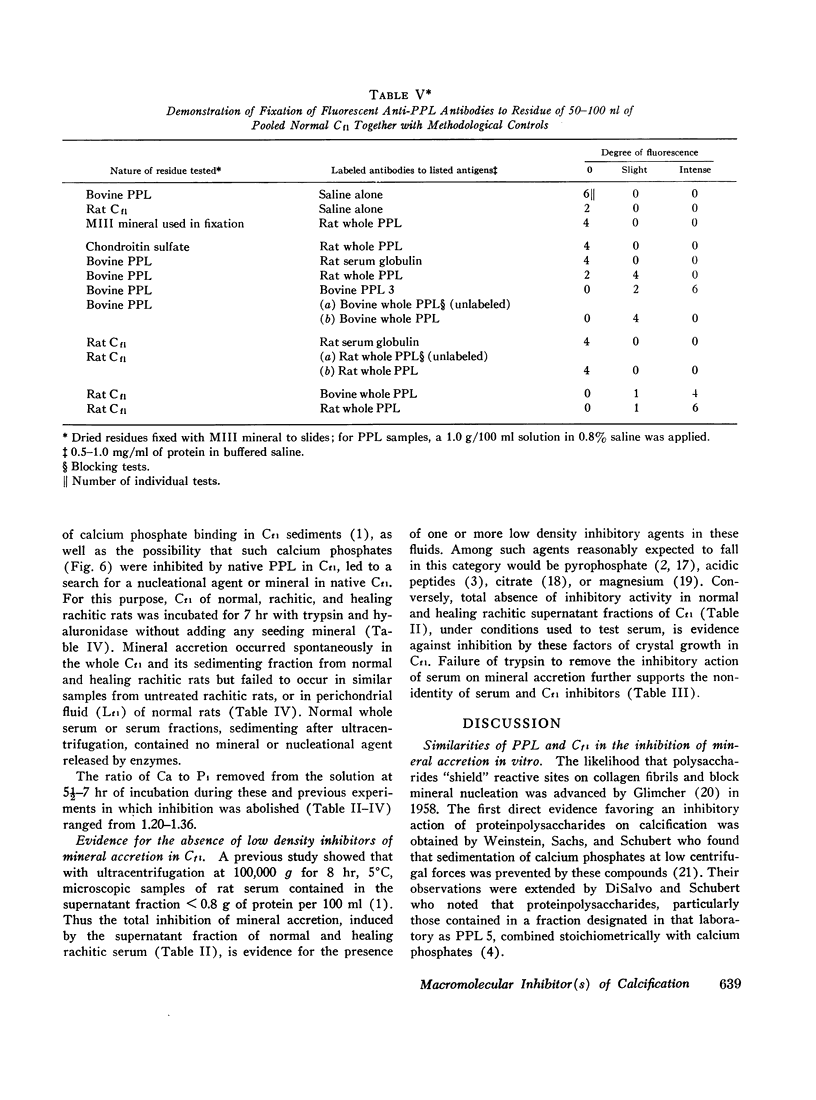
Abstract
An extracellular fluid phase (Cf1), aspirated by micropuncture techniques from the hypertrophic cell zone of calcifying epiphyseal certilage, has been characterized in a calcifying system in vitro in respect to the behavior of sedimenting and supernatant fractions after high speed ultracentrifugation. To perform these tests on the starting samples of 20 nl of Cf1, macroscopic analytical methods were scaled down for the identification of relevant organic components, including hexuronic acid and proteinpolysaccharides (PPL). The mineral accretion system was designed to simulate physiologic conditions in the calcifying cartilage septa of normal rats, and the mineral used for seeding was an immature calcium phosphate similar to native cartilage mineral. Normal Cf1 or its dilutions in synthetic lymph up to 1:4 completely prevented mineral accretion in vitro. The inhibitory action was localized to the sedimented fractions after ultracentrifugation and could be destroyed by incubation with trypsin or hyaluronidase. The sediment of Cf1 contained 2 mg of hexuronic acid per ml of Cf1 and gave a strong reaction of identification for a light fraction of PPL by fluorescent antibodies to rat PPL. PPL fractions were tested in the same mineral accretion systems as Cf1 and exhibited responses similar to those of Cf1. Also, there was evidence of a mineral phase in Cf1 of normal rats, in Cf1 of rats with healing rickets, but not in Cf1 of untreated rachitic rats. These results are interpreted to indicate that certain PPLs function as an inhibitor of crystal growth at extracellular sites premonitory to calcification. Evidence for a low density inhibitor of mineral accretion was found in normal serum but not in Cf1.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachra B. N. Some molecular aspects of tissue calcification. Clin Orthop Relat Res. 1967 Mar-Apr;51:199–222. [PubMed] [Google Scholar]
- Bowness J. M. Present concepts of the role of ground substance in calcification. Clin Orthop Relat Res. 1968 Jul-Aug;59:233–247. [PubMed] [Google Scholar]
- Di Salvo J., Schubert M. Specific interaction of some cartilage proteinpolysaccharides with freshly precipitating calcium phosphate. J Biol Chem. 1967 Feb 25;242(4):705–710. [PubMed] [Google Scholar]
- Dziewiatkowski D. D. The role of sulfated protein-polysaccharides in calcification. Clin Orthop Relat Res. 1964 Jul-Aug;35:189–201. [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol. 1962 Oct;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol. 1962 Oct;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- Hirschman A., Dziewiatkowski D. D. Protein-polysaccharide loss during endochondral ossification: immunochemical evidence. Science. 1966 Oct 21;154(3747):393–395. doi: 10.1126/science.154.3747.393. [DOI] [PubMed] [Google Scholar]
- Howard J. E., Thomas W. C., Jr, Barker L. M., Smith L. H., Wadkins C. L. The recognition and isolation from urine and serum of a peptide inhibitor to calcification. Johns Hopkins Med J. 1967 Mar;120(3):119–136. [PubMed] [Google Scholar]
- Howell D. S., Carlson L. Alterations in the composition of growth cartilage septa during calcification studied by microscopic x-ray elemental analysis. Exp Cell Res. 1968 Jul;51(1):185–195. doi: 10.1016/0014-4827(68)90169-9. [DOI] [PubMed] [Google Scholar]
- Howell D. S., Pita J. C., Marquez J. F., Madruga J. E. Partition of calcium, phosphate, and protein in the fluid phase aspirated at calcifying sites in epiphyseal cartilage. J Clin Invest. 1968 May;47(5):1121–1132. doi: 10.1172/JCI105801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Termine J. D., Wuthier R. E., Posner A. S. Amorphous-crystalline mineral changes during endochondral and periosteal bone formation. Proc Soc Exp Biol Med. 1967 May;125(1):4–9. doi: 10.3181/00379727-125-31999. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN G. M., SACHS C. R., SCHUBERT M. PROTEIN POLYSACCHARIDE IN CONNECTIVE TISSUE: INHIBITION OF PHASE SEPARATION. Science. 1963 Nov 22;142(3595):1073–1075. doi: 10.1126/science.142.3595.1073. [DOI] [PubMed] [Google Scholar]