Abstract
Angelman syndrome (AS) is due to deficient ubiquitin protein ligase 3a, the gene for which (UBE3A) maps to chromosome 15q11–q13 and is imprinted such that only the maternally inherited gene is expressed. The paternally inherited UBE3A gene is silenced, a process mediated by an antisense transcript. We conducted a trial using methylation-promoting dietary supplements (betaine, Metafolin, creatine and vitamin B12) in an attempt to reduce antisense transcript production, increase UBE3A expression and ameliorate the symptoms of AS. Neuropsychological evaluations, biochemical testing, and assessment of DNA methylation were performed at the beginning and at the end of one year of supplementation. The primary outcome measures were changes in the level of developmental function (cognitive, motor, and language) as measured using standardized instruments. The secondary outcomes measures were changes in biochemical parameters and global DNA methylation. These data were compared to those of a control group from a previous randomized double-blind trial using folic acid and betaine. There were no statistically significant changes in the developmental performance of children treated with supplements. There were no unexpected changes in biochemical parameters and no change in site-specific DNA methylation when comparing samples from before and after treatment. There were 10 adverse events that resulted in study withdrawal of 7 participants (worsening of seizures, onset or worsening of sleep problems, constipation, and anorexia). Supplementation with betaine, Metafolin, creatine and vitamin B12 appears safe but ineffective in decreasing the severity of AS.
Keywords: Angelman syndrome, Clinical Trial, Methylation, Dietary Supplements
INTRODUCTION
Angelman syndrome (AS) is a neurodevelopmental disorder characterized by functionally severe intellectual disability, minimal or absent speech, seizures, ataxic gait, sleep disturbances, and a distinctive behavioral profile that includes happy demeanor, easily-provoked laughter, excitability, hypermotoric activity, fascination with water, and excessive mouthing behaviors [Williams and Frias, 1982; Williams et al., 1995a,b; Williams et al., 2006; Zori et al., 1992]. Treatment for AS is supportive and mainly consists of controlling seizures and employing rehabilitative therapies.
There are four known molecular mechanisms that lead to the deficiency of maternal UBE3A expression: deletion of 5–6 Mb in the AS critical region on maternal chromosome 15q11–q13 (in 70% of AS individuals), paternal uniparental disomy (UPD) for chromosome 15q11–q13 (3–5%), imprinting defects causing lack of maternal imprint and expression of UBE3A (3–5%), and loss-of-function mutations in the maternally-inherited copy of UBE3A (10%) [Lossie et al., 2001; Clayton-Smith and Laan, 2003].
AS is caused by a lack of functional UBE3A (ubiquitin protein ligase E3A) in the brain. In the mouse, Ube3a is expressed from both paternal and maternal chromosomes in most tissues except in neurons where only the maternally-inherited copy of the gene is expressed [Rougelle et al., 1997; Vu and Hoffman, 1997; Jiang et al., 1998a,b; Jiang et al., 1999; Kashiwagi et al., 2003; Dindot et al., 2008; Gustin et al., 2010]; the situation in humans is presumed to be analogous. Silencing of the paternally-inherited UBE3A is believed to be regulated by a non-coding antisense RNA, the UBE3A anti-sense transcript (UBE3A-ATS). Expression of UBE3A-ATS is controlled by one element of a bipartite imprinting center (IC), the Prader-Willi Syndrome-IC (PWS-IC) [Chamberlain and Lalande, 2010]. The maternally-inherited copy of PWS-IC is methylated, repressing the UBE3A-ATS and allowing UBE3A to be transcribed. The paternally-inherited copy of PWS-IC is unmethylated, which permits UBE3A-ATS expression, thereby repressing transcription of UBE3A.
Patients with AS due to UPD or imprinting defects exhibit a milder phenotype than those with AS due to deletion [Freeman et al., 1993; Saitoh et al., 1997; Lossie et al., 2001]. Two potential explanations, which are not mutually exclusive, have been offered to explain this observation: 1) haploinsufficiency for other genes at 15q11–q13 may contribute to a more severe phenotype in deletion patients and 2) silencing of the paternally-inherited UBE3A allele is incomplete, so patients with two copies of a paternally-imprinted allele have slightly more UBE3A expression that mitigates the phenotype. If the paternally-inherited UBE3A allele could be activated, even if only minimally, increased gene expression might reduce the severity of the AS phenotype. Thus, pharmacological or molecular strategies to increase UBE3A expression might be a viable treatment strategy. We hypothesized that by increasing global DNA methylation through dietary supplements, the paternally-inherited copy of PWS-IC would become methylated, thereby decreasing the amount of UBE3A anti-sense transcript and increasing UBE3A expression from the paternal allele.
In our previous double-blind placebo-controlled trial, we attempted this strategy using high-dose oral folic acid and betaine supplements to increase methyl donors available for DNA methylation, but the outcomes were not significantly different between the treatment and placebo groups [Peters et al., 2010]. However, there were trends suggesting that younger patients and those without a co-morbid diagnosis of autism were more likely to respond [Peters et al., 2010]. We therefore conducted a follow up trial with younger patients, restricting enrollment to those less than 6 years of age. Due to concerns that the accumulation of unmetabolized folic acid might be detrimental, we used 5-methyltetrahydrofolate (Metafolin), which is the reduced and active metabolite in the folic acid / methionine-homocysteine cycle, instead of folic acid. Two additional supplements were added to the treatment regimen, both designed to increase the availability of S-adenosylmethionine (SAM) for DNA methylation: creatine (because a large fraction of SAM is used to synthesize creatine), and Vitamin B12 (a co-factor for methionine synthase, the enzyme involved in the re-methylation of homocysteine to methionine in the methionine-homocysteine cycle that leads to the production of SAM).
MATERIALS AND METHODS
Study Design
This was a one-year non-randomized open-labeled prospective clinical trial in which all subjects received the same combination of study medications (ClinicalTrials.gov Identifier: NCT00348933). Participants were enrolled at one of four institutions (Baylor College of Medicine/Texas Children’s Hospital, Children’s Hospital Boston, Greenwood Genetic Center, Rady Children’s Hospital San Diego), all of which are members of the NIH Rare Diseases Clinical Research Network Angelman, Rett, and Prader-Willi Syndromes Consortium. Approval for the protection of human subjects was granted by a Data and Safety Monitoring Board convened by the NIH and by the Institutional Review Board at each participating site.
Participants who had received placebo in the previous double-blind placebo-controlled trial of folic acid and betaine [Peters et al., 2010] served as the control group for this treatment trial. The primary outcome measures were changes in the level of developmental function (cognitive, motor, and language) as measured by the standardized instruments described below. The secondary outcomes measures were changes in biochemical parameters and global DNA methylation.
Description of Participants
Subjects were recruited through parent support groups such as the Angelman Syndrome Foundation and the Angelman syndrome listserv, and referrals from professional colleagues. In addition, the study was registered on the public web site, http://ClinicalTrials.gov/, through which parents and healthcare professionals were able to contact the investigators. Participants between the ages of 1 day and 5 years with a molecularly confirmed diagnosis of AS were enrolled between July 2006 and March 2008. The exclusion criteria were: 1) extreme prematurity, 2) treatment with high dose folic acid or derivatives within the last 12 months, 3) uncontrolled seizures or other unstable medical problem, or 4) liver or kidney disease. Written informed consent was granted by the legal guardian of each participant. Four participants who had received placebo in the previously reported folate-betaine trial [Peters et al., 2010] also participated in the trial reported herein. Therefore, these four individuals are represented in both the control and treatment groups. These four individuals participated in the open-label year of the folate-betaine study (following their initial year as placebo-receiving individuals), but had been off of supplements for at least 1 year prior to enrolling in the trial reported herein.
Treatment Protocol
Participants received L-5-methyltetrahydrofolate (0.5 mg/kg/day divided into 2 doses, maximum 8 mg per day) (Metafolin, Source Naturals), creatine (200 mg/kg/day divided into 2 doses, maximum 5 g per day) (NutraBio, Nutrabiotics Research Labs, Inc., Middlesex, NJ, USA), betaine (100–200 mg/kg/day divided into 2 doses) (Cystadane®, Rare Disease Therapeutics, Inc., Franklin, TN, USA) and vitamin B12 (1 mg per day) (various generic manufacturers). This combination of supplements is hereafter referred to as the “methylation regimen.” Compliance was monitored by logs documenting doses given to each participant, which were submitted by the guardians.
Evaluations
Participants were evaluated at baseline and after 1 year of treatment (range: 11–15 months) with complete medical histories, physical and neurological examinations, formal developmental assessments, laboratory investigations, and EEG studies.
Psychometric Evaluations
Developmental and behavioral assessments were conducted by child psychologists (JG, LH, RB-W, AB-C, SW, SP) using standardized instruments: the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) [Bayley, 2005]; Preschool Language Scale, Fourth Edition (PLS-4) [Zimmerman et al., 2002]; and Vineland Adaptive Behavior Scales, Second Edition (VABS-II) [Sparrow et al., 2005].
The BSID-III [Bayley, 2005] is a developmental tool that is appropriate for individuals with a developmental age between birth-42 months, and assesses cognitive, language, and motor functioning based on a set of standardized tasks administered by a psychologist to the child. The PLS-4 [Zimmerman et al., 2002] assesses auditory comprehension (receptive language) and expressive communication (expressive language) in children up to a developmental age of 6 years 11 months. BSID-III and PLS-4 raw scores were converted to developmental age, and a developmental quotient (DQ) was calculated by dividing the developmental age by the chronological age and multiplying by 100. This controlled for chronological age as well as differences in the ages of participants with different molecular subtypes.
The VABS-II [Sparrow et al., 2005] evaluates a child’s ability to perform specific tasks in 4 domains as a measure of overall adaptive skills (Communication, Daily Living Skills, Socialization, and Motor Skills) based on a set of questions administered to the parents/caregivers by a psychologist in a semi-structured interview. The VABS-II has been validated in children and adults with intellectual disabilities.
Some children with AS merit a co-morbid diagnosis of autism, which may impact developmental abilities. We administered the Autism Diagnostic Observation Schedule, Module 1 (ADOS) [Lord et al., 2002] to all children enrolled in this trial. The ADOS is a semi-structured, standardized assessment of communication, social interaction, and play for use with individuals with possible autism spectrum disorders. The ADOS has been shown to be reliable and valid for distinguishing children with only cognitive and language delays from those with autism spectrum disorders. The Autism Diagnostic Interview – Revised (ADI-R) [Lord et al., 1998] was administered to the parent of any participant who exceeded the cut-off on the ADOS for autism or autistic spectrum disorder.
Laboratory Evaluations
Laboratory investigations were performed at baseline and at the end of 1 year of treatment (range 11–15 months). Complete blood count (CBC), red blood cell (RBC) folate, blood urea nitrogen (BUN), creatinine, alanine transaminase (ALT), aspartate transaminase (AST) and urinalysis were performed at the CLIA-approved clinical laboratories at individual study sites using standard techniques while plasma homocysteine (Hcy), methionine, betaine (trimethylglycine), dimethylglycine (DMG, a by-product of betaine metabolism), creatine, and guanidinoacetate (GAA) were analyzed at a central facility (Baylor College of Medicine) using a Quattro Micro tandem mass spectrometer (Waters Corporation, Milford, MA). Creatine and GAA were measured to monitor the potential shuttling of methyl groups to the synthesis of creatine, normally driven by the conversion of GAA to creatine. Quantitative determinations were made using selected mass transitions with the addition of appropriate stable isotopes when possible.
Changes in DNA methylation after 1 year of treatment were analyzed in samples from 16 participants using the Infinium HumanMethylation27 BeadChip array which examines methylation at 27,578 CpG dinucleotide sites in the genome. DNA was extracted from whole blood samples using standard techniques. Of the 16 individuals studied, nine were male and seven were female. Average beta values, which reflect the level of methylation for the individual CpG sites, were compared between samples taken before and after treatment; CpG sites with a differential score of greater than +13 or less than −13 were considered significantly hypermethylated or hypomethylated respectively.
Data Analysis
Participants receiving the methylation regimen were evaluated using the BSID-III, VABS-II, and PLS-4, whereas participants in the control group from the prior treatment trial using folic acid and betaine were evaluated with previous editions of these tools (BSID-II, VABS, PLS-3). Since the scales are not identical in the two different editions of the same assessment tool (e.g. BSID-II and BSID-III), we could not compare the raw scores, or changes in the raw scores, between the placebo and the treatment groups. Therefore, we compared the standard scores for each of the domains of the VABS (Communication, Daily Living Skills, Socialization, Motor Skills) since standard scores are normalized and therefore comparable between different editions of the VABS. For the PLS, we compared the difference in DQs in the Auditory Comprehension and Expressive Communication domains. For the BSID, standard scores could not be used in the analyses because nearly all of our participants scored at the floor of the test resulting in a lack of variability in the standard scores in this population; hence DQs were used instead. The domains in BSID-II and BSID-III are not identical, but the BSID-II Mental Developmental Index (MDI) is a measure of both cognitive and language skills (based on correlations between editions), while the Psychomotor Developmental Index (PDI) is a measure of motor skills [Bayley, 2005]. Thus, we computed a “MDI DQ” for each subject in the current trial by calculating the mean of the cognitive, receptive language and expressive language DQs, and a “PDI DQ” by taking the mean of the gross motor and fine motor DQs.
It has been shown that developmental quotients (DQ) can be used to monitor the developmental progress of intellectually disabled children against their chronological ages and to compare their levels of functioning in different developmental domains [Dykens et al., 1994]. Therefore, we consider the use of DQs as part of our primary outcome measures as valid.
Statistical Design
The primary outcome variables were differences between the treatment cohort and the control group in: 1) changes in either the standard or DQ scores for each developmental domain at the end of the treatment period relative to baseline scores; and 2) changes in biochemical parameters at the end of the treatment period relative to baseline scores. The Student t-test was used to compare these differences between the treatment and control groups. Bonferonni correction for multiple comparisons was applied to the p values within each group of closely-related variables such as the psychometric testing results (Table II) and biochemical analyte results (Table III) to maintain an overall level of statistical significance (type 1 error, α) at 0.05. The differences in the ages of participants between the treatment and control groups were compared using the Mann-Whitney test due to the skewing of the age distribution within these groups.
Table II. Psychometric testing results.
VABS, BSID, and PLS scores (mean±SD) in both placebo and treatment groups at baseline and at the end of the study, and the differences in the magnitude of the changes within each group (expressed as mean and 95% confidence intervals in parentheses).
| Placebo group | Methylation regimen treatment group | Placebo vs. treatment | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Final | Change from Baseline | Baseline | Final | Change from Baseline | Difference in Change from Baseline | |
| VABS Communication | 61.3±11.5 | 54.6±8.4 | −6.6±4.5 | 60.0±10.9 | 54.2±10.8 | −5.8±8.3 | 0.84 (−1.98, 3.66) |
| VABS Daily Living Skills | 60.2±14.5 | 50.8±12.9 | −9.5±5.9 | 62.1±10.8 | 58.4±10.3 | −3.7±7.9 | 5.72 (2.51, 8.92) § |
| VABS Socialization | 66.5±11.4 | 61.5 8.5 | −5.0±6.6 | 70.2±8.8 | 65.5±8.0 | −4.7±5.2 | 0.34 (−2.84, 3.52) |
| VABS Motor Skills | 54.4±15.1 | 45.8±13.4 | −8.6±6.0 | 59.5±8.4 | 57.4±7.5 | −2.0±6.1 | 6.54 (3.55, 9.54) § |
| Bayley MDI DQ | 31.5±14.0 | 26.5±10.8 | −5.1±6.0 | 33.8±14.4 | 28.1±11.7 | −5.7±7.8 | −0.6 (−3.81, 2.62) |
| Bayley PDI DQ | 33.8±13.5 | 27.6±10.9 | −6.2±6.3 | 34.8±12.7 | 30.2±11.7 | −4.7±8.6 | 1.49 (−1.98, 4.95) |
| PLS Auditory | 25.4±14.6 | 20.8±9.8 | −4.5±11.1 | 28.9±13 | 26.9±14.8 | −2±10.8 | 2.56 (−2.96, 8.08) |
| PLS Expressive | 18.3±11.9 | 15.1±8.8 | −3.2±8.5 | 29.5±16.3 | 24±10.9 | −5.5±9.6 | −2.29 (−6.7, 2.11) |
p value <0.001
Table III. Biochemical Analyte Results.
values (mean±SD) in both placebo and treatment groups at baseline and at study completion, and the differences in the magnitude of the changes within each group (expresed as mean and 95% confidence intervals in brackets).
| Placebo group | Methylation regimen treatment group | Placebo vs. treatment | |||||
|---|---|---|---|---|---|---|---|
| Analyte* | Baseline | Final | Change from Baseline | Baseline | Final | Change from Baseline | Difference in Change from Baseline |
| BUN | 13.8±3.4 | 13.9±2.7 | 0.5±3.4 | 15.1±4.4 | 16.6±4.6 | 1.5±5.3 | 1.07 (−0.94, 3.08) |
| Creatinine | 0.34±0.06 | 0.35±0.07 | 0.02±0.1 | 0.36±0.08 | 0.37±0.10 | 0.03±0.1 | 0.01 (−0.04, 0.06) |
| Betaine | 49.7±39.0 | 33.6±19.0 | −18.1±36.3 | 24.8±22.4 | 231.6±226.6 | 206.9±224.5 | 224.9 (165.8, 284.1)§ |
| Creatine | 60.3±30.8 | 48.0±15.0 | −13.4±32.7 | 54.0±19.6 | 137.5±169.0 | 83.3±165.9 | 96.7 (52.9, 140.5)§ |
| DMG | 9.2±5.1 | 9.0±6.1 | −0.4±0.73 | 9.3±8.0 | 102.0±127.6 | 93.7±129 | 94.1 (61.2, 126.9)§ |
| GAA | 1.13±0.82 | 1.03±0.42 | −0.14±0.73 | 0.61±0.26 | 0.51±0.31 | −0.11±0.34 | 0.03 (−0.32, 0.38) |
| Hcy | 5.6±2.6 | 3.9±1.7 | −1.8±3.0 | 4.7±1.8 | 2.5±1.4 | −2.3±2.1 | −0.45 (−1.93, 1.03) |
| Met | 20.0±8.5 | 19.3±6.8 | −1.0±10.7 | 19.8±5.2 | 25.3±11.5 | 5.5±12.1 | 6.54 (0.78, 12.3) |
| RBC Folate | 659±257 | 731±194 | 54±298 | 710± 168 | 788±207 | 77±267 | 23.5 (−145.9, 192.8) |
Normal ranges for BUN and creatinine (mg/dL) are age adjusted. The normal values for the biochemical analytes studied were as follows: creatine 30–120 mmol/L, GAA 0.4–4.0 mmol/L, methionine 9–45 mmol/L, total plasma Hcy 4–14 mmol/L, RBC folate 280–903 ng/ml. No standardized values for normal ranges exist for betaine and DMG (values expressed in millimoles per liter).
p value <0.0001
RESULTS
Participant characteristics
We enrolled a total of 90 participants, 65 of whom (30 females and 35 males) completed the study. The remaining 25 failed to complete the study because of non-compliance with treatment regimen (n=2), lost to follow-up (n=6), adverse events (n=7, detailed in Table I), social or financial reasons that prevented continuance (n=9), or illness that prevented continuance (n=1). Study completers ranged in age from 5 months to 64 months at time of enrollment (mean±SD: 34.9±14.7 months) and from 17 months to 76 months (mean±SD: 47.5±14.8 months) at the end of the study. Among the 65 completers, 48 had deletions (age range: 11–59 months at entry), 7 had UBE3A mutations (age range: 5–61 months at entry), and 10 had either UPD or an imprinting defect (age range: 28–64 months at entry).
Table I. Adverse events.
| Events resulting in study withdrawal* | # of events | related to treatment? |
|---|---|---|
| Seizures - new or significantly worse | 1 | possibly |
| Sleep problems - new or significantly worse | 5 | probably not (1), possibly (2), probably (1), definitely (1) |
| Constipation | 2 | probably not (1), possibly (1) |
| Anorexia/loss of appetite | 2 | Possibly (2) |
| Events not resulting in study withdrawal | # of events | related to treatment? |
|---|---|---|
| Seizures - new or significantly worse | 17 | probably not (15), possibly (2) |
| Sleep problems - new or significantly worse | 9 | probably not (2), possibly (6), probably (1) |
| Increased appetite | 1 | possibly |
| Behavior disturbance - new or significantly worse | 2 | probably not (1), possibly (1) |
| Laboratory abnormalities @ 6 months | 3 | probably not (1), possibly (1), probably (1) |
| Body odor | 1 | definitely |
| Events probably or definitely not related to treatment, and not resulting in study withdrawal | # of events | related to treatment? |
|---|---|---|
| Infection | 7 | definitely not (1), probably not (6) |
| Serum sickness | 1 | probably not |
| Adult Respiratory Distress Syndrome | 1 | probably not |
| Loose stools/diarrhea | 1 | probably not |
total # subjects = 7, some reported more than one adverse effect
There were a total of 53 adverse events affecting 43 participants, 31 of which were judged to be either definitely or probably not related to the treatment (Table I). Of the 22 events that were possibly, probably, or definitely related to treatment, 8 resulted in the withdrawal of seven participants from the study (1 for new-onset or significant worsening of seizures, 4 for new-onset or significant worsening of sleep problems, 1 for constipation, and 2 for anorexia).
Twenty-two participants from the previous folate-betaine trial who had received placebo were in the comparable age range to serve as controls for the methylation regimen study completers. The placebo group consisted of 6 females and 16 males, and ranged in age from 15 months to 59 months (mean±SD: 30.5±14.4 months) at time of enrollment and 26 months to 73 months (mean±SD: 42.6±14.6) at the end of the study. Of these 22 participants in the placebo group, 17 had deletions (age range 15–59 months at entry), 1 had a UBE3A mutation (23 months at entry), and 4 had either UPD or an imprinting defect (age range 26–56 months at entry).
There was no significant difference in the age of the methylation regimen group and the placebo group at enrollment (p=0.18) and at the end of the study (p=0.15). Within the methylation regimen group, participants with deletions were younger than the non-deletion participants at study entry (p=0.031) and study completion (p=0.037). Within the placebo group, there were no differences in the ages between the deletion and non-deletion participants both at study entry (p=0.27) and study completion (p=0.31).
Autism testing was accomplished on 64 participants in the methylation regimen group, 29 of whom were assigned as having an autism spectrum disorder. Autism testing was accomplished in 20 participants in the placebo group, 7 of whom scored in the autistic range. There were no significant differences in the proportion of children who merited a co-morbid diagnosis of autism spectrum disorder between the methylation regimen group and the placebo group (Fisher’s exact test: p=0.45).
Psychometric Investigations
Table II shows the mean ± standard deviations of the VABS-II standard scores, BSID-III DQs and PLS-4 DQs at baseline and at the final evaluation for the placebo and the methylation regimen groups, as well as the changes in the scores from baseline to the end of the study for each group. The differences in these changes between the placebo and the treatment groups are expressed as mean with 95% confidence intervals.
There were no differences in the baselines scores between the two groups. All the scores were lower at the end of the study than they were at baseline because the participants developed more slowly than their age-matched peers during this time. There were no differences between the placebo and the methylation regimen groups in the magnitude of these changes on the BSID-III and PLS-4, but on the VABS-II scale, the treatment group fell less far behind their age-matched non-AS peers than the placebo group did in the Daily Living Skills (self-help skills) and Motor Skills domains.
Analyses of these developmental measures against the Communication and Social Interaction raw scores on the ADOS by linear, cubic, and quadratic regression modeling did not identify any significant correlation (R2 less than 0.3 for all variables analyzed). This suggests that these developmental outcome measures are not influenced by the presence or severity of autistic features in our study population, a finding supported in Peters et al., [in press].
Laboratory Investigations
AST and ALT were not measured in the placebo group because these investigations were not part of the previous folic acid-betaine study from which this placebo group was drawn. In the methylation regimen group, there were no clinically significant changes in the mean AST and ALT values at the end of the study compared to the baseline values (data not shown).
Table III shows the mean values for the biochemical analytes at baseline and after 1 year of supplementation for participants in the placebo and methylation regimen groups. There were no significant differences in baseline BUN or creatinine values among participants on placebo compared to those on the methylation regimen, nor were there any significant changes in these values between the baseline and the final measurements.
As expected, after 1 year of supplementation, the treatment group had significantly higher betaine, creatine and DMG levels (p<0.0001). The difference between placebo and treatment groups in their changes in methionine levels was of borderline significance (p=0.027). There were no differences in the hematologic parameters (white blood cell count, red blood cell count, hemoglobin concentration, hematocrit, platelet count) (data not shown).
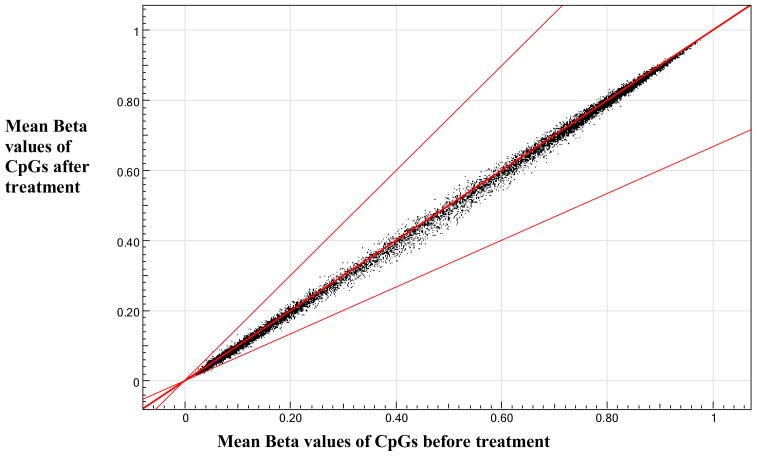
Figure 1 shows the pooled methylation data from a subset (n=16) of treated participants. We observed no significant changes in methylation at the 27,578 CpG sites tested by the assay when comparing pooled pre-treatment data with pooled post-treatment data. Four individuals showed significant changes in the methylation (a differential score greater than +13 or less than −13) at a few CpG sites, but neither the sites nor the direction of change (i.e. hyper- vs. hypomethylation) were consistent among other individuals, suggesting that treatment with the methylation regimen did not result in any specific changes in methylation levels of the CpG dinucleotides represented in the array.
Figure 1. Pooled Methylation Data.
Pooled methylation data from 16 subjects. Samples taken before and after 1 year of treatment with methylation regimen exhibited no difference in methylation.
Bold center line indicates no change in methylation comparing samples taken after treatment with samples taken before treatment. Lower line indicates two-fold decrease and upper line indicates two-fold increase in methylation comparing samples after treatment with before treatment.
DISCUSSION
AS is a neurodevelopmental disorder with severe intellectual disability and lack of speech for which there is no treatment other than standard rehabilitative therapies. We employed a regimen of dietary supplements intended to promote DNA methylation in the hopes of altering UBE3A gene expression and ameliorating the AS phenotype.
This study found no statistically significant changes in cognitive or language abilities in those who received the methylation regimen compared with those who received placebo. The treatment group demonstrated a modest benefit in daily living (self-help) skills and motor skills as evidenced by scores on the VABS that fell less far behind age-matched peers compared to those in the placebo group. However, the motor component of the BSID showed no differences. Since the BSID relies on observed skills and the VABS on caretaker report, the apparent inconsistency may be explained by differences in objective and subjective reporting. Many of the daily living skills are predicated on motor abilities, so improvements in this area are likely to be related to improvements in motor skills. The improvement in motor abilities may be related to creatine administration, as many parents reported a subjective impression of increased muscle tone and strength in children on the methylation regimen.
The only changes in biochemical parameters between those who received placebo and those who received the methylation regimen were those consistent with betaine and creatine supplementation. We think that compliance with the methylation regimen was good, in view of the positive changes in average levels of betaine, DMG, and creatine. Although in a handful of participants, one of the biochemical parameters failed to show the expected magnitude or direction of change, no individual exhibited a pattern for all analytes that suggested non-compliance. We do not feel that lack of response to the methylation regimen is due to non-compliance.
This study found no consistent changes in global DNA methylation when comparing pre-treatment to post-treatment samples. We do not have an explanation for why methylation was not different, but there are at least 2 possible interpretations: 1) methylation cannot be influenced by the dietary administration of pro-methylation agents or 2) DNA methylation cannot be influenced beyond fetal life or early infancy. Since we were unable to change global DNA methylation levels, the lack of developmental improvement in our study participants is not surprising, given our hypothesis.
The strengths of this study are 1) having a biological correlate (DNA methylation) for the proposed mechanism of action and 2) assessing the participants using standardized instruments administered by child psychologists who have expertise with AS. One weakness is that the version of these instruments changed between the folic acid-betaine study and the present study, which made direct comparisons impossible. However, we believe that the methods we used to address this issue, and hence our results are valid.
A significant weakness of this study is the lack of concomitant controls to control for unrecognized factors that may have influenced the outcome of this trial. Trials using historical controls are widely regarded as less preferable than randomized controlled trials. However, in comparing studies using historical controls with studies using randomized controls, Sacks and colleagues [1982] determined that studies using historical controls rarely come to false negative conclusions but frequently report false positive results (i.e. historical controlled studies overestimate effectiveness of treatment). The study reported herein, using historical controls, reports negative results; given the findings of Sacks et al. [1982], they are likely to be reliable.
The methylation regimen was generally well tolerated with few unexpected or adverse effects on clinical or biochemical parameters, and no obvious short-term harm from supplementation with vitamin B12, 5-methyltetrahydrofolate, betaine and creatine. The most common adverse effects reported, seizures and sleep disturbances, are integral components of AS, so in most cases, these symptoms were adjudicated to be unrelated to treatment. We were unable to determine the safety of long-term administration of this regimen in this one-year study. This study, in conjunction with the previous trial, suggests that it may not be possible to alter DNA methylation in humans through dietary supplementation. Given the general lack of benefit in most areas of development, the authors cannot endorse the use of this methylation regimen as a treatment for AS.
Acknowledgments
This study would not have been possible without the AS participants and their parents’ and guardians’ interest and devotion to this long-term study. We also express our appreciation to the General Clinical Research Centers (GCRC) at Children’s Hospital Boston and Texas Children’s Hospital for their support. The authors specifically thank: the study coordinators/nurses for their invaluable assistance – Beverly M. Feldman (Baylor College of Medicine), Janette Z. Lawrence (Children’s Hospital Boston), Fran Annese and Joy Graham (Greenwood Genetic Center), Marla Hashiguchi (Rady Children’s Hospital San Diego); the clinical research staff at the Data Management Coordinating Center (DMCC) (Jennifer Pilger, Rachel Richesson, June T. Tran, Hye-Seung Lee) for their technical support; and Alan K. Percy (University of Alabama at Birmingham) and Mary Lou Oster-Granite (National Institute of Child Health and Human Development) for their leadership and support.
The Angelman, Rett and Prader-Willi syndromes consortium is a part of NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided in part by Grant Number NIH U54 RR019478 (awarded to ALB) from the National Center for Research Resources (NCRR) and Grant Number NIH U54HD061222 (awarded to Alan K Percy, MD) from National Institute of Child Health and Human Development, components of the National Institutes of Health (NIH); the NIH Office of Rare Diseases Research (ORDR); and the Angelman Syndrome Foundation – Western Area Chapter. Manuscript preparation, review, and approval were solely that of the authors. The views expressed herein do not represent the official views of NCRR, ORDR, NICHD, NIH, or the Angelman Syndrome Foundation; reflect the official policies of the Department of Health and Human Services; or imply endorsement of trade names, commercial practices, or organizations by the U.S. Government.
References
- Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Harcourt Assessment, Inc; 2005. (Bayley-III) [Google Scholar]
- Chamberlain SJ, Lalande M. Angelman syndrome, a genomic imprinting disorder of the brain. J Neurosci. 2010;30(30):9958–63. doi: 10.1523/JNEUROSCI.1728-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17(1):111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Evans DW. Profiles and development of adaptive behavior in children with Down syndrome. Am J Ment Retard. 1994;98:580–587. [PubMed] [Google Scholar]
- Freeman SB, May KM, Pettay D, Fernhoff PM, Hassold TJ. Paternal uniparental disomy in a child with a balanced 15;15 translocation and Angelman syndrome. Am J Med Genet. 1993;45(5):625–630. doi: 10.1002/ajmg.1320450522. [DOI] [PubMed] [Google Scholar]
- Gustin RM, Bichell TJ, Bubser M, Daily J, Filonova I, Mrelashvili D, Deutch AY, Colbran RJ, Weeber EJ, Haas KF. Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol Dis. 2010;39(3):283–291. doi: 10.1016/j.nbd.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lev-Lehman E, Bressler J, Tsai TF, Beaudet AL. Genetics of Angelman syndrome. Am J Hum Genet. 1999;65(1):1–6. doi: 10.1086/302473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Tsai TF, Bressler J, Beaudet AL. Imprinting in Angelman and Prader-Willi syndromes. Curr Opin Genet Dev. 1998a;8(3):334–342. doi: 10.1016/s0959-437x(98)80091-9. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998b;21(4):799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, Meguro M, Hoshiya H, Haruta M, Ishino F, Shibahara T, Oshimura M. Predominant maternal expression of the mouse Atp10c in hippocampus and olfactory bulb. J Hum Genet. 2003;48:194–198. doi: 10.1007/s10038-003-0009-3. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview – Revised. Los Angeles: Western Psychological Services; 1998. [Google Scholar]
- Lossie AC, Whitney MM, Amidon D, Dong HJ, Chen P, Theriaque D, Hutson A, Nicholls RD, Zori RT, Williams CA, Driscoll DJ. Distinct phenotypes distinguish the molecular classes of Angelman syndrome. J Med Genet. 2001;38(12):834–845. doi: 10.1136/jmg.38.12.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Bird LM, Kimonis V, Glaze DG, Shinawi LM, Bichell TJ, Barbieri-Welge R, Nespeca M, Anselm I, Waisbren S, Sanborn E, Sun Q, O’Brien WE, Beaudet AL, Bacino CA. Double-blind therapeutic trial in Angelman syndrome using betaine and folic acid. Am J Med Genet A. 2010;152:1994–2001. doi: 10.1002/ajmg.a.33509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Horowitz L, Barbieri-Welge R, Taylor JL, Hundley RJ. Longitudinal follow-up of autism spectrum features and sensory behaviors in Angelman syndrome by deletion class. J Child Psychol Psychiatry. 2011 doi: 10.1111/j.1469-7610.2011.02455.x. doi: 10.1111/j.1469-7610.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- Rougeulle C, Glatt H, Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat Genet. 1997;17(1):14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- Sacks H, Chalmers TC, Smith H., Jr Randomized versus historical controls for clinical trials. Am J Med. 1982;72(2):233–240. doi: 10.1016/0002-9343(82)90815-4. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Buiting K, Cassidy SB, Conroy JM, Driscoll DJ, Gabriel JM, Gillessen-Kaesbach G, Glenn CC, Greenswag LR, Horsthemke B, Kondo I, Kuwajimi K, Niikawa N, Rogan PK, Schwartz S, Seip J, Williams CA, Nicholls RD. Clinical spectrum and molecular diagnosis of Angelman and Prader-Willi syndrome patients with an imprinting mutation. Am J Med Genet. 1997;68(2):195–206. [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Upper Saddle River, NJ: Pearson Education, Inc; 2005. (Vineland-II) [Google Scholar]
- Vu TH, Hoffman AR. Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nat Genet. 1997;17(1):12–13. doi: 10.1038/ng0997-12. [DOI] [PubMed] [Google Scholar]
- Williams CA, Angelman H, Clayton-Smith J, Driscoll DJ, Hendrickson JE, Knoll JH, Magenis RE, Schinzel A, Wagstaff J, Whidden EM, Zori RT. Angelman syndrome: consensus for diagnostic criteria. Angelman Syndrome Foundation. Am J Med Genet. 1995a;56(2):237–238. doi: 10.1002/ajmg.1320560224. [DOI] [PubMed] [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J, Knoll JH, Kyllerman M, Laan LA, Magenis RE, Moncla A, Schinzel AA, Summers JA, Wagstaff J. Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A. 2006;140(5):413–418. doi: 10.1002/ajmg.a.31074. [DOI] [PubMed] [Google Scholar]
- Williams CA, Frias JL. The Angelman (“happy puppet”) syndrome. Am J Med Genet. 1982;11(4):453–460. doi: 10.1002/ajmg.1320110411. [DOI] [PubMed] [Google Scholar]
- Williams CA, Zori RT, Hendrickson J, Stalker H, Marum T, Whidden E, Driscoll DJ. Angelman syndrome. Curr Probl Pediatr. 1995b;25(7):216–231. doi: 10.1016/s0045-9380(06)80036-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale. 4. NC Pearson; San Antonio, TX: 2002. [Google Scholar]
- Zori RT, Hendrickson J, Woolven S, Whidden EM, Gray B, Williams CA. Angelman syndrome: clinical profile. J Child Neurol. 1992;7(3):270–280. doi: 10.1177/088307389200700307. [DOI] [PubMed] [Google Scholar]