Summary
Objective
To investigate the epidiomology of HPV infection in Malian women, for whom cervical cancer is the most common cancer and the second most common cause of cancer-related mortality.
Methods
Pilot study of 202 women aged 15 to 65 to determine the prevalence rate of high-risk HPV infection among unscreened Malian women. Information on risk factors was collected through a standardized, structured interview and clinical examination. High-risk HPV DNA was detected using signal amplification methods (hybrid capture-II).
Results
High-risk (HR) HPV DNA was detected in 12% of unscreened women, while visual inspection after application of acetic acid and Lugol’s iodine (VIA/VILI) identified suspicious abnormalities in 2.5% of un-screened women. Histopathological evaluation of VIA/VILI positive biopsies revealed no evidence of cervical intraepithelial neoplasia or cervical cancer. The majority of infections occurred among women in the 15-24 year old range. Compared to women who were married or widowed, single women were 3.5 times more likely to be infected with HR HPV.
Conclusions
The prevalence of infection with cancer causing types of HPV in this study was 12%. These prevalence estimates are consistent with what has been reported previously for other west African countries.
Keywords: cervical cancer, human papillomavirus, Africa, epidemiology
Introduction
Approximately 99% of cervical cancer is attributable to persistent infection with one or more high risk (HR) types of human papillomavirus (HPV)(1). Age-adjusted incidence rates of cervical cancer in Africa are heterogeneous, ranging from a low of 1.6/100,000 among women from Egypt to a high of 56.3/100,000 among women from Guinea (2). While much is known about general risk factors for cervical cancer, particularly in developed countries, relatively little is known about risk factors for HR HPV infection in developing countries, especially in sub-Saharan Africa. Schiffman and Wacholder (3)recently noted that country-specific epidemiologic data are critical for planning, implementation and evaluation of cervical cancer control programs in developing countries.
As part of our efforts to develop culturally and economically sustainable strategies for cervical cancer prevention in poor settings, we are developing a research agenda and evidence base to inform cancer prevention and control efforts in Mali, a landlocked country in sub-Saharan West Africa with an estimated population of 14.2 million, Mali, which is one of the poorest countries in the world (4), has low utilization and coverage rates for key preventive and primary curative interventions, in part because of poor physical and financial access to care (5). Its tiered healthcare system includes community health centers, district health centers, and regional hospitals. Presently, Mali lacks the economic or healthcare resources to offer routine cervical cancer screening to women. Instead, midwives and physicians at a limited number of district health centers may offer screening with visual inspection after acetic acid or Lugol’s iodine (VIA.VILI) if requested. Treatment for cervical abnormalities (e.g., cryotherapy, electrocautery) can only be obtained at a district health center or regional hospital; hysterectomy for cervical cancer must be obtained at a regional hospital, and presently neither chemotherapy nor radiation are available for more advanced disease. All treatment costs must be borne by the woman and her family.
Cervical cancer is the most common cancer in Mali, with an age-adjusted incidence rate of 37.7 per 100,000 women, and it is the leading cause of cancer-related mortality (2). Rates of cervical cancer in Malian women are among the highest for western Africa, thus prevention of HR HPV infection and cervical cancer is of major public health importance for Malian women. Cervical cancer screening for Malian women is extremely limited and done on a passive basis. A comprehensive cervical cancer control program is not available to Malian women, despite their perennially high incidence rates. Identification of risk factors associated with HR HPV infection will help identify women at greatest risk of developing persistent HR HPV infection that may lead to cervical cancer. Characterization of the epidemiology of HPV infection will provide data for evaluating success of prevention and intervention efforts. Finally, unlike many African countries, Mali has relatively low prevalence of HIV, thus allowing us to characterize risk factors associated with HR HPV apart from the additional risk HIV confers. The purposes of the study were to: 1) evaluate the feasibility of study methods; 2) obtain data on sexual and reproductive history to correlate with HPV infection; 3) estimate the prevalence of HR HPV infection; and 4) identify correlates of high-risk HPV infection in Malian women.
Material and Methods
Study design
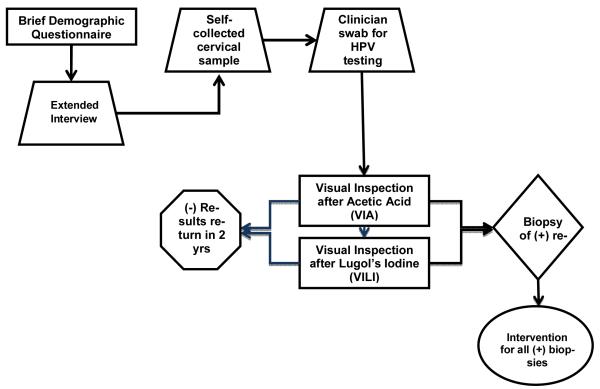
The study used a cross-sectional design and was conducted as an auxiliary study to a study to evaluate the accuracy of visual screening techniques (i.e., visual inspection after acetic acid [VIA] and visual inspection after Lugol’s iodine [VILI]) for cervical neoplasia in low resource settings. A description of the parent study was previously published (6). All women completed a standardized interview, clinical examination, and clinician collection of a cervical specimen (Figure 1 for overview of design and procedures). Women also received screening with VIA and VILI according to the parent protocol; biopsies were taken in all women with abnormal or suspicious findings of VIA/VILI, and appropriate treatment was offered based on biopsy results. Our protocol was approved by the local institutional review committee and the Institutional Review Board of the University of Maryland School of Medicine. Participants were offered the US dollar equivalent of $5.00 to defray transportation costs associated with participation.
Figure 1.
Study protocol flow diagram
Recruitment
An age-stratified random sample of women was selected from a census registry sampling frame. Women were selected to represent 5 age bands: 15-24, 25-34, 35-44, 45-54, and 55 and older. To be eligible for participation, women had to: 1) be age 15 or older; 2) be sexually active; 3) provide informed consent. Women were excluded from participation if they had: 1) history of conization for treatment of cervical cancer; 2) history of hysterectomy with removal of the cervix; or 3) history of ano-genital, breast, oral, esophagus, lung, bladder, liver, or cervical cancers.
Standardized interview
A standardized interview was used to obtain detailed information on socio-demographic, sexual, reproductive, and gynecologic histories. The interview was a modified version of a more extensive interview used by the International Agency for Research on Cancer (IARC) in an international multi-site study of the epidemiology of cervical cancer(7). All interviews were conducted by trained interviewers prior to clinical exam and specimen collection.
Self-collected cervical samples
Self-collected samples were obtained from the first 100 enrollees to evaluate feasibility and acceptability of this method of sampling. After completion of the interview, women were escorted individually to a private restroom where each was individually supervised by a trained research assistant on self-collection of a cervical sample according to procedures outlined by Gravitt et al.(8, 9).
Clinical examination and specimen collection
Clinical examinations and specimen collection were done according to standardized procedures (6, 10). Prior to examination and specimen collection, the clinician inspected perineal, vulvar, vaginal, and cervical regions for evidence of warts, ulcers, discharge, inflammation, or tenderness. Clinicians also noted presence/absence of female circumcision and circumcision type. The clinician then inserted an unlubricated vaginal speculum and examined the cervix with the aid of a focus lamp. Cervical cells were collected with a Digene female cervical swab collection kit according to standardized procedures (6). Swabs were then placed into a 5-ml vial containing 1 ml of specimen transport medium. Samples were stored at − 80 °C at the CVD-Mali microbiology laboratory until shipped for testing.
VIA/VILI screening
After clinician exam and collection of cervical cells, colposcopic-assisted VIA and VILI were performed. Punch biopsies were obtained for any cervical areas assessed as abnormal by colposcopy. Clinicians were blind to HPV test results at the time of VIA/VILI. Women who had biopsies taken were scheduled for a follow-up visit 2 weeks later to discuss biopsy results and treatment as needed (e.g., cyrotherapy, electrocautery, hysterectomy).
HPV DNA testing
HPV DNA testing was performed by the University of Maryland Medical Center Pathology Laboratory using Digene Hybrid Capture II (HC2; Digene, Rockville, MD) and following the manufacturer’s instructions. Each sample was determined to be positive/negative for one of the cancer causing types of HPV (i.e., HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). The HC2 is a nucleic acid hybridization assay with signal amplication that uses microplate chemiluminescent detection. Samples were considered positive if the RLU was greater than or equal to the mean RLU of the positive control value supplied by the HC2 kit; the specified positive threshold was 1.- pg/ml, corresponding to 5,000 or more viral copies. Personnel at the UMMC Pathology laboratory were blind to VIA/VILI test results. As primary clinical assessment and management of abnormalities was based on results of VIA/VILI screening according to the parent study protocol, results of HPV DNA testing were not provided to participants.
Procedures
By local custom, community meetings were held prior to recruitment to obtain consent from community leaders and to allow members of the community to ask questions about the purposes of the study and the procedures that would be take place during the study visit. Subsequently, community recruiters visited the homes of each of the randomly selected women to invite each to participate in the pilot study. If a woman verbally agreed to participate, she was provided with an appointment time to appear at the CVD-Mali research clinic. Women were scheduled in groups of 10 so that the purpose of the study and study procedures could be explained in group format prior to individual informed consent and enrollment. Women were then individually assigned to an interviewer who discussed the study in greater detail, answered any questions, and obtained informed consent in the privacy of an interview room. A printed consent form was read and/or translated by a trained investigator into the local language to the study participant and her signature or index print was obtained as indication of consent to participate.
Quality Control, Data Management, and Statistical methods
Participants were assigned a project ID to protect confidentiality. This number was used to identify samples and interview forms. Interview and clinical examination data were recorded on standardized, scannable data collection forms designed using the Teleform software system (Cardiff; Sunnyvale, CA). All study team members were oriented and trained by the first and last authors prior to the start of the study. Quality control review of all data collection instruments was performed by the first author at the time of data collection. HPV samples were tested according to defined good laboratory practices for HPV testing by the University of Maryland Medical System pathology laboratory. Laboratory technicians were blind to the results of VIA and VILI results. Data entry/capture was performed by the first author’s research staff. Data were stored in a password protected database.
Statistical analyses were performed by the first author using Stata 9.0 (Stata Corp., College Station, TX). Data consistency and completeness were checked before completion of planned analyses. Data were examined for normality and completeness. Univariate analyses were performed to evaluate group differences between women who were HR HPV positive (HR HPV+) and those who were negative (HR HPV-). Group differences were assessed using Student’s t tests for continuous variables and Chi-Square/Fisher’s exact test for categorical variables. Multivariate logistic regression analyses were used to determine the association between HR HPV status (positive v, negative) and potential risk factors. Statistical significance was inferred when p ≤ 0.05.
Results
Participants
Participants were 223 healthy, asymptomatic Malian women from the Djikoroni neighborhood in the capital city of Bamako, Mali. 177 women were recruited via random selection from a census registry and 46 women presented voluntarily at the research clinic on day 2 of data collection and expressed interest in participation. To foster positive relations with the local community of women and facilitate community engagement in support of this research, the 46 women who presented voluntarily were included in the study; these women were from the same geographic catchment area and did not differ from the randomly selected participants with respect to age, marital status, education, or proportion of HPV positivity.
Of the 223 women screened for participation, 4 refused enrollment following further explanation of study procedures and 9 completed the interview but refused completion the clinical examination and collection of cervical samples. An additional 8 samples were unusable due to sample handling problems related to transit; thus, our final analytic sample consisted of data for 202 women. The remainder of data reported herein applies to the 202 women who constituted the analytic sample.
Table 1 lists the socio-demographic and reproductive health characteristics of participants. Women ranged in age from 15 to 65. The majority of participants were married, and the median age of first marriage was 16 years; 42% were involved in polygamous marriage arrangements. The majority of women reported household incomes of less than 50,000 CFA (USD 101) per year. Mean age of sexual debut was 16 years (range 10-32), and the median number of sexual partners was 1 (range of 0-5). Only 29% of women reported using any form of contraception; the majority used either oral, implant, or injectable methods (85%) and/or condoms (26%). Median number of pregnancies among participants was 4 (range 0-14).
Table 1.
Characteristics of study participants
| Characteristic | Mean(SD) | HR HPV− Mean(SD) |
HR HPV+ Mean (SD) |
p val- ue |
|---|---|---|---|---|
|
| ||||
| Age: mean(sd) | 34.2(11.9) | 34.6 (11.9) | 31.2 (12.0) | 0.182 |
|
| ||||
| Age distribution | ||||
| • 15-24 | 51(23) | 33 | 22 | 0.713 |
| • 25-34 | 74(34) | 33 | 33 | |
| • 35-44 | 39(18) | 17 | 19 | |
| • 45-54 | 32(15) | 8 | 16 | |
| • 55-65 | 23(11) | 8 | 10 | |
|
| ||||
| Literacy rate | 29.2% | 29.2% | 33.3% | 0.678 |
|
| ||||
| School attendance | ||||
| • any school | 38% | 37% | 46% | 0.529 |
|
| ||||
| Marriage | ||||
| • Marital status (% married) | 69% | 91.4% | 74.2% | 0.020 |
| • Age of first marriage (median) | 16 yrs | 17.5(4.3) | 15.9 (8.1) | 0.201 |
| • Polygamous marriage (%) | 41% | 35 | 42% | 0.564 |
|
| ||||
| Household income below 50,000 cfa (USD = $100) |
59% | 40% | 47.6% | 0.503 |
|
| ||||
| Work outside the home (%) | 45.5% | 44.8% | 43.5% | 0.907 |
|
| ||||
| Age of sexual debut: mean(SD) | 16.1(2.47) | 13.1(3.0) | 14.9(1.9) | 0.609 |
|
| ||||
| Number of sexual partners (median) | 1 | 1 | 1 | |
|
| ||||
| Previous cervical cancer screening (%) | 2.73 | 3% | 0% | 0.490 |
|
| ||||
| Smoking (%) | <1 | 0 | 4.6% | 0.004 |
|
| ||||
| Condom use (%) | 26 | 10% | 25% | 0.037 |
|
| ||||
| Number of pregnancies | 4.7 (3.1) | 4.8(3.1) | 3.2(2.3) | 0.025 |
Knowledge of cervical cancer and previous participation in screening
Participation in previous cervical cancer screening was extremely low among study participants. Only 2.73% of participants reported a previous Pap smear. Knowledge of cervical cancer, Pap smears, and appropriate prevention methods was relatively low with only 14.5% of women having adequate understanding of the purpose of cervical cancer screening as assessed by trained interviewers.
VIA, VILI, and HR HPV DNA testing
VIA and VILI identified suspicious abnormalities suggestive of dysplasia in 2.5% (n=6) of participants. Women with a positive test results on VIA/VILI had colposcopically-directed biopsies collected according to the parent study’s protocol. Histopathological evaluation of biopsies revealed no evidence of cervical intraepithelial neoplasia (CIN) or cervical cancer in any of the VIA/VILI positive women. Among positive VIA/VILI cases, 1 woman was diagnosed with schistosomiasis (Schistosoma haematobium), 3 were diagnosed with benign cervical polyps, and 2 were diagnosed with non-specific cervicitis.
HR HPV was detected in 12% (95% CI: 7.4 to 16.4) of clinician collected samples and 14.1% (95% CI: 7.1 to 21.1st) of self-collected samples. Cohen’s Kappa was computed to quantify agreement between clinician and self-collected samples. Moderate agreement was observed between the two methods of sampling for identification of HR HPV (κ= 0.457, SE=0.102, CI: 0.187 to 0.727). The majority of infections occurred among women in the 15-24 year age range. There was no significant difference in HPV positivity across age groups (see Table 1).
Correlates of HPV infection
Table 2 provides results of simple and multivariable regression analyses performed to evaluate correlates of HR HPV infection. Compared to women who were married or widowed, single women were 3.5 times more likely to be infected with HR HPV. Number of pregnancies was protective. Factors unrelated to infection with HR HPV included age, number of lifetime partners, age at first marriage, and participation in a polygamous marital relationships. In a multivariable model that controlled for marital status, increasing numbers of pregnancies continued to be significant.
Table 2.
Results of Regression Analyses Comparing HR HPV+ and HR HPV− Women
| Characteristic | N | # HR HPV+ |
Crude Odds Ratio (95% CI) |
Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
| Marital status (sin- gle) |
201 | 24 | 3.5 (1.38 - 8.79) | 2.55* (.789 - 8.22) |
| # of pregnancies | 183 | 21 | .822 (.689 - .979) | .831† (.699 -- .990) |
| Age | 202 | 24 | .974 (.937 - 1.01) | -- |
| # of partners | 201 | 24 | 1.23 (.959 - 1.58) | -- |
| Age at first mar- riage |
176 | 18 | .936 (.847 - 1.03) | -- |
| Polygamous | 183 | 20 | .752 (.285 - 1.98) | -- |
Adjusted for number of pregnancies.
Adjusted for marital status
Discussion
This study, although preliminary in nature, provides important data regarding the epidemiology of high risk HPV infection in Mali. The prevalence of HR HPV infection was 12% in clinician collected specimens and 14% in the subset of women who provided both self- and clinician-collected samples. These prevalence estimates are consistent with what has been reported for other West African countries (6). Schiffman and Wacholder (3) recently noted that regional, age-specific HPV prevalence patterns are an important next step toward widespread implementation of screening with HPV testing in low resource settings. Our study is a foundational step toward characterizing these epidemiologic patterns for Mali.
While VIA/VILI appear to adequately identify cervical inflammatory processes, the lack of specificity limits enthusiasm for this strategy on a national scale for many countries. Recently Sankaranarayanan et al. (11) reported results of a randomized clinical trial of screening for cervical cancer involving more than 130,000 women in India and conclusively showed that a single screening visit for HPV testing dramatically reduced the incidence of advanced cervical cancer and cervical-cancer mortality within 8 years; reduction was far greater than a single conventional cytologic test or VIA. Future studies should focus on identifying logistically and economically feasible strategies that use rapid HPV tests to provide at least one lifetime screening. At present, cost-effective methods for HR HPV DNA detection in low resource settings are not yet available, although field trials of a rapid HR HPV DNA test kit (e.g, Care HPV) are promising (12).
Lack of association between HPV infection and age, number of lifetime partners, and age at first marriage is not consistent with reports in the extant literature. There are several possible explanations for this. First, only 33% of the Malian population of women are 25 years or older and HPV prevalence appears to be relatively flat across age groups. Second, the median number of sexual partners among sexually active Malian women was 1, thus unlike what is observed in countries like the United States, number of partners was not a compelling risk factor as few had more than one lifetime sexual partner and even fewer had more than 2 lifetime partners. Similarly, age at first marriage lacked explanatory power because the distribution for age of marriage was significantly skewed to younger ages. These contradictory findings highlight the importance of characterizing risk factors for HR HPV infection and cervical cancer that are regionally and culturally specific so that prevention efforts can be optimally targeted to risk factors that most salient for geographic and/or cultural groups of women.
The protective association noted between HPV positivity and number of pregnancies is also counter to previously published findings (13). Given that the women included in our study were urban residents with greater access to community-based health care, it is possible that gynecologic health was monitored during previous prenatal or antenatal encounters with the local health system. Our study did not expressly evaluate previous prenatal care, however, so this hypothesis is speculative and requires further investigation.
This study provides evidence that self-collected samples yield valid test results for identifying women infected with HR HPV and at risk of developing cervical cancer. In developing country settings such as Mali, self-collected samples offer a cost-effective method for screening large numbers of women. Further, based on qualitative interviews conducted by the first author, self-collection, in contrast to clinician-collected sampling, is preferred by local women, thus increasing the number of women who would be receptive to cervical cancer screening. A strategy that combines self-collected sampling and rapid HR HPV DNA testing combined with appropriate triage and evaluation may offer the most economically viable and sustainable option for screening in low resource settings such as Mali. Promising data have recently been published for the careHPV test(12), a rapid HPV screening test designed for use in low resource settings. CareHPV tests for 14 cancer causing strains of HPV and samples can be tested in almost any environment with minimal technical training. Further, test results are available within a matter of hours allowing women to be informed immediately of test results and recommendations for follow-up (e.g., repeated testing after a defined interval, screen-and-treat, watchful waiting). CareHPV has a reported sensitivity of 90% on cervical specimens and represents a vast improvement over VIA/VILI methods for identifying women at increased risk of developing cervical cancer. Given the logistical and economic challenges of implementing widespread cervical cancer screening in Mali, an approach that combines HPV vaccination for young girls prior to sexual debut with a targeted HR HPV screening with rapid HPV testing may offer the most effective strategy for reducing cervical cancer in Mali.
While this study provides important findings related to the acceptability of cervical self-sampling and the basic epidemiology of HR HPV infection in Malian women, it is not without limitations. Our cross-sectional design allows us to characterize HR HPV prevalence in Malian women and to explore correlates of prevalent infection. However, we are unable to characterize persistent infection or to explore correlates of persistent HR HPV infection in this population of women. Future studies should include repeated sampling to allow for characterization of HR HPV clearance rates and identification of factors associated with persistent infection in Malian women. This study also focused on women from the urban region of Bamako; therefore, epidemiologic patterns for rural areas may or may not be similar to what was observed in this study. Analyses are currently in progress for a second study that screened women from a rural village. Data from these two pilot studies are being analyzed to assess variations in epidemiologic patterns between urban and rural women in Mali. These data will be valuable for evolving plans by the Malian Ministry of Health to design and implement a cervical cancer prevention and control program in Mali.
Cervical cancer is a preventable disease that disproportionately affects women from developing countries like Mali. Although current models for HPV testing are cost prohibitive, rapid HPV testing is expected to be available soon and will ideally offer a safe and cost-effective HPV testing strategy for cervical cancer screening in the developing world. As innovations in cervical cancer screening and prevention continue to emerge, it is critical that the population be informed about strategies for cervical cancer prevention such as HPV vaccines and the importance of cervical cancer screening and how to access treatment. As indicated by our assessment of knowledge of cervical cancer, rates of participation in previous cervical cancer screening efforts were extremely low among women who participated in our study. Only 2.73% of participants reported a previous Pap smear. Knowledge of cervical cancer, Pap smears, and appropriate prevention methods was relatively low with only 14.5% of women having adequate understanding of the purpose of cervical cancer screening as assessed by trained interviewers. Regardless of the effectiveness of HPV vaccination and cervical cancer screening, these efforts will fail to reduce cervical cancer incidence and mortality if the population remains unaware of the methods or the value of these methods to prevent cervical cancer.
Acknowledgement
The work was supported in part by grants from the University of Maryland School of Medicine and the National Institutes of Health.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.GLOBOCAN . 2008, cancer incidence and mortality worldwide [Internet] International Agency for Research on Cancer (IARC); Lyon, France: 2010. Available from: http://globocan.iarc.fr/ [Google Scholar]
- 3.Schiffman M, Wacholder S. From india to the world--a better way to prevent cervical cancer. N Engl J Med. 2009 Apr 2;360(14):1453–5. doi: 10.1056/NEJMe0901167. [DOI] [PubMed] [Google Scholar]
- 4.The world factbook--mali [Internet] Central Intelligence Agency; Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/ml.html. [Google Scholar]
- 5.Franco LM, Diop FP, Burgert CR, Kelley AG, Makinen M, Simpara CH. Effects of mutual health organizations on use of priority health-care services in urban and rural mali: A case-control study. Bull World Health Organ. 2008 Nov;86(11):830–8. doi: 10.2471/BLT.08.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in india and africa. Int J Cancer. 2004 Jul 20;110(6):907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 7.Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: A pooled analysis. Lancet. 2005 Sep 17-23;366(9490):991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 8.Gravitt PE, Lacey JV, Jr, Brinton LA, Barnes WA, Kornegay JR, Greenberg MD, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001 Feb;10(2):95–100. [PubMed] [Google Scholar]
- 9.Safaeian M, Kiddugavu M, Gravitt PE, Ssekasanvu J, Murokora D, Sklar M, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in rakai, uganda. Sex Transm Dis. 2007 Jul;34(7):429–36. doi: 10.1097/01.olq.0000243623.67673.22. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79(10):954–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural india. N Engl J Med. 2009 Apr 2;360(14):1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 12.Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: A cross-sectional study of clinical accuracy in rural china. Lancet Oncol. 2008 Oct;9(10):929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 13.Bayo S, Bosch FX, de Sanjose S, Munoz N, Combita AL, Coursaget P, et al. Risk factors of invasive cervical cancer in mali. Int J Epidemiol. 2002 Feb;31(1):202–9. doi: 10.1093/ije/31.1.202. [DOI] [PubMed] [Google Scholar]