Abstract
Background and Objectives
Depression is common among acute coronary syndrome (ACS) patients and is associated with poor prognosis. Cardiac side effects of older antidepressants were well-known, but newer antidepressants are generally thought of as safe to use in patients with heart disease. The objective was to assess rates of antidepressant use or prescription to patients within a year of an ACS.
Methods
PubMed, PsycINFO, and CINAHL databases searched through May 29, 2009; manual searching of 33 journals from May 2009 to September 2010. Articles in any language were included if they reported point or period prevalence of antidepressant use or prescription in the 12 months prior or subsequent to an ACS for ≥100 patients. Two investigators independently selected studies for inclusion/exclusion and extracted methodological characteristics and outcomes from included studies (study setting, inclusion/exclusion criteria, sample size, prevalence of antidepressant prescription/use, method of assessing antidepressant prescription/use, time period of assessment).
Results
A total of 24 articles were included. The majority were from North America and Europe, and most utilized chart review or self-report to assess antidepressant use or prescription. Although there was substantial heterogeneity in results, overall, rates of antidepressant use or prescription increased from less than 5% prior to 1995 to 10–15% after 2000. In general, studies from North America reported substantially higher rates than studies from Europe, approximately 5% higher among studies that used chart or self-report data.
Conclusions
Antidepressant use or prescription has increased considerably, and by 2005 approximately 10% to 15% of ACS patients were prescribed or using one of these drugs.
Introduction
Major depressive disorder (MDD) is present in approximately 20% of patients with coronary heart disease (CHD), including acute coronary syndrome (ACS) patients [1], [2]. Depression impacts quality of life post-ACS [3], and many studies have linked MDD or depressive symptoms to poor prognosis [4].
The rate of self-reported antidepressant use increased from approximately 7% to 12% among US adults from 1996 to 2005 [5]. In Europe, just under 4% of respondents from 6 countries surveyed between 2001 and 2003 reported 12-month antidepressant use [6]. The potential for tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) to cause serious and potentially fatal side effects has limited their use in cardiac populations [7], [8]. On the other hand, selective serotonin reuptake inhibitors (SSRIs) have generally been accepted as safe in cardiac patients because they appear to lack these side effects [9]–[11]. Several clinical trials have examined the efficacy of SSRIs in cardiac populations [9], [10], [12]–[14] and have reported effect sizes that are similar to those from non-cardiac populations [15]–[18]. Concerns have been raised in recent years, however, about previously unrecognized adverse side effects of SSRIs potentially relevant to CHD patients [19]–[21], as well as potentially dangerous interactions between SSRIs and commonly used cardiac medications [22]–[26].
No systematic reviews have characterized the rate of antidepressant use or prescription in ACS patients, which was the objective of this review.
Methods
Reporting of the study was based on guidelines established by the Meta-analysis of Observational Studies in Epidemiology statement [27].
Data Sources and Searches
Potentially eligible articles were identified from the PubMed, PsycINFO, and CINAHL databases, searched on May 29, 2009. Search strategies (Supporting Information S1) were designed to identify articles that reported on ACS patients in association with depression or antidepressant use. In addition, manual searching of 33 cardiology, psychiatry, and general medicine journals (Supporting Information S2) was conducted to identify articles published subsequent to the electronic database search (May 1, 2009 to September 30, 2010).
Study Selection
Eligible articles included published articles of original research in any language that reported point or period prevalence of antidepressant use or prescription in the 12 months prior or subsequent to an ACS, defined as unstable angina, non-ST-elevation myocardial infarction (MI), or ST-elevation MI, for at least 100 patients. Antidepressants included SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), TCAs, MAOIs, and atypical antidepressants. Studies with data on only a single antidepressant class were excluded. Studies with inclusion criteria that required antidepressant use or a condition associated with antidepressant use (e.g., depressive disorders) and studies that utilized antidepressants as part of an intervention were excluded unless they reported data on antidepressant prescription or use for all patients assessed for study eligibility. Non-published studies, studies published in abstract form only, letters, editorials, and case series or case reports were excluded. When multiple articles were published on the same cohort or portions of the same cohort, the article with the most complete data was included. Studies with mixed populations were included if data for ACS patients were reported separately.
Two investigators independently reviewed articles for eligibility. If either deemed an article potentially eligible based on title/abstract review, then a full-text review was completed. Disagreements after full-text review were resolved by consensus of three investigators, including the two reviewers. Non-English articles were evaluated by one investigator with the assistance of a translator. As necessary, authors were contacted to clarify information relevant to determining eligibility.
Data Extraction
Two investigators independently extracted data from included studies into a standardized spreadsheet with discrepancies resolved via consensus. Extracted data included the study setting (North America, Europe, other), study inclusion/exclusion criteria, sample size, prevalence of antidepressant prescription/use (total and by antidepressant class), method of assessing antidepressant prescription/use, and time period of assessment. Methods of assessment were classified as administrative database, review of medical record, observation of medication containers, and patient self-report. The time period was recorded as the midpoint of the range of years when data were collected. When antidepressant use was assessed at multiple time points for the same patients, the time point closest to the index ACS was used. Authors were contacted as necessary for clarification.
One included article [28] used province-wide administrative data from Ontario, Canada to determine the percentage of post-MI patients aged 65 or older who were prescribed an antidepressant in the first 6 months post-MI. Antidepressant prescriptions were reported on a quarterly basis from the second calendar quarter of 1993 to the first calendar quarter of 2002. Numerical data were provided for the first and last quarters of the study, with scatterplot data provided for other quarters. Original data were no longer available (personal communication, Muhammad Mamdani, May 17, 2010). Therefore, two investigators estimated quarterly prevalence data from the published scatterplot, and all estimates were within 0.1% of each other. For the purposes of the present study, data are presented for one calendar quarter per year (second quarter 1993 through second quarter 2001).
Data Synthesis and Analysis
Included studies were evaluated to determine if there was sufficient clinical and methodological similarity to support a pooled meta-regression of the rate of antidepressant prescription or use over time, factoring in setting (North America versus Europe and other) and method of assessment (administrative data versus self-report or chart review). However, excluding the study by Benazon et al. [28] that provided quarterly data over an approximately 10-year period, there were too few studies overall and not enough coverage across time, setting, and assessment method to reasonably pool all of the data from the other studies with meta-regression. Therefore, a qualitative synthesis was done for all studies in the review and a quantitative meta-analysis for a subset of studies.
Specifically, we determined that there was a sufficient number of studies to assess the association of time and setting with antidepressant prescription or use among studies that ascertained rates using non-administrative data (e.g., self-report or chart review) with the midpoint of data collection from October 1997 or later. There was not enough coverage of studies to extend the analysis prior to October 1997 or to include studies with administrative data.
For this subset of studies, we first performed an overall meta-analysis and then proceeded to fit meta-regressions controlling for region and for time. The I2 statistic was used to assess heterogeneity for the meta-analysis [29], and radial and funnel plots were generated to assess model assumptions. The R statistical software was used to analyze the results via the metafor library [30], [31]. The main meta-regression model was fit using Restricted Maximum Likelihood estimators with the rate of antidepressant usage as the outcome.
Results
Search Results
The database search yielded 1,619 unique citations (Figure 1), including 666 selected for full text review and 35 that met inclusion criteria. Of the 35 eligible articles, 12 reported on the same cohort, leaving 23 unique articles for review. One additional eligible article was identified through manual search, resulting in a total of 24 included articles [28], [32]–[54].
Figure 1. Flow Diagram of Study Selection Process.
Characteristics of Included Studies
As shown in Table 1, the 24 included studies reported data on antidepressant prescription or use from April 1993 through December 2005. Data from one published study by Benazon et al. [28] reported antidepressant prescription rates in the 6 months following hospital discharge for patients surviving at least 6 months from the 2nd quarter of 1993 to the 1st quarter of 2002. There were 13 studies from North America, 8 from Europe, and 1 each from Japan, Australia, and New Zealand. As shown in Table 2, only 3 studies used data from administrative databases, including the Benazon et al. study [28]. One study reviewed patients' medication containers, and 20 used self-report, medical chart review, or a combination of the two. Sample sizes in the 3 studies that used administrative data ranged from 1,637 to 8,887 patients. For other studies, the range was from 126 to 2,509 patients. Of the 24 studies, 15 reported antidepressant prescription or use during the index ACS admission or at discharge, 4 reported pre-admission prescription or use, and 5 studies reported post-ACS prescription or use. One study reported antidepressant use among women aged <65 years [44], 2 studies did not report gender composition [28], [32], and the rest of the studies included predominantly males (57% to 98%).
Table 1. Characteristics of Studies Included in the Systematic Review.
| First Author, Year | Country | Patients | N | % MI | MeanAge(Years) | % Male | % White | Years of Study |
| Benazon, 2005a | Canada | MI admission, survived ≥6 months postdischarge; ≥65 years; no MI 3 years prior to index MI | 1637–2231 | 100% | NR | NR | NR | 1993–2002 |
| Grunau, 2006 | Canada | MI admission; ≥66 years | 5559 | 100% | NR | NR | NR | 1994–1995 |
| Lesperance, 2000 | Canada | Unstable angina admission; documented CAD | 430 | 0% | 62 | 71% | NR | 1994–1996 |
| Parakh, 2008 | USA | MI admission | 284 | 100% | 65 | 57% | 87% | 1995–1996 |
| Sauer, 2001 | USA | First MI admission; 30–65 years | 653 | 100% | 52b | 68%b | NR | 1995–1997 |
| Lauzon, 2003 | Canada | MI admission | 464 | 100% | 60c | 79%c | 96%c | 1996–1998 |
| Grace, 2005 | Canada | ACS admission | 913 | 53% | 62 | 65% | NR | 1997–1999 |
| Rumsfeld, 2003 | USA | ACS admission | 1957 | 50% | 65 | 98% | 89% | 1998–1999 |
| Frasure-Smith, 2008 | Canada | ACS admission; cardiac catherization | 804 | NR | 60 | 81% | NR | 1999–2001 |
| Corser, 2006 | USA | ACS admission | 521 | NR | 60 | 64% | 84% | 2002–2003 |
| Mallik, 2006 | USA | MI admission | 2361 | 100% | 61 | 68% | NR | 2003–2004 |
| Grace, 2008 | Canada | ACS admission | 505 | NR | 62 | 77% | 84% | 2003–2004 |
| Huffman, 2006 | USA | MI admission | 131 | 100% | 62 | 80% | NR | 2003–2005 |
| Zimmermann-Viehoff, 2010 | Sweden | Women <65 years admitted for ACS | 266 | 36% | 56 | 0% | NR | 1991–1993 |
| Monster, 2004 | Denmark | First MI admission | 8887 | 100% | 72 | 62% | NR | 1994–2002 |
| Dickens, 2007 | UK | MI admission | 507 | 100% | 59d | 71%d | 95%d | 1997–1999 |
| Kaptein, 2006 | Netherlands | MI admission | 475 | 100% | 61 | 81% | NR | 1997–2000 |
| Pitzalis, 2001 | Italy | MI admission <70 years | 128 | 100% | 54e | 86%e | NR | 1998–2000 |
| Sorensen, 2005 | Denmark | MI admission <70 years | 889 | NR | 60 | 73% | NR | 1999–2000 |
| Nakatani, 2005 | Japan | MI admission | 2509 | 100% | 64 | 77% | NR | 1999–2003 |
| Costa Dias, 2005 | Portugal | ACS admission | 240 | 71% | 59 | 85% | NR | 2001–2002 |
| Bhattacharyya, 2007 | UK | ACS admission; 18 to 90 years with paid employment | 126 | NR | 55 | 88% | 85% | 2001–2004 |
| Parker, 2008 | Australia | ACS admission | 489 | 65% | 66 | 70% | NR | 2001–2003 |
| Wong, 2008 | NZ | ACS admission | 276 | 63% | 64 | 71% | NR | 2005 |
ACS = acute coronary syndrome; CAD = coronary artery disease; MI = myocardial infarction; NR = Not reported; NZ = New Zealand; UK = United Kingdom; USA = United States of America.
Provincial database with quarterly data evaluated from 2nd quarter of 1993 to 1st quarter of 2002.
Excluding 5 patients with “other” antidepressant use.
Based on 550 patient enrolled in the study at index hospitalization.
Based on 527 patients enrolled in study.
Based on 103 patients.
Table 2. Antidepressant Prescription or Use in Included Studies.
| First Author, Year | Country | Method of Antidepressant Use Assessment | Time of Antidepressant Use Assessment | N (%) withAntidepressant | Classes of AntidepressantsUsed |
| Benazon, 2005a | Canada | Administrative database | Within 6 months post-MI | 128–357 (8% to 16%) | 1993, Q2: SSRI = 29; TCA = 106. 2002, Q1: SSRI = 217; TCA = 126 |
| Grunau, 2006 | Canada | Administrative database | Within 12 months pre-MI | 471 (9%) | NR |
| Lesperance, 2000 | Canada | Chart review | Discharge | 18 (4%) | NR |
| Parakh, 2008 | USA | Chart review | Discharge | 14 (5%) | NR |
| Sauer, 2001 | USA | Self-report | Within 1 week pre-MI | 18 (3%) | SSRI = 13; Other = 5 |
| Lauzon, 2003 | Canada | Self-report | 12 months post-MI | 13 (3%) | NR |
| Grace, 2005 | Canada | Self-report | During admission | 42 (5%) | NR |
| Rumsfeld, 2003 | USA | Chart review | Discharge | 254 (13%) | NR |
| Frasure-Smith, 2008 | Canada | Medicine containers | 2 months post-ACS | 68 (8%) | NR |
| Corser, 2006 | USA | Chart review | Discharge | 61 (12%) | NR |
| Mallik, 2006 | USA | Chart review | Discharge | 231 (10%) | NR |
| Grace, 2008 | Canada | Self-report | 9 months post-ACS | 48 (10%) | SSRI = 27; TCA = 6; SNRI = 8; Other = 7 |
| Huffman, 2006 | USA | Chart review | During admission | 16 (12%) | NR |
| Zimmermann-Viehoff, 2010 | Sweden | Chart review/Self-report | During admission | 0 (0%) | 0 |
| Monster, 2004 | Denmark | Administrative database | Within 90 days pre-MI | 540 (6%) | SSRI = 289; Other = 251 |
| Dickens, 2007 | UK | Chart review | Discharge | 21 (4%) | NR |
| Kaptein, 2006 | Netherlands | Self-report | 3 months post-MI | 15 (3%) | NR |
| Pitzalis, 2001 | Italy | Self-report | During admission | 0 (0%) | 0 |
| Sorensen, 2005 | Denmark | Chart review | Discharge | 39 (4%) | NR |
| Nakatani, 2005 | Japan | Chart review/Self-report | During admission | 124 (5%) | NR |
| Costa Dias, 2005 | Portugal | Self-report | Prior to admission | 2 (1%) | NR |
| Bhattacharyya, 2007 | UK | Chart review | During admission | 8 (6%) | NR |
| Parker, 2008 | Australia | Self-report | During admission | 32 (7%) | SSRI = 17; TCA = 12; MAOI = 4 |
| Wong, 2008 | NZ | Self-report | During admission | 11 (4%) | NR |
MAOI = monoamine oxidase inhibitor; NR = Not reported; NZ = New Zealand; Q1 = 1st quarter; Q2 = 2nd quarter; SNRI = serotonin-norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant;
Provincial database with quarterly data evaluated from 2nd quarter of 1993 to 1st quarter of 2002.
Prevalence of Antidepressant Prescription or Use
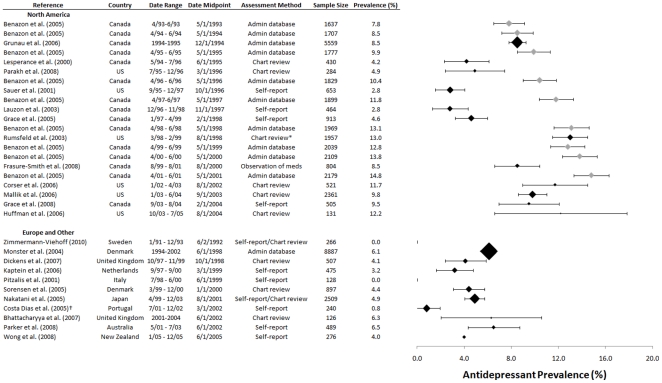
As shown in Figure 2, the rate of patients prescribed or using antidepressants increased from 1993 to 2005. Results from the Benazon et al. study are shown separately for 9 cohorts/years (1993 to 2001) [28]. Generally, studies from North America reported substantially higher rates than studies from Europe. Based on self-report or chart review, the only study from North America from 1995 or earlier [33] reported a rate of 4.2%, whereas the only pre-1995 European study, which reported on women under age 65 from Sweden, reported that no patients were using antidepressants upon admission for ACS [44]. One other study, which reported on Italian patients under the age of 70 admitted for MI from 1998 to 2000, similarly reported that no patients were using antidepressants at the time of admission [48]. Rates of antidepressant use or prescription among North American studies that used self-report or chart review from 2000 onward ranged from 9.5% to 13.0%, compared to 0.8% to 6.5% among non-North American studies in the same time period.
Figure 2. Prevalence of Antidepressant Use in ACS Patients.
Studies are grouped by geographic region and sorted by midpoint of data collection period. Benazon et al. [28] rates are denoted with grey markers. Error bars are +/−1.96 standard errors, and marker sizes are weighted according to the inverse of the squared standard error. *Medication information was abstracted from patient charts but only prescriptions actually filled were included. †Study reported, “only two patients were taking antidepressants prior to hospitalization,” which was coded as being assessed at hospital admission.
Among studies of administrative databases, rates in North America ranged from 7.8% to 9.9% from 1993 to 1995 and increased to 13.8% to 14.8% in studies from 2000 onward. The only European study that used administrative data was a Danish study that reported a rate of 6.1% of 8,887 patients with antidepressant prescriptions in the years 1994 to 2002 [45]. During approximately the same period, rates reported by Benazon et al. from Ontario, Canada increased from 7.8% in the second quarter of 1993 to 15.7% by the first quarter of 2002 (Benazon data in Figure 2 through the second quarter of 2001) [28].
Only five studies reported rates of use or prescription by antidepressant class 28,35,42,45,53], so analysis of rates by class was not possible. However, in the study by Benazon et al. [28] the percentage of patients prescribed an SSRI increased five-fold from approximately 2% in the second quarter of 1993 to approximately 10% in the first quarter of 2002. TCA prescription, on the other hand, remained stable at approximately 6% across the study period.
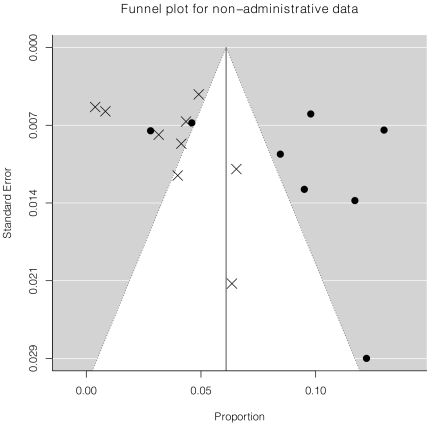
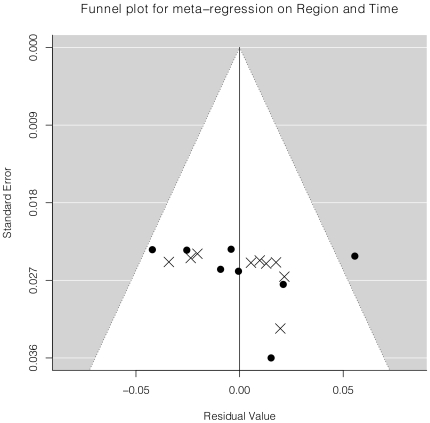
There were 17 studies that assessed prevalence of antidepressant prescription or use using non-administrative databases with study midpoints of October 1997 onward and were included in the meta-analysis, including 8 studies from North America [36]–[43] and 9 studies from Europe [46]–[49], [51], [52], Japan [50], Australia [53], or New Zealand [54]. Overall, the random effects model estimate for antidepressant use for all 17 studies was 6.1% (95% confidence interval (CI) 4.3% to 7.9%). However, the I2 statistic was extremely large (95.7%), indicating a substantial amount of between study heterogeneity, which was also seen in the funnel plot (Figure 3). Meta-regression was done with setting (North American versus Europe and other) and time (year of midpoint of data collection) as predictor variables. The estimated difference between rate of use in North America versus Europe and other was 5.1% (95% CI 2.5% to 7.8%, p<0.001). In addition, the rate of use increased by approximately 0.5% per year (95% CI 0.0% to 1.1%, p = 0.065). Adjusting for setting and time accounted for 50% of heterogeneity in prevalence between studies from the overall meta-analysis, and the funnel plot reflected less structured heterogeneity and better model fit (Figure 4). Results did not change if transformations of the raw proportions (arcsin and log) were modeled.
Figure 3. Heterogeneity of Unadjusted Prevalence Estimates.
The funnel plot shows individual study prevalence estimates (x-axis) plotted against estimated standard errors (y-axis). The white cone inside the gray region indicates a 95% probability region that points would be expected to fall into assuming no heterogeneity and no publication bias. The North American studies are indicated by the filled circles and the non-North American studies are indicated by the × symbols.
Figure 4. Heterogeneity of Adjusted Prevalence Estimates.
The funnel plot shows the residuals from the meta-regression on setting and time (x-axis) plotted against estimated standard errors (y-axis). The white cone inside the gray region indicates a 95% probability region that points would be expected to fall into assuming the assumptions of the meta-regression were valid. The North American studies are indicated by the filled circles and the other studies are indicated by the × symbols.
Discussion
Consistent with general population trends [5], [6], [55] the prescription or use of antidepressants has risen dramatically among ACS patients since the early 1990s. Although there was substantial heterogeneity in results, overall, rates of antidepressant prescription or use increased from less than 5% prior to 1995 to 10–15% after 2000. One study that tracked antidepressant prescribing to post-MI patients using province-wide administrative data from Ontario, Canada found that the percentage of patients with an antidepressant prescription in the 6 months following hospital discharge increased from 7.8% in 1993 to 15.7% by 2002 [28]. In the meta-regression, there was an increase of approximately 0.5% per year in studies that used non-administrative data between 1997 and 2005. As with general population data, the rate of antidepressant prescription or self-reported use was notably higher in North America than in other parts of the world, primarily Europe, with an estimated difference of approximately 5.1% among studies from 1997 onward that used self-report or chart data to establish usage rates.
There has been a striking increase in the use of antidepressant medications in the general population in the past two decades, due in large part to a significant increase in the prescribing of antidepressant medications by nonpsychiatrists [55]. One reason for this trend is likely to be the perception that antidepressants are safe to use even in patients with medical comorbidities, including heart disease [56]. Based on the results of several studies [9], [10], [12] including post hoc analysis of the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study [57], it has been concluded that SSRIs are safe to use in patients with heart disease [58]. Consistent with this, a recent study of antidepressant use in primary care notes “…the preponderance of evidence suggests that it is not necessary to be overly cautious when prescribing for patients who have medical comorbidities. Most studies have demonstrated that SSRIs are safe and effective in persons who have both CAD (coronary artery disease) and stroke” [56]. The side effects of the older antidepressants, however, limited their use in heart disease. TCAs can increase heart rate, produce orthostatic hypotension, decrease heart rate variability, prolong cardiac conduction, and trigger potentially lethal ventricular arrhythmias [9]. MAOIs can also produce orthostatic hypotension, but their potential to cause hypertensive crisis in patients who ingest tyramine-containing food or certain other drugs is the most concerning of their cardiovascular side effects [59].
The increased use of antidepressant drugs in ACS patients in recent years may not only reflect greater comfort with these agents, but also a greater awareness of the burden of depression in patients with CHD [2], [4]. Over the years, many informal calls have been made for increased depression treatment in heart disease patients, and recently an AHA Science Advisory recommended routine depression screening for all heart patients in order to increase the treatment rate [58]. Indeed, the problem of under-diagnosed and under-treated depression has been recognized as an important public health issue outside of cardiovascular care settings, and increasing depression treatment, including antidepressant prescribing, has been prioritized [60].
Whether depression in ACS patients is under-treated despite the more widespread prescription or use of antidepressants documented in this study is not known. Distinguishing under-use of antidepressants from appropriate use or overuse is beyond the scope of this systematic review. Nonetheless, it should be noted that overuse occurs when the risk of potential harm of using a drug outweighs the likely benefits for some patients. The overuse of antidepressants among populations who are unlikely to benefit meaningfully, is well-documented [61]. In the context of ACS, it should be pointed out that as antidepressant use increases, the potential for harm, even if small, also increases. Although the SSRIs appear to have few direct adverse cardiovascular effects, clinically important drug-drug interactions may be encountered with more widespread use in ACS patients since many of these drugs inhibit hepatic cytochrome P450 isoenzymes [62], which are involved in the metabolism of many cardiac drugs. The safety of SNRIs in ACS patients has not been as well-studied, which is important to consider since these drugs can increase blood pressure and heart rate [63]. It should be noted that recent reports raise persistent concern about potential adverse cardiovascular effects of antidepressant drugs [20], [21], [25], [26], [64]–[66], suggesting that additional studies that evaluate cardiovascular side effects of antidepressant drugs in greater numbers of patients followed for longer time periods may be warranted.
There are limitations that should be considered in interpreting the results of this study. First, although the search that was conducted was broad and resulted in the review of more than 600 full-texts of articles, it is possible that some studies that reported on the rate of antidepressant use or prescription may not have been identified. Second, there are limitations in the methods used by original studies to ascertain the rate of antidepressant prescription or use. It is well-known, for instance, that self-report methods, which were used in a number of the studies we reviewed, tend to underestimate rates of prescription and use [67]–[69].
There were not enough studies from North America versus Europe and that used administrative data versus self-report or chart data across the time frame of the studies we reviewed to synthesize all data quantitatively. Thus, we were only able to estimate the prevalence quantitatively using non-administrative database studies that were conducted from 1997 onward. With only 17 studies included in the meta-analysis and limited information on some of these, we were not able to adjust for study characteristics beyond setting and time, such as patient gender or age. Even after adjusting for these variables, there was a substantial amount of heterogeneity between studies. Thus, the meta-regression results should be interpreted with caution.
Additionally, very few studies provided information on the classes of antidepressants prescribed, which did not permit specific analysis. Furthermore, no studies with data on antidepressant prescription or use since 2005 were available, so it is not known whether or not prescription rates have continued to increase beyond the rates reported in the studies reviewed. Finally, the protocol for this review was not registered prior to initiating work on the review.
In summary, the results of this systematic review showed that the rate of antidepressant prescriptions and use has risen dramatically in recent years among ACS patients and, as in the general population, tends to be higher in North America than in other parts of the world. The fact that by 2005 between 10% and 15% of ACS patients were prescribed or using an antidepressant suggests that more patients are receiving treatment for depression, which is clearly an important problem in patients with heart disease. Alternatively, this prevalence of antidepressant use raises the possibility that antidepressants are being used by some ACS patients who may not benefit meaningfully despite being exposed to the possibility of adverse effects of the drugs themselves or to clinically significant drug-drug interactions. Careful consideration needs to be given to the balance between potential benefits and harms in prescribing antidepressant medications to patients with heart disease.
Supporting Information
Search Strategies.
(DOC)
Journals Included in Manual Searches.
(DOC)
Acknowledgments
We would like to acknowledge Gertrude Brichwood, Jewish General Hospital, Montreal, Canada, Karolina Konieczna, MA, McGill University, Montreal, Canada, Katherine Milette, McGill University, Montreal, Canada, Ilya Razykov, McGill University, Montreal, Canada, Anna Seccareccia, Jewish General Hospital, Montreal, Canada, Andrea Szabo, PhD, Collège Montmorency, Laval, Quebec, Canada, Philipp Weber, LLB, Rijksuniversiteit Groningen, The Netherlands, and Yue Zhao, MSc, Concordia University, Montréal, Québec, Canada for assistance with translation of non-English titles/abstracts and articles.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Dr. Ziegelstein was supported by grant number R24AT004641 from the National Center for Complementary & Alternative Medicine and by the Miller Family Scholar Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health. Dr. Thombs was supported by a New Investigator Award from the Canadian Institutes of Health Research and an Établissement de Jeunes Chercheurs award from the Fonds de la Recherche en Santé Québec. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 2.Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21:30–38. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111:250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 4.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 6.Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, et al. The ESEMeD/MHEDEA 2000 investigators. Psychotropic drug utilization in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand. 2004;109:55–64. doi: 10.1111/j.1600-0047.2004.00331.x. [DOI] [PubMed] [Google Scholar]
- 7.Glassman AH, Roose SP, Bigger JT., Jr The safety of tricyclic antidepressants in cardiac patients. Risk-benefit reconsidered. JAMA. 1993;269:2673–2675. [PubMed] [Google Scholar]
- 8.Murray JB. Cardiac disorders and antidepressant medications. J Psychol. 2000;134:162–168. doi: 10.1080/00223980009600859. [DOI] [PubMed] [Google Scholar]
- 9.Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor D. Antidepressant drugs and cardiovascular pathology: a clinical overview of effectiveness and safety. Acta Psychiatr Scand. 2008;118:434–442. doi: 10.1111/j.1600-0447.2008.01260.x. [DOI] [PubMed] [Google Scholar]
- 12.Lespérance F, Frasure-Smith N, Koszycki D, Laliberté M-A, van Zyl LT, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease. JAMA. 2007;297:367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 13.Strik JJMH, Honig A, Lousberg R, Lousberg AHP, Cheriex EC, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: Findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000;62:783–789. doi: 10.1097/00006842-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, et al. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86:527–532. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 15.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 16.Dowlati Y, Herrmann N, Swardfager WL, Reim EK, Lanctôt KL. Efficacy and tolerability of antidepressants for treatment of depression in coronary artery disease: a meta-analysis. Can J Psychiatry. 2010;55:91–99. doi: 10.1177/070674371005500205. [DOI] [PubMed] [Google Scholar]
- 17.Mazza M, Lotrionte M, Biondi-Zoccai G, Abbate A, Sheiban I, et al. Selective serotonin reuptake inhibitors provide significant lower re-hospitalization rates in patients recovering from acute coronary syndromes: evidence from a meta-analysis. J Psychopharmacol. 2010;24:1785–1792. doi: 10.1177/0269881109348176. [DOI] [PubMed] [Google Scholar]
- 18.Delisle VC, Arthurs E, Ziegelstein RC, Thombs BD. A meta-analysis of selective serotonin reuptake inhibitors in acute coronary syndrome: cause for concern. J Psychopharmacol. Epub ahead of print doi: 10.1177/0269881111408464. [DOI] [PubMed] [Google Scholar]
- 19.Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and reconsideration of mechanisms. J Clin Psychiatry. 2010;71:1565–1575. doi: 10.4088/JCP.09r05786blu. [DOI] [PubMed] [Google Scholar]
- 20.Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2462–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Celexa (citalopram hydrobromide): drug safety communication - abnormal heart rhythms associated with higher doses. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm269481.htm. Accessed September 6, 2011.
- 22.Tibold A, Feher G, Csejtei A, Tettinger A, Kiss I. Selective serotonin reuptake inhibitors may interfere with the antiplatelet effect of clopidogrel. Am J Cardiol. 2007;99:1025–1026. doi: 10.1016/j.amjcard.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Ziegelstein RC, Meuchel J, Kim TJ, Latif M, Alvarez W, et al. Selective serotonin reuptake inhibitor use by patients with acute coronary syndromes. Am J Med. 2007;120:525–530. doi: 10.1016/j.amjmed.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, et al. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- 25.Fosbol EL, Gislason GH, Poulsen HE, Hansen ML, Folke F, et al. Prognosis in heart failure and the value of {beta}-blockers are altered by the use of antidepressants and depend on the type of antidepressants used. Circ Heart Fail. 2009;2:582–590. doi: 10.1161/CIRCHEARTFAILURE.109.851246. [DOI] [PubMed] [Google Scholar]
- 26.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses' Health Study. J Am Coll Cardiol. 2009;53:950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 28.Benazon NR, Mamdani MM, Coyne JC. Trends in the prescribing of antidepressants following acute myocardial infarction, 1993–2002. Psychosom Med. 2005;67:916–920. doi: 10.1097/01.psy.0000188399.80167.aa. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.R Development Core Team. R: A language and environment for statistical computing. 2011. R Foundation for Statistical Computing: Vienna. http://www.R-project.org/
- 31.Viechtbauer W. Conducting meta-anlayses in R with the metafor package. J Stat Softw. 2010;36:August 2010. [Google Scholar]
- 32.Grunau GL, Ratner PA, Goldner EM, Sheps S. Is early- and late-onset depression after acute myocardial infarction associated with long-term survival in older adults? A population-based study. Can J Cardiol. 2006;22:473–478. doi: 10.1016/s0828-282x(06)70263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med. 2000;160:1354–1360. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 34.Parakh K, Thombs BD, Fauerbach JA, Bush DE, Ziegelstein RC. Effect of depression on late (8 years) mortality after myocardial infarction. Am J Cardiol. 2008;101:602–606. doi: 10.1016/j.amjcard.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104:1894–1898. doi: 10.1161/hc4101.097519. [DOI] [PubMed] [Google Scholar]
- 36.Lauzon C, Beck CA, Huynh T, Dion D, Racine N, et al. Depression and prognosis following hospital admission because of acute myocardial infarction. Can Med Assoc J. 2003;168:547–552. [PMC free article] [PubMed] [Google Scholar]
- 37.Grace SL, Abbey SE, Pinto R, Shnek ZM, Irvine J, et al. Longitudinal course of depressive symptomatology after a cardiac event: effects of gender and cardiac rehabilitation. Psychosom Med. 2005;67:52–58. doi: 10.1097/01.psy.0000151486.28349.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rumsfeld JS, Magid DJ, Plomondon ME, Sales AE, Grunwald GK, et al. History of depression, angina, and quality of life after acute coronary syndromes. Am Heart J. 2003;145:493–499. doi: 10.1067/mhj.2003.177. [DOI] [PubMed] [Google Scholar]
- 39.Frasure-Smith N, Lesperance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 40.Corser W, Hanak M, Stommel M, Olomu A, Yang Z, et al. Association of cardiac drugs with depression after acute coronary syndrome. J Pharm Technol. 2006;22:319–325. [Google Scholar]
- 41.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, et al. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006;166:876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 42.Grace SL, Leung YW, Stewart DE. A prospective examination of antidepressant use and its correlates in patients with acute coronary syndrome. Psychosomatics. 2008;49:199–207. doi: 10.1176/appi.psy.49.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huffman JC, Smith FA, Blais MA, Beiser ME, Januzzi JL, et al. Rapid screening for major depression in post-myocardial infarction patients: an investigation using Beck Depression Inventory II items. Heart. 2006;92:1656–1660. doi: 10.1136/hrt.2005.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann-Viehoff F, Orth-Gomer K, Wang HX, Deter HC, Merswolken M, et al. Depressive symptoms and heart rate variability in younger women after an acute coronary event. Eur J Cardiovasc Prev Rehabil. 2010;17:509–513. doi: 10.1097/HJR.0b013e328337b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monster TB, Johnsen SP, Olsen ML, McLaughlin JK, Sorensen HT. Antidepressants and risk of first-time hospitalization for myocardial infarction: a population-based case-control study. Am J Med. 2004;117:732–737. doi: 10.1016/j.amjmed.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 46.Dickens C, McGowan L, Percival C, Tomenson B, Cotter L, et al. Depression is a risk factor for mortality after myocardial infarction: fact or artifact? J Am Coll Cardiol. 2007;49:1834–1840. doi: 10.1016/j.jacc.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 47.Kaptein KI, de Jonge P, van den Brink RH, Korf J. Course of depressive symptoms after myocardial infarction and cardiac prognosis: a latent class analysis. Psychosom Med. 2006;68:662–668. doi: 10.1097/01.psy.0000233237.79085.57. [DOI] [PubMed] [Google Scholar]
- 48.Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, et al. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J. 2001;141:765–771. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- 49.Sorensen C, Brandes A, Hendricks O, Thrane J, Friis-Hasche E, et al. Psychosocial predictors of depression in patients with acute coronary syndrome. Acta Psychiatr Scand. 2005;111:116–124. doi: 10.1111/j.1600-0447.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakatani D, Sato H, Sakata Y, Shiotani I, Kinjo K, et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J. 2005;150:652–658. doi: 10.1016/j.ahj.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 51.Costa Dias C, Mateus PS, Mateus C, Bettencourt N, Santos L, et al. Acute coronary syndrome and depression. Rev Port Cardiol. 2005;24:507–516. [PubMed] [Google Scholar]
- 52.Bhattacharyya MR, Perkins-Porras L, Whitehead DL, Steptoe A. Psychological and clinical predictors of return to work after acute coronary syndrome. Eur Heart J. 2007;28:160–165. doi: 10.1093/eurheartj/ehl440. [DOI] [PubMed] [Google Scholar]
- 53.Parker GB, Hilton TM, Walsh WF, Owen CA, Heruc GA, et al. Timing is everything: the onset of depression and acute coronary syndrome outcome. Biol Psychiatry. 2008;64:660–666. doi: 10.1016/j.biopsych.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Wong CK, Tang EW, Herbison P, Birmingham B, Barclay L, et al. Pre-existent depression in the 2 weeks before an acute coronary syndrome can be associated with delayed presentation of the heart attack. Q J Med. 2008;101:137–144. doi: 10.1093/qjmed/hcm153. [DOI] [PubMed] [Google Scholar]
- 55.Mojtabai R, Olfson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Affairs. 2011;30:1434–1442. doi: 10.1377/hlthaff.2010.1024. [DOI] [PubMed] [Google Scholar]
- 56.Gill JM, Klinkman MS, Chen YX. Antidepressant medication use for primary care patients with and without medical comorbidities: a national electronic health record (EHR) network study. J Am Board Fam Med. 2010;23:499–508. doi: 10.3122/jabfm.2010.04.090299. [DOI] [PubMed] [Google Scholar]
- 57.Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 58.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 59.Blackwell B, Marley E, Price J, Taylor D. Hypertensive interactions between monoamine oxidase inhibitors and foodstuffs. Br J Psychiatry. 1967;113:349–365. doi: 10.1192/bjp.113.497.349. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Preventive Services Task Force. Screening for depression in adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;151:784–792. doi: 10.7326/0003-4819-151-11-200912010-00006. [DOI] [PubMed] [Google Scholar]
- 61.Jureidini J, Tonkin A. Overuse of antidepressant drugs for the treatment of depression. CNS Drugs. 20:623–632. doi: 10.2165/00023210-200620080-00002. [DOI] [PubMed] [Google Scholar]
- 62.Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: An update. Clin Ther. 2008;30:1206–1227. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]
- 63.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 64.Krantz DS, Whittaker KS, Francis JL, Rutledge T, Johnson BD, et al. Psychotropic medication use and risk of adverse cardiovascular events in women with suspected coronary artery disease: outcomes from the Women's Ischemia Syndrome Evaluation (WISE) study. Heart. 2009;95:1901–1906. doi: 10.1136/hrt.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women's Health Initiative Study. Arch Intern Med. 2009;169:2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong GL, Jiang W, Clare R, Shaw LK, Smith PK, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol. 2006;98:42–47. doi: 10.1016/j.amjcard.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 67.Haapea M, Miettunen J, Lindeman S, Joukamaa M, Koponen H. Agreement between self-reported and pharmacy data on medication use in the Northern Finland 1966 Birth Cohort. Int J Methods Psychiatr Res. 2010;19:88–96. doi: 10.1002/mpr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boudreau DM, Daling JR, Malone KE, Gardner JS, Blough DK, et al. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am J Epidemiol. 2004;159:308–317. doi: 10.1093/aje/kwh038. [DOI] [PubMed] [Google Scholar]
- 69.Kwon A, Bungay KM, Pei Y, Rogers WH, Wilson IB, et al. Antidepressant use: concordance between self-report and claims records. Med Care. 2003;41:368–374. doi: 10.1097/01.MLR.0000053019.79054.B6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategies.
(DOC)
Journals Included in Manual Searches.
(DOC)