Abstract
Objectives
The benefit of improved health outcomes for blacks receiving highly active antiretroviral therapy (HAART) lags behind that of whites. This project therefore sought to determine whether the reason for this discrepancy in health outcomes could be attributed to disparities in use of antiretroviral therapy between black and white patients with HIV.
Materials and Methods
The 1996–2006 National Hospital Ambulatory Medical Care Surveys were used to identify hospital outpatient visits that documented antiretrovirals. Patients younger than 18 years, of nonblack or nonwhite race, and lacking documentation of antiretrovirals were excluded. A multivariable logistic regression model was constructed with race as the independent variable and use of HAART as the dependent variable.
Results
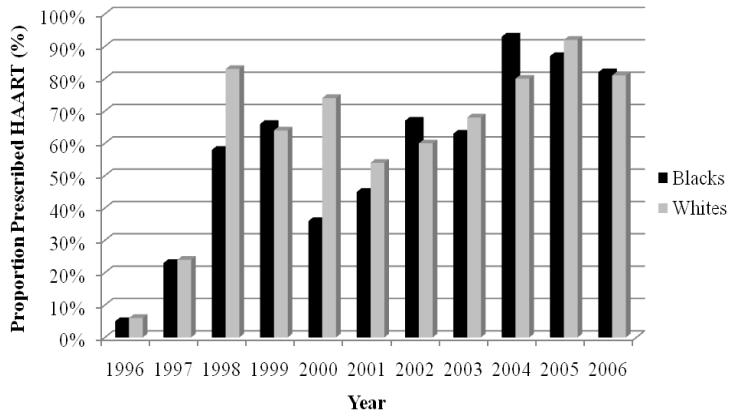
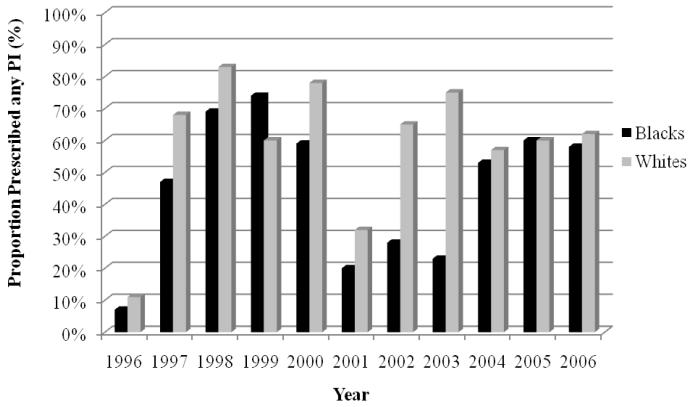
Approximately 3 million HIV/AIDS patient visits were evaluated. Blacks were less likely than whites to use HAART and protease inhibitors (odds ratio, 95% CI 0.81 [0.81–0.82] and 0.67 [0.67–0.68], respectively). More blacks than whites used non-nucleoside reverse transcriptase inhibitors (odds ratio, 95% CI 1.18 [1.17–1.18]). In 1996, the crude rates of HAART were relatively low for both black and white cohorts (5% vs 6%). The rise in HAART for blacks appeared to lag behind that of whites for several years, until 2002, when the proportion of blacks receiving HAART slightly exceeded the proportion of whites receiving HAART. In later years, the rates of HAART were similar for blacks and whites (81% vs 82% in 2006). Blacks appeared less likely than whites to use protease inhibitors and more likely than whites to use non-nucleoside reverse transcriptase inhibitors from 2000 to 2004.
Conclusions
Blacks experienced a lag in the use of antiretrovirals at the beginning of the study; this discrepancy dissipated in more recent years.
Keywords: HIV/AIDS, highly active antiretroviral therapy, racial disparities
Highly active antiretroviral therapy (HAART) has been proven to reduce HIV/AIDS–related morbidity and mortality1; however, it has been postulated that not all patients use antiretroviral therapy equally.2,3 Studies have suggested that racial disparities existed in the years after the introduction of these medications.4,5 Emerging studies suggest that although there may be a convergence of some of these disparities over time, differences may still exist.4,6–8 It has been proposed that higher rates of mortality among blacks with HIV/AIDS compared to whites, may be attributable to less use of protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and combination therapy.5,8–10
Disparities in care may compound the likelihood that certain subgroups may be disadvantaged for receiving optimal treatment. This study examined potential racial differences in usage patterns of antiretrovirals in the HAART era by using the 1996–2006 National Hospital Ambulatory Medical Care Surveys (NHAMCS). This study compared the use of HAART, PIs, and NNRTIs between blacks and whites and evaluated their uptake between blacks and whites from 1996 to 2006.
Materials and Methods
Data Source
Data were retrieved from the NHAMCS, a series of annual national surveys conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. Data were collected to characterize the utilization and provision of hospital-based ambulatory care services in nonfederal hospital outpatient departments. Such survey data have been used to evaluate other areas of racial disparities, including opioid-prescribing patterns, length of emergency department stays, and obesity prevention counseling.11–13
Field representatives from the US Census Bureau trained hospital providers and staff to collect the clinical data contained in the NHAMCS. Patient visits to hospital clinics were systematically sampled via a multistage process during a randomly assigned 4-week reporting period. Clinics in nonfederal, short-stay hospital outpatient departments (average length of stay <30 days) across the United States were identified. The sampling unit for the NHAMCS was an individual patient visit, a visit or encounter for which the patient sought medical care. Therefore, a potential limitation was that some patients may be sampled multiple times. Because the reporting period was only 4 weeks, however, only a patient returning to the clinic within the reporting interval could be sampled more than once. Patient visits were then weighted within the surveys to provide national estimates.
The NHAMCS provided three data entry fields for the healthcare provider completing the survey to indicate the patient’s medical diagnoses. Medication information also was collected within the NHAMCS survey instrument. Providers were instructed to record all of the “new or continued” medications during the patient’s visit. Medications were classified as any drug or medication that was ordered, supplied, or administered during the patient’s visit, or any medication that a patient was instructed to continue. In 2003, the survey medication entry fields were increased from 6 to 8 medications. Computer edits for code ranges and inconsistencies, clerical edits, and audits for data collection completeness were performed by field staff. Medication data were coded by a classification scheme developed by the National Center for Health Statistics. Quality control measures for medication coding were performed centrally by Constella Group Inc (Durham, NC).
Study Definitions
HIV/AIDS ambulatory visits were defined as any visit with a primary diagnosis of at least 1 of the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for HIV diagnosis: 042 (HIV disease), 043 (AIDS-related complex), 044 (other HIV disease), V08 (asymptomatic HIV infection), and 079.53 (HIV-2 illness).14 A primary diagnosis of HIV/AIDS therefore indicated an ambulatory visit primarily related to HIV/AIDS. All of the HIV/AIDS related visits were then limited further to those that documented at least one antiretroviral. US Food and Drug Administration (FDA)–approved antiretrovirals available during the study’s timeframe were identified by generic name code and/or and therapeutic ingredient code and are listed in Table 1.
Table 1.
Differences in Prescribing Patterns by Types of Antiretroviral Medication between Black vs. Whites HIV/AIDS Patients
| Type of Antiretroviral Therapy Prescribed |
Total (N=2,970,222) |
Race | OR (95% CI) | |
|---|---|---|---|---|
| Blacks (58%) |
Whites (42%) |
|||
| HAART | 2,012,991 | 66% | 70% | 0.81 (0.81-0.82) |
| Any PI | 1,652,902 | 52% | 61% | 0.67 (0.67-0.68) |
| Any NNRTI | 886,060 | 31% | 28% | 1.18 (1.17-1.18) |
HAART = Highly active antiretroviral therapy
PI = Protease Inhibitor
NNRTI = Non-nucleoside reverse transcriptase inhibitor
HAART was defined as regimens that contained specific combinations of antiretrovirals. Combinations included the following: ≥3 NRTIs, ≥1 PI + ≥2 NRTIs, ≥1 NNRTI + ≥2 NRTIs, ≥1 NRTI + ≥1 PI + ≥1 NNRTI, or enfuvirtide in combination with other antiretrovirals. Any regimen containing enfuvirtide (HIV fusion inhibitor, FDA approved in 2003) was classified as HAART. All mono- or dual-antiretroviral regimens were excluded from the HAART definition. Similar definitions of HAART have been used in other studies retrospectively evaluating antiretroviral therapy.7, 8 Specific guideline recommendations for antiretroviral combinations change as new guidelines are published based on emerging HIV literature; however, the general recommendation for combination therapy with HAART has been the standard of care since 1996, when PIs and NNRTIs first became available.15 Later antiretroviral guidelines list specific regimens as “preferred” or “alternative.”16 They also discourage the use of certain combinations of antiretrovirals because of the potential for adverse effects.16 The present study did not evaluate specific antiretroviral combinations; rather, it evaluated class components of regimens. Two newer classes of antiretrovirals are also approved for HIV treatment: CCR5 antagonist (maraviroc, FDA approved in 2007) and integrase inhibitor (raltegravir, FDA approved in 2007). These medications were not approved until after the present study’s time frame. For the purposes of data analysis, the different forms of insurance were defined as uninsured (no charge or self- pay), government insurance (Medicare, Medicaid, or other government form of payment), private insurance (health maintenance organization/preferred provider organization, BlueCross BlueShield, or other private insurance), and other/unknown insurance (workers’ compensation, other, or not stated). Age at time of outpatient visit was also collapsed into categorical variables and into the following groups: 18 to 34 years, 35 to 49 years, and 50 years old and older.
Study Design
This study was a cross-sectional retrospective analysis of HIV/AIDS–related outpatient, hospital-based physician visits in the United States from 1996 to 2006 with documented use of an antiretroviral medication. The University of Texas at Austin institutional review board reviewed and approved the study protocol. Data included age, race, sex, year of ambulatory visit, insurance status, geographic region, and antiretroviral medication. Inclusion criteria included any outpatient clinic visit that occurred between 1996 and 2006 and a primary ICD-9-CM code for HIV/AIDS at the time of clinic visit. Exclusion criteria included age younger than 18 years, no documented antiretroviral therapy, and any documented race other than black or white.
Statistical Analysis
The primary study outcome included use of HAART at clinic visit and secondary outcomes included use of any PI or any NNRTI at clinic visit. Patient visit weights were incorporated into the analysis to provide national estimates for patients using therapy. For bivariable analysis, χ2 tests were used to investigate differences in categorical visit characteristics with respect to race between the two cohorts (black vs white). P values that were less than a prespecified alpha level of 0.05 were considered to be of statistical significance. Statistically significant variables from χ2 analyses were entered into a multivariable logistic regression model using normalized weights to assess black race (independent variable) as a predictor of HAART (dependent variable). To trend black race as a predictor of HAART throughout the study time frame, cross-sectional logistic regression analyses were individually performed for the following years: 1996–1997, 1998–1999, 2000–2001, 2002–2003, and 2004–2005, and 2006. Parameter estimates from logistic regressions were transformed into odds ratios and are presented with the accompanying independent variables and covariates. All of the data were analyzed using JMP 7.0 (SAS Institute Inc, Cary, NC).
Results
From 1996 to 2006, there were an estimated 926.7 million visits made to hospital outpatient departments, according to the NHAMCS surveys. HIV/AIDS–related visits represented 24.4 million (3%) of all of the visits. Of all of the HIV-related hospital outpatient department visits, 45% of the patients were white and 38% were black; the remaining 17% were of various racial groups. An estimated 12.4 million of these HIV-related visits were associated with antiretroviral therapy. The remainder of the data presented here pertains to the study cohort—black and white patients with a principal diagnosis of HIV/AIDS who used antiretroviral therapy.
A total of 2,970,222 patient visits met the study inclusion criteria; 58% of the patients were black and 42% were white. Of all of the patients on any antiretroviral medication, most (68%) documented HAART. Most used at least 1 PI (56%) and many used 1 NNRTI (30%). The mean patient age (years, 95% CI) was 42 years (range 41–43) years, and 73% of all of the visits were made by men. A large proportion of patients held some form of government insurance (49%), followed by private insurance (24%) or no insurance (16%); the remainder were either other/unknown forms of insurance. A majority of the patients had been examined in the clinic previously (96%) and most patients were examined in a general medicine clinic (93%).
Antiretroviral Differences between Blacks and Whites
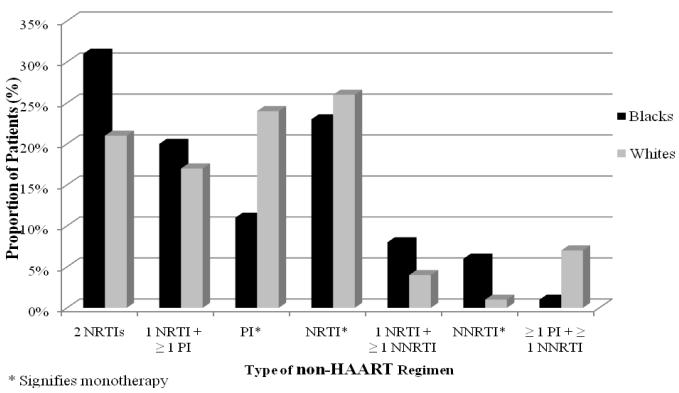
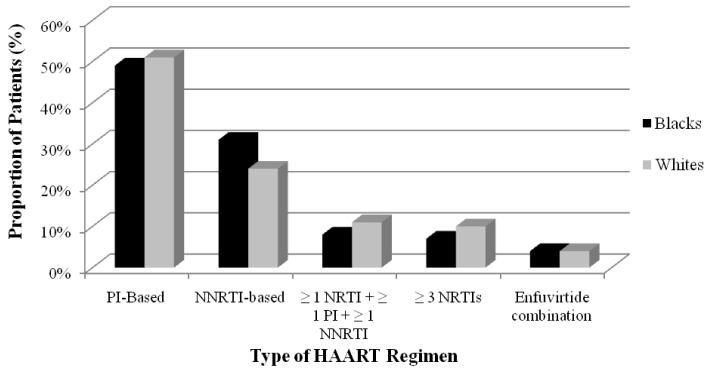
Differences in antiretroviral patterns for HAART, Pis, and NNRTIs between blacks and whites are displayed in Table 2. Blacks were less likely than whites to use HAART and PIs (odds ratio [OR], 95% confidence interval [CI] 0.81 [0.81–0.82] and 0.67 [0.67–0.68]), respectively. In contrast, a greater proportion of blacks used an NNRTI compared to whites (OR, 95% CI 1.18 [1.17–1.18]). Components of HAART and non-HAART regimens are illustrated in Figures 1 and 2, respectively. When comparing components of HAART and non-HAART regimens, the lower rates of PIs and higher rates of NNRTIs for blacks were consistent.
Table 2.
Selected Characteristics between Black and White HIV/AIDS Patients Prescribed Antiretroviral Therapy
| Characteristic | Total (N=2,970,222) |
Race |
P-Value | |
|---|---|---|---|---|
| Blacks (58%) |
Whites (42%) |
|||
| Age (years) | < 0.001 | |||
| 18-34 | 579,771 | 22% | 16% | |
| 35-49 | 1,857,380 | 60% | 66% | |
| ≥ 50 years | 533,071 | 18% | 18% | |
|
| ||||
| Gender | < 0.001 | |||
| Male | 2,168,597 | 67% | 81% | |
| Female | 801,625 | 33% | 19% | |
|
| ||||
| Insurance Status | < 0.001 | |||
| Government | 1,442,976 | 51% | 47% | |
| Private | 708,705 | 19% | 30% | |
| Uninsured | 482,023 | 18% | 13% | |
| Other | 336,518 | 12% | 10% | |
|
| ||||
| Geographic Region | < 0.001 | |||
| Northeast | 923,245 | 31% | 31% | |
| Midwest | 851,879 | 26% | 33% | |
| South | 985,497 | 41% | 22% | |
| West | 209,601 | 2% | 14% | |
Fig. 1.
Composition of HAART regimens for black and white patients with HIV/AIDS.
Fig. 2.
Composition of non-HAART regimens for black and white patients with HIV/AIDS.
Trends in Usage Patterns between Blacks and Whites
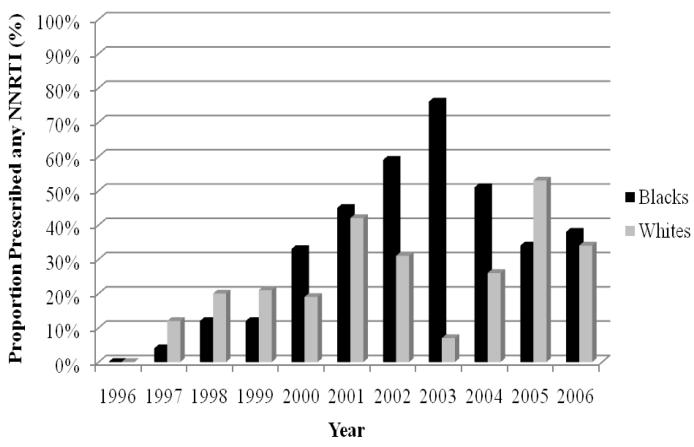
The trends in use of HAART are depicted in Figure 3. In 1996, the crude rates of HAART were relatively low for black and white cohorts (5% vs 6%). The rise in HAART for blacks appeared to lag behind that of whites for several years. By 2002, the proportion of blacks receiving HAART slightly exceeded the proportion of whites receiving therapy. In later years, the rates of HAART were similar for blacks and whites (81% vs 82% in 2006). The patterns for PIs and NNRTIs are illustrated in Figures 4 and 5, respectively. The rate of PIs was, in general, lower for blacks from 1996 to 2003. This discrepancy seemed to dissipate from 2004 to 2006. This pattern was in contrast to that of the NNRTIs. In the first few years after NNRTIs were made available, their use among blacks consistently lagged behind that of whites, until 2000 to 2004, when blacks had higher rates of NNRTIs. The racial gap reversed once more in 2005 and rates of NNRTI use were relatively similar in 2006.
Fig. 3.
Trends in HAART for black and white patients with HIV/AIDS, 1996–2006.
Fig. 4.
Trends in protease inhibitors (PIs) for black and white patients with HIV/AIDS, 1996– 2006.
Fig. 5.
Trends in non-nucleoside reverse transcriptase inhibitor (NNRTIs) for black and white patients with HIV/AIDS, 1996–2006.
Demographic Predictors of HAART
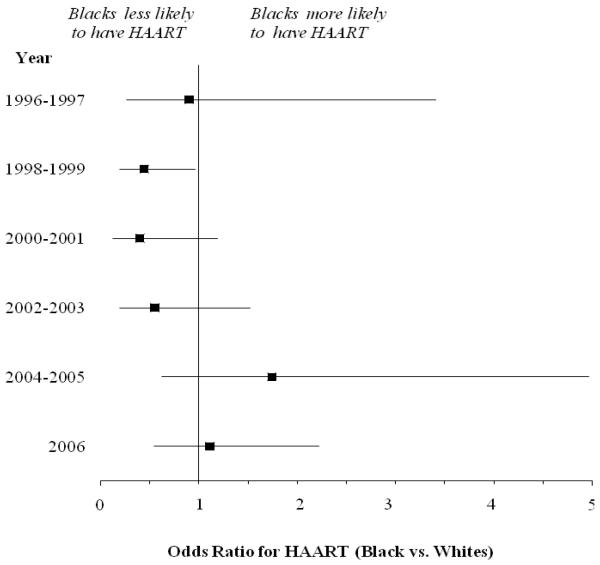
When discrepancies in baseline characteristics were considered, black race was not found to be associated with HAART (vs white race; OR, 95% CI 0.84 [0.61–1.16], Table 3). Significant predictors included logistic regression χ2, year (218; P < 0.001), and insurance status (14.6; P = 0.002). Multivariable logistic regression analysis was repeated from 1996 to 2006 to assess black race as an independent predictor of HAART within specific time frames. The results of these analyses are illustrated in Figure 6. For each individual time frame, black race was associated only with a decreased likelihood of HAART from 1998 to 1999. No significant associations were detected for any other years.
Table 3.
Demographic Predictors of HAART from Multivariable Logistic Regression Analysis
| Characteristic | OR (95% CI) | LR Chi-Square | P-value |
|---|---|---|---|
| Race | 1.06 | 0.3 | |
| White | 1.00 (ref.) | ||
| Black | 0.84 (0.61-1.16) | ||
|
| |||
| Year | 218.0 | < 0.001 | |
| 1996-1997 | 1.00 (ref.) | ||
| 1998-1999 | 1.49 (0.80-2.79) | ||
| 2000-2001 | 0.33 (0.14-0.76) | ||
| 2002-2003 | 1.32 (0.67-2.66) | ||
| 2004-2005 | 1.39 (12.99-65.30) | ||
| 2006 | 5.95 (3.18-11.30) | ||
|
| |||
| Geographic Region | 2.9 | 0.4 | |
| Northeast | 1.00 (ref.) | ||
| Midwest | 0.73 (0.37-1.42) | ||
| South | 1.26 (0.72-2.19) | ||
| West | 1.46 (0.61-3.55) | ||
|
| |||
| Age (years) | 0.9 | 0.6 | |
| 18-34 | 1.00 (ref.) | ||
| 35-49 | 1.12 (0.75-1.69) | ||
| ≥ 50 | 1.14 (0.66-1.99) | ||
|
| |||
| Gender | 2.0 | 0.2 | |
| Female | 1.00 (ref.) | ||
| Male | 1.28 (0.91-1.79) | ||
|
| |||
| Insurance Status | 14.6 | 0.002 | |
| Private | 1.00 (ref.) | ||
| Uninsured | 1.03 (0.74-1.99) | ||
| Government | 0.41 (0.26-0.67) | ||
| Other | 1.42 (0.70-2.97) | ||
Fig. 6.
Adjusted predictors for use of HAART for black versus white patients with HIV/AIDS, 1996–2006.
Discussion
The present study describes differences in antiretroviral use between black and white patients with HIV/AIDS in ambulatory clinics across the United States through 2006 of the HAART era. The data estimate that on average 270,000 patients in care each year were receiving antiretroviral therapy. This number is comparable to estimates from 2003 national data that identified that 268,000 (95% CI 253,000–284,000) individuals used antiretroviral therapy in a given year.17 In addition, the study demonstrates that HAART was well disseminated among patients soon after combination therapy was recommended for routine practice. At the beginning of the study, few patients used HAART—only 5% in 1996. Patients demonstrated rapid uptake of HAART in the years following its availability, with the rate increasing to 82% in 2006; similar proportions of HAART use have been reported elsewhere.1,18,19 Although a majority of patients overall used HAART, blacks were less likely overall to use HAART as compared with whites (66% vs 70%). After adjusting for variables including visit year, sex, insurance status, and geographic location, these disparities no longer existed. Study results also failed to identify a negative association between black race and use of HAART in the individual study years, except from 1998 to 1999.
Disparities in HAART have been characterized in the literature. Demographic factors including black race/nonwhite ethnicity, older age, and female sex have been associated with a decreased likelihood of HAART.7,8,20–22 In contrast, a lack of treatment disparities among blacks and whites has been demonstrated as patients continue to seek care in referral centers.23,24 The present study suggests that blacks were disadvantaged initially for using therapy, but may no longer be disadvantaged. Similarities between the present study and the two aforementioned studies include adjustments for age, sex, race, and defining therapy according to antiretroviral availability during each study.23,24 Similar to the two studies, the present investigation could not account for medication adherence or correlate use of therapy with health outcomes.23,24 The current investigation, however, carried the advantage of evaluating national data for approximately 3 million patient visits associated with antiretroviral therapy for more than 10 years immediately after HAART introduction. This investigation also was able to evaluate how black race changed as a predictor of HAART during that time. Unlike the previous studies, this study was able to trend use of PIs and NNRTIs between blacks and whites. An unexpected disparity was noted in the use of PIs; use of PIs for blacks tended to lag behind that of whites for much of the study. The reverse was noted for the use of NNRTIs. Future investigations should explore differences in medication adherence, treatment costs, and the burden of drug–drug/drug– disease interactions as possible explanations for these race-based differences in antiretroviral use.
This study was able to classify components of non-HAART regimens by race. Dual NRTI therapy use, a non-HAART regimen, was greater among blacks than whites (10% vs 6%). Specific NRTIs can be used as dual NRTI therapy but only for the treatment of chronic HBV monoinfection.25 The antiretroviral guidelines recommend patients requiring hepatitis B virus treatment be started on fully suppressive HAART regimens using agents that are active against both viruses.16 PI monotherapy was lower for blacks than for whites (4% vs 7%). The use of PI monotherapy is under investigation to prevent some of the toxicities of NRTIs while effectively maintaining virological suppression.26,27 NRTI monotherapy was 8% for both blacks and whites. None of the above regimens are considered to be highly active and have not been recommended for use by guidelines since 1996.15 The only exception is the endorsed recommendation for zidovudine (an NRTI) monotherapy in HIV-positive pregnant women for the prevention of perinatal transmission.28 In this study, zidovudine monotherapy among females was documented in only 1% of all of the patients. It is hoped that identifying specific components of non-HAART regimens in this study will facilitate changes in clinical practice, whereby clinicians can identify the patients who may be at risk for using suboptimal regimens.
This study identified a disparity in the proportion of black and white women (33% vs 19%). Black women continue to bear an increasingly disproportionate burden of HIV/AIDS in the United States. Although gender disparities among races were not addressed in this study, future efforts are needed to curb the epidemic of HIV/AIDS among black women. This epidemic also has been documented to affect certain regions of the United States more than others.29 Forty percent of cumulative AIDS patients are individuals residing in the south, followed by 29% in the northeast, 20% in the west, and 11% in the midwest.29 A lesser percentage of blacks in this study were from the west compared with whites (2% vs 14%). The data represented in this study may not provide accurate information for the epidemic among blacks in the western United States.
Simply using antiretrovirals may not be sufficient for improving some of the cited disparities in health outcomes.5,29–31 There may be underlying explanations beyond therapy that may explain differences in black-white mortality that were not assessed in the present investigation. Minorities, specifically blacks, tend to bear a disproportionate burden of HIV/AIDS transmission risk factors, comorbidities, and delays in presentation.29,32–36 These factors may have a deleterious impact on black patients, thereby increasing disease burden and potentially placing them at a higher risk for detrimental outcomes even when using HAART.32–36 These factors were not assessed in the present study because of the scope of data collected in the NHAMCS, but they may have a role in explaining some of the differences in health outcomes that have been documented in other studies.
Certain limitations should be considered when interpreting these results. HAART use could not be correlated with objective laboratory markers of disease progression that indicate when patients should be started on antiretroviral therapy because this information is not available within the NHAMCS.16 Furthermore, the present study is representative only of patients who are already receiving HIV/AIDS care. Therefore, it was not possible to evaluate the potential racial disparities that may exist regarding timely diagnosis and treatment delays postdiagnosis. In addition, rates of antiretroviral therapy were based on documentation of therapy but neither take medication adherence nor the appropriateness of individual components of HAART regimens into consideration. Finally, other variables such as intravenous drug abuse, comorbid conditions, and social and economic status may influence HAART use, but these variables were not factored into the regression analysis because these were not encompassed within the NHAMCS surveys.
Conclusions
The study potentially dispels one of the theories surrounding the cause of health disparities among black and white patients with HIV/AIDS, namely that disparities in outcomes cited by other studies may not result solely from differences between blacks and whites in antiretroviral use. Several alternative theories remain as to why black patients fail to attain the optimal benefits of antiretroviral therapy.
Key Points.
The benefit of improved health outcomes for blacks with HIV/AIDS receiving highly active antiretroviral therapy (HAART) typically lags behind that of whites with HIV/AIDS.
This study sought to determine whether discrepancies in health outcomes between blacks and whites could be attributable to differences in the use of HAART.
Blacks were initially less likely to use HAART compared with whites; however, this disparity has dissipated in recent years.
Several alternative theories remain as to why black patients with HIV/AIDS fail to attain the optimal benefits of HAART.
Brief Description.
Historically, health outcomes for blacks with HIV/AIDS have lagged behind those of whites with HIV/AIDS, possibly because of a decreased likelihood of receiving antiretroviral therapy. The results of this study indicate that the disparities in outcomes may not be attributable to receipt of therapy because disparities in therapy between blacks and whites existed after 1996 but gradually dissipated. Prescribing antiretroviral therapy does not appear to be enough for black patients with HIV/AIDS to experience improved health outcomes.
Acknowledgments
The authors thank Kyllie Ryan-Hummel for assistance with this project.
Funding for this project was provided in part by the National Institutes of Health (NIH) Loan Repayment Program and a graduate fellowship from the University of Texas at Austin, both granted to Dr Oramasionwu.
Dr Frei is supported by the NIH in the form of a NIH/KL2 career development award (3UL1RR025767). In addition, Dr Frei has received research grants and/or served as a scientific consultant/advisor for AstraZeneca, Forest Laboratories, Ortho McNeil Janssen Pharmaceuticals, and Pfizer.
Footnotes
The other authors have no financial relationships to disclose and no conflicts of interest to report.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palella FJ, Jr., Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Palacio H, Kahn JG, Richards TA, Morin SF. Effect of race and/or ethnicity in use of antiretrovirals and prophylaxis for opportunistic infection: a review of the literature. Public Health Rep. 2002;117:233–251. doi: 10.1093/phr/117.3.233. discussion 231-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oramasionwu CU, Skinner J, Ryan L, Frei CR. Disparities in antiretroviral prescribing for blacks and whites in the United States. J Natl Med Assoc. 2009;101:1140–1144. doi: 10.1016/s0027-9684(15)31110-x. [DOI] [PubMed] [Google Scholar]
- 4.Sambamoorthi U, Moynihan PJ, McSpiritt E, Crystal S. Use of protease inhibitors and non-nucleoside reverse transcriptase inhibitors among Medicaid beneficiaries with AIDS. Am J Public Health. 2001;91:1474–1481. doi: 10.2105/ajph.91.9.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RS, Briggs NC, Kilbourne BS, et al. Black-White mortality from HIV in the United States before and after introduction of highly active antiretroviral therapy in 1996. Am J Public Health. 2007;97:1884–1892. doi: 10.2105/AJPH.2005.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair JM, Fleming PL, Karon JM. Trends in AIDS incidence and survival among racial/ethnic minority men who have sex with men, United States, 1990-1999. J Acquir Immune Defic Syndr. 2002;31:339–347. doi: 10.1097/00126334-200211010-00011. [DOI] [PubMed] [Google Scholar]
- 7.King WD, Minor P, Kitchen C Ramirez, et al. Racial, gender and geographic disparities of antiretroviral treatment among US Medicaid enrolees in 1998. J Epidemiol Community Health. 2008;62:798–803. doi: 10.1136/jech.2005.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-Infected persons in care. J Infect Dis. 2009;199:991–998. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oramasionwu CU, Hunter JM, Skinner J, et al. Black race as a predictor of poor health outcomes among a national cohort of HIV/AIDS patients admitted to US hospitals: a cohort study. BMC Infect Dis. 2009;9:127. doi: 10.1186/1471-2334-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oramasionwu CU, Brown CM, Lawson KA, Ryan L, Frei CR. Evaluating HIV/AIDS disparities for Blacks in the United States: a review of antiretroviral and mortality studies. J Natl Med Assoc. 2009;101:1221–1229. doi: 10.1016/s0027-9684(15)31133-0. [DOI] [PubMed] [Google Scholar]
- 11.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 12.Pines JM, Localio A Russell, Hollander JE. Racial disparities in emergency department length of stay for admitted patients in the United States. Acad Emerg Med. 2009 doi: 10.1111/j.1553-2712.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- 13.Branner CM, Koyama T, Jensen GL. Racial and ethnic differences in pediatric obesity-prevention counseling: national prevalence of clinician practices. Obesity (Silver Spring) 2008;16:690–694. doi: 10.1038/oby.2007.78. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed March 29, 2009];Official Authorized Addenda: Human Immunodeficiency Virus Infection Codes and Official Guidelines for Coding and Reporting ICD-9-CM. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00032908.htm.
- 15.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276:146–154. [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed Feb 7, 2011]. Jan 10, 2011. pp. 1–161. Available at: http://www.aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 17.Teshale E, Kamimoto L, Harris N, et al. Estimated number of HIV-Infected persons eligible for and receiving HIV antiretroviral therapy, 2003--United States. Conf Retrovir Opportunistic Infect. 2005 Feb 22-25;:12. abstract no. 167. [Google Scholar]
- 18.Lillie-Blanton M, Stone VE, Jones A Snow, et al. Association of race, substance abuse, and health insurance coverage with use of highly active antiretroviral therapy among HIV-infected women, 2005. Am J Public Health. 2009;100:1493–1499. doi: 10.2105/AJPH.2008.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elford J, Ibrahim F, Bukutu C, Anderson J. Uptake of antiretroviral treatment among people living with HIV in London: ethnicity, gender and sexual orientation. Sex Transm Infect. 2008;84:176–178. doi: 10.1136/sti.2007.029249. [DOI] [PubMed] [Google Scholar]
- 20.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 21.McNaghten AD, Hanson DL, Dworkin MS, Jones JL. Differences in prescription of antiretroviral therapy in a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:499–505. doi: 10.1097/00126334-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Holodniy M, Hornberger J, Rapoport D, et al. Relationship between antiretroviral prescribing patterns and treatment guidelines in treatment-naive HIV-1-infected US veterans (1992-2004) J Acquir Immune Defic Syndr. 2007;44:20–29. doi: 10.1097/01.qai.0000248354.63748.54. [DOI] [PubMed] [Google Scholar]
- 23.Moore RD, Stanton D, Gopalan R, Chaisson RE. Racial differences in the use of drug therapy for HIV disease in an urban community. N Engl J Med. 1994;330:763–768. doi: 10.1056/NEJM199403173301107. [DOI] [PubMed] [Google Scholar]
- 24.Reif S, Whetten K, Thielman N. Association of race and gender with use of antiretroviral therapy among HIV-infected individuals in the Southeastern United States. South Med J. 2007;100:775–781. doi: 10.1097/SMJ.0b013e3180f626b4. [DOI] [PubMed] [Google Scholar]
- 25.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 26.Delfraissy JF, Flandre P, Delaugerre C, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. AIDS. 2008;22:385–393. doi: 10.1097/QAD.0b013e3282f3f16d. [DOI] [PubMed] [Google Scholar]
- 27.Pulido F, Arribas JR, Delgado R, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and two nucleosides for maintenance therapy of HIV. AIDS. 2008;22:F1–9. doi: 10.1097/QAD.0b013e3282f4243b. [DOI] [PubMed] [Google Scholar]
- 28.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention [Accessed Oct 26, 2010];Diagnoses of HIV Infection and AIDS in the United States and dependent areas. 2008 Available at: http://www.cdc.gov/hiv/surveillance/resources/reports/2008report/index.htm.
- 30.Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100:1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine RS, Rust GS, Pisu M, et al. Increased Black-White disparities in mortality after the introduction of lifesaving innovations: a possible consequence of US federal laws. Am J Public Health. 2010;100:2176–2184. doi: 10.2105/AJPH.2009.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention HIV infection among young black men who have sex with men--Jackson, Mississippi, 2006-2008. MMWR Morb Mortal Wkly Rep. 2009;58:77–81. [PubMed] [Google Scholar]
- 33.Late versus early testing of HIV--16 Sites, United States, 2000-2003. MMWR Morb Mortal Wkly Rep. 2003;52:581–586. [PubMed] [Google Scholar]
- 34.Estrada AL. Health disparities among African-American and Hispanic drug injectors-- HIV, AIDS, hepatitis B virus and hepatitis C virus: a review. AIDS. 2005;19(Suppl 3):S47–52. doi: 10.1097/01.aids.0000192070.95819.7c. [DOI] [PubMed] [Google Scholar]
- 35.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman LM, Berman SM. Epidemiology of STD disparities in African American communities. Sex Transm Dis. 2008;35:S4–12. doi: 10.1097/OLQ.0b013e31818eb90e. [DOI] [PubMed] [Google Scholar]