One ether-concentration | Positive | 4 | 2 | 9 | 0 | 64 | 1 | ||||||
| Negative | 44 | 96 | 0.08 | 0.044 | 60 | 61 | 0.12 | 0.002 | 92 | 13 | 0.09 | 0.008 | |
The study was carried out in three settings in south Côte d'Ivoire in October and November 2010. The κ-agreement of a single Kato-Katz thick smear from the first collected stool sample, triplicate Kato-Katz thick smears from the first collected stool sample, one CCA-A from the first collected urine sample, three CCA-A tests from three collected urine samples, one CCA-B test from the first collected urine sample, and one ether-concentration test on SAF-fixed stool samples from the second collected stool sample versus our diagnostic ‘gold’ standard of nine Kato-Katz thick smears (triplicate Kato-Katz thick smears from each of three stool samples) for the diagnosis of S. mansoni was calculated.
Sensitivity and specificity of the diagnostic techniques
The sensitivity and specificity of the different diagnostic tests were determined for each of our two ‘gold’ standards (Table 4). In setting A, if the combined results of nine Kato-Katz thick smears were used as ‘gold’ standard, very low sensitivities of only 8.3% for a single ether-concentration test and 10.4% for a single CCA-B were determined. The highest sensitivity was revealed for triplicate CCA-A tests (66.7%), followed by duplicate (60.4%) and a single CCA-A test (56.3%).
Table 4. Sensitivity and specificity of different tests for the diagnosis of S. mansoni.
| Setting A | Setting B | Setting C | ||||
| Nine Kato-Katz thick smears as ‘gold’ standard | Sensitivity, % (CI*) | Specificity, % (CI*) | Sensitivity, % (CI*) | Specificity, % (CI*) | Sensitivity, % (CI*) | Specificity, % (CI*) |
| Single Kato-Katz | 25.0 (13.6, 39.6) | 100 (96.3, 100) | 46.4 (34.3, 58.8) | 100 (94.1, 100) | 82.7 (75.8, 88.3) | 100 (76.8, 100) |
| Duplicate Kato-Katz | 29.2 (17.0, 44.1) | 100 (96.3, 100) | 55.1 (42.6, 67.1) | 100 (94.1, 100) | 86.5 (80.2, 91.5) | 100 (76.8, 100) |
| Triplicate Kato-Katz | 47.9 (33.3, 62.8) | 100 (96.3, 100) | 73.9 (61.9, 83.7) | 100 (94.1, 100) | 94.2 (89.3, 97.3) | 100 (76.8, 100) |
| Single CCA-A | 56.3 (41.2, 70.5) | 93.9 (87.1, 97.7) | 69.6 (57.3, 80.1) | 91.8 (81.9, 97.3) | 89.6 (83.5, 93.7) | 84.6 (54.6, 98.6) |
| Duplicate CCA-A | 60.4 (45.3, 74.2) | 88.7 (80.6, 94.2) | 77.6 (65.8, 86.9) | 84.2 (72.1, 92.5) | 91.5 (85.7, 95.3) | 76.9 (46.2, 95.0) |
| Triplicate CCA-A | 66.7 (51.6, 79.6) | 82.5 (73.4, 89.4) | 77.3 (65.3, 86.7) | 84.2 (72.1, 92.5) | 91.4 (85.7, 95.3) | 76.9 (46.9, 95.0) |
| Single CCA-B | 10.4 (3.4, 22.7) | 100 (96.3, 100) | 29.9 (19.3, 42.3) | 96.7 (88.7, 100) | 75.0 (67.4, 81.6) | 100 (76.8, 100) |
| Single ether-concentration | 8.3 (2.3, 20.0) | 98.0 (92.9, 99.8) | 13.0 (6.1, 23.3) | 100 (94.1, 100) | 41.0 (33.2, 49.2) | 92.9 (66.1, 99.8) |
| Combined results as ‘gold’ standard | ||||||
| Single Kato-Katz | 17.9 (9.6, 29.2) | 100 (95.4, 100) | 40.0 (28.9, 52.0) | 100 (92.3, 100) | 80.5 (73.4, 86.5) | 100 (69.2, 100) |
| Duplicate Kato-Katz | 20.9 (11.9, 32.6) | 100 (95.4, 100) | 48.0 (36.3, 59.8) | 100 (92.3, 100) | 84.4 (77.7, 89.8) | 100 (69.2, 100) |
| Triplicate Kato-Katz | 34.3 (23.2, 46.9) | 100 (95.4, 100) | 64.0 (52.1, 74.8) | 100 (92.3, 100) | 92.2 (86.8, 95.9) | 100 (69.2, 100) |
| Single CCA-A | 47.8 (35.4, 60.3) | 100 (95.4, 100) | 65.3 (53.5, 76.0) | 100 (92.3, 100) | 89.0 (82.9, 93.4) | 100 (69.2, 100) |
| Duplicate CCA-A | 59.7 (47.0, 71.5) | 100 (95.4, 100) | 77.3 (66.2, 86.2) | 100 (92.3, 100) | 91.6 (86.0, 95.4) | 100 (69.2, 100) |
| Triplicate CCA-A | 73.1 (60.9, 83.2) | 100 (95.4, 100) | 77.3 (66.2, 86.2) | 100 (92.3, 100) | 91.6 (86.0, 95.4) | 100 (69.2, 100) |
| Single CCA-B | 7.5 (2.5, 16.6) | 100 (95.4, 100) | 28.0 (18.2, 39.6) | 100 (92.3, 100) | 72.7 (65.0, 79.6) | 100 (69.2, 100) |
| Single ether-concentration | 8.9 (3.4, 18.5) | 100 (95.4, 100) | 10.7 (4.7, 19.9) | 100 (92.3, 100) | 39.6 (31.8, 47.8) | 100 (69.2, 100) |
The study was carried out in three epidemiological settings of south Côte d'Ivoire in October and November 2010. Two different diagnostic ‘gold’ standards were applied to calculate sensitivity and specificity, namely (i) the combined results of nine Kato-Katz thick smears, and (ii) the combined results of all tests able to diagnose S. mansoni infections (i.e., nine Kato-Katz thick smears, one ether-concentration, three CCA-A, and one CCA-B).
*Exact 95% confidence interval.
In setting B, the lowest sensitivity of 13.0% was determined for a single ether-concentration test. The highest sensitivity was found for duplicate or triplicate CCA-A (both = 77%). Single CCA-A (69.6%), showed a slightly lower sensitivity than triplicate Kato-Katz thick smears (73.9%) from a single stool sample.
In setting C, the ether-concentration showed the lowest sensitivity (41.0%) for diagnosis of S. mansoni. A single (89.6%) and duplicate or triplicate CCA-A tests (both = 91%) did not show a higher sensitivity than triplicate Kato-Katz thick smears (94.2%).
Considering nine Kato-Katz thick smears as ‘gold’ standard, the specificity of triplicate CCA-A test was 82.5% (setting A), 84.2% (setting B), and 76.9% (setting C). The respective specificity of the CCA-B test was 100%, 96.7% and 100%.
Even when using a more rigorous diagnostic ‘gold’ standard (i.e., the combined results of nine Kato-Katz thick smears, plus triplicate CCA-A, a single CCA-B, and a single ether-concentration), very similar sensitivities were obtained.
Effect of Concurrent S. haematobium Infection
Table 3 shows that, if only S. mansoni-negative children were included in a logistic regression analysis and adjustments were made for S. haematobium egg counts and infection intensity classes, no significant association between the CCA-A positivity rate and S. haematobium egg counts was found (OR = 1.09; p = 0.121). There was also no significant association between the CCA-A positivity rate and microhematuria classes detected (p>0.05). Due to the small number of children found positive with the CCA-B test no logistic regression analysis was performed.
Discussion
For the rapid identification of populations at highest risk of schistosomiasis and other helminth infections that warrant preventive chemotherapy, as well as for monitoring progress of control interventions and new efforts toward elimination, assessment of drug efficacy, and improved patient management, the importance of an accurate diagnosis at the individual and population level must be emphasized [6], [7], [31]. The widely used Kato-Katz technique for the diagnosis of S. mansoni (and S. japonicum) has several shortcomings: in low endemicity settings this technique considerably underestimates the ‘true’ prevalence of infection [8], [11], [32]–[34]. Moreover, a minimum of equipment and well trained laboratory technicians are needed for quality results. Promising results have been reported with a CCA urine test for the diagnosis of S. mansoni in different settings [17], [35]. Some of the previous investigations, however, lacked a rigorous diagnostic ‘gold’ standard, as CCA test results were compared with singe or duplicate Kato-Katz thick smears from one or two stool samples [21], [36].
Within the frame of a SCORE-funded multi-country study, we have now assessed the accuracy of a commercially available CCA urine cassette assay (CCA-A, batch 32727) and an experimental formulation (CCA-B, batch 32686) provided by the same manufacturer and tuned to have a higher specificity, which was run in parallel with the commercially available test in three epidemiological settings of south Côte d'Ivoire. Results of the CCA tests were compared with nine Kato-Katz thick smears (three stool samples, each subjected to triplicate Kato-Katz thick smears). Additionally, we performed a single ether-concentration test using SAF-fixed stool samples. The influence of S. haematobium infection and presence of microhematuria on the performance of the CCA test was determined. In all three settings, a single CCA-A showed a similarly high sensitivity than triplicate Kato-Katz thick smears from a single stool sample, but both approaches missed a considerable number of infections when considering nine Kato-Katz thick smears as ‘gold’ standard. As expected, CCA-B showed a higher specificity than CCA-A, but the sensitivity of CCA-B was considerably lower than that of CCA-A. Indeed, a single CCA-B showed a significantly lower sensitivity than a single CCA-A, and triplicate Kato-Katz thick smears, particularly in settings A and B where the endemicity of S. mansoni was lower than in setting C. We were surprised by the low sensitivity of the ether-concentration method for S. mansoni diagnosis, which warrants follow-up investigations.
The CCA-A seems to be an appropriate test for the diagnosis of S. mansoni in our study area in south Côte d'Ivoire where the prevalence of S. mansoni is above 25% and no recent control efforts have been implemented. Importantly, the co-endemicity of S. haematobium did not influence the accuracy of the CCA-A for the diagnosis of S. mansoni. Additionally, a concurrent infection with soil-transmitted helminths showed no negative influence on the accuracy of the CCA urine test for S. mansoni diagnosis, confirming recent observations made by Shane and colleagues in a study done in Kenya [17]. Furthermore, our study did not reveal a significant association between CCA-A positive results and microhematuria, as determined by Hemastix dipsticks, which relaxes the manufacturer's indication that false-positive results can occur if an individual presents microhematuria. However, further studies in different settings are warranted to confirm that microhematuria or urinary tract infections are not negatively impacting on CCA test results. Also the ability of the CCA test to detect antigen of juvenile Schistosoma worms, which are not yet producing eggs, needs further investigation. Noteworthy, the sensitivity of 56.3% of a single CCA-A in the setting A with a S. mansoni prevalence of 32.9% (based on nine Kato-Katz thick smears) is considerably lower than the sensitivity of 96.3% detected with a single CCA cassette of the same manufacturer in a Kenyan setting with a similar prevalence (38.8%) [17]. This difference might be explained by our more rigorous diagnostic approach, i.e., triplicate instead of duplicate Kato-Katz thick smears of three consecutive stool samples as ‘gold’ standard and by working in a slightly lower endemicity area. The sensitivity of a single CCA-A for S. mansoni diagnosis increased from 56.3% (setting A) to 69.6% (setting B) and 89.6% (setting C) in parallel to increasing prevalence (32.9% to 53.1% and finally to 91.8%), and corresponding mean FECs (17.4 EPG to 62.4 EPG and finally to 482.8 EPG). These findings emphasize the impact of higher prevalences and infection intensities on the positivity rate of the CCA. The strong association between the intensity of the color reaction of the CCA-A band and S. mansoni infection intensities according to FECs by the Kato-Katz method in our studies is in line with previous reports of the CCA dipstick and cassette [37], [38]. The results of the experimental CCA-B formulation, which has been tested on a single urine sample from all children, are suboptimal. Indeed, only low sensitivities and a poor agreement with results of the Kato-Katz method were found, particularly in the lower endemicity areas (settings A and B). In our hands, despite high specificity, the CCA-B in its current formulation cannot be recommended for S. mansoni diagnosis in south Côte d'Ivoire.
The following issues speak for or against the application of the CCA-A versus the Kato-Katz method in helminth control programs or public health centers: at first view, in moderate-to-high-risk communities for S. mansoni infections as found in our study in Côte d'Ivoire (i.e., prevalence above 25%), the collection of a single stool sample and its examination with triplicate Kato-Katz thick smears seems to be an acceptable approach for S. mansoni diagnosis. The advantage of the Kato-Katz method is that it can concurrently detect other helminth species, such as the three main soil-transmitted helminths (i.e., A. lumbricoides, hookworm, and T. trichiura), which is not possible with the CCA. However, the Kato-Katz method requires a minimum of equipment, including a microscope, and well trained laboratory technicians who can identify helminth species-specific eggs in the thick smears. For application of the CCA-A, no additional equipment and only a minimum of training are needed. However, it only detects S. mansoni and no concurrent soil-transmitted helminth infections. The cost of a single cassette (approximately US$ 2) is currently still out of reach of people at highest risk of intestinal schistosomiasis (i.e., poor rural dwellers in sub-Saharan Africa) [17]. However, the cost of triplicate Kato-Katz thick smears are likely higher than a single CCA test [10]. From a convenience and logistical point of view, the collection of urine samples for the CCA is more straightforward than collection of stool for the Kato-Katz method. Indeed, urine production is more convenient for the patient and can be done without special efforts on the spot and at the same day resulting in high compliance rates, while stool production is inconvenient and collection can render a second consultation necessary and thus further exacerbate costs [8], [39].
The performance and sensitivity of the CCA test in low-risk communities (prevalence below 10%), identified by the application of multiple Kato-Katz thick smears on stool samples collected over multiple days, remains to be elucidated. Noteworthy, our study intended to test the CCA in a setting with a S. mansoni prevalence of 10–24% as requested by SCORE. However, we observed a considerable increase in the prevalence of S. mansoni when not only applying triplicate Kato-Katz from a single stool sample as in the pre-screening, but nine Kato-Katz thick smears overall from three stool samples: the observed prevalence increased from 17% to 34% in setting A, and from 36% to 54% in setting B. Due to this rigorous diagnostic approach we ended up with higher prevalences. Retrospectively, this had to be expected, as predicted by mathematical modeling and field observations [11], [13]. If the CCA test proves to be more sensitive than multiple Kato-Katz thick smears in settings characterized by low prevalence and intensity of S. mansoni infection intensities, it will be a most useful test. For example, in areas where intense helminth control efforts have diminished the prevalence and intensity of S. mansoni infections and control programs are focusing elimination, population screenings are necessary to identify remaining S. mansoni hot-spots for targeted anthelmintic treatment and other interventions. For these large-scale screenings the CCA-A would be an excellent tool due to its fast and easy application.
We conclude that in the current study area of south Côte d'Ivoire, where the prevalence and intensity of S. mansoni are still high, partially explained by the prior lack of control efforts, the CCA-A can become a useful method for S. mansoni diagnosis in health centers at the periphery and schistosomiasis control programs. On the other hand, while the specificity of the CCA-B test was high, its current formulation cannot be recommended for S. mansoni diagnosis. Clearly, there is a need to evaluate the CCA test in settings characterized by low S. mansoni prevalences and infection intensities to assess its potential role in schistosomiasis control programs progressing toward transmission control and local elimination and for reliable individual diagnosis.
Supporting Information
Genauigkeit des im Urin zirkulierenden kathodischen Antigen (CCA) Tests für die Diagnose von Schistosoma mansoni Infektionen in verschiedenen Gebieten der Côte d'Ivoire - Translation of abstract into German by Stefanie Knopp.
(DOC)
Précision d'un test basé sur la détection d'antigènes cathodiques circulants (ACC) dans l'urine pour le diagnostic de Schistosoma mansoni dans différents foyers en Côte d'Ivoire - Translation of abstract into French by Jean T. Coulibaly and Kigbafori D. Silué.
(DOC)
STARD checklist.
(DOC)
Acknowledgments
We thank the SCORE secretariate for giving us the opportunity to participate in this multi-country evaluation of the CCA cassette test for the diagnosis of S. mansoni. We are grateful to the district health and education authorities of Azaguié for their support and for facilitating the implementation of our study. We are indebted to Prof. Bassirou Bonfoh, Director-General of the Centre Suisse de Recherches Scientifiques en Côte d'Ivoire for continued support, and to Dr. Govert J. van Dam for a series of excellent comments on this manuscript. We thank the teachers of the schools involved in this study for their deep commitment throughout. Last but not least, we are grateful to the children for their enthusiastic participation.
Footnotes
The authors have declared that no competing interests exist.
This study received financial support from the University of Georgia Research Foundation Inc, which is funded by the Bill & Melinda Gates Foundation for this SCORE project. JTC acknowledges financial support from the Carolito Foundation for a Ph.D. fellowship. SK is grateful to the “Forschungsfonds der Universität Basel” for a personal research grant. TF is associated to the National Centre of Competence in Research (NCCR) North-South and grateful for financial support through a Pro*Doc Research Module from the Swiss National Science Foundation (SNSF; project no. PDFMP3-123185). The research of EKN and JU is financially supported by Fairmed and the Swiss National Science Foundation (project no. IZ70Z0_123900). With the exception of the University of Georgia Research Foundation Inc, the aforementioned funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Ehrlich Sachs S, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, MacArthur C, Mubila L, Baker S. Control of neglected tropical diseases needs a long-term commitment. BMC Med. 2010;8:67. doi: 10.1186/1741-7015-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utzinger J, Raso G, Brooker S, de Savigny D, Tanner M, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859–1874. doi: 10.1017/S0031182009991600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. 2006. World Health Organization, Geneva.
- 5.Hotez PJ, Molyneux DH, Fenwick A, Savioli L, Takeuchi T. A global fund to fight neglected tropical diseases: is the G8 Hokkaido Toyako 2008 summit ready? PLoS Negl Trop Dis. 2008;2:e220. doi: 10.1371/journal.pntd.0000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Johansen MV, Sithithaworn P, Bergquist R, Utzinger J. Towards improved diagnosis of zoonotic trematode infections in Southeast Asia. Adv Parasitol. 2010;73:171–195. doi: 10.1016/S0065-308X(10)73007-4. [DOI] [PubMed] [Google Scholar]
- 8.Utzinger J, N'Goran EK, Caffrey CR, Keiser J. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl 1):S121–S137. doi: 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sâo Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 10.Speich B, Knopp S, Mohammed KA, Khamis IS, Rinaldi L, et al. Comparative cost assessment of the Kato-Katz and FLOTAC techniques for soil-transmitted helminth diagnosis in epidemiological surveys. Parasit Vectors. 2010;3:71. doi: 10.1186/1756-3305-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vlas SJ, Gryseels B. Underestimation of Schistosoma mansoni prevalences. Parasitol Today. 1992;8:274–277. doi: 10.1016/0169-4758(92)90144-q. [DOI] [PubMed] [Google Scholar]
- 12.Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, et al. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis. 2010;4:e754. doi: 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utzinger J, Booth M, N'Goran EK, Müller I, Tanner M, et al. Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology. 2001;122:537–544. doi: 10.1017/s0031182001007752. [DOI] [PubMed] [Google Scholar]
- 14.Utzinger J, N'Goran EK, N'Dri A, Lengeler C, Tanner M. Efficacy of praziquantel against Schistosoma mansoni with particular consideration for intensity of infection. Trop Med Int Health. 2000;5:771–778. doi: 10.1046/j.1365-3156.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 15.van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, et al. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol. 2004;42:5458–5461. doi: 10.1128/JCM.42.12.5458-5461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stothard JR. Improving control of African schistosomiasis: towards effective use of rapid diagnostic tests within an appropriate disease surveillance model. Trans R Soc Trop Med Hyg. 2009;103:325–332. doi: 10.1016/j.trstmh.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, et al. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in western Kenya. PLoS Negl Trop Dis. 2011;5:e951. doi: 10.1371/journal.pntd.0000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000;77:69–80. doi: 10.1016/s0001-706x(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 19.De Jonge N, Rabello ALT, Krijger FW, Kremsner PG, Rocha RS, et al. Levels of the schistosome circulating anodic and cathodic antigens in serum of schistosomiasis patients from Brazil. Trans R Soc Trop Med Hyg. 1991;85:756–759. doi: 10.1016/0035-9203(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 20.Polman K, Diakhate MM, Engels D, Nahimana S, Van Dam GJ, et al. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop Med Int Health. 2000;5:534–537. doi: 10.1046/j.1365-3156.2000.00600.x. [DOI] [PubMed] [Google Scholar]
- 21.Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, et al. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop. 2006;97:219–228. doi: 10.1016/j.actatropica.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 23.Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]
- 24.Raso G, Vounatsou P, McManus DP, N'Goran EK, Utzinger J. A Bayesian approach to estimate the age-specific prevalence of Schistosoma mansoni and implications for schistosomiasis control. Int J Parasitol. 2007;37:1491–1500. doi: 10.1016/j.ijpara.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legesse M, Erko B. Field-based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Trans R Soc Trop Med Hyg. 2007;101:668–673. doi: 10.1016/j.trstmh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Utzinger J, Botero-Kleiven S, Castelli F, Chiodini PL, Edwards H, et al. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin Microbiol Infect. 2010;16:267–273. doi: 10.1111/j.1469-0691.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 28.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 29.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinmann P, Du ZW, Wang LB, Wang XZ, Jiang JY, et al. Extensive multiparasitism in a village of Yunnan province, People's Republic of China, revealed by a suite of diagnostic methods. Am J Trop Med Hyg. 2008;78:760–769. [PubMed] [Google Scholar]
- 31.Becker SL, Lohourignon LK, Speich B, Rinaldi L, Knopp S, et al. Comparison of the Flotal-400 dual technique and the formalin-ether concentration technique for diagnosis of human intestinal protozoon infection. J Clin Microbiol. 2011;49:2183–2190. doi: 10.1128/JCM.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enk MJ, Lima AC, Drummond SC, Schall VT, Coelho PM. The effect of the number of stool samples on the observed prevalence and the infection intensity with Schistosoma mansoni among a population in an area of low transmission. Acta Trop. 2008;108:222–228. doi: 10.1016/j.actatropica.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Lin DD, Liu JX, Liu YM, Hu F, Zhang YY, et al. Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: a case study in an endemic area of the People's Republic of China. Parasitol Int. 2008;57:281–286. doi: 10.1016/j.parint.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Stothard JR, Sousa-Figuereido JC, Betson M, Adriko M, Arinaitwe M, et al. Schistosoma mansoni infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable? PLoS Negl Trop Dis. 2011;5:e938. doi: 10.1371/journal.pntd.0000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashton RA, Stewart BT, Petty N, Lado M, Finn T, et al. Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan. Trop Med Int Health. 2011;16:1099–1103. doi: 10.1111/j.1365-3156.2011.02815.x. [DOI] [PubMed] [Google Scholar]
- 37.Legesse M, Erko B. Field-based evaluation of a reagent strip test for diagnosis of schistosomiasis mansoni by detecting circulating cathodic antigen (CCA) in urine in low endemic area in Ethiopia. Parasite. 2008;15:151–155. doi: 10.1051/parasite/2008152151. [DOI] [PubMed] [Google Scholar]
- 38.Standley C, Lwambo N, Lange C, Kariuki H, Adriko M, et al. Performance of circulating cathodic antigen (CCA) urine-dipsticks for rapid detection of intestinal schistosomiasis in schoolchildren from shoreline communities of Lake Victoria. Parasit Vectors. 2010;3:7. doi: 10.1186/1756-3305-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayele B, Erko B, Legesse M, Hailu A, Medhin G. Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia. Parasite. 2008;15:69–75. doi: 10.1051/parasite/2008151069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genauigkeit des im Urin zirkulierenden kathodischen Antigen (CCA) Tests für die Diagnose von Schistosoma mansoni Infektionen in verschiedenen Gebieten der Côte d'Ivoire - Translation of abstract into German by Stefanie Knopp.
(DOC)
Précision d'un test basé sur la détection d'antigènes cathodiques circulants (ACC) dans l'urine pour le diagnostic de Schistosoma mansoni dans différents foyers en Côte d'Ivoire - Translation of abstract into French by Jean T. Coulibaly and Kigbafori D. Silué.
(DOC)
STARD checklist.
(DOC)