Abstract
Treatment of pea (Pisum sativum L.) hypocotyl segments with indole-3-butyric acid, which promotes segment elongation, increased the solubilization of both xyloglucan and cello-oligosaccharides in the apoplast of auxin-treated pea stems. The cello-oligosaccharides were isolated from the apoplastic solution with a charcoal/Celite column and were identified as cellobiose, cellotriose, and cellotetraose after subsequent thin-layer chromatography and paper electrophoresis. Cello-oligosaccharides in the apoplastic fraction were monitored using cellobiose dehydrogenase. Both xyloglucan and cello-oligosaccharides appeared to be formed concurrently within 30 min after treatment with the auxin, and the cello-oligosaccharides increased with stem elongation even after 2 h. The total activity of cellulase did not increase for up to 4 h.
Studies of auxin-induced cell elongation have been focused on the effects of auxin on metabolic and structural changes in the cell wall polysaccharides. In pea (Pisum sativum L.) epicotyl segments, Labavitch and Ray (1974) showed an auxin-promoted solubilization of xyloglucans into the water-soluble fraction in pea stem segments, in which they proposed involvement of xyloglucan metabolism during auxin-induced extension growth. In fact, solubilization begins within 15 min after auxin treatment, increases with increasing auxin concentration, and corresponds to the increase in elongation rate. The phenomenon may be generated by the release of xyloglucan from the xyloglucan-cellulose network into the apoplastic free space (Terry and Bonner, 1980). Although there are several enzyme candidates responsible for xyloglucan solubilization, i.e. xyloglucanase (Matsumoto et al., 1997), xyloglucan endotransglycosylase (Fry and Matthews, 1992), expansin (McQueen-Mason and Cosgrove, 1994), and cellulase, the mechanism of xyloglucan solubilization has not yet been clarified. An endo-1,4-β-glucanase activity responsible for degradation and/or solubilization of xyloglucan was certainly associated during the auxin-induced lateral expansion of pea epicotyls (Hayashi and Maclachlan, 1984), but there are at least two types of endo-1,4-β-glucanases, differing in their specificities to cellulose and xyloglucan.
Auxin-treated pea epicotyl segments have been used because of xyloglucan turnover (solubilization) and because of an increase in cellulase transcripts that may be closely related to auxin-induced cell growth in peas (Verma et al., 1975). Pea cellulase is localized in the inner surface of the cell wall (Bal et al., 1976), where the synthesis and assembly of cellulose microfibrils in association with xyloglucan may occur. Addition of 2,4-D induced the expression of the gene encoding cellulase and subsequently solubilized xyloglucan in suspension-cultured poplar cells (T. Hayashi and T. Takeda, unpublished results), suggesting that cellulase mediates auxin-induced wall elongation in poplar cells. Xyloglucan solubilization may be caused by the hydrolysis of cellulose microfibrils, which substantially evokes the weakening of the cellulose microfibril framework by releasing xyloglucans, and subsequent turgor-driven wall expansion (Hayashi, 1991). In this paper we describe the occurrence of cello-oligosaccharides at the early stage of stem elongation in the apoplastic solution of auxin-treated pea stems, accompanying xyloglucan solubilization during the elongation of pea stem segments.
Either partial hydrolysis or loosening of cellulose microfibrils involving the solubilization of xyloglucan must be responsible for cell wall loosening because it causes weakening of the cellulose microfibril framework. Xyloglucans in the pea xyloglucan-cellulose network potentially function as cross-linking bridges between microfibrils (Hayashi, 1989). The intercalated and anchored xyloglucan may contribute to the cross-linking of each cellulose microfibril to make a rigid framework in the cell wall. We propose here that an auxin-induced xyloglucan turnover is accompanied by the turnover of cellulose.
MATERIALS AND METHODS
Materials
β-Glucosidase (chromatographically purified) from almonds and α-glucosidase from Brewer's yeast were obtained from Sigma and invertase was obtained from Wako (Osaka, Japan). The invertase preparation is sugar free and has no cellulase or β-glucosidase activity. Cello-oligosaccharides were from Seikagaku (Tokyo, Japan). Xyloglucan-oligosaccharides were endoglucanase hydrolysates of tamarind or pea (Pisum sativum L.) xyloglucans.
Seeds of pea var Alaska were soaked for 10 h in water and sown in moistened vermiculite, and seedlings were grown in darkness at 28°C. When the third internodes reached 2 to 3 cm in length (7 d after sowing), epicotyl segments (10 mm long) were excised from the region, 5 mm below the hook. They were soaked in ice-cold water for 30 min and incubated at 25°C in the dark with shaking in a Petri dish (10 cm in diameter) containing 20 μm indole-3-butyric acid (Merck, Darmstadt, Germany) in 10 mL of water.
Isolation of Cello-Oligosaccharides from Apoplastic Solution
An apoplastic solution was collected from pea segments by centrifugation according to the procedure reported by Terry and Bonner (1980). About 40 segments that had been auxin treated were packed into a plastic syringe barrel fitted with a disc of nylon mesh. The segments were infiltrated with ice-cold water for 3 min under a vacuum and then centrifuged at 1500g at 20°C for 10 min to collect the apoplastic solution. This procedure was repeated twice. About 200 mL of wall solution obtained from 40,000 segments (1.6 kg) was boiled for 5 min and incubated with an invertase preparation (4 units) in 20 mm sodium acetate buffer, pH 5.0, at 40°C for 12 h. The reaction was stopped by boiling the mixture for 5 min and the mixture was applied to a column (4.5 × 15 cm) containing 160 g of charcoal and 40 g of Celite (Nakalai, Kyoto, Japan). The sugars were sequentially eluted stepwise each with 2 L of 2.5%, 5%, 10%, 15%, and 20% ethanol after washing with water. Each eluate was collected and evaporated, and oligosaccharide fractions were obtained. Each fraction was subjected to a preparative paper chromatography with solvent A and oligosaccharides mobile with cellobiose, cellotriose, and cellotetraose were excised and eluted with water. Each oligosaccharide was further purified by using a preparative paper chromatography with solvent A. Each cello-oligosaccharide fraction was again excised, eluted with water, and freeze-dried.
Determination of Stem Length and Cello-Oligosaccharide Content after Auxin Treatment
The length of 20 segments was measured using a binocular microscope after incubation with indole-3-butyric acid. About 40 segments were infiltrated with ice-cold water for 3 min under a vacuum and then centrifuged at 1500g at 20°C for 10 min to collect the apoplastic solution. This procedure was repeated twice. About 0.5 mL of wall solution obtained from 40 segments was boiled for 5 min and left at room temperature for 24 h to equilibrate the anomer configuration between α- and β-type-reducing sugars. The amount of cello-oligosaccharides was determined by using cellobiose dehydrogenase purified from conidia spores of Phanerochaete chrysosporium, according to the method previously reported (Samejima and Eriksson, 1992). The reaction mixture contained 90 milliunits (10 μL) of cellobiose dehydrogenase, 50 μm Cyt c (10 μL), and sample solution (70 μL) in 100 μL of 100 mm sodium acetate buffer, pH 4.2. After incubation for 5 min at room temperature, the A550 was determined. A linear standard curve was obtained with a standard cellobiose solution and an absorbance of 0.5 corresponded to approximately 270 ng per 100 μL of reaction mixture for cellobiose.
Carbohydrate Analysis
Paper chromatography was performed on Whatman 3MM filter paper by the multiple ascending method with the following solvent system: 1-butanol:pyridine:water, 4:3:4 (v/v; solvent A). The zone corresponding to the desired sugar was excised and eluted with water, and the solution was freeze-dried. TLC was performed on a TLC plate (Merck) by the multiple ascending method with the following solvent system: 1-butanol:acetone:water, 4:5:1 (v/v; solvent B). Paper electrophoresis was performed on a Whatman 3MM filter paper at 250 V for 20 h with 0.1 m sodium tetraborate (pH 9.3). Sugars were visualized using a silver nitrate reagent (Robyt and French, 1963). Carbohydrate was determined by the phenol/sulfuric acid method (Dubois et al., 1956). Xyloglucan was determined by the iodine-sodium sulfate method (Kooiman, 1960). Ten microliters of a solution (0.5% I2 in 1% KI and 100 μL of 20% Na2SO4) was added to 20 μL of apoplastic solution. The mixture was vortexed and the tube was allowed to stand for 60 min at 4°C in the dark. The A640 was measured. A linear standard curve was obtained with xyloglucan and an absorbance of 1 corresponded to approximately 6 μg per 20 μL of sugar solution for pea xyloglucan.
Enzymatic Hydrolysis
Each oligosaccharide (5 μg) was incubated at 37°C with α-glucosidase (5 units for p-nitrophenyl-α-glucoside) in 10 mm phosphate buffer (pH 6.8) or with β-glucosidase (5 units for salicin) in 10 mm acetate buffer (pH 5.6) in a total volume of 30 μL. During the time course aliquots (5 μL) of the reaction mixture were determined for Glc by the mutarotase-Glc-oxidase method (Keston and Brandt, 1963; Okuda and Miwa, 1971). The sample was incubated with 100 μL of Glucose CII-test solution (Wako) for 10 min at 37°C, and the A505 was determined. A linear standard curve was obtained with a standard Glc solution and an absorbance of 1 corresponded to approximately 0.6 μg per 5 μL of sugar solution for Glc.
Preparation of Cellulase from Pea Stems
Pea stem segments were homogenized in a mortar with 2 volumes of 20 mm sodium phosphate buffer (pH 6.2) containing 1 m NaCl. The homogenate was centrifuged, the enzymes in the supernatant were concentrated by precipitation with solid ammonium sulfate to 65% saturation, and portions were used as the enzyme source.
Assay of Cellulase
Cellulase activity was assayed viscometrically at 35°C for 2 h with 0.1 mL of enzyme preparation plus 0.9 mL of 10 mm sodium phosphate buffer (pH 6.2) containing 0.65% (w/v) carboxymethylcellulose in Cannon semimicroviscometers (Cannon Instrument, State College, PA). One unit of activity is defined as the amount of enzyme required to cause 0.1% loss in viscosity in 2 h under such conditions (Nakamura and Hayashi, 1993). Protein was determined using the Coomassie Plus protein assay reagent (Pierce), according to the method of Smith et al. (1985).
RESULTS
Isolation and Identification of Cello-Oligosaccharides in the Apoplastic Solution
Fractionation of the apoplastic solution of auxin-treated pea stems (40,000 segments) by charcoal/Celite column chromatography yielded many kinds of oligosaccharides. Each oligosaccharide was isolated by preparative paper chromatographies with solvent A and the following yields: disaccharide, 212 μg; trisaccharide, 66 μg; and tetrasaccharide, 60 μg (Table I). Of these oligosaccharides, the disaccharide (Rg = 0.74, where Rg is the distance of the substance from the origin divided by the distance of the Glc from the origin) mobile with cellobiose was obtained mainly from both 5% and 10% ethanol eluates, the trisaccharide (Rg = 0.41) mobile with cellotriose was obtained mainly from both 10% and 15% ethanol eluates, and the tetrasaccharide (Rg = 0.19) mobile with cellotetraose was obtained mainly from both 15% and 20% ethanol eluates by charcoal/Celite column chromatography.
Table I.
Isolation of cello-oligosaccharides from apoplastic solution
| Ethanol Fractionation | Total Sugara | Cello-Oligosaccharidesb | Isolated
Cello-Oligosaccharides
|
||
|---|---|---|---|---|---|
| Cellobiose | Cellotriose | Cellotetraose | |||
| % | μg | ||||
| 0 | 5,100 | 0 | 0 | 0 | 0 |
| 2.5 | 186,000 | 0 | 0 | 0 | 0 |
| 5 | 2,200 | 362 | 113 | — | — |
| 10 | 480 | 284 | 99 | 19 | — |
| 15 | 780 | 213 | — | 47 | 28 |
| 20 | 2,500 | 242 | — | — | 32 |
Values were determined by the phenol/sulfuric acid method (Dubois et al., 1956).
Values were determined by using cellobiose dehydrogenase (Samejima and Eriksson, 1992).
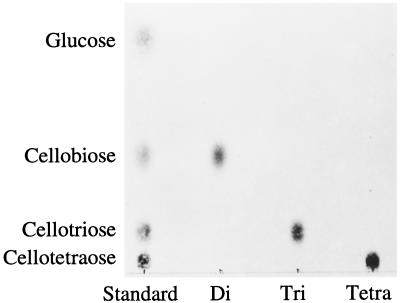
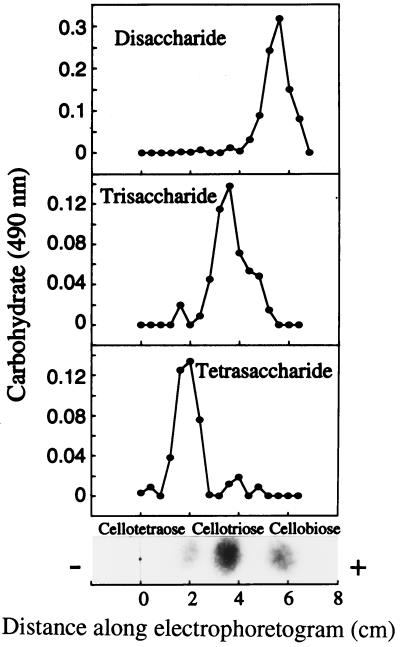
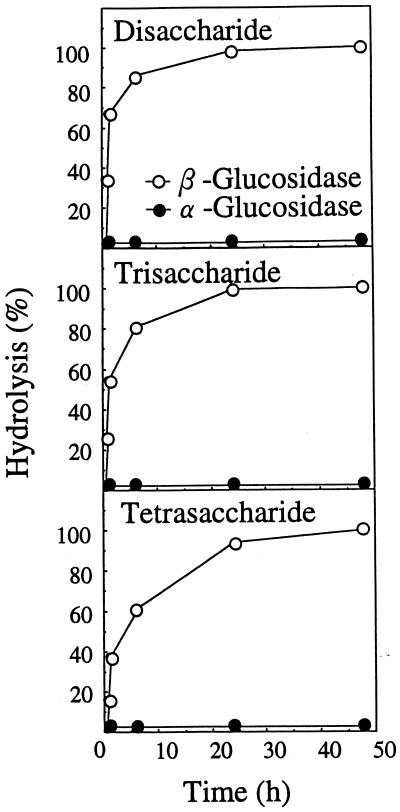
The di-, tri-, and tetrasaccharides had a mobility similar to those of authentic cellobiose (Rg = 0.51), cellotriose (Rg = 0.17), and cellotetraose (Rg = 0.06) on TLC with solvent B, respectively (Fig. 1). Figure 2 also shows that the di-, tri-, and tetrasaccharides had a mobility similar to those of authentic cellobiose, cellotriose, and cellotetraose on paper electrophoresis, respectively. They could be hydrolyzed and release Glc by almond β-glucosidase but not by yeast α-glucosidase (Fig. 3). This confirms that Glc is the only monosaccharide present and that the oligosaccharides are composed of β-glucosyl residues.
Figure 1.
TLC of disaccharides (Di), trisaccharides (Tri), and tetrasaccharides (Tetra) obtained from apoplastic solution. Sugars were visualized by spraying 10% H2SO4 in ethanol followed by heating at 100°C.
Figure 2.
Paper electrophoresis of disaccharides, trisaccharides, and tetrasaccharides. Each oligosaccharide (50 μg) was applied on Whatman 3MM filter paper. Sugar was eluted with water and aliquots were determined by the phenol/sulfuric acid method (Dubois et al., 1956). Cellobiose has a mobility (Mg = 0.28, where Mg is the distance of substance from origin divided by distance of Glc from origin) that is different from that of gentiobiose (Mg = 0.75), laminaribiose (Mg = 0.69), and sophorose (Mg = 0.24; Bourne et al., 1956).
Figure 3.
Hydrolysis of disaccharides, trisaccharides, and tetrasaccharides with α- and β-glucosidases. The level of hydrolysis is expressed as a percentage of Glc liberated per total sugar.
Structural evidence for the isolated oligosaccharides was also obtained from the relation between log(Rg/1 − Rg) and degree of polymerization value (French and Wild, 1953). A straight-line relationship was observed from the mobility of oligosaccharides on paper chromatography with solvent A and on TLC with solvent B, when the logarithm value of Glc, disaccharides, trisaccharides, and tetrasaccharides were plotted against their degree of polymerization values. This shows that the oligosaccharide line represents the β-(1→4)-linked Glc series. These data show that the di-, tri-, and tetrasaccharides are cellobiose, cellotriose, and cellotetraose, respectively, and that cello-oligosaccharides occur in the apoplastic solution of auxin-treated pea stems.
Specificity of Cellobiose Dehydrogenase
Ayers et al. (1978) examined the substrate specificity of cellobiose dehydrogenase against several oligosaccharides. The enzyme specifically oxidized cello-oligosaccharides but acted minimally on gentiobiose and sophorose.
The specificity of cellobiose dehydrogenase was again examined for several plant sugars as a substrate (Table II). The activity was higher for cellobiose and decreased gradually in its increased degree of polymerization. The enzyme probably does not recognize β (1→3) linkage (laminari-oligosaccharides) and α-(1→4) linkage (maltose). The major sugars raffinose, Suc, Fru, and Glc in the apoplastic solution were ineffective. Xyloglucan oligosaccharides, which appear to be formed after treatment with auxin from pea stems, were also ineffective for the action of cellobiose dehydrogenase. These results suggest that the cellobiose dehydrogenase is specific for cello-oligosaccharides. Cellobiose dehydrogenase was used to monitor the amount of cello-oligosaccharides.
Table II.
Substrate specificity of cellobiose dehydrogenase
| Sugar (2 μm) | Relative Activity |
|---|---|
| % | |
| Cellobiose | 100.0 |
| Cellotriose | 88.1 |
| Cellotetraose | 79.0 |
| Cellopentaose | 72.4 |
| Cellohexaose | 72.0 |
| Laminaribiose | 0 |
| Laminaritriose | 0 |
| Maltose | 0.1 |
| Suc | 0 |
| Raffinose | 0.1 |
| Glc | 0.7 |
| Fru | 0 |
| Isoprimeverose | 0 |
| XXG | 0 |
| XXXG | 0 |
| XXXGXXXG | 0 |
The reaction mixtures contained 90 milliunits of cellobiose dehydrogenase, 5 nm Cyt c, 2 nmol of substrate, and 0.1 m sodium acetate (pH 4.2) in a total volume of 100 μL. After incubation for 5 min, the A550 was determined.
Formation of Cello-Oligosaccharides during Stem Elongation
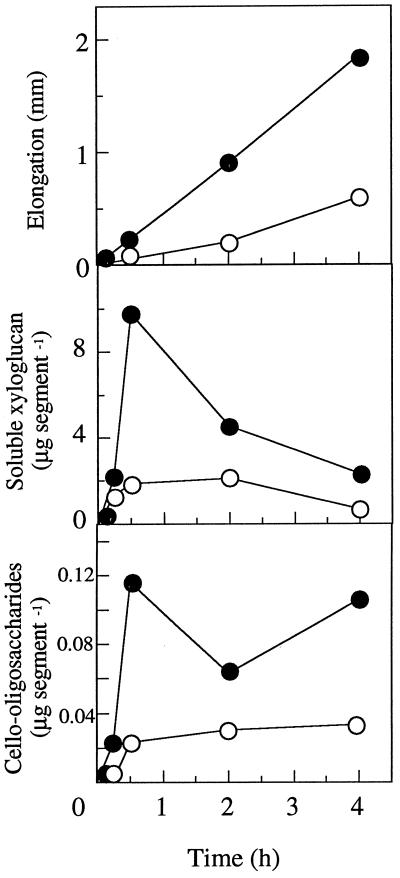
Treatment of the segments of pea stems with 20 μm indole-3-butyric acid promoted elongation of the segments and increased the amount of soluble xyloglucan and cello-oligosaccharides (Fig. 4). Both xyloglucan and cello-oligosaccharides appeared to be formed concurrently within 30 min after treatment with the auxin. The amount of xyloglucan determined by the iodine-sodium sulfate method (Kooiman, 1960) was decreased after 2 h, whereas the cello-oligosaccharides increased with stem elongation even after 2 h. This is in agreement with the earlier observation (Matsumoto et al., 1997) that the amount of xyloglucan in the apoplastic solution was highest within 2 h and declined thereafter with the auxin treatment. These findings suggest that the elongation of the segments is associated not only with solubilization of xyloglucan but also with that of cello-oligosaccharides.
Figure 4.
Formation of cello-oligosaccharides and soluble xyloglucan in the apoplastic solution. Segments were incubated in water in the presence (•) or absence (○) of 20 μm indole-3-butyric acid. The amount of cello-oligosaccharides was determined by using cellobiose dehydrogenase (Samejima and Eriksson, 1992). Means ± se are shown (n = 3). ses fall within the respective symbols.
The activity of total cellulase did not change in auxin-treated stems, compared with the control (Table III). This is consistent with the earlier observations in pea stems (Davies and Maclachlan, 1968) that cellulase activity is not increased during incubation until 24 h. This also indicates that the elongation of pea stems does not correspond to the level of cellulase activity extracted from their whole tissues.
Table III.
Effect of indole-3-butyric acid (IBA) on cellulase activity in pea stem segments
| Treatment | Time (min)
|
||||
|---|---|---|---|---|---|
| 0 | 15 | 30 | 120 | 240 | |
| units mg−1 protein | |||||
| Control | 90 ± 6 | 119 ± 4 | 93 ± 5 | 128 ± 10 | 112 ± 8 |
| IBA | 124 ± 5 | 101 ± 8 | 119 ± 12 | 97 ± 7 | |
DISCUSSION
To our knowledge, this is the first identification of the cello-oligosaccharides in the apoplastic solution of auxin-treated pea stems. The occurrence of the oligosaccharides was accompanied with xyloglucan solubilization, namely xyloglucan turnover. The cello-oligosaccharides that we isolated were cellobiose, cellotriose, and cellotetraose, which might not be due to the fragmented oligosaccharides derived from xyloglucan. The degradation of xyloglucan proceeds by the alternate splitting of the α-xylosidic and β-glucosidic linkages from fragmented oligosaccharides, in which the release of xylosyl and glucosyl residues occurs sequentially at the nonreducing terminal of the backbone (Koyama et al., 1983).
α-Xylosidase isolated from pea stem extracts hydrolyzed the glycosidic linkage of only one of the three xylosyl residues of the heptasaccharide (XXXG; O'Neill et al., 1987). Therefore, the probable hydrolysates of xyloglucan oligosaccharide in pea stems are XXXG, GXXG, XXG, GXG, XG, and GG (cellobiose) as the sequential fragments. In fact, XXG and XG were detected only in the hydrolysate of the fragment XXXG with an enzyme preparation from the soybean cell wall (Koyama et al., 1983). Cellobiose might be derived from both xyloglucan and cellulose, but cellotriose and cellotetraose are not hydrolysates of xyloglucan. Alternately, the cello-oligosaccharides obtained here are probably not derived from the precursor of xyloglucan because cello-oligosaccharides are not served as acceptors by soybean xyloglucan synthase (Hayashi and Matsuda, 1981). The biosynthesis of xyloglucan proceeds by the concurrent transfer of β-glucosyl and α-xylosyl residues, in which xylosyl transfer does not occur with the preformed 1,4-β-glucan backbone. Therefore, the cello-oligosaccharide is probably not derived from xyloglucan.
Is xyloglucan turnover accompanied by cellulose degradation in the xyloglucan-cellulose network during stem elongation? Unfortunately, the results showed that auxin induced the formation of cello-oligosaccharides at the early stage of stem elongation (Fig. 4), although the activity of total cellulase did not increase during this time (Table III). Nevertheless, we speculate that the auxin-promoted elongation growth is accompanied by the degradation of cellulose to form cello-oligosaccharides rather than the biosynthesis of cellulose to produce new 1,4-β-glucans, which are not derived from cellulose microfibrils. Because cello-oligosaccharides still form with stem elongation in 4 h, when both auxin and 2,6-dichlorobenzonitrile, a specific inhibitor for cellulose biosynthesis, were provided together (data not shown). This is also consistent with the earlier observation (Brummell and Hall, 1985) that auxin-induced cell elongation is not inhibited by 2,6-dichlorobenzonitrile at the early stage of incubation. The cell wall property drastically changed within 30 min after auxin treatment (Fig. 4), and we think that the change induces cell elongation during incubation. Auxin may temporarily activate an H+ pump in the membranes through the action on the permeability of plasma membranes. The H+ excreted is presumed to activate cellulase in intact pea stems (Hayashi, 1991). Although an increase in the activity of total cellulase extracted was not observed for up to 4 h after treatment with auxin of pea stems, we hypothesize that in situ cellulase activity may increase rapidly in response to the H+ in the apoplastic space.
A xyloglucan-specific endo-1,4-β-glucanase was isolated from the apoplast fraction of auxin-treated pea stems in which both the rate of stem elongation and the amount of xyloglucan solubilized were high. Both the endo-1,4-β-glucanase and xyloglucan transglycosylase potentially generate free xyloglucan fragments from the xyloglucan-cellulose network either by one or synergistically by two. Expansin may also release xyloglucans because the protein loosens the hydrogen bondings between cellulose microfibrils (McQueen-Mason and Cosgrove, 1994). The overall in situ actions of cellulase, xyloglucanase, xyloglucan endotransglycosylase, and expansin may be required for the solubilization of xyloglucan, as well as the formation of cello-oligosaccharides. Although an increase in cellulase mRNA accumulation was not observed until at least 6 h in tomato stem segments incubated with 2,4-D (Catalá et al., 1997), cello-oligosaccharides might be formed. It should be noted that the expression of genes encoding cellulase, xyloglucanase, xyloglucan transglycosylase, and expansin does not always explain the cell wall elongation.
Cellobiose dehydrogenase is a useful tool for studying cellulase action and the occurrence of cello-oligosaccharides (Samejima and Eriksson, 1992), because the dehydrogenase is specific for cello-oligosaccharides and highly sensitive for detecting the oligosaccharides (Table II). Determination of cello-oligosaccharides may help monitor the in situ action of cellulase in tissues. Even for studies of cellulose biosynthesis, one might specifically detect nascent cellulose, the reducing end of which seems to be apart from cellulose synthase.
LITERATURE CITED
- Ayers A, Ayers S, Eriksson KE. Eur J Biochem. 1978;90:171–181. doi: 10.1111/j.1432-1033.1978.tb12588.x. [DOI] [PubMed] [Google Scholar]
- Bal AK, Verma DPS, Byrne H, Maclachlan GA. Subcellular localization of cellulases in auxin-treated pea. J Cell Biol. 1976;69:97–105. doi: 10.1083/jcb.69.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne EJ, Foster AS, Grant PM (1956) Ionophoresis of carbohydrates. Part IV. Separations of carbohydrates on fiberglass sheets. J Chem Soc 4311–4314
- Brummell DA, Hall JL. The role of cell wall synthesis in sustained auxin-induced growth. Physiol Plant. 1985;63:406–412. [Google Scholar]
- Catalá C, Rose JKC, Bennett AB. Auxin regulation and spatial localization of an endo-1,4-β-d-glucanase and a xyloglucan endotransglycosylase in expanding tomato hypocotyls. Plant J. 1997;12:417–426. doi: 10.1046/j.1365-313x.1997.12020417.x. [DOI] [PubMed] [Google Scholar]
- Davies E, Maclachlan GA. Effects of indoleacetic acid on intracellular distribution of β-glucanase activities in the pea epicotyl. Arch Biochem Biophys. 1968;128:595–600. doi: 10.1016/0003-9861(68)90068-4. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- French D, Wild GM. Correlation of carbohydrate structure with papergram mobility. J Am Chem Soc. 1953;75:2612–2616. [Google Scholar]
- Fry SC, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T. Xyloglucans in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:139–168. [Google Scholar]
- Hayashi T. Biochemistry of xyloglucans in regulating cell elongation and expansion. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. San Diego, CA: Academic Press; 1991. pp. 131–144. [Google Scholar]
- Hayashi T, Maclachlan G. Pea xyloglucan and cellulose. II. Hydrolysis by pea endo-1,4-β-glucanases. Plant Physiol. 1984;75:605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Matsuda K. Biosynthesis of xyloglucan in suspension-cultured soybean cells. J Biol Chem. 1981;256:11117–11122. [PubMed] [Google Scholar]
- Keston AS, Brandt R. Method for analysis of the anomers of glucose. Anal Biochem. 1963;6:461–467. doi: 10.1016/0003-2697(63)90098-8. [DOI] [PubMed] [Google Scholar]
- Kooiman P. A method for the determination of amyloid in plant seeds. Recl Trav Chim Pay-Bas Belg. 1960;79:675–678. [Google Scholar]
- Koyama T, Hayashi T, Kato Y, Matsuda K. Degradation of xyloglucan by wall-bound enzymes from soybean tissue. II. Degradation of the fragment heptasaccharide from xyloglucan and the characteristic action pattern of the α-d-xylosidase in the enzyme system. Plant Cell Physiol. 1983;24:155–162. [Google Scholar]
- Labavitch JM, Ray PM. Relationship between promotion of xyloglucan metabolism and induction of elongation by indoleacetic acid. Plant Physiol. 1974;54:499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Sakai F, Hayashi T. A xyloglucan-specific endo-1,4-β-glucanase isolated from auxin-treated pea stems. Plant Physiol. 1997;114:661–667. doi: 10.1104/pp.114.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Cosgrove DJ. Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proc Natl Acad Sci USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Hayashi T. Purification and properties of an extracellular endo-1,4-β-glucanase from suspension-cultured popular cells. Plant Cell Physiol. 1993;34:1009–1013. [PubMed] [Google Scholar]
- Okuda J, Miwa I. Polarographic microdetermination of d-glucose anomers with beta-d-glucose oxidase. Anal Biochem. 1971;39:387–394. doi: 10.1016/0003-2697(71)90428-3. [DOI] [PubMed] [Google Scholar]
- O'Neill RA, Darvill AG, Albersheim P(1987) A xylosidase from pea stems hydrolyzes selected xylosidic linkages of xyloglucan oligosaccharides (abstract no. 640). Plant Physiol 83: S-106
- Robyt JF, French D. Arch Biochem Biophys. 1963;106:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- Samejima M, Eriksson K-EL. A comparison of the catalytic properties of cellobiose:quinone oxidoreductase and cellobiose oxidase from Phanerochaete chrysosporium. Eur J Biochem. 1992;207:103–107. doi: 10.1111/j.1432-1033.1992.tb17026.x. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Terry ME, Bonner BA. An examination of centrifugation as a method of extracting an extracellular solution from peas, and its use for the study of indoleacetic acid-induced growth. Plant Physiol. 1980;66:321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma DPS, Maclachlan GA, Byrne H, Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975;250:1019–1026. [PubMed] [Google Scholar]