Abstract
Objective
To establish associations between leg strength and mortality in men and women with lower extremity peripheral arterial disease (PAD).
Design
Observational, prospective study.
Setting
Chicago area medical centers.
Subjects
Participants were 410 men and women with PAD age 55 and older followed for a mean of 60.0 months.
Interventions
Isometric knee extension, knee flexion, hip extension, and hip flexion were measured at baseline. Primary outcomes were all-cause and cardiovascular disease mortality. Cox proportional hazards models were used to assess relations between leg strength and all-cause and cardiovascular disease mortality among men and women, adjusting for age, race, comorbidities, smoking, body mass index, and the ankle brachial index.
Results
Among the 246 male participants, poorer baseline strength for knee flexion (P trend = .029), knee extension (P trend =.010), and hip extension (P trend = .013) were each associated independently with higher all-cause mortality. Poorer strength for knee flexion (P trend = .042) and hip extension (P trend = .029) were associated with higher cardiovascular mortality. Compared to those in the fourth (best) baseline knee flexion quartile, Hazard Ratios for all-cause and cardiovascular disease mortality among men in the 1st (poorest) knee flexion quartile were 2.23 (95% Confidence Interval (CI) = 1.02–4.87, P=.045) and 4.20 (95% CI = 1.12–15.79, P=.043), respectively. No significant associations of leg strength and all-cause mortality were identified among women.
Conclusions
Poorer leg strength is associated with increased mortality in men, but not women, with PAD. Future study is needed to determine whether interventions that increase leg strength improve survival in men with PAD.
Eight million people in the United States have lower extremity peripheral arterial disease (PAD), which is associated with increased cardiovascular events and greater functional impairment compared to individuals without PAD1,2. PAD is also associated with adverse calf muscle characteristics and reduced lower extremity strength, compared to those without PAD3,4. Smaller calf muscle area and reduced lower extremity strength are associated with greater functional impairment, increased rates of mobility loss, and/or functional decline in men and women with PAD3–6.
The prognostic significance of leg strength for all-cause and cardiovascular mortality is unknown in PAD. This prospective cohort study assessed the relationship between leg strength and all-cause as well as cardiovascular disease mortality in men and women with PAD. We hypothesized that poorer leg strength in PAD participants would be associated with increased all-cause and cardiovascular mortality rates.
METHODS
Participant Identification
The Institutional Review Boards at Northwestern University and Catholic Health Partners Hospital in Chicago approved the study protocol. Participants were men and women age 55 and older at baseline with PAD in the Walking and Leg Circulation Study (WALCS) cohort1. Participants provided written informed consent.
Methods for the WALCS have been described1,6. PAD was defined as an ankle brachial index (ABI) < 0.903–6. Most participants were identified from among consecutive patients diagnosed with PAD in three Chicago-area non-invasive vascular laboratories. A small number were identified from among patients age 55 and older in a large general internal medicine practice who were screened with the ABI and found to have an ABI < 0.90.
Exclusion Criteria
Exclusion criteria for the WALCS cohort have been reported previously and are summarized briefly here1,6. Patients with severe functional impairment at baseline, including nursing home residents, wheelchair bound persons, and individuals with leg amputations were excluded. To ensure accuracy of patient-reported medical history, potential participants with dementia were excluded. Since the staff was not fluent in languages other than English, non-English-speaking patients were excluded. Participants with life expectancy < one year were excluded.
Ankle Brachial Index Measurements
Participants rested in the supine position for five minutes prior to ABI measurements. Using a handheld Doppler Probe (Nicolet Vascular Pocket Dop II, Nicolet Biomedical Inc., Golden, Colorado), systolic blood pressures were measured in the following order: right brachial artery, right dorsalis pedis and posterior tibial arteries, left dorsalis pedis and posterior tibial arteries, and left brachial artery.1,3–7 Two sets of measurements for each pressure were obtained: first in the order listed and then in reverse order. The mean of the dorsalis pedis and posterior tibial pressures in each leg was divided by the mean of all four brachial pressures to obtain the ABI value7. Subclavian stenosis was suspected if the same brachial pressure was higher in both measurement sets, and there was a difference of at least 10 mm Hg between the brachial pressures in either measurement set. In these cases, the average brachial pressure in the arm with highest pressure was used to calculate the ABI value.8
Comorbidities
Comorbidities assessed were diabetes, cardiac or cerebrovascular disease (angina, myocardial infarction, stroke, and heart failure), pulmonary disease, and cancer, using comorbidity algorithms from the Women's Health and Aging Study9. These comorbidity algorithms combine data from patient report, medical record review, physical examination, medications, laboratory values, and questionnaires completed by the participant's primary care physician9. Algorithms from the American College of Rheumatology were used to determine presence of knee and hip arthritis10,11.
Body Mass Index
Height (kilograms) and weight (meters) were measured. Body mass index (BMI) was calculated as kilograms/(meter)2.
Leg Strength
A Musculoskeletal Fitness Evaluation (MFE) chair was used to measure isometric lower extremity knee flexion, knee extension, hip flexion, and hip extension strength4,6. The MFE chair has strain gauges connected to a computerized data collecting unit that records isometric strength. Participants sat in the MFE chair and were instructed to build to their maximum strength by pushing against leg attachments. Strength was recorded in Newton-meters over five seconds. The highest recorded strength during the final three seconds was used for analyses. Strength measurements were performed twice and averaged. Pearson correlation coefficients between the first and second trials ranged from 0.90 (P<.001) to 0.96 (P<.001) for each strength measure. The leg strength value for the leg with the lowest ABI was used in analyses4,6. If the ABI value was the same in both legs, strength from the right leg was considered to be the index leg.
Six-minute walk
Following a standardized protocol1,5,6 participants walked up and down a 100-foot hallway for six minutes after instructions to cover as much distance as possible. The distance achieved at the end of six-minutes was recorded.
Cigarette Smoking and Physical Activity Measures
Cigarette smoking history was obtained using self-report. Participants' physical activity was measured with a questionnaire derived from the Harvard Alumni Activity Survey that has been previously validated12–13.
Death
The Social Security Administration death database was used to search for deaths. Additional information on death was acquired from family members, proxies, and physicians of study participants. The International Classification of Disease-10 codes in the range of 101.0 through 199.9 were used by a certified nosologist to identify deaths from cardiovascular disease. The State of Illinois or medical records of the patient were used to obtain death certificates13.
Statistical Analyses
Baseline characteristics between survivors and decedents were compared using analyses of covariance, adjusting for age and sex. Among decedents, baseline characteristics between those with vs. without death certificates were compared using t-tests for continuous variables and chi-square tests for dichotomous variables.
Prior study demonstrated distinct associations of leg strength with functional decline among men and women with PAD6. Therefore, analyses were performed separately in men and women with PAD. For the leg with lowest ABI, leg strength measurements were categorized into four quartiles ranging from poorest (1st quartile) to highest strength (4th quartile). Baseline characteristics of men and women, respectively, were compared across quartiles of leg strength using t-tests for continuous variables and chi-square tests for dichotomous variables. A P value for trend was used for these associations, which has more statistical power to detect linear associations than a traditional P value. Cox proportional hazard analyses were performed to compare differences in all-cause and cardiovascular mortality across the leg strength quartiles among men and women, respectively, adjusting for age, race, BMI, smoking history, diabetes, cardiac and cerebrovascular disease, pulmonary disease, cancer, ABI, and physical activity. Analyses were repeated with additional adjustment for six-minute walk performance, to determine whether significant associations of leg strength with mortality were independent of functional performance.
In analyses for cardiovascular disease mortality, we imputed the cause of death when death certificates were unobtainable, using methods proposed by Lu and Tsiatis14. The probability of cardiovascular disease death was modeled using logistic regression. Results were used to impute the unknown cause of death (cardiovascular vs. non-cardiovascular death). The Cox regression analysis was performed using the imputed cause of death. The imputation and corresponding regression analyses were repeated five times and the average of the ten estimated regression coefficients was used to determine the hazard ratios for cardiovascular death.
Because knee arthritis can potentially influence leg strength, participants with knee arthritis were excluded from analyses of knee flexion and extension. Similarly, participants with hip arthritis were excluded from analyses of hip flexion and extension. Two tailed P-values were used, and analyses were considered statistically significant if the P-value was less than .05. SAS statistical software version 9.2 (SAS Institute Inc, Cary, North Carolina) was used to perform all analyses.
RESULTS
Of 460 WALCS participants with PAD, 422 (91.7%) completed one or more baseline strength measure with the MFE. Of these, three (0.7%) died or were lost to follow-up before the first annual follow-up visit. Eight participants were excluded because they had both hip and knee arthritis and were therefore ineligible for inclusion in any analyses. An additional participant was excluded because of missing data for physical activity. Of the remaining 410 PAD participants, 246 were men.
Of the 410 PAD participants eligible, 126 (30.7%) died during a mean follow-up of 60 months ± 22.6. Of these, cause of death was not available for 22 (17.4%). Among PAD participants with known cause of death, 41 (39%) were due to cardiovascular disease and 31 (28%) were due to cancer. Among decedents, those without a death certificate included a higher prevalence of women (55% vs. 30%, P=.026) and a lower prevalence of angina pectoris (22.7% vs. 49.0%, P=.024), compared to those with a death certificate. Survival time was greater for decedents without a death certificate (66.8 vs. 35.8 months, P < .001). This is attributable to the time required to obtain death certificates and have them interpreted by the nosologist. There were no other differences in characteristics of decedents with vs. without death certificates (data not shown).
Average ages of survivors and decedents were 71.07 years ± 8.06 and 73.71 years ± 8.63, respectively (P=.003). Fifty-seven percent of survivors vs. 69.5% of decedents were men (P=.106). Table 1 shows baseline characteristics of survivors and decedents among men and women, respectively, adjusting for age. Among men, decedents had lower baseline ABI and BMI values, included a higher proportion of African-Americans, and had higher prevalences of diabetes mellitus, heart failure, cancer, and pulmonary disease as compared to survivors (Table 1). Among men, decedents had poorer baseline six-minute walk performance and lower baseline hip flexion, knee extension, and knee flexion strength compared to decedents (Table 1). Among women, decedents had a higher prevalence of diabetes mellitus as compared to survivors. There were no other differences in clinical characteristics between women survivors and decedents (Table 1).
Table 1.
Age-Adjusted Baseline Characteristics Associated with Survivor and Decedent Status in Men and Women with Lower Extremity Peripheral Arterial Disease (N=410)
| MEN | WOMEN | |||||
|---|---|---|---|---|---|---|
| Survivors (N=163) | Decedents (N=83) | P value | Survivors (N=121) | Decedents (N=43) | P-value | |
| Ankle brachial index | 0.67 (0.01) | 0.63 (0.02) | 0.019 | 0.64 (0.01) | 0.63 (0.02) | .694 |
| Body mass index (kg/M2) | 27.47 (0.37) | 26.08 (0.53) | 0.034 | 25.99 (0.56) | 25.92 (0.94) | .953 |
| African American (%) | 9.1 | 20.8 | 0.014 | 21.5 | 19.3 | .767 |
| Diabetes mellitus (%) | 28.0 | 45.8 | 0.009 | 18.1 | 52.7 | <.001 |
| Angina pectoris (%) | 42.2 | 47.2 | 0.458 | 22.3 | 37.2 | .063 |
| Myocardial infarction (%) | 27.6 | 39.8 | 0.056 | 18.9 | 23.2 | .541 |
| Heart failure (%) | 25.1 | 38.8 | 0.031 | 18.3 | 27.4 | .211 |
| Pulmonary disease (%) | 25.2 | 42.0 | 0.009 | 28.5 | 24.2 | .594 |
| Cancer (%) | 11.1 | 23.5 | 0.014 | 15.6 | 28.3 | .076 |
| Current cigarette smoking (%) | 16.6 | 25.5 | 0.151 | 20.0 | 11.9 | .249 |
| Number of city blocks walked during the past week | 42.47(5.12) | 38.53 (7.21) | 0.658 | 21.9(3.8) | 33.0 (6.4) | .138 |
| Six minute walk (feet) | 1297(28) | 1036 (39) | <0.001 | 1,038 | 1,039 | .992 |
| Hip extension strength (Newton-Meters) | 75.1 (2.8) | 65.7 (3.9) | 0.053 | 48.1 (2.0) | 44.7 (3.5) | .412 |
| Hip flexion strength (Newton-Meters) | 67.6 (1.5) | 61.6 (2.1) | 0.021 | 42.1 (1.4) | 40.5 (2.3) | .540 |
| Knee extension strength (Newton-Meters) | 56.1 (1.6) | 46.3 (2.3) | 0.001 | 38.5 (1.1) | 37.5 (2.4) | .707 |
| Knee flexion strength (Newton-Meters) | 38.3 (1.3) | 33.8 (1.8) | 0.037 | 21.8 (1.1) | 20.4 (1.8) | .502 |
Tables 2 and 3 show baseline characteristics of male PAD participants across quartiles of each strength measure. Older age and poorer six-minute walk performance were associated with poorer strength for all strength measures among men (Tables 2 and 3). Lower BMI was associated with poorer hip extension and lower ABI values were associated with poorer knee flexion among men (Table 2). Higher prevalences of myocardial infarction and heart failure were associated with poorer hip flexion and poorer knee extension among men (Tables 2 and 3). Lower physical activity levels were associated with lower hip flexion and a higher prevalence of angina was associated with poorer knee extension at baseline.
Table 2.
Associations of Baseline Leg Strength Measures with Clinical Characteristics among Men with Peripheral Arterial Disease (n=246).
| 1st Quartile (5.15 – 46.45 Newton-Meters) (N=59) | 2nd Quartile (47.65 – 68.10 Newton- Meters) (N=61) | 3rd Quartile (68.50 – 92.05 Newton-Meters) (N=59) | 4th Quartile (92.25 – 243.85 Newton-Meters) (N=60) | Trend P-value | |
|---|---|---|---|---|---|
| Hip Extension | |||||
| Age (years) | 74.1 (7.5) | 72.1 (8.5) | 70.7 (7.6) | 67.8 (8.8) | <.001 |
| Ankle brachial index | 0.62(0.15) | 0.67(0.15) | 0.67(0.12) | 0.67(0.13) | .191 |
| Body mass index (kg/M2) | 26.4 (5.4) | 26.4 (4.6) | 26.9 (4.8) | 28.6(4.1) | .029 |
| African American (%) | 15.3 | 6.6 | 17.0 | 15.0 | .33 |
| Diabetes mellitus (%) | 35.6 | 31.2 | 33.9 | 36.7 | .93 |
| Angina pectoris (%) | 45.8 | 47.6 | 40.7 | 40.0 | .80 |
| Myocardial infarction (%) | 35.6 | 36.1 | 28.8 | 26.7 | .60 |
| Heart failure (%) | 35.6 | 31.2 | 28.8 | 25.0 | .64 |
| Pulmonary disease (%) | 32.2 | 32.8 | 28.8 | 28.3 | .93 |
| Cancer (%) | 18.6 | 16.4 | 15.3 | 13.3 | .88 |
| Current cigarette smoker (%) | 20.3 | 18.0 | 27.1 | 16.7 | .50 |
| Number of city blocks walked during the past week | 32.46 (55.8) | 40.13 (65.2) | 34.98 (45.8) | 60.0 (88.6) | .095 |
| Six minute walk distance (feet) | 1120 (404) | 1135 (371) | 1251 (353) | 1324 (380) | .009 |
| Hip Flexion | |||||
|---|---|---|---|---|---|
| 1st Quartile (14.50 – 49.90 Newton-Meters) (N=60) | 2nd Quartile (49.95 – 63.45 Newton-Meters) (N=60) | 3rd Quartile (64.05 – 79.35 Newton-Meters) (N=60) | 4th Quartile (80.70 – 135.60 Newton-Meters) (N=60) | P-value | |
| Age (years) | 74.7( 8.2) | 72.4 (7.2) | 71.1 (8.3) | 66.6 (7.9) | <.0001 |
| Ankle brachial index | 0.62 (0.15) | 0.67 (0.15) | 0.68 (0.11) | 0.66 (0.13) | .082 |
| Body mass index (kg/M2) | 26.1 (5.4) | 27.0 (5.8) | 27.3 (3.7) | 27.8 (3.9) | .254 |
| African American (%) | 10.0 | 18.3 | 5.0 | 20.0 | .051 |
| Diabetes mellitus (%) | 28.3 | 40.0 | 33.3 | 35.0 | .604 |
| Angina pectoris (%) | 46.7 | 40.0 | 48.3 | 38.3 | .623 |
| Myocardial infarction (%) | 40.0 | 25.0 | 40.0 | 21.7 | .049 |
| Heart failure (%) | 28.3 | 45.0 | 26.7 | 20.0 | .022 |
| Pulmonary disease (%) | 36.7 | 23.3 | 31.7 | 31.7 | .461 |
| Cancer (%) | 20.0 | 18.3 | 15.0 | 10.0 | .453 |
| Current cigarette smoker (%) | 18.3 | 21.7 | 15.0 | 28.3 | .316 |
| Number of city blocks walked during the past week. | 35.1 (39.4) | 32.3 (62.5) | 37.3 (54.1) | 63.0 (93.6) | .039 |
| Six minute walk distance (feet) | 1122 (402) | 1143 (364) | 1269 (344) | 1299 (390) | .022 |
Table 3.
Associations of Baseline Leg Strength Measures with Clinical Characteristics among Men with Peripheral Arterial Disease (n=246)
| Knee Extension | |||||
|---|---|---|---|---|---|
| 1st Quartile (5.50 – 37.65 Newton-Meters) (N=91) | 2nd Quartile (38.65 – 50.95 Newton-Meters) (N=57) | 3rd Quartile (51.30 – 66.30 Newton-Meters) (N=57) | 4th Quartile (66.70 – 120.55 Newton-Meters) (N=57) | Trend P-value | |
| Age (years) | 73.4 (8.6) | 72.5( 7.7) | 71.8(8.4) | 67.0( 7.9) | <.001 |
| Ankle brachial index | 0.63 (0.16) | 0.67 (0.14) | 0.64 (0.12) | 0.68 (0.13) | .176 |
| Body mass index (kg/M2) | 26.0 (5.4) | 26.8 (4.2) | 26.7 (3.9) | 27.8 (4.2) | .212 |
| African American (%) | 12.5 | 14.0 | 15.8 | 8.8 | .713 |
| Diabetes mellitus (%) | 39.3 | 22.8 | 36.8 | 40.4 | .173 |
| Angina pectoris (%) | 60.7 | 47.4 | 38.6 | 35.1 | .031 |
| Myocardial infarction (%) | 46.4 | 38.6 | 22.8 | 26.3 | .028 |
| Heart failure (%) | 53.6 | 29.8 | 14.0 | 17.5 | <.001 |
| Pulmonary disease (%) | 35.7 | 28.1 | 24.6 | 35.1 | .500 |
| Cancer (%) | 19.6 | 12.3 | 15.8 | 14.0 | .732 |
| Current cigarette smoker (%) | 17.9 | 21.1 | 19.3 | 29.8 | .413 |
| Number of city blocks walked in the past week | 33.5 (44.1) | 26.4 (28.9) | 50.2(81.2) | 46.3 (78.9) | .149 |
| Six minute walk distance (feet) | 1106 (341) | 1186 (394) | 1277 (364) | 1330 (328) | .005 |
| Knee Flexion | |||||
|---|---|---|---|---|---|
| 1st Quartile (3.65 – 24.25) (N=56) | 2nd Quartile (24.45 – 35.75) (N=57) | 3rd Quartile (35.80 – 46.60) (N=57) | 4th Quartile (46.65 – 96.65) (N=57) | P-value | |
| Age (years) | 73.4 (8.8) | 73.2 (8.1) | 71.7 (8.0) | 66.4 (7.3) | <.0001 |
| Ankle brachial index | 0.61 (0.15) | 0.67 (0.14) | 0.68 (0.12) | 0.66 (0.13) | .043 |
| Body mass index (kg/M2) | 25.9 (4.2) | 26.9 (5.1) | 26.7 (4.2) | 27.8 (4.2) | .141 |
| African American (%) | 21.4 | 12.3 | 7.0 | 10.5 | .126 |
| Diabetes mellitus (%) | 26.8 | 40.4 | 38.6 | 33.3 | .428 |
| Angina pectoris (%) | 46.4 | 43.9 | 52.6 | 38.6 | .504 |
| Myocardial infarction (%) | 32.1 | 36.8 | 38.6 | 26.3 | .509 |
| Heart failure (%) | 32.1 | 36.8 | 24.6 | 21.1 | .233 |
| Pulmonary disease (%) | 30.4 | 35.1 | 24.6 | 33.3 | .635 |
| Cancer (%) | 14.3 | 19.3 | 14.0 | 14.0 | .830 |
| Current cigarette smoker (%) | 19.6 | 24.6 | 21.1 | 22.8 | .930 |
| Number of city blocks walked in the past week. | 27.6 (33.5) | 36.7 (64.6) | 35.8 (44.0) | 56.1 (91.0) | .096 |
| Six minute distance (feet) | 1118 (369) | 1206 (386) | 1233 (335) | 1343 (343) | .011 |
Among women, poorer six-minute walk performance was associated with poorer strength for all strength measures and older age was associated with poorer strength for hip flexion, knee extension, and knee flexion (data not shown). Among women, there was a lower prevalence of African-Americans among participants with poorer hip extension strength. Lower ABI values were associated with poorer hip flexion and hip extension (data not shown). Lower BMI values were associated with poorer hip flexion and knee flexion strength among women (data not shown).
Leg Strength and All-Cause Mortality among Men with PAD
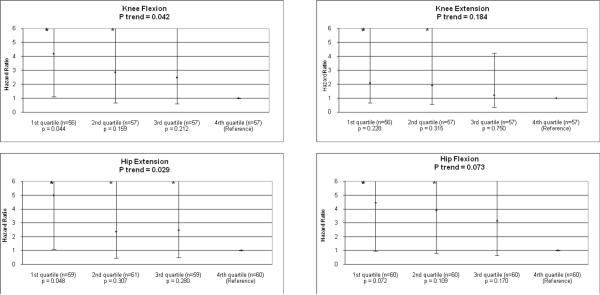
Among men, poorer baseline strength for knee flexion (P trend =.029), knee extension (P trend = .010), and hip extension (P trend = .013) were each associated with higher all-cause mortality, adjusting for age, race, BMI, smoking history, comorbidities, ABI, and physical activity (Figure 1). In these adjusted analyses, men in the 1st and 2nd quartiles of knee flexion strength had hazard ratios (HR) for all-cause mortality of 2.23 (95% Confidence Interval (CI) = 1.02–4.87, P = .045) and 2.69 (95% CI = 1.23–5.88, P = .013), respectively, compared to men in the 4th (best) quartile of knee flexion strength (Figure 1). Men in the 1st and 2nd quartiles of knee extension strength at baseline had HRs for all-cause mortality of 2.91 (95% Confidence Interval (CI) = 1.30–6.53, P = .009) and 2.84 (95% CI = 1.20–6.70, P = .017), respectively, compared to participants in the 4th (best) strength quartile (Figure 1). Findings were similar when analyses were repeated among participants without death certificates (data not shown).
Figure 1.
Adjusted associations of baseline lower extremity strength with all-cause mortality among men with peripheral arterial disease (n=246).*
* Analyses adjust for age, race, body mass index, comorbidities, smoking, ankle brachial index, and physical activity level.
Number of deaths per quartile:; Knee Flexion: 1st: 24, 2nd: 25, 3rd : 18, 4th: 10; Knee Extension: 1st: 30, 2nd: 18, 3rd : 20, 4th: 9; Hip Extension: 1st : 27, 2nd: 26, 3rd: 15, 4th: 12; Hip Flexion: 1st: 27, 2nd: 26, 3rd: 14, 4th: 13
*indicates that the upper bound of the error bar is not within the y-axis of the graph. P values under each quartile represent results of a pairwise comparison with the reference group.
The overall associations of hip extension strength and knee extension strength with all-cause mortality remained statistically significant even after additional adjustment for six-minute walk performance (P trend = .013 and P trend = .028, respectively). The association of lower knee flexion strength with higher all-cause mortality was no longer significant when this analysis was additionally adjusted for six-minute walk performance (P trend = .060). The adjusted pairwise comparisons for the associations of the 1st and 2nd quartiles of knee extension strength and for the 2nd quartile of knee flexion strength with higher all-cause mortality, compared to the reference (highest) quartiles of strength, remained statistically significant and were not substantially attenuated after additional adjustment for six-minute walk performance (data not shown). These findings indicate that associations of poorer leg strength with higher all-cause mortality were largely independent of six-minute walk performance.
Leg Strength and Cardiovascular Mortality among Men with PAD
Among men, poorer baseline strength for hip extension (P trend =.029) and knee flexion (P trend = .042) were associated with increased cardiovascular mortality, adjusting for age, sex, race, BMI, smoking history, comorbidities, ABI, and physical activity (Figure 2). In these adjusted analyses, men in the 1st (poorest) strength quartiles for hip extension strength had an HR for cardiovascular mortality of 5.00 (95% CI=1.09–22.93, p=0.048) and men in the 1st strength quartile for knee flexion strength had an HR for cardiovascular mortality of 4.20 (95% CI=1.12–15.79, p=0.044) compared to those in the 4th (best) quartile of hip extension and knee flexion, respectively (Figure 2).
Figure 2.
Adjusted associations of baseline lower extremity strength with cardiovascular disease mortality among men with peripheral arterial disease (n=246).*
* Analyses adjust for age, race, body mass index, comorbidities, ankle brachial index, smoking, and physical activity level.
Number of deaths per quartile is not provided because imputations were performed due to missing death certificates for some participants. * Indicates that the upper bound of the error bar is not within the y-axis of the graph. P values under each quartile represent results of a pairwise comparison with the reference group.
Among male PAD participants, the overall association of hip extension strength with cardiovascular disease mortality remained statistically significant even after additional adjustment for six-minute walk performance (P trend = .031). The association of knee flexion strength with cardiovascular disease mortality was no longer statistically significant when analyses shown in Figure 2 were additionally adjusted for six-minute walk performance (P trend = .65). After additional adjustment for six-minute walk performance, neither pairwise association between the 1st and 4th quartiles for hip extension strength and knee flexion strength remained statistically significant (data not shown).
Leg Strength and Mortality in Women
Among women, there were no significant associations of leg strength with all-cause mortality (Table 4). There were no significant interactions of gender with the associations between leg strength and mortality (data not shown). There were too few cardiovascular deaths among women (N=9) to assess associations of leg strength with cardiovascular deaths among women.
Table 4.
Adjusted Associations of Leg Strength with All-Cause Mortality among Female Participants with Peripheral Arterial Disease (N=164)*
| Strength Measure | Number of Female Participants | Number of Deaths | Hazard Ratio (95% Confidence Interval) | Trend P Value |
|---|---|---|---|---|
| Hip Extension | ||||
| 1st quartile | 39 | 9 | 0.87 (0.30–2.52) | .597 |
| 2nd quartile | 41 | 14 | 1.68 (0.63–4.48) | |
| 3rd quartile | 41 | 11 | 1.97 (0.67–5.82) | |
| 4th quartile | 41 | 8 | 1.00 (reference) | |
| Hip Flexion | ||||
| 1st quartile | 39 | 11 | 0.63 (0.23–1.77) | .515 |
| 2nd quartile | 41 | 13 | 1.41 (0.54–3.67) | |
| 3rd quartile | 41 | 10 | 0.88 (0.34–2.28) | |
| 4th quartile | 41 | 9 | 1.00 (reference) | |
| Knee Extension | ||||
| 1st quartile | 34 | 11 | 0.81 (0.28–2.30) | .195 |
| 2nd quartile | 36 | 6 | 0.33 (0.09–1.20) | |
| 3rd quartile | 36 | 13 | 1.79(0.62–5.19) | |
| 4th quartile | 35 | 7 | 1.00 (reference) | |
| Knee Flexion | ||||
| 1st quartile | 34 | 9 | 1.12 (0.37–3.40) | .740 |
| 2nd quartile | 36 | 9 | 0.67 (0.23–1.98) | |
| 3rd quartile | 36 | 12 | 1.59 (0.57–4.40) | |
| 4th quartile | 36 | 7 | 1.00 (reference) |
Analyses adjust for age, race, body mass index, comorbidities (diabetes, angina, myocardial infarction, heart failure, pulmonary disease, cancer), ankle brachial index, and number of blocks walked during the prior week.
DISCUSSION
Prior calf muscle biopsy data demonstrate that lower extremity ischemia is associated with smaller Type 1 and Type 2 muscle fibers and muscle dennervation.15 Among individuals without lower extremity revascularization, PAD is associated with reduced lower extremity strength compared to those without PAD.4 To our knowledge, associations of lower extremity strength with mortality have not been reported previously among men and women with PAD, who have more adverse calf muscle characteristics and poorer lower extremity strength compared to people without PAD.
Findings reported here demonstrate that poorer strength for knee flexion, knee extension, and hip extension, respectively, are associated significantly and independently with higher all-cause mortality at five-year follow-up among men with PAD. Poorer hip extension and knee flexion strength are associated with increased cardiovascular disease mortality among men with PAD. These findings were independent of the ABI, BMI, comorbidities, and other confounders. In contrast, we found no significant associations of baseline leg strength with mortality among women with PAD.
Poorer lower extremity strength may predict increased mortality in men with PAD because it reflects overall health status. Lower extremity strength could be a sensitive measure of systemic atherosclerosis or may reflect total comorbidity burden. In support of these theories, lower baseline strength for some measures was associated with lower ABI values and more prevalent comorbidities among men.
Our prior work demonstrates that poorer lower extremity functional performance, such as six-minute walk performance, is associated independently with increased risk of all-cause and cardiovascular disease mortality in participants with PAD16. In the current analyses, associations of poorer lower extremity strength with greater all-cause mortality were largely unchanged, even after adjustment for six-minute walk performance. These findings indicate that leg strength adds to and is independent of six-minute walk performance as a predictor of all-cause mortality in men with PAD. However, associations of leg strength with cardiovascular disease mortality were largely attenuated and no longer statistically significant after additional adjustment for six-minute walk performance. Furthermore, the six-minute walk test is likely to be a more practical measurement in the clinical setting than leg strength measures.
Reasons for differences in the association of leg strength with mortality among men and women with PAD are unclear. Leg strength may be a better surrogate measure of overall health among men than among women. In addition, the larger sample size of men and greater number of deaths among male as compared to female participants increased statistical power to detect an association among men.
This study has limitations. First, participants were identified from non-invasive vascular laboratories and a large general internal medicine practice. PAD was defined based on an ABI < 0.90. Results may not be generalizable to PAD populations in other settings or to PAD patients who have ABI values > 0.90. Second, data are observational. A causal association between reduced leg strength and mortality cannot be established based on these data. Third, data available to us do not allow us to identify the causal pathway for associations of poorer leg strength with increased mortality in persons with PAD. Fourth, we did not collect data on physical activity levels prior to study entry or participation in leg strengthening exercises, both of which may have influenced baseline leg strength. Fifth, we did not collect data allowing us to determine whether decrements in leg strength among the PAD participants were primarily related to an intrinsic skeletal muscle pathology vs. motor nerve conduction pathology. Sixth, older age was associated strongly with both leg strength and mortality. Residual confounding by age may influence the associations of leg strength and mortality in adjusted analyses. To address this, we performed additional analyses of associations of leg strength with mortality, stratified by age. These analyses were consistent with the main study findings, though results were slightly attenuated.
Men and women with PAD have poorer leg strength than those without PAD. Yet even across the spectrum of reduced leg strength in men with PAD, poorer leg strength is associated with higher mortality. Recent study demonstrates that lower extremity resistance training significantly improves treadmill walking performance and quality of life among participants with PAD.17. . Further study is needed to determine whether interventions that improve lower extremity strength reduce all-cause and cardiovascular disease mortality rates in men with PAD.
Acknowledgments
SOURCES OF FUNDING This work was supported by the National Heart Lung and Blood Institute, National Institutes of Health [R01-HL58099, R01-HL64739, R01-HL071223, and R01-HL076298] and the National Center for Research Resources, National Institutes of Health [#RR-00048]and by the Intramural Research Program, National Institutes on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, et al. The ankle brachial index is associated with leg function and physical activity: the walking and leg circulation study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Criqui MH, Greenland P, Guralnik JM, Liu K, Pearce L, et al. Leg Strength in Peripheral Arterial Disease: Associations with Disease Severity and Lower Extremity Performance. Journal of Vascular Surgery. 2004;39(3):523–30. doi: 10.1016/j.jvs.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Ferrucci L, Guralnik JM, Tian L, Liu K, Hoff F, et al. Pathophysiologic changes in calf muscle predict mobility loss at two-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman SD, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral arterial disease. J Am Geriatr Soc. 2009 Oct 24; doi: 10.1111/j.1532-5415.2009.02562.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott MM, Criqui MH, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and associations with functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 8.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Fried LP, Simonsick EM, kasper JD, Lafferty ME, editors. The Women's Health and Aging Study: health and social characteristics of older women with disability. National Institute on Aging; Bethesda, MD: 1995. NIH publication 95-4009, Appendix E. [Google Scholar]
- 10.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 12.Lee IM, Paffenbarger RS, Hsieh CC. Time trends in physical activity among college alumni, 1962–1988. Am J Epidemiol. 1992;135:912–925. doi: 10.1093/oxfordjournals.aje.a116387. [DOI] [PubMed] [Google Scholar]
- 13.Garg PK, Tian L, Criqui MH, Liu K, Ferrucci L, Guralnik JM, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu K, Tsiatis AA. Multiple imputation methods for estimating regression coefficients in the competing risks model with missing cause of failure. Biometrics. 2001;57:1191–1197. doi: 10.1111/j.0006-341x.2001.01191.x. [DOI] [PubMed] [Google Scholar]
- 15.Regensteiner JG, Wolfel EE, Brass EP, et al. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation. 1993;87:413–421. doi: 10.1161/01.cir.87.2.413. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, et al. Prognostic value of functional performance for mortality in patients with peripheral arterial disease. Journal of American College of Cardiology. 2008;51:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]